Abstract
Conditions, where the patient's immune system is compromised are the main risk factor for mucormycosis. Approximately 23% of the world's population is estimated to have a latent Mycobacterium tuberculosis infection and more than 10 million new cases were estimated in 2017. Pulmonary mucormycosis and tuberculosis co-infections are very rare. We present the case of a 56-year-old insulin-dependent diabetic patient with a pulmonary mucormycosis and tuberculosis co-infection. While the patient did not suffer from ketoacidosis, she had poor glycemic control. A chest X-ray and a computed tomography showed nodular and cavitary lesions in both lungs. The patient was diagnosed through a biopsy of the bronchial mucosa and an RT-PCR for M. tuberculosis from bronchoalveolar lavage. The patient was treated with the recommended 4-drug regimen for TB (i.e. isoniazid, rifampin, pyrazinamide, and ethambutol); concurrently, amphotericin B deoxycholate was administered to treat the mucormycosis infection. Thirty days after initial hospital admission the patient underwent a lobectomy on the right lung. The case described here is only the sixth case reported in the literature of concomitant pulmonary tuberculosis and mucormycosis and the third case associated with a TB and mucormycosis co-infection involving an uncontrolled DM patient to survive.
Keywords: Diabetes, Immunosuppression, Pulmonary mucormycosis, Tuberculosis
1. Introduction
Immunosuppressed patients are at high risk of opportunistic infections. Mucormycosis is a group of diseases caused by infection of angiotrophic fungi in the order Mucorales [1]. Most mucormycoses are life-threatening, representing a medical emergency and usually involve immunocompromised patients [1], [2]. The estimated prevalence of mucormycoses among hospital discharges is 0.12 per 10,000 [3]. Mucormycosis can be classified based on anatomical localization: (1) rhinocerebral (i.e. rhino-orbito-cerebral); (2) pulmonary; (3) cutaneous; (4) gastrointestinal; (5) disseminated; and (6) uncommon presentations (e.g. endocarditis, mediastinitis, peritonitis, osteomyelitis, and renal abscesses) [1]. Conditions, where the patient's immune system is compromised are the main risk factor for mucormycosis. Among these risk factors are: (1) hematologic neoplasms; (2) neutropenia; (3) uncontrolled diabetes mellitus (DM), especially with ketoacidosis; (4) body trauma and wound contamination; (5) glucocorticoid use; (6) intravenous (IV) drugs; (7) iron overload; and (8) extreme malnutrition [2], [4]. Immunocompromised patients are also at risk of developing tuberculosis (TB). About 1.7 billion people (i.e. 23% of the world's population) are estimated to have a latent Mycobacterium tuberculosis infection and more than 10 million new cases were estimated in 2017 [5]. The clinical picture associated with pulmonary (i.e. endobronchial) TB includes thoracic pain, cough, fever, weight loss, hemoptysis, and dyspnea [6]. The diagnosis of pulmonary TB is based on the combination of clinical suspicion, clinical findings, imaging studies, and analysis of tissue and secretions [7].
The case of a patient with pulmonary TB and mucormycosis is reported here. The immunocompromised patient (i.e. long evolution and uncontrolled DM) was screened for autoimmune diseases and human immunodeficiency viruses (HIV) with negative results. After a bronchoscopy with bronchoalveolar lavage the histopathological diagnosis of mucormycosis and a reverse transcription polymerase chain reaction (RT-PCR) for M. tuberculosis from the bronchial secretions, the patient was diagnosed with pulmonary tuberculosis and mucormycosis. After medical treatment and lobectomy, the patient was discharged due to clinical improvement and consecutive negative bacilloscopies with directly observed therapy (DOT). Concomitant pulmonary mucormycosis and TB is very rare and has a poor prognosis. An opportune diagnosis and treatment are necessary in order to reduce the mortality of concomitant pulmonary mucormycosis and TB in immunocompromised patients.
2. Case presentation
A 56-year-old female arrived at the Emergency Department after complaining of localized pleuritic chest pain with an intensity of 7/10 on the visual analog scale for pain (VAS), that did not improve with the use of nonsteroidal anti-inflammatory drugs during the past 30 days. The patient reported profuse diaphoresis, dry cough, and dyspnea with mild to minimal activity for the previous three months to her hospital admission. During this time the patient experienced a 10 kg weight-loss and the patient noticed hemoptysis on several occasions, as well as fever reaching 39 °C and diaphoresis with predominance during the evening, improving with antipyretics. The patient previously attended another hospital, where she was managed with intramuscular (IM) ceftriaxone 1 g every (quaque, q) 12 h for four days without improvement. The patient's family history included a mother with type 2 diabetes (DM2) and arterial hypertension; other relevant aspects of family history were questioned and denied. The patient reported being diagnosed with DM2 for 15 years, currently treated with metformin 850 mg orally (per os, PO) q12 h, as well as 15 units of insulin glargine subcutaneously every night. The patient did not have optimal glycemic control, having two elevated hemoglobin A1c tests (i.e. 7.8 and 8.0%) within the previous 6 months. Among the patient's personal history, she denied tobacco or controlled substance use, allergies, past blood transfusions, traveling to regions with endemic diseases within the last three months, tattoos and body piercings. The patient had no history of lung disease or asthma during childhood.
Upon initial physical examination, we found a patient recumbent with freely chosen body position, Glasgow coma score of 15, without focal neurologic deficits nor meningeal sings, aware of his environment, with reference to place, time, and people. The patient's integumentary system was diaphoretic with skin and mucosal membranes dehydrated +/+++, while the head and neck exploration had no alterations. Upon inspection, the respiratory apparatus with oral ventilation, tachypnea with thoracic and abdominal dissociation. The thorax had decreased expansion without vibrations or fremitus during palpation. No asymmetries or abnormal findings in tone intensity, pitch, duration, and quality through direct percussion. Upon auscultation, disseminated bilateral crepitant crackles and decreased inspiratory breath sounds at the right hemithorax base were found. Precordial auscultation revealed heart sounds of good intensity without extra heart sounds. Abdominal exploration without visceromegaly nor abnormalities upon light and deep palpation. The extremities had filiform pulse augmented in frequency and decreased in amplitude without trophic changes. Upon admission, the patient had the following vital signs: blood pressure 130/80 mmHg; heart rate 90 bpm; respiratory rate 17 rpm; SO2 with noninvasive ventilation at a rate of 3 L/min of 94%; temperature 37 °C; weight 70 kg; height 165 cm; body mass index 25.7 kg/m2; arterial blood gas test: pH = 7.4, PaO2 = 58 mmHg, PaCO2 = 24 mmHg, [HCO3−] = 16.9 mEq/L, O2 content= 88%, base excess= −7.0 mmol/L, lactate= 0.9 mmol/L.
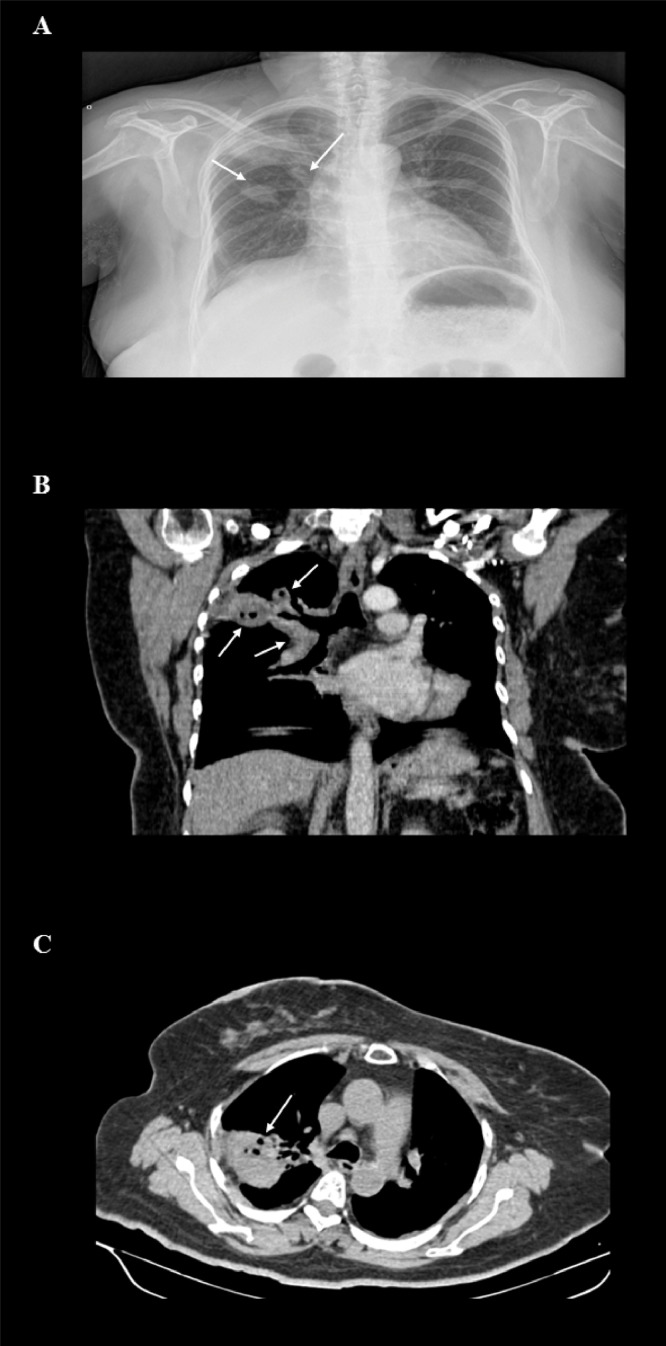
Laboratory results at admission are presented in Table 1 and the supplementary laboratory results in Table 2. Upon admission, a chest X-ray, posterior-anterior projection, shows right alveolar infiltrate at the base and presence of ipsilateral nodules (Fig. 1a). A computed tomography (CT) of the thorax, was performed showing three nodular images with a heterogeneous appearance (i.e. post-contrast enhancement, relating to a probable necrotic lesion) in the right superior lung (Fig. 1b); as well as a cavitary lesion in the left inferior lung (Fig. 1c). Due to the clinical and imaging presentation, as well as the initial laboratory results a lung and urinary tract (i.e. leukocyturia, hematuria and proteinuria) infections were integrated. Initial antibiotic management consisted of ceftriaxone 1 g intravenously (IV) q12 h and clarithromycin 500 mg PO q12 h for seven days.
Table 1.
Laboratory test results upon admission the emergency department.
| Full blood count | |
|---|---|
| Hemoglobin at admission | 11.6 g/dL |
| Hematocrit | 37.1% |
| Erythrocyte count | 4500 µL |
| Platelet count | 268,000 µL |
| Mean corpuscular volume | 81.9 fL |
| Mean corpuscular hemoglobin concentration | 25.60 pg |
| Leukocyte count | 10,500 µL |
| Lymphocytes | 8.0% |
| Neutrophils | 89.1% |
| Monocytes | 2.9% |
| Eosinophils | 0.0% |
| Basophils | 0.0% |
| Blood chemistry | |
| Glucose | 95 mg/dL |
| Creatinine | 0.9 mg/dL |
| Urea nitrogen | 43 mg/dL |
| Blood urea nitrogen | 17 mg/dL |
| Uric acid | 6.5 mg/dL |
| Cholesterol | 120 mg/dL |
| Triglycerides | 150 mg/dL |
| Liver function enzymes | |
| Aspartate transaminase | 25 U/L |
| Alanine transaminase | 27 U/L |
| Lactate dehydrogenase | 285 U/L |
| Albumin | 3.4 g/dL |
| Alkaline phosphatase | 85 U/L |
| Gamma-glutamyl transpeptidase | 40 U/L |
| Blood coagulation | |
| Prothrombine time | 13.4 s |
| Partial thromboplastin time | 32.3 s |
| International normalized ratio | 1.08 |
| Electrolytes | |
| Sodium | 144 mEq/L |
| Potassium | 4 mEq/L |
| Chlorine | 107 mEq/L |
| Calcium | 8.6 mg/dL |
| Phosphorus | 3.6 mg/dL |
| Magnesium | 2 mg/dL |
Table 2.
Supplementary laboratory test results.
| Antibodies | |||||
|---|---|---|---|---|---|
| Cytoplasmic antineutrophil cytoplasmatic antibodies (cANCA) | 0.9 | ||||
| Perinuclear antineutrophil cytoplasmatic antibodies (pANCA) | 0.1 | ||||
| Anti-nuclear antibodies | 1.0 | ||||
| Anti-double-stranded deoxyribonucleic acid | 2.61 U/mL | ||||
| Anti-cardiolipin IgM antibody | 7.7 U/mL | ||||
| Anti-cardiolipin IgG | 2 U/mL | ||||
| Cyclic citrullinated peptide antibody | 1 < U/mL | ||||
| Anti. SSB (LA) | Negative | ||||
| Anti –SSA(RO) | Negative | ||||
| Anti-SM | Negative | ||||
| Complement C3 | 1 g/L | ||||
| Complement C4 | 0.5 g/L | ||||
| Viral panel | |||||
| Hepatitis B virus | Negative | ||||
| Hepatitis C virus | Negative | ||||
| Human immunodeficiency virus 1 and 2 | Negative | ||||
| Urinalysis | |||||
| Appearance | Crystalline | ||||
| pH | 5.0 | ||||
| Specific gravity | 1.005 | ||||
| Proteins | 250 | ||||
| Ketones, glucose, and nitrite | Negative | ||||
| Leukocytes | 500 per high power field | ||||
| Erythrocytes | 111 per high power field | ||||
| Bacteria | Limited | ||||
| Tuberculosis specific tests | |||||
| PCR for M. tuberculosis from expectoration | Negative | ||||
| Xpert MTB/RIF for M. tuberculosis from bronchial secretion | Positive | ||||
| Resistance to rifampicin | Negative | ||||
| Xpert MTB/RIF for M. tuberculosis from urine | Negative | ||||
| Follow-up blood chemistry | |||||
|---|---|---|---|---|---|
| Day 10 | Day 15 | Day 20 | Day 30 | Day 120 | |
| Glucose (mg/dL) | 95 | 98 | 100 | 88 | 104 |
| Creatinine (mg/dL) | 0.6 | 1.33 | 2.36 | 3.04 | 1.1 |
| Urea nitrogen (mg/dL) | 15.4 | 27.58 | 30 | 35 | 27 |
| Blood urea nitrogen (mg/dL) | 33 | 59 | 53 | 17 | 58 |
| Uric acid (mg/dL) | 6.6 | 7 | 7.2 | 7 | 7.5 |
| Electrolytes | |||||
| Sodium (mEq/L) | 141 | 138.7 | 140 | 144 | 138.7 |
| Potassium (mEq/L) | 4 | 4.4 | 3.4 | 3 | 4.5 |
| Chlorine (mEq/L) | 105 | 105 | 108 | 108 | 105 |
| Calcium (mg/dL) | 8.7 | 9.1 | 8.9 | 8.7 | 9.1 |
| Phosphorus (mg/dL) | 4 | 3.9 | 3.1 | 3 | 3.9 |
| Magnesium (mg/dL) | 2 | 1.76 | 1.5 | 1.5 | 1.76 |
Fig. 1.
Posterior-anterior X-ray and computerized tomography of thorax
(A) Chest X-ray, posterior-anterior projection. Right alveolar infiltrate at the base and presence of ipsilateral masses with irregular borders in superior and middle segments (arrows). (B) Computed tomography (CT) of the thorax with contrast, coronal reconstruction with viewing window in the arterial phase. Three nodular images (arrows) with a heterogeneous appearance, post-contrast enhancement with air density within the lesion corresponding to a probable necrotic lesion, in the right superior lung. (C) CT of thorax with contrast, axial reconstruction with viewing window in the contrast phase. Subpleural mass with circumscribed edges and post-contrast enhancement in the left inferior lung (arrow), corresponding to a cavitary lesion.
2.1. Clinical evolution
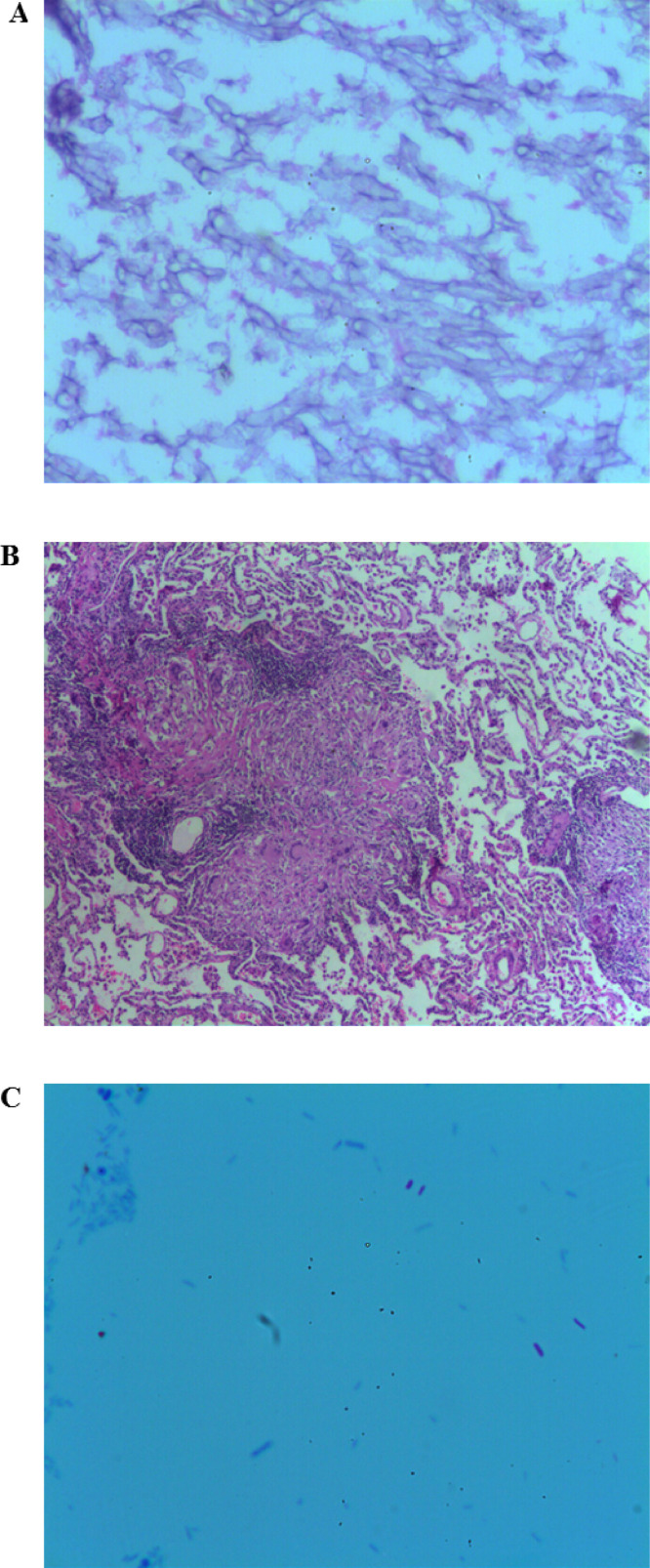
Upon admission to the Department of Internal Medicine, consecutive blood cultures of each extremity, three bacilloscopies, IgG antibodies against Mycoplasma pneumonie, and a galactomannan assay to exclude aspergillosis were performed; all being reported negative. Serum procalcitonin levels of 0.02 ng/mL were reported. In search of an autoimmune etiology, immunoassays were requested and reported as negative (Table 2). An enzyme-linked immunosorbent assay (ELISA) to detect antibodies to HIV-1 and HIV-2 were performed and reported negative (Table 2). Due to the high likelihood of pulmonary tuberculosis even after the three negative bacilloscopies, the patient was isolated and remained in isolation throughout her hospitalization. An initial RT-PCR for M. tuberculosis from sputum and urine were performed, both reported as negative. During the second week of hospitalization and without complete symptom improvement a bronchoscopy with bronchoalveolar lavage was performed, as well as a biopsy of the bronchial mucosa. An RT-PCR for M. tuberculosis from the bronchial secretions was performed with a positive result. An epidemiological survey was carried out among the patient's contacts, without the discovery of new cases. The histopathological report reported minimal inflammatory infiltrate, with the presence of abundant thick and pale hyphae without septa (Fig. 2a), compatible with a pulmonary mucormycosis diagnosis.
Fig. 2.
Bronchial secretion and lung biopsy histopathology
Histopathology. Lung. (A) Bronchial secretion, 4x, hematoxylin and eosin staining. Minimal inflammatory infiltrate, with presence of abundant thick and pale hyphae without septa. (B) Lung mucosa biopsy, 4x, hematoxylin and eosin staining. Large caseous necrotic regions are seen in the center of the image surrounded by epithelial cells, Langhans giant cell and lymphocytes that coalesce into granulomas. Lung parenchyma can be seen in the periphery (C) Lung mucosa biopsy, 10x, Ziehl-Neelsen staining. Acid-resistant bacilli stained bright red on a blue background compatible with M. tuberculosis.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Initial empiric treatment was initiated with rifampicin 600 mg PO q24 h, isoniazid 300 mg PO q24 h, pyrazinamide 1600 mg PO q24 h, and ethambutol 1200 mg PO q24 h all administered on weekdays. Amphotericin B deoxycholate was administered IV (i.e. calculated at 1.0 mg/kg/d the first 7 days and 1.5 mg/kg/d for 23 days) to treat the pulmonary mucormycosis with a total dose of 2.9 g, which was suspended after 30 days of treatment due to nephrotoxicity (i.e. creatinine 3.04 mg/dL) and electrolyte imbalance (i.e. persistent hypokalemia and hypomagnesemia; Table 2) even after adequate reposition. After nephrotoxicity remitted a lobectomy was performed on the right lung (i.e. 35 days after initial hospital admission). The surgical piece biopsy report reported large caseous necrotic regions, surrounded by epithelial cells that cluster into granulomas with lymphocytic infiltrate (Fig. 2b); furthermore, under acid-fast staining acid-resistant bacilli compatible with M. tuberculosis were identified (Fig. 2c). Directly observed therapy (DOT) as previously mentioned was continued and no antifungal medications were administered as no histopathological findings of pulmonary mucormycosis were reported in the surgical piece biopsy report.
After 40 days of hospital stay, the patient was discharged due to clinical improvement. The patient continued with the DOT management at her community clinic with rifampicin 600 mg PO q72 h, isoniazid 800 mg PO q72 h completing 45 total doses, without adverse effects and improved kidney function (Table 2). Ninety days after being released from the hospital the patient was clinically asymptomatic and with three consecutive, negative bacilloscopies the patient was discharged from the pulmonary medicine outpatient clinic.
3. Discussion
Concomitant pulmonary mucormycosis and tuberculosis is very rare and has a poor prognosis. The case presented above describes the rare case of an immunosuppressed patient secondary to uncontrolled diabetes. Only nine previous cases of concomitant mucormycosis and TB (i.e. five cases of pulmonary mucormycosis and TB) have been reported in the literature. While the first case of pulmonary co-infection of TB and mucormycosis reported in the literature is in Japanese, the four other cases available in English are from India [8], [9], [10], [11]. Three cases involved uncontrolled diabetic patients (i.e. one with ketoacidosis and two with previous history and treatment of TB) [8], [9], [10], while the fourth case was an immunocompromised patient with aplastic anemia post allogeneic stem cell transplantation [11]. The case described above is only the sixth case described in the literature of pulmonary co-infection by mucormycosis and TB and the third case involving an uncontrolled DM patient to survive. Furthermore, despite medical and surgical treatment, invasive pulmonary mucormycosis has mortality rates as high as 70% [12]; which highlights the importance of an opportune diagnosis and treatment of concomitant pulmonary mucormycosis and TB in immunocompromised patients.
Pulmonary mucormycosis can have a similar presentation to pneumonia, which is often refractory to initial antibiotic treatment and a key feature is hemoptysis. Upon clinical follow-up, arterial aneurysms, pseudoaneurysms, bronchial obstruction, and solitary cavitary lesions are associated with pulmonary mucormycosis [1]. Pulmonary mucormycosis is highly lethal, with mortality ranging between 51–91% [13], [14]. Among the most frequent tomographic findings for pulmonary mucormycosis are consolidation and nodular patterns, as well as intrapulmonary masses with a halo sign [15]. Diagnosis is made through histopathological detection of hyphae ranging 3–25 mm in length, obtained from samples obtained through bronchoscopies or fine needle biopsies [3], [16], [17]. Meanwhile, pulmonary tuberculosis should be suspected in patients with cough and persistent fever greater than two weeks. Other clinical findings associated with pulmonary TB are weight-loss, hemoptysis, and nocturnal diaphoresis [18], [19]. There are three main validated methods for the detection of active tuberculosis: (1) microscopic identification of acid-fast bacilli; (2) nucleic acid amplification tests; and (3) culture from sputum [20].
Our patient underwent three consecutive bacilloscopies, all reported negative. However, due to the persistent clinical picture suggesting TB a bronchoscopy with bronchoalveolar lavage and mucosa biopsy was performed in order to perform an RT-PCR in search of M. tuberculosis mycobacterial ribosomal RNA. Due to the presence of hemoptysis, fever, cough, and the cavitary pulmonary lesions, differential diagnosis was made with vasculitis (e.g., granulomatosis with polyangiitis, eosinophilic granulomatosis polyangiitis, and lupus erythematosus); consequently, requesting immunoassays which were reported as negative. Other differential diagnoses considered where neoplastic processes such as lymphoma and metastasis from an unknown primary but these were ruled out after obtaining the results from the lung biopsy.
Pulmonary mucormycosis treatment hinges on the administration of antifungal medication (e.g. amphotericin B deoxycholate, liposomal and lipid complex amphotericin B, isavuconazole, and posaconazole), treatment of underlying risk factors (e.g. metabolic control of DM, glucocorticoid suspension, and interruption of deferoxamine therapy), and surgical management [21]. Furthermore, surgical lobectomy should be considered in the presence of lung cavitation secondary to TB. Surgical success, reduction in mortality rate, ranges between 75 and 98% among TB patients [22]. In the case presented above, the patient responded well to the recommended 4-drug regimen for TB treatment with isoniazid, rifampin, pyrazinamide, and ethambutol. The only adverse reaction noted due to this treatment regimen was the elevation of uric acid, which was related to pyrazinamide. This side effect ceased after pyrazinamide was suspended.
3.1. Limitations
One of the limitations of this case report is related to the treatment of mucormycosis. Although the liposomal formulation of amphotericin B is the drug of choice based on efficacy and safety data [21], [23], we only had amphotericin B deoxycholate at our disposal in our hospital. This is a limitation since the deoxycholate formulation is nephrotoxic; consequently, treatment had to be suspended when the total dose reached 2.9 g and the creatinine serum levels reached 3.04 mg/dL in association with electrolyte imbalance. In this regard, although a rare complication, ethambutol can induce nephrotoxicity and could be a contributing factor to the renal insufficiency. Furthermore, the patient's renal function (e.g. creatinine clearance, mean of urea and creatinine clearance, radioisotopic methods) was not quantified before, during or after the administration of tuberculosis and mucormycosis treatment, hence the association with the exact time of renal failure or premorbid renal damage cannot be established. Careful monitoring of renal function is advisable in chronic diabetic patients undergoing treatment with nephrotoxic agents such as ethambutol and amphotericin B. Another limitation to the case presented was the lack of confirmatory assessment of mucormycosis remission after treatment through a follow-up bronchoscopy with bronchoalveolar lavage and mucosa biopsy since the patient refused to undergo this procedure prior to her discharge. However, the patient improved clinically and no new lesions were identified through imaging after medical and surgical treatment.
3.2. Conclusion
Pulmonary mucormycosis and tuberculosis co-infections are very rare. When confronted with an uncontrolled diabetic patient with a clinical picture that includes hemoptysis, fever, and dyspnea, associated with consolidation or cavitary lesions and nodules as imaging findings, we are obligated as clinicians to test for tuberculosis and mucormycosis.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgments
This study was supported by CONACyT (Consejo Nacional de Ciencia y Tecnología) grant #440591. This research did not receive any specific grant from funding agencies in the commercial sector. We would like to commend the work of the medical staff (i.e. specialists, medical residents, and nursing staff) of the Internal Medicine Department and Juana Rosalba García from the Pathology Department at Hospital General León.
Availability of data and materials
The clinical data supporting the conclusions of this article is included in the article.
Authors’ contributions
Study concept and design: O.J.Z. and J.D.M. Acquisition of data: M.M.R., O.J.Z., and M.A.L. Analysis and interpretation of data: J.D.U.A., G.A.F.S., L.N.V.R., and J.D.M. Critical revision of the manuscript for important intellectual content: All authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approval from the ethical committee was not required due to the nature of this case report. Abiding by the Declaration of Helsinki, patient anonymity was guaranteed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2019.100105.
Appendix. Supplementary materials
References
- 1.Farmakiotis D, Kontoyiannis D.P. Mucormycoses. Infect Dis Clin North Am. 2016;30(1):143–163. doi: 10.1016/j.idc.2015.10.011. PMID 26897065. [DOI] [PubMed] [Google Scholar]
- 2.Hamilos G, Samonis G, Kontoyiannis D.P. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693–702. doi: 10.1055/s-0031-1295717. PMID 22167397. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis D.P, Lewis R.E. How I treat mucormycosis. Blood. 2011;118(5):1216–1224. doi: 10.1182/blood-2011-03-316430. PMID 21622653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh T.J, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–S34. doi: 10.1093/cid/cir866. PMID 22247442. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global Tuberculosis Report2018. Geneva: World Health Organization; 2018. https://www.who.int/tb/publications/global_report/en/.
- 6.Lee J.H, Park S.S, Lee D.H, Shin D.H, Yang S.C, Yoo B.M. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest. 1992;102(4):990–994. doi: 10.1378/chest.102.4.990. PMID 1395814. [DOI] [PubMed] [Google Scholar]
- 7.Siow W.T, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis. 2017;9(1):E71–E77. doi: 10.21037/jtd.2017.01.49. PMID: 28203440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal D, Chander J, Janmeja A.K, Katyal R. Pulmonary tuberculosis and mucormycosis co-infection in a diabetic patient. Lung India. 2015;32(1):53–55. doi: 10.4103/0970-2113.148452. PMID 25624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube P, Saroa R, Palta S. Coinfections in intensive care unit with pulmonary tuberculosis and mucormycosis: a clinical dilemma. Indian J Crit Care Med. 2016;20(3):191–193. doi: 10.4103/0972-5229.178187. PMID 27076735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg R, Marak R.S, Verma S.K, Singh J, Sanjay Prasad R. Pulmonary mucormycosis mimicking as pulmonary tuberculosis: a case report. Lung India. 2008;25(3):129–131. doi: 10.4103/0970-2113.59595. PMID 20165666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S.K, Agarwal N, Mukherjee A, Seth T, Mishra P, Xess I, Mahapatra M, Sharma S. Coexisting pulmonary tuberculosis and mucormycosis in a patient with aplastic anemia post allogenic stem cell transplantation. Mediterr J Hematol Infect Dis. 2011;3(1) doi: 10.4084/MJHID.2011.0036. PMID 22084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden M.M, Zaoutis T.E, Buchanan W.L, Knudsen T.A, Sarkisova T.A, Schaufele R.L, Sein M, Sein T, Chiou C.C, Chu J.H, Kontoyiannis D.P, Walsh T.J. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. PMID 16080086. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. PMID 16020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marty F.M, Ostrosky-Zeichner L, Cornely O.A. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. PMID 26969258. [DOI] [PubMed] [Google Scholar]
- 15.Nam B.D, Kim T.J, Lee K.S, Kim T.S, Han J, Chung M.J. Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol. 2018;28(2):788–795. doi: 10.1007/s00330-017-5007-5. PMID 28812135. [DOI] [PubMed] [Google Scholar]
- 16.He R, Hu C, Tang Y, Cao L, Niu R. Report of 12 cases with tracheobronchial mucormycosis and a review. Clin Respir J. 2018;12(4):1651–1660. doi: 10.1111/crj.12724. PMID 29028140. [DOI] [PubMed] [Google Scholar]
- 17.Chamilos G, Luna M, Lewis R.E, Bodey G.P, Chemaly R, Tarrand J.J, Safdar A, Raad I.I, Kontoyiannis D.P. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91(7):986–989. PMID 16757415. [PubMed] [Google Scholar]
- 18.Miller L.G., Asch SM, Yu EI. A population-based survey of tuberculosis symptoms: how atypical are atypical presentations? Clin Infect Dis. 2000;30(2):293–299. doi: 10.1086/313651. PMID 10671331. [DOI] [PubMed] [Google Scholar]
- 19.Lewinsohn D.M, Leonard M.K, LoBue P.A. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):111–115. doi: 10.1093/cid/ciw778. PMID 28052967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pai M, Nicol M.P, Boehme C.C. Tuberculosis diagnostics: state of the art and future directions. Microbiol Spectr. 2016;4(5):1–15. doi: 10.1128/microbiolspec.TBTB2-0019-2016. PMID 27763258. [DOI] [PubMed] [Google Scholar]
- 21.Skiada A, Lanternier F, Groll A.H, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL. European Conference on Infections in Leukemia. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3) Haematologica. 2013;98(4):492–504. doi: 10.3324/haematol.2012.065110. PMID 22983580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yablonskii P.K, Kudriashov G.G, Avetisyan A.O. Surgical resection in the treatment of pulmonary tuberculosis. Thorac Surg Clin. 2019;29(1):37–46. doi: 10.1016/j.thorsurg.2018.09.003. PMID 30454920. [DOI] [PubMed] [Google Scholar]
- 23.Spellberg B, Walsh T.J, Kontoyiannis D.P, Edwards J, Ibrahim A.S. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–1751. doi: 10.1086/599105. PMID 19435437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data supporting the conclusions of this article is included in the article.