Abstract
Background: Optimal screening and management of latent tuberculosis infection (LTBI) and active tuberculosis (TB) in solid organ transplant (SOT) candidates and recipients is necessary to prevent morbidity and mortality.
Methods: We conducted a cross-sectional survey of TB and transplant experts across the United States reviewing the clinical practice preferences on key management issues related to LTBI and TB in SOT candidates and recipients.
Results: Thirty TB and 13 SOT experts were surveyed (response rate = 53.8%). Both groups agreed that tuberculin skin test (TST) and chest x-ray screening in SOT candidates was useful (78.6% and 84.6%, respectively). TST after SOT was not useful for most transplant experts and TB experts (0% vs. 32.1%, respectively), but both groups were split on usefulness of interferon gamma release assays (IGRA) in SOT recipients (42.9% TB experts vs. 46.2% SOT experts). Most experts recommend LTBI treatment prior to SOT if close monitoring is assured (82.1% TB experts vs. 76.9% transplant experts). LTBI treatment with isoniazid was preferred for patients on calcineurin inhibitors. Evaluation for suspected TB in SOT recipients varied, but most TB experts favored sputum testing (88.9%) whereas most transplant experts favored bronchoscopic testing (69.2%). Preferred TB treatment regimens in SOT recipients were similar to regimens recommended for immunocompetent patients.
Conclusions: Most TB and transplant experts recommend evaluation and treatment for LTBI in SOT candidates. Liver transplant candidates, however, should only be treated if close monitoring can be assured and after consulting with a hepatologist. Practice preferences varied regarding the initial diagnostic approach for suspected TB in SOT recipients; however, most experts agreed that SOT recipients should receive similar treatments as immunocompetent patients.
Keywords: Tuberculosis, Transplantation, Survey
Introduction
Tuberculosis (TB) incidence in solid organ transplant (SOT) recipients is reported between 0.25 to 13.7%, and occurs more often in countries and settings with high prevalence of TB [1], [2], [3], [4], [5]. Moreover, TB in SOT recipients carries high TB-related and SOT-related morbidity and mortality. The most recent TB consensus guidelines in the United States addressed the diagnosis in immunosuppressed individuals and some aspects of TB treatment in SOT recipients, such as drug-to-drug interactions with anti-TB medications. They do not, however, provide a dedicated section with recommendations for the diagnosis and management of LTBI and TB in various types of SOT candidates and recipients [6], [7], [8], [9]. The Spanish Society of Infectious Diseases and Clinical Microbiology previously put together a consensus statement to provide clinical guidelines specifically for the management of SOT recipients in 2009 [10]. Several of those recommendations are based on expert opinion and retrospective data [11]. However, it is unclear if consensus between TB and SOT experts exists, if there are TB management practice variations between Spain and the US, and some key clinical issues remain unaddressed.
Important considerations in SOT candidates are clinical evaluation of latent tuberculosis infection (LTBI) with the tuberculin skin testing (TST) and/or interferon gamma release assays (IGRAs), the utility of routine screening chest x-rays (CXR), and LTBI treatment regimens (especially in liver transplant candidates). In SOT recipients, important considerations are diagnostic accuracy of testing for LTBI, diagnostic approach for suspected active TB, and treatment regimens for LTBI and active TB.
Anti-TB medications, specifically rifamycins (rifampicin, rifabutin, or rifapentine) interact with calcineurin inhibitors (i.e. cyclosporine and tacrolimus), mammalian target of rapamycin (mTOR) inhibitors (i.e. sirolimus and everolimus), and corticosteroids by reducing serum levels of these medications potentially precipitating graft rejection and dysfunction [6], [12], [13]. For these reasons, the use of rifamycin-based regimens for the treatment of LTBI and active TB in SOT recipients is controversial [14]. Moreover, common LTBI regimens have associated risk of hepatotoxicity. Isoniazid and rifamycins can cause drug-induced hepatitis, and the previously used combination of rifampicin/pyrazinamide can cause severe liver toxicity [6], [10]. This raises the issue of how to best treat liver transplant candidates with LTBI or liver transplant recipients with LTBI or active TB.
To help address these key clinical issues, the American College of Chest Physicians (ACCP) sponsored a project to conduct a national survey that invited United States experts in TB and SOT to compare medical preferences for common TB diagnostic and management questions in SOT candidates and recipients. After reviewing the recently released American Thoracic Society(ATS), Centers for Disease Control and Prevention(CDC), and Infectious Disease Society of America(IDSA) diagnostic and treatment guidelines, we decided to publish this original work and discuss our findings in comparison with the most recent published data [7], [9].
Material and methods
The study was reviewed and approved by the Mayo Clinic Institutional Review Board and was research exempt.
We conducted a web-based survey amongst TB and transplant experts from August 18, 2009 to June 21, 2010.
Development of survey questionnaire
No validated questionnaire regarding TB practices in SOT existed. Therefore, a seven-member steering committee representing pulmonary and infectious diseases TB experts and transplant experts reviewed the literature and developed potential survey questions utilizing the Delphi method. The survey questions were focused on the following clinical themes: (1) diagnostic utility of TST and IGRAs, before and after SOT, (2) usefulness of routine CXR in assessment of candidates undergoing SOT evaluations, (3) LTBI treatment alternatives for patients taking calcineurin and/or mTOR inhibitors, (4) management of LTBI, before or after liver transplantation, (5) diagnostic work-up and treatment alternatives for active TB after SOT. Responses were formatted on a 5-point Likert scale or for priority ranking, and comments were allowed. The proposed questionnaire and survey instructions were reviewed by non-TB experts and non-transplant experts from the ACCP Chest Infection Network for clarity and comments. The questionnaire was revised accordingly. One question, in which consensus guidelines exists for the treatment of patients with active TB in general, was included for internal validation. The final questionnaire had 11 questions (see Appendix A).
Selection of study participants
We sought a representative sample from the sampling frame of TB and SOT experts of varied institutions and geographic regions from the United States to avoid bias based on regional practice variation and differences in TB prevalence. TB and SOT experts were identified in three ways: (1) nomination by the study's steering committee based on known contributions in the field of TB or SOT; (2) through a PubMed publication search using terms “tuberculosis” and “solid organ transplantation” and/or; (3) practitioner in a TB or SOT referral center in the United States obtained from the ACCP and other professional organizations databases. Inclusion criteria for TB experts included physicians who care for TB patients in a referral practice and/or have published one or more research articles on TB. TB experts were comprised of the following specialties: Pulmonary Medicine, Infectious Diseases, and Internal Medicine. Transplant experts included physicians who manage SOT patients in referral centers and/or published one or more research articles related to SOT. SOT experts were comprised of Pulmonary Medicine and Infectious Diseases specialists. Experts were sorted by their contributions to the respective fields (SOT or TB) and current practice setting, not by specialty training.
Survey procedure
Need for consent was waived by the IRB. The self-administered, web-based questionnaire was sent to participants via an email invitation. The email included survey instructions, voluntary nature of the study, a statement regarding the purpose of the study, identification of the study sponsor and principal investigator, and an option to accept or decline participation as previously described [14]. If no response was received within 2 weeks, a subsequent e-mail invitation was sent. If there was no response by four weeks, the e-mail address was reconfirmed and a follow-up invitation was sent at 6 weeks and if necessary 8 weeks. On failure to obtain response after the fourth invitation, experts were deemed non-participators. No incentive or remuneration was offered.
Measurable outcomes
The measurable outcomes were level of agreement amongst experts and order of preference on priority ranking.
Definitions
Agreement was defined as responding either “agree” or “strongly agree”, and disagreement was defined as responding “disagree” or “strongly disagree”. Neutral responses were counted in the denominator. Consensus level was defined when 80% of respondents in a category were in agreement or disagreement.
Data management
We used the web-based Survey Monkey™ platform to gather and compile the information from the invited experts as previously described [14]. Individual information was confidentially maintained on a password secured folder on a password secured network, and the responses were deidentified.
Statistical analysis
Responses were calculated as percent agreement with each expert group. Categorical data were calculated as percent frequency of occurrence. The Fisher Exact test was used to compare responses between the two groups—SOT and TB experts. A P value of <0.05 was considered significant. All statistical analysis was performed using JMP®, Version JMP Pro 10. (SAS Institute Inc., Cary, NC, 1989–2007).
Results
Survey response
Fifty-eight TB experts and 22 SOT experts were identified as potential participants. The overall survey response rate was 53.8% with 43 out of 80 experts responding. This included 13 SOT experts out of 22 (59.1%) and 30 TB experts out of 58 (51.7%) who received the survey. Geographic areas of primary practice for TB experts included the following states: Arkansas, California, Colorado, Florida, Georgia, Illinois, Maryland, Massachusetts, Minnesota, New York, North Carolina, Tennessee, Texas, Virginia, and Washington. Areas of primary practice for SOT experts included California, Illinois, Michigan, Minnesota, New York, Ohio, Oklahoma, and Texas.
Utility of TST for LTBI screening, before and after SOT in average risk patients
Most TB experts and SOT experts (consensus level) agreed that TST was a useful screening tool before SOT (78.6% and 84.6% agreement, respectively; p = 1.0) (Table 1). Following SOT, however, some TB experts but none of the SOT experts indicated that TST was a useful screening tool (32.1% vs 0% agreement in transplant experts; p = 0.038).
Table 1.
Comparison between TB and transplant experts for LTBI diagnosis and management questions surrounding solid organ transplantation.
| Question | TB experts | Transplant experts | |
|---|---|---|---|
| % agreement | % agreement | p-value | |
| (N = 29) | (N = 13) | ||
| TST prior to SOT | 78.6% (N = 28) | 84.6% (N = 13) | 1.0 |
| TST after SOT | 32.1% (N = 28) | 0% (N = 13) | 0.038* |
| CXR prior to SOT | 76.9% (N = 26) | 69.2% (N = 13) | 0.704 |
| IGRA after SOT | 42.9% (N = 28) | 46.2% (N = 13) | 1.0 |
| LTBI therapy before liver transplantation | 78.6% (N = 28) | 76.9% (N = 13) | 1.0 |
| LTBI therapy after liver transplantation | 55.2% (N = 29) | 53.9% (N = 13) | 1.0 |
TB = mycobacterium tuberculosis; SOT = solid organ transplant; TST = Tuberculin skin test; CXR = Chest radiograph; IGRA = interferon gamma release assay; LTBI: Latent TB infection; % agreement includes agree and strongly agree answers to questionnaire. Comparison by Fisher's Exact test. (*) p ≤ 0.05 is considered statistically significant.
Routine CXR in the assessment of patients prior to SOT in average risk patients
The majority of TB experts and SOT experts agreed with CXR for evaluation of SOT candidates, 76.9% and 69.2% agreement, respectively (p = 0.70) (Table 1). Neither group, however, reached consensus.
Diagnostic value of IGRA for LTBI after SOT in patients with risk factors for TB
No consensus was reached by either group on whether IGRAs represent a useful diagnostic test for LTBI in SOT recipients who had risk factors for TB (42.9% TB experts and 46.2% SOT experts, p = 1.0). However, IGRA diagnostic utility was felt to be higher in comparison to TST in this patient population (Table 1).
Treatment of LTBI in liver transplant candidates and recipients
The majority of TB experts (78.6%) and SOT experts (76.9%) agreed in the utility of treating LTBI prior to liver transplantation in patients with end-stage liver disease (p = 1.0), provided that close monitoring is assured for those patients (Table 1). Likewise, the majority of TB experts (55.2%) and SOT experts (53.9%) agreed that LTBI should be treated following liver transplantation (p = 1.0).
Treatment regimens for LTBI in patients receiving calcineurin inhibitors
TB experts and SOT experts ranked a regimen of daily isoniazid (INH) for 9 months as the first choice for treatment of LTBI in SOT recipients on calcineurin inhibitors (Consensus level: 96.4% and 83.3%, respectively, p = 0.209). The second most common treatment regimen chosen was INH for 6 months (TB experts = 41.4%, transplant experts = 58.3%, p = 0.493). No other single regimen was preferred over another by either expert group.
Diagnostic workup for possible pulmonary TB in patient with cough, fever, and non-cavitary lung infiltrates after SOT
TB experts agreed (consensus level) that the first priority in working up SOT recipients with suspected TB was sputum acid fast bacillus (AFB) smear and culture (82.8%), followed by sputum nucleic acid amplification testing (NAA) (44.8%). However, the majority of SOT experts chose diagnostic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsies (TBBx) (69.3%) as first priority with 30.7% choosing sputum NAA and AFB smear/ culture as either second or third priority. Beyond those priorities, no other diagnostic testing was preferred by SOT experts.
Treatment regimens for non-cavitary pulmonary TB with negative sputum culture at 2 months of treatment in SOT recipients
Intensive phase with daily RIPE therapy (i.e. rifampin or rifabutin), isoniazid, pyrazinamide, ethambutol) followed by 4 months of continuation therapy with daily INH and rifampin or rifabutin were the preferred treatment regimens by both TB (55.1%) and SOT experts (76.9%). In addition, some TB experts (20.9%) and SOT experts (15.4%) preferred the same daily antimicrobial treatments options but with a 7 months of continuation phase.
Treatment regimens for cavitary TB with positive culture at 2 months of treatment in SOT recipients
Intense phase with daily RIPE therapy (with rifampin or rifabutin), followed by 7 months of continuation therapy with daily INH and rifampin or rifabutin were the preferred treatment regimens by TB experts in patients with cavitary TB. Over 80% of TB experts chose this regimen as their first or second choice. All SOT experts chose RIPE therapy with daily INH and rifabutin for 7 months as their first or second preference.
Discussion
TB is a serious infection in SOT recipients, which can lead to significant morbidity and mortality [1], [5], [13], [15]. SOT candidates with unrecognized LTBI can also develop atypical and disseminated forms of TB due to chronic illness and/or exposure to immunosuppressing medications. These severe and sometimes difficult-to-diagnose forms of TB are associated with poor outcomes [1], [5], [13], [15]. Thus, TB diagnosis and management in this patient population can be challenging leading to significant practice variation. Survey methodology of current practices can assist to show areas of disagreement and areas of agreement enough to potentially reduce practice variation. This information can be important for practice standardization.
In this context, the most recent international guidelines for TB in SOT candidates and recipients are from the 2009 consensus document from the Spanish Society of Infectious Diseases and Clinical Microbiology. Despite a recent European experts follow-up review, the current ATS/CDC/IDSA guidelines do not fully standardize surveillance, diagnosis, and treatment strategies in SOT candidates and recipients [7], [8], [9], [12], [13]. Moreover, current recommendations for TB management in SOT candidates and recipients are based mostly on retrospective data and expert opinion. In this context, we utilized data from surveyed TB experts and SOT experts across the United States to not only quantify their medical preferences but also to review critical issues in the diagnosis and management of LTBI and active TB in SOT candidates and recipients.
LTBI screening with TST and IGRA in SOT candidates and recipients
TST was the main diagnostic test for LTBI for almost 100 years. About 10 years ago, IGRAs were introduced and became widely available in clinical practice [19]. TST can be flawed with errors in intradermal administration of tuberculin and variability in interpretation [20]. Anergy to the tuberculin antigen is an additional concern in chronically-ill and immunosuppressed SOT candidates and recipients, which can cause false negative results. False-positive TST can occur with prior vaccination with BCG (Bacillus Calmette–Guérin) or infection with other non-tuberculous mycobacteria such Mycobacterium avium complex [21]. IGRA uses more specific M. tuberculosis antigens than TST and has been recommended in populations with low or intermediate risk, especially in subjects with prior BCG vaccination [7].
In the current study, both TB and SOT experts were in agreement that TST was a useful test for LTBI screening in SOT candidates but the majority of experts felt a limited diagnostic utility of TST in SOT recipients. This is likely secondary to the belief that SOT recipients will likely develop anergy and false-negative TST results. However, in immunocompromised patients, such as HIV patients with low CD4 counts, IGRAs have not been proven consistently superior to TST [22]. Moreover, indeterminate and false negative results can also occur with IGRAs in immunocompromised patients, and some experts suggest using both TST and IGRA in those individuals to maximize the sensitivity of both tests for LTBI screening purposes in high risk populations [8], [22], [23], [24], [25]. In fact, both TST and IGRA have a low predictive value for TB reactivation and both tests can also yield negative results in immunosuppressed patients who actually progressed to active TB [22], [26]. At the time of the survey, we found no clear consensus among TB and SOT experts regarding the diagnostic utility of IGRA after SOT. However, these preferences could have changed over more recent years with increased familiarity with the strengths and limitations of these immunodiagnostic tests. Moreover, a positive TST and/or IGRA test can be diagnostically useful, but negative results need to be interpreted with caution, in particular if patients have prior exposure to TB and/or have CXR findings suggestive of previously untreated TB [13].
Routine CXR in the assessment of SOT candidates
Radiographic presentation of pulmonary TB can be atypical in immunocompromised individuals [12]. While most SOT candidates are not immunosuppressed from a pharmacologic standpoint, many are relatively immunocompromised secondary to their underlying disease and/or chronic illness (i.e. renal or liver failure). In other immunosuppressed populations, such as those with HIV infection, routine use of CXR to assess for active pulmonary TB is not recommended as the test sensitivity varies [27], [28]. In our survey, TB experts reached near consensus level agreement on the utility of routine CXR in patient assessment prior to SOT. The majority of transplant experts also agreed with routine CXR prior to SOT, but at lower level of agreement. In clinical practice, most patients undergoing SOT, in particular lung transplant candidates, have chest imaging performed for a variety of reasons other than evaluating for LTBI and TB.
Treatment of LTBI in liver transplant candidates and recipients
A systematic review in liver and renal transplant recipients suggested that treatment of LTBI reduces the risk of TB reactivation [16], [17]. Diagnostic strategies that accurately detect and treat LTBI in SOT candidates and recipients can prevent subsequent morbidity and mortality associated with TB reactivation [18]. End-stage liver disease patients are at risk for liver decompensation with LTBI treatment or TB treatment. They are also at risk of developing disseminated TB following transplantation, which is likely secondary to immunosuppressing medications required to prevent graft rejection [5]. Treatment of LTBI in patients with end-stage liver disease is challenging secondary to the risk of drug related hepatotoxicity that may have a significant impact on marginally reserved liver function [6]. Despite this potential risk, some studies suggest that these patients can be safely treated for LTBI with INH or rifamycin based regimens prior to transplantation [31], [32]. In our survey, both TB and SOT experts reached near consensus level of agreement that liver transplant candidates with LTBI should be treated prior to transplantation provided very close monitoring is ensured. However, neither TB nor SOT experts reached consensus on preference for any treatment regimen, and no hepatologist completed the survey. Rifampin daily for 4 months and INH daily for 9 months were the most frequently preferred regimens. Treatment with INH for at least 6 months significantly decreases the risk of developing active TB, and a very small number of patients need to discontinue this regimen secondary to drug induced hepatitis or acute liver failure [17]. However, a more recent report from Canada found that a significant proportion of liver transplant candidates and recipients do not tolerate standard LTBI therapy [18].
Treatment regimens for LTBI in patients receiving with calcineurin inhibitors
SOT recipients receiving calcineurin inhibitors and other anti-rejection drugs are at risk of reactivation of TB, which can be associated with high mortality and morbidity [33]. In immunocompetent patients, LTBI is treated with one of the following regimens: INH daily for 9 months, rifamycin daily for 4 months, or weekly rifapentin and isoniazid for 3 months [6]. Rifamycins are potent inducer of cytochrome P-450 oxidative enzymes leading to decreased serum concentration of calcineurin inhibitors; however, rifabutin has a lower cytochrome inducer effect [9], [34]. This important drug-to-drug interaction can potentially lead to graft-versus-host disease (GVHD) or graft rejection and organ failure [12]. Likely for this reason, both TB and SOT experts reached a consensus on INH daily for 9 months as the preferred treatment regimen in patients with LTBI who are additionally receiving a calcineurin inhibitor. This recommendation is also shared by some European experts [12], [13]. However, rifabutin is also an effective therapy for LTBI and usually well-tolerated therapeutic option [29], [30]. Moreover, rifabutin can be less hepatotoxic than INH and faster to complete with 4 months of treatment, and thus, with accumulating experience using rifabutin along with calcineurin inhibitors or mTOR inhibitors, practice seems to be changing to more often use of rifabutin as the initial therapeutic choice for LTBI as long as careful evaluation, close drug monitoring, and adjustment of the immunosuppression is assured [14], [31].
Management of active TB following SOT
Diagnosis of active TB infection in immunosuppressed patients is challenging secondary to atypical clinical features including extra-pulmonary involvement and presence of concomitant infections. Confirmation of diagnosis by isolating M. tuberculosis in liquid or solid cultures is time consuming, but NAA and other rapid molecular tests can detect the organism in hours. NAA diagnostic performance does not depend on the immune status of the patients, but false negative results are possible if the organism is not or rarely present in the sample. In suspected active TB after SOT, TB experts reached consensus that 3 AFB sputum smears with subsequent mycobacterial cultures is the preferred initial diagnostic test approach. Interestingly, SOT experts had consensus agreement on diagnostic bronchoscopy with broncho-alveolar lavage and trans-bronchial biopsy to diagnose active TB in this setting. It is unclear why the two group of experts had a different initial diagnostic approach for suspected TB, but it is possible that transplant experts have a lower threshold for bronchoscopic testing to not only diagnose TB but also detect other opportunistic infections that are often in differential diagnosis.
Moreover, no widely accepted treatment regimen for active TB infection in SOT recipients exists. Our study found no consensus amongst TB and SOT experts for treatment regimens in this population. The initial intensive phase with daily RIPE (with rifampin or rifabutin) followed by a combination of INH and rifamycin or rifabutin daily for 4 months were the preferred regimens by both TB and SOT experts. Given the interactions with calcineurin inhibitors and rifamycins, immunosuppressive drug levels need to be closely monitored and adjusted to prevent organ rejection and dysfunction [17]. The Spanish Society of Infectious Diseases and Clinical Microbiology guidelines favor the use of prolonged anti-TB treatment regimens without rifamycins in SOT with localized and non-severe forms of TB [13]. For cavitary TB in SOT recipients, a daily intensive phase with RIPE(with rifampin or rifabutin) followed by INH and rifabutin or rifabutin monotherapy daily for 7 months were preferred regimens by both TB and SOT experts. Once again, continuous monitoring of immunosuppressive drugs levels for patients on calcinurin inhibitors, mTOR inhibitors, and/or corticosteroids is recommended [12], [13].
Strengths and limitations of the study
The study had several strengths, including a careful design and review of the questionnaire by an external steering committee of Pulmonary Medicine and Infectious Disease TB experts from most of the United States’ regional TB centers. Questionnaire items were also reviewed for clarity by pulmonary physicians from the ACCP who were not experts in TB or SOT. This study also included a large number of TB and SOT experts from various geographic locations and academic centers in the United States, and we had a high response rate of over 50%. However, the diversity in the types of medical subspecialties represented was low, including lack of hepatologist participation in the survey, but a hepatologist was part of the study data analysis and manuscript writing. Although this survey data was obtained in 2010, there have been very few changes in the management of LTBI and TB in SOT candidates and recipients since that time [7], [8], [9], [13]. Important areas of regarding TB in SOT candidates and recipients were not addressed by this survey. In the future, researchers should explore attitudes and preferences of SOT practitioners toward reduction in immunosuppression during treatment for active TB. In addition, management issues unique to lung transplant should be queried (e.g. singe versus bilateral transplant), and management issues regarding the treatment of LTBI based on the degree of liver failure as determined by liver failure stratification tools.
Conclusions
TB and SOT experts agree that SOT candidates should have appropriate pre-transplant evaluation for LTBI and active TB with either TST or IGRA along with a CXR. Treatment for LTBI in SOT candidates, even in advanced liver disease, should be considered if very close monitoring can be assured and after consulting with a hepatologist. To avoid important drug-to-drug interactions, patients taking calcineurin inhibitors or mTOR inhibitors should receive INH for LTBI therapy, but rifabutin can be also used with close drug monitoring. No consensus was reached regarding the initial diagnostic evaluation for SOT recipients suspected to have active TB; however, both groups of experts did agree that SOT recipients with TB should be treated with anti-TB treatment regimens commonly used for immunocompetent TB patients.
Author contributions
Concept/design: PE; Data analysis/Interpretation: KMP, CCK, PE; Drafting article: KMP, CCK, SC, PE; Critical Revision of article: KMP, CCK, SC, ML, BTM, MB, DEG, BJS, PE; Approval of article: KMP, CCK, SC, ML, BTM, MB, DEG, BJS, PE; Statistics: PE, CCK; Funding secured by: PE
Acknowledgments
The authors want acknowledge the wonderful enthusiasm and detailed data analysis work from the late Dr. Kate E. Birkenkamp from the Mayo Clinic, who passed away unexpectedly before the writing process of this manuscript. We also appreciate the valuable input and original support from Dr. Bonita T. Mangura from the UMDNJ-New Jersey Medical School and Global TB Institute, Newark, NJ, and Dr. Jeremy A. Falk from the Lung Transplant Program at Cedars Sinai Hospital, Los Angeles, CA. The survey was approved as a Chest Infections Network project and supported by the American College of Chest Physicians. Support included funding for a face-to-face meeting and on-line survey engine support. No other financial or material support was provided to the authors and participants. The sponsors had no role in analysis of the data or drafting of the manuscript.
Footnotes
This study was reviewed by Mayo Clinic Institutional Review Board and was research exempted.
All authors have reviewed and contributed to this manuscript. No authors have any financial disclosures or competing interests.
Appendix A
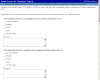 .
.
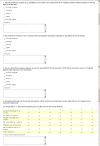

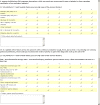
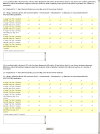
References
- 1.Bodro M, Sabe N, Santin M. Clinical features and outcomes of tuberculosis in solid organ transplant recipients. Transplant Proc. 2012;44(9):2686–2689. doi: 10.1016/j.transproceed.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Shu KH, Ho HC. A nationwide population-based study of the risk of tuberculosis in different solid organ transplantations in Taiwan. Transplant Proc. 2014;46(4):1032–1035. doi: 10.1016/j.transproceed.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 3.John GT, Shankar V. Mycobacterial infections in organ transplant recipients. Semin Respir Infect. 2002;17(4):274–283. doi: 10.1053/srin.2002.36445. [DOI] [PubMed] [Google Scholar]
- 4.Klote MM, Agodoa LY, Abbott K. Mycobacterium tuberculosis infection incidence in hospitalized renal transplant patients in the United States, 1998-2000. Am J Transplant. 2004;4(9):1523–1528. doi: 10.1111/j.1600-6143.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 5.Povoas D, Machado J, Perdigoto R. Tuberculosis in liver transplant recipients: a report of eight cases during a five year period. Acta Med Port. 2017;30(1):41–46. doi: 10.20344/amp.7052. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg HM, Burman WJ, Chaisson RE. American Thoracic society/centers for disease control and prevention/infectious diseases society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 7.Lewinsohn DM, Leonard MK, LoBue PA. Official American thoracic society/infectious diseases society of America/centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):111–115. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection - United States, 2010. MMWR. Recommend Rep. 2010;59(Rr-5):1–25. [PubMed] [Google Scholar]
- 9.Nahid P, Dorman SE, Alipanah N. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offermann G, Keller F, Molzahn M. Low cyclosporin A blood levels and acute graft rejection in a renal transplant recipient during rifampin treatment. Am J Nephrol. 1985;5(5):385–387. doi: 10.1159/000166968. [DOI] [PubMed] [Google Scholar]
- 11.Dromer C, Nashef SA, Velly JF, Martigne C, Couraud L. Tuberculosis in transplanted lungs. J Heart Lung Transplant. 1993;12(6 Pt 1):924–927. [PubMed] [Google Scholar]
- 12.Aguado JM, Torre-Cisneros J, Fortun J. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish society of infectious diseases and clinical microbiology. Clin Infect Dis. 2009;48(9):1276–1284. doi: 10.1086/597590. [DOI] [PubMed] [Google Scholar]
- 13.Meije Y, Piersimoni C, Torre-Cisneros J, Dilektasli AG, Aguado JM. Mycobacterial infections in solid organ transplant recipients. Clinical Microbiol Infect. 2014;20(Suppl 7):89–101. doi: 10.1111/1469-0691.12641. [DOI] [PubMed] [Google Scholar]
- 14.Birkenkamp KE, Lauzardo M, Mangura BT. Diagnosis and management of tuberculosis in candidates for tumor necrosis factor alpha antagonists: an experts survey. J Mycob Dis. 2014;04(05) [Google Scholar]
- 15.Anand M, Nayyar E, Concepcion B, Salani M, Schaefer H. Tuberculosis in kidney transplant recipients: A case series. World J Transp. 2017;7(3):213–221. doi: 10.5500/wjt.v7.i3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie AC, Knight SR, Morris PJ. Tuberculosis in renal transplant recipients: the evidence for prophylaxis. Transplantation. 2010;90(7):695–704. doi: 10.1097/TP.0b013e3181ecea8d. [DOI] [PubMed] [Google Scholar]
- 17.Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transpl. 2009;15(8):894–906. doi: 10.1002/lt.21709. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu A, Verma G, Humar A, Kumar D. Outcome of latent tuberculosis infection in solid organ transplant recipients over a 10-year period. Transplantation. 2014;98(6):671–675. doi: 10.1097/TP.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 19.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131(6):1898–1906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 20.Pouchot J, Grasland A, Collet C, Coste J, Esdaile JM, Vinceneux P. Reliability of tuberculin skin test measurement. Ann Intern Med. 1997;126(3):210–214. doi: 10.7326/0003-4819-126-3-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11):1192–1204. [PubMed] [Google Scholar]
- 22.Pai M, Denkinger CM, Kik SV. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartalesi F, Vicidomini S, Goletti D. QuantiFERON-TB Gold and the TST are both useful for latent tuberculosis infection screening in autoimmune diseases. Eur Respir J. 2009;33(3):586–593. doi: 10.1183/09031936.00107608. [DOI] [PubMed] [Google Scholar]
- 24.Escalante P, Kooda KJ, Khan R. Diagnosis of latent tuberculosis infection with T-SPOT((R)).TB in a predominantly immigrant population with rheumatologic disorders. Lung. 2015;193(1):3–11. doi: 10.1007/s00408-014-9655-9. [DOI] [PubMed] [Google Scholar]
- 25.Winthrop KL, Weinblatt ME, Daley CL. You can't always get what you want, but if you try sometimes (with two tests–TST and IGRA–for tuberculosis) you get what you need. Ann Rheum Dis. 2012;71(11):1757–1760. doi: 10.1136/annrheumdis-2012-201979. [DOI] [PubMed] [Google Scholar]
- 26.Sester M, van Leth F, Bruchfeld J. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Res Critical Care Med. 2014;190(10):1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 27.Logan PM, Primack SL, Staples C, Miller RR, Muller NL. Acute lung disease in the immunocompromised host. Diagnostic Accuracy Chest Radiograph. Chest. 1995;108(5):1283–1287. doi: 10.1378/chest.108.5.1283. [DOI] [PubMed] [Google Scholar]
- 28.Schneider RF, Hansen NI, Rosen MJ. Lack of usefulness of radiographic screening for pulmonary disease in asymptomatic HIV-infected adults. pulmonary complications of HIV infection study group. Arch Intern Med. 1996;156(2):191–195. [PubMed] [Google Scholar]
- 29.Soborg B, Ruhwald M, Hetland ML. Comparison of screening procedures for Mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol. 2009;36(9):1876–1884. doi: 10.3899/jrheum.081292. [DOI] [PubMed] [Google Scholar]
- 30.Vassilopoulos D, Stamoulis N, Hadziyannis E, Archimandritis AJ. Usefulness of enzyme-linked immunosorbent assay (Elispot) compared to tuberculin skin testing for latent tuberculosis screening in rheumatic patients scheduled for anti-tumor necrosis factor treatment. Addendum J Rheumatol. 2008;35(7):1464. [PubMed] [Google Scholar]
- 31.Singh N, Wagener MM, Gayowski T. Safety and efficacy of isoniazid chemoprophylaxis administered during liver transplant candidacy for the prevention of posttransplant tuberculosis. Transplantation. 2002;74(6):892–895. doi: 10.1097/01.TP.0000027945.73198.66. [DOI] [PubMed] [Google Scholar]
- 32.Jahng AW, Tran T, Bui L, Joyner JL. Safety of treatment of latent tuberculosis infection in compensated cirrhotic patients during transplant candidacy period. Transplantation. 2007;83(12):1557–1562. doi: 10.1097/01.tp.0000266578.45634.4f. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27(5):1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 34.Baciewicz AM, Chrisman CR, Finch CK, Self TH. Update on rifampin and rifabutin drug interactions. Am J Med Sci. 2008;335(2):126–136. doi: 10.1097/MAJ.0b013e31814a586a. [DOI] [PubMed] [Google Scholar]


