Abstract
Objective: To describe frequency and predictors of use of pharmacological therapies for osteoporosis in persons with a spinal cord injury (SCI).
Design: Retrospective cohort study.
Setting: United States Veterans Health Administration (VA) national databases.
Participants: 11,048 persons with a traumatic SCI who received VA health care between Fiscal Years (FY) 2005–2015. Pharmacy data from VA’s Corporate Data Warehouse were used to identify prescriptions for Food and Drug Administration-approved pharmacological treatments for osteoporosis including bisphosphonates, calcitonin, denosumab, raloxifene and teriparatide.
Outcome Measures: Demographics, clinical and SCI-related characteristics, receipt of a dual energy x-ray absorptiometry (DXA), and prevalent lower extremity fractures were examined to determine factors related to receiving a pharmacological agent for osteoporosis.
Results: 1,041 persons (9.4%) had a prescription for a pharmacological agent for osteoporosis; the majority (n = 964, 93.0%) were bisphosphonates. There was a significant decline in the number of these prescriptions from FY 2005 (13.0%) to FY 2015 (2.2%). In multivariable analysis, age (>50 years) (OR = 1.60, 95% CI 1.31–1.94); female sex (OR = 4.09, 95% CI 2.74–6.09); opioid (OR = 1.24, 95% CI 1.01–1.51) or corticosteroid (OR = 1.92, 95% CI 1.01–1.51) prescriptions; complete injury (OR = 1.26, 95% CI 1.04–1.53); receipt of a DXA scan (OR = 84.03, 95% CI 59.80–118.07) and prevalent fracture (OR = 5.43, 95% CI 4.13–7.15) were positive predictors. Black race (OR = 0.43, 95% CI 0.33–0.57) and obese BMI (OR = 0.59, 95% CI 0.45–0.76) were negative predictors.
Conclusions: Prescriptions for osteoporosis medications for persons with a SCI declined in recent years. The strongest predictors for having filled these prescriptions were having had a DXA or a prevalent fracture.
Keywords: Spinal cord injury, Osteoporosis
Introduction
Sublesional loss of bone mass is almost universally present in persons with a spinal cord injury (SCI).1,2 In men with a SCI of at least two years’ duration, the rate of lower extremity fractures, the most common fractures in persons with a SCI, was estimated in one report to be 2.14 per 100 patient-years at risk for at least one fracture.3 These fractures are associated with significant morbidity4 and excess mortality,3 with more than a threefold increased risk for mortality in older men with complete injuries.3
There are a number of pharmacological therapies available for treatment of osteoporosis which are efficacious for fracture prevention in the general healthy population.5–7 However, no studies have reported on whether they reduce fracture incidence in persons with a SCI. Use of osteoporosis medications is recommended to treat postmenopausal women and elderly men in the general population without a SCI when there is a DXA-derived diagnosis of osteoporosis or osteopenia, osteopenia on DXA with certain clinical risk factors, or a history of a hip or vertebral fracture.8 Information characterizing treatment patterns of osteoporosis in persons with a SCI is limited to one previous report, an online survey including 92 practitioners, which indicated that approximately half of these practitioners treat osteoporosis in persons with a SCI in the VA health care system.9
The purpose of this study was to describe frequency and predictors of use of pharmacological therapies for osteoporosis in persons with a traumatic SCI in a large cohort of Veterans with SCI treated in VA facilities.
Methods
Participants
A national retrospective cohort study was performed utilizing data from persons with a SCI receiving care in the VA health care system. Persons with a SCI were identified from the Allocation Resource Center (ARC), a cumulative file of Veterans who used VA care between FY2005 and FY2015. Those individuals who were in the ARC file prior to FY2005 were considered to have entered the study cohort as of Oct. 1, 2004: others entered the cohort when they began using VA care after Oct. 1, 2004. The VA Spinal Cord Dysfunction (SCD) Registry and/or the SCI/D Outcomes Database (SCIDO) from the same time periods were utilized to determine SCI-level variables. The SCD Registry included all U.S. Veterans with SCI who received care at a VA. This clinical administrative database was maintained by individual VA medical centers to track the population of Veterans with SCI followed by each center with data aggregated at a national level. The Registry included information about SCI characteristics such as etiology, date of onset, and level of injury.10 VA implemented SCIDO, a national outcomes-based registry, in 2011 to capture information on all persons with SCI who received care within the VA system as a replacement for the Registry. We used both sources of data to ensure we captured all Veterans with SCI seen in VA. National Corporate Data Warehouse (CDW) information was used to capture other patient characteristics, including non-SCI related predictor and outcome variables.11
Prescriptions for a Food and Drug Administration (FDA) approved pharmacological agent for osteoporosis including a bisphosphonate [alendronate, ibandronate (intravenous or oral), risedronate, and zoledronic acid], calcitonin, denosumab, raloxifene, and teriparatide were ascertained from the VA national CDW pharmacy files. Persons with no VA health care utilization during the study time period, and those with a nontraumatic or unknown etiology of SCI were excluded. Those with a history of a malignant neoplasm (ICD-9 codes 140–208 and 230–239) or Paget’s disease (ICD-9 code 731.0) were also excluded as several of these osteoporosis medications can be used for indications other than osteoporosis, i.e. to treat malignancy-related complications or Paget’s disease of bone.
Predictors of prescriptions for pharmacological therapies for osteoporosis
Potential predictors of a prescription for a pharmacological therapy for osteoporosis including demographic, clinical and SCI-related characteristics were determined as close to the beginning of the study time period as possible. Demographic risk factors for osteoporosis examined included age, race, ethnicity, and sex. Body mass index (BMI) and medication use (filled prescriptions for opioids, corticosteroids, and anticonvulsants in the year prior to the first osteoporosis medication12–14) were examined as clinical risk factors. We limited medications that may be risk factors for fracture in SCI that have been documented in the literature.3,4,15 SCI – specific characteristics examined include level and extent of injury.12 Receipt of a DXA [CPT code 76075 (before calendar year 2007) or CPT codes 77080–77081 (calendar year 2007 and after)] in the year prior to receipt of the first pharmacologic therapy for osteoporosis and presence of a lower extremity (ICD-9 codes 820-829) or pathological lower extremity fracture (ICD-9 codes 733.10, 733.14–733.16, and 733.19) that occurred in the year prior to receipt of the first pharmacologic therapy for osteoporosis were also examined as potential predictors.
For the comparison group of patients who did not receive an osteoporosis medication, we determined the median point between when patients in the pharmacological treatment group entered the study to when they received their first prescription for an osteoporosis medication (median = 905 days) as the anchor or starting point for control patients. For each comparison group patient, we identified the date that was 905 days from his/her study entry and then looked back one year from that point to see whether these patients had received an opioid, corticosteroid, or anticonvulsant prescription, received a DXA scan, and/or experienced a lower extremity or pathological lower extremity fracture.
Statistical analysis
Baseline characteristics of the study population including age (<50 and >=50 years), race, ethnicity, sex, BMI (underweight, normal, overweight, and obese), level (paraplegia or tetraplegia) and extent of injury (complete or incomplete) by whether or not the patient filled any prescriptions for pharmacological therapies for osteoporosis were examined using Pearson’s Chi-Square Statistic. To account for multiple comparisons we used a Bonferroni Adjustment with adjusted P = 0.004.
These analyses used the odds ratio (OR) with its 95% confidence interval (CI) as the measure of association. ORs and 95% CIs for filled prescriptions for osteoporosis and for baseline demographics, clinical and SCI-related factors, receipt of DXA, and prevalent lower extremity fractures were estimated using a logistic regression model.16
Statistical analyses were conducted using SAS release 9.4 (SAS Institute, Cary, NC) with two-sided p-values reported.
The VA Institutional Review Boards at the Charlie Norwood VA Hospital, Augusta, Georgia, and the Hines VA Hospital, Hines, Illinois approved the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of humans in research were followed during the course of this research.
Results
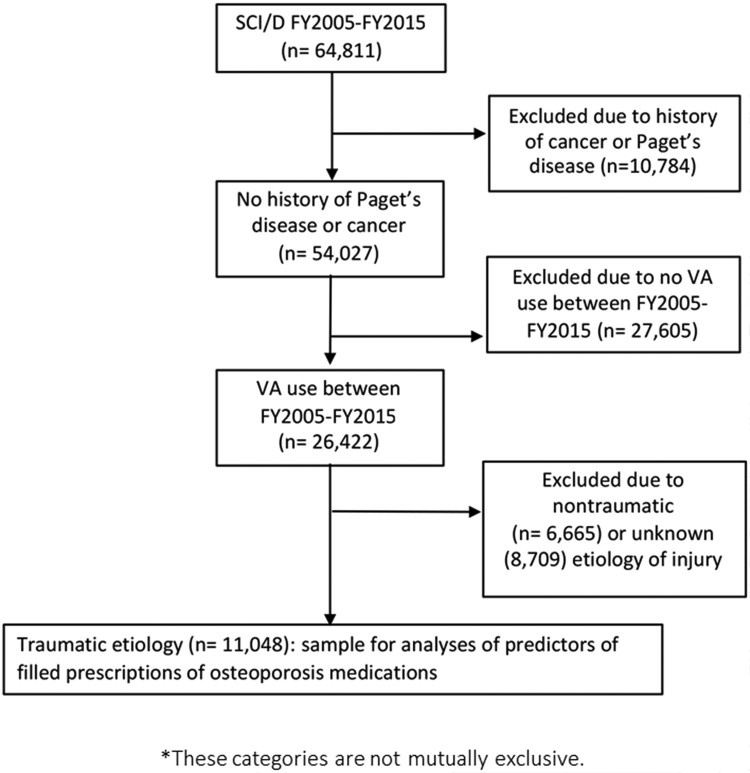
There were 64,811 unique persons in the ARC list combined with the SCD and/or SCIDO Registry between FY 2005 and FY 2015; of these, 10,784 had a history of Paget’s disease or a malignant neoplasm and were excluded. Additional exclusions included no VA health care during the time period (n = 27,605), nontraumatic spinal cord injury/condition (n = 6,665) and unknown etiology of injury (n = 8,709). The final cohort included 11,048 persons with a traumatic SCI (Fig. 1).
Figure 1.
Flowchart of patient population included in study.
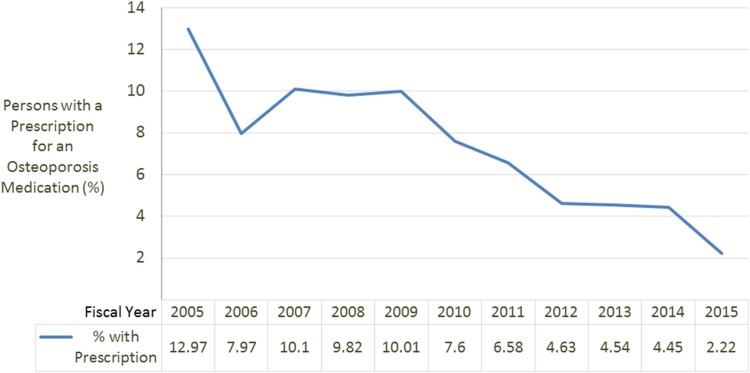
Fewer than 10% (n = 1,041, 9.4%) of the SCI cohort received a pharmacological therapy for osteoporosis over the study time period. 964 (93.0%) patients received a bisphosphonate [alendronate (n = 863, 76.9%), ibandronate (n = 4, <0.1%), risedronate (n = 4,<0.1% and zoledronic acid (n = 6, <0.1%)]. Many fewer had prescriptions for calcitonin (n = 173, 15.4%), denosumab (n = 6, 0.5%), raloxifene (n = 2, 0.1%), or teriparatide (n = 18, 1.6%). Of patients who received any osteoporosis medication, 11.7% (n = 122) changed medications at least once during the study period. For those who changed medications (exclusive of any changes from teriparatide to another agent after 24 months since this is the maximum recommended duration of treatment and it is recommended to follow this medication by an anti-resorptive agent),17 the most common medication prescribed first was alendronate (n = 115). Most of these patients were switched to calcitonin (n = 77) followed by risedronate (n = 25). Over the time period of the study, there was a significant decline in the number of filled prescriptions for osteoporosis medications. FY2005, 13.0% patients had a prescription for an osteoporosis medication, by FY2010 10.0% had a prescription for osteoporosis medication and in the last year of our study, FY2015, only 2.2% had prescriptions for osteoporosis medications (Z = −6.91; P < .0001; Fig. 2).
Figure 2.
Trends in osteoporosis prescriptions over time.
Baseline characteristics of the study population by receipt of osteoporosis medication prescriptions are provided in Table 1. A list of all the opioid and anticonvulsant medications prescribed during the study time period are provided in Appendix A. Persons with any prescriptions for an osteoporosis pharmacological therapy were more likely to be older (P = 0.0009), female (P < 0.0001), have a lower BMI (P < 0.0001), have a paraplegic-level injury (P = 0.0008) and have a complete injury (P < 0.0001), compared to those without a prescription. There were also significant differences in race among those who filled a prescription for a pharmacological agent for osteoporosis compared to those who did not (P < 0.0001).
Table 1. Patient characteristics by medication receipt (N = 11,048).
| Variable | No filled prescription (N = 10,007) | Filled prescription (N = 1,041) | P-valueƚ |
|---|---|---|---|
| Demographics | |||
| Age, n (%) | |||
| >50 | 5,751 (90%) | 654 (10%) | 0.0009 |
| ≤ 50 | 4,256 (92%) | 387 (8%) | |
| Race, n (%) | |||
| White | 6,519 (89%) | 793 (11%) | <0.0001 |
| Black | 2,010 (95%) | 116 (5%) | |
| Asian | 65 (92%) | 6 (8%) | |
| American Indian/Alaskan | 106 (91%) | 10 (9%) | |
| Hawaiian/Pacific Islander | 104 (87%) | 16 (13%) | |
| Ethnicity, n (%) | |||
| Hispanic | 504 (91%) | 49 (9%) | |
| Non-Hispanic | 8,434 (90%) | 918 (10%) | 0.4621 |
| Sex, n (%) | |||
| Female | 224 (75%) | 75 (25%) | <0.0001 |
| Male | 9,783 (91%) | 966 (9%) | |
| Clinical Characteristics | |||
| BMI, n (%) | |||
| Underweight | 519 (88%) | 68 (12%) | <0.0001 |
| Normal | 3,472 (89%) | 438 (11%) | |
| Overweight | 3,245 (90%) | 354 (10%) | |
| Obese | 2,299 (93%) | 170 (7%) | |
| Opioid 1 Year Prior to Index Date*, n (%) | |||
| Yes | 3,863 (87%) | 570 (13%) | <0.0001 |
| No | 6,144 (93%) | 471 (7%) | |
| Corticosteroids 1 Year Prior to Index Date*, n (%) | |||
| Yes | 295 (74%) | 101 (26%) | <0.0001 |
| No | 9,712 (91%) | 940 (9%) | |
| Anticonvulsants 1 Year Prior to Index Date*, n (%) | |||
| Yes | 2,334 (87%) | 335 (13%) | <0.0001 |
| No | 7,673 (92%) | 706 (8%) | |
| SCI-Related Characteristics | |||
| Level of Injury, n (%) | |||
| Paraplegia | 4,319 (90%) | 472 (10%) | 0.0008 |
| Tetraplegia | 4,987 (92%) | 431 (8%) | |
| Extent of Injury, n (%) | |||
| Complete | 3,904 (90%) | 452 (10%) | <0.0001 |
| Incomplete | 4,595 (93%) | 371 (7%) | |
| DXA 1 Year Prior to Index Date*, n (%) | |||
| Yes | 61 (14%) | 364 (85%) | <0.0001 |
| No | 9,946 (94%) | 677 (6%) | |
| Lower Extremity or Pathological Fracture 1 Year Prior to Index Date*, n (%) | |||
| Yes | 340 (62%) | 206 (38%) | <0.0001 |
| No | 9,667 (92%) | 835 (8%) | |
ƚPearson's Chi-Square.
*Index date for those with a filled prescription was the date of their first filled prescription. Index date for those without a filled prescription was the date of their first utilization plus 905 days.
Using multiple logistic regression analysis, we examined the relationship among patient demographics, clinical and SCI-related characteristics (i.e. age, race, ethnicity, sex, BMI, receipt of any prescriptions for opioids, corticosteroids or anticonvulsants in the prior year, and level and extent of injury), receipt of a DXA scan in the year prior and incident of lower extremity or pathological lower extremity fracture in the year prior to first receipt of an osteoporosis medication. We looked for the same data in the year prior to the anchor date for the comparison patients. Receipt of an opioid or corticosteroid prescription, having had a DXA, or history of a lower extremity or pathological lower extremity fracture in the year prior were predictive of receiving an osteoporosis medication after adjusting for demographic, clinical and SCI-related characteristics. Receiving an opioid prescription (OR = 1.24, 95% CI 1.01–1.51) increased the likelihood of an osteoporosis medication prescription by 24%, and receiving a corticosteroid prescription (OR = 1.92, 95% CI: 1.27–2.92) increased the likelihood of an osteoporosis medication prescription by 92%. In particular, having had a DXA scan increased the likelihood of an osteoporosis medication prescription by approximately 84-fold (OR = 84.03; 95% CI 59.80–118.07). Having experienced a lower extremity or pathological fracture in the year prior also was highly related to receiving an osteoporosis medication (OR = 5.43, 95% CI 4.13–7.15). Persons over age 50, women and those with complete injuries were more likely to have received a pharmacological medication for osteoporosis while black and obese persons were less likely to have received one of these medications (Table 2).
Table 2. Predictors of use of pharmacological treatment for osteoporosis (comparator is all others who are not given any pharmacological therapy for osteoporosis, i.e. entire cohort from FY2005 – FY2015).
| Predictors | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Demographics | ||||
| Age (> 50 vs. ≤ 50) | 1.25 | 1.10−1.43 | 1.6 | 1.31−1.94 |
| Race | ||||
| (Black vs. White) | 0.47 | 0.39−0.58 | 0.43 | 0.33−0.57 |
| (Asian vs. White) | 0.76 | 0.33−1.76 | 0.85 | 0.28−2.57 |
| (American Indian/Alaskan vs. White) | 0.78 | 0.40−1.49 | 0.64 | 0.24−1.69 |
| (Hawaiian/Pacific Islander vs. White) | 1.27 | 0.74−2.15 | 1.14 | 0.52−2.51 |
| Ethnicity (Hispanic vs. Non-Hispanic) | 0.89 | 0.66−1.21 | 0.75 | 0.49−1.15 |
| Sex (Female vs. Male) | 3.39 | 2.59−4.44 | 4.09 | 2.74−6.09 |
| Clinical Factors | ||||
| BMI (Underweight vs Normal) | 1.04 | 0.79−1.36 | 1.14 | 0.78−1.66 |
| BMI (Overweight vs Normal) | 0.87 | 0.75−1.00 | 0.84 | 0.67−1.04 |
| BMI (Obese vs Normal) | 0.59 | 0.49−0.71 | 0.59 | 0.45−0.76 |
| Opioid 1 Year Prior to Index* Date (Yes vs. No) | 1.93 | 1.69−2.19 | 1.24 | 1.01−1.51 |
| Corticosteroids 1 Year Prior to Index* Date (Yes vs. No) | 3.54 | 2.80−4.48 | 1.92 | 1.27−2.92 |
| Anticonvulsants 1 Year Prior to Index* Date (Yes vs. No) | 1.56 | 1.36−1.79 | 1.14 | 0.92−1.41 |
| SCI-Related Factors | ||||
| SCI Level (Paraplegia vs. Tetraplegia) | 1.27 | 1.10−1.45 | 1.1 | 0.91−1.34 |
| SCI Extent (Complete vs. Incomplete) | 1.43 | 1.24−1.66 | 1.26 | 1.04−1.53 |
| DXA 1 Year Prior to Index Date* (Yes vs. No) | 87.62 | 66.09−116.17 | 84.03 | 59.80−118.07 |
| Lower Extremity or Pathological Fracture 1 Year Prior to Index Date* (Yes vs. No) | 7.02 | 5.82−8.46 | 5.43 | 4.13−7.15 |
CI, confidence interval; OR, odds ratio.
*Index date for those with a filled prescription was the date of their first filled prescription.
Index date for those without a filled script was the date of their first utilization plus 905 days.
Discussion
In clinical practice within the VA health system, less than 10% of persons with a traumatic SCI filled a prescription for a pharmacological therapy for osteoporosis over the last decade, most commonly for a bisphosphonate (93%). Receipt of a DXA or a history of a prior lower extremity fracture and medications including corticosteroids and opioids were significant factors in whether a person with SCI received pharmacological therapy for osteoporosis. Patients with complete injuries also were more likely to have been prescribed one of these medications; complete injury is a well-established risk factor for fracture.12 Predictors of osteoporosis medication use in the general population (i.e. older age, white, female, and not obese)18 were also predictors for the SCI population, suggesting that providers who do prescribe osteoporosis medications incorporate information on high risk populations for fracture from the general population without a SCI.19
That few patients had prescriptions for pharmacological therapy for osteoporosis is curious, as a prior study of VA providers in which 54% of respondents indicated they prescribed medications for SCI-induced bone loss with 43% reporting that they prescribed bisphosphonates.9 While that study did not ask whether they prescribe for most or fewer patients with osteoporosis, the data suggest that osteoporosis medication was used more frequently than was found in our study. In agreement with this study,9 alendronate was the most commonly filled bisphosphonate (93.6%) and there were very few filled prescriptions for other medications such as denosumab, teriparatide or raloxifene (less than 4% combined). Use of bisphosphonates as first line treatment for osteoporosis in SCI is in accord with guidelines for treatment of osteoporosis in the general healthy population without a SCI; however, denosumab may also be used as a first line therapy17 but was infrequently used in this cohort. Similar to reports in the general population without a SCI, there was a significant decline in prescriptions for osteoporosis medications over the time period of this study.20,21 A recent study of osteoporosis treatment after hip fracture during the same study period as ours found a very similar trend from 9.8% in 2004 to 3.3% in 2015.22 It is not clear why rates have dropped, especially since studies have found reduced fracture risk, regardless of pretreatment bone density.23 It is unclear whether lack of knowledge, concerns about drug tolerability and side effects, or other factors may account for this trend, although public perception about long-term potential possible, albeit rare, side effects, is likely one reason.24
In persons with a SCI, low bone mass and/or osteoporosis by DXA,2,25 and a history of prevalent lower extremity fractures12 are the risk factors best able to predict incident lower extremity osteoporotic fractures. Our study suggests that clinicians are using these fracture risk factors to appropriately target pharmacological therapies for osteoporosis to those at highest risk for fracture. Moreover, in the general healthy population without a SCI, there is a time dependent risk of future fractures, with a recent fracture within a year increasing the risk for future fractures much more so than remote fractures26 and fractures in the one-year prior time period were considered in this cohort.
Medications including corticosteroids and opioids also predicted receipt of pharmacological therapy for osteoporosis. It has been hypothesized that corticosteroids, which substantially increase the risk of fracture in the general population without a SCI27 may increase risk of fractures in persons with a SCI,15 although there are no large-scale studies that have specifically examined this question. Use of opioids is common in persons with a SCI,28 and opioids increase risk of fracture in this population.13
This study has a number of important strengths. To our knowledge, this is the first comprehensive study to characterize what factors trigger pharmacological management of osteoporosis and what treatments are being prescribed in persons with a SCI. Although the number of women included was small (n = 299), both men and women, and persons of all races and ethnicities were included. Further, this study used a static analytic dataset that was derived using information from multiple data points over time.
There are also a number of limitations to consider. We were unable to include duration of injury or ambulatory status in our models as this information was not available in the administrative data, nor were we able to examine ASIA scores as these were missing on over 5,000 cases and 1,726 cases, respectively. We excluded those with a history of cancer because bisphosphonates and denosumab may be used to treat bone metastasis or hypercalcemia in these persons and not osteoporosis, but the possibility exists that these medications were being used for osteoporosis.29,30 In addition, we did not separate out short courses of opioid or steroid use in this cohort, which would be unlikely to affect osteoporosis and fracture risk. We did not consider care outside the VA; approximately 12% of Veterans with a SCI/D in one report were receiving medication outside the VA system.29 We did not have information from the DXAs as to whether there was osteopenia or osteoporosis, although the majority would be expected to have some degree of low bone mass.2
In conclusion, although pharmacological therapies for osteoporosis are not commonly prescribed for Veterans with a SCI, it appears that clinicians do rely on factors that predict fracture risk in this population including information from DXA measurements, prior fracture history and use of medications associated with bone loss, as well as traditional clinical risk factors, to target treatments with pharmacological therapies for osteoporosis in persons with SCI.
Appendix A
Prescriptions by medication class.
| Opioids | # of prescriptions |
|---|---|
| Hydrocodone/Acetaminophen | 7970 |
| Acetaminophen/Oxycodone | 3487 |
| Tramadol/Tramadol HCL | 3238 |
| Oxycodone/Oxycodone HCL | 2590 |
| Morphine/Morphine sulfate | 2533 |
| Acetaminophen/Codeine | 1672 |
| Methadone/Methodone HCL | 1112 |
| Fentanyl | 722 |
| Hydrocodone/APAP | 573 |
| Hydromorphone | 555 |
| Propoxyphene APAP | 439 |
| Propoxyphene/Propoxyphene HCL | 197 |
| Meperidine | 124 |
| Codeine | 119 |
| Hydrocodone Bitartrate | 70 |
| Belladonna/opium | 61 |
| Buprenorphine/Naloxone | 44 |
| Aspirin/Oxycodone | 17 |
| Propoxyphene/ Acetaminophen | 10 |
| Naloxone/Pentazocine | 7 |
| Buprenorphine | 6 |
| Codeine Phospate | 5 |
| Tapentadol | 5 |
| Levorphanol | 4 |
| Nalbuphine | 4 |
| Oxycodone/acetaminophen | 4 |
| Propoxyphene Napsylate | 4 |
| Darvocet | 3 |
| Oxycodone APAP | 3 |
| CPD Baclfen/Bupv/Clond/Hydromorphone INJ | 2 |
| Percocet | 2 |
| Alfentanil | 1 |
| Butorphanol | 1 |
| Clonodine/hydromorphone inj | 1 |
| CPD Baclfen/Clond/Hydromorphone | 1 |
| CPD Bupiv/Hydromorphone | 1 |
| Hydromorphone & Bacolfen | 1 |
| Hydrocodone/Ibuprofen | 1 |
| Hydromorphone Lyphilized PWD | 1 |
| Pain Cocktail | 1 |
| Roxicet oral solultion | 1 |
| Sufentanil | 1 |
| Tylenol #3 | 1 |
| Vicodin | 1 |
| White Hydrocodone/APAP | 1 |
| Anticonvulsants | # of prescriptions |
|---|---|
| Gabapentin | 5385 |
| Divalproex | 431 |
| Topriramate | 403 |
| Carbamzepine | 339 |
| Levetiracetam | 330 |
| Phenytoin | 287 |
| Lamotrigine | 266 |
| Valproic acid | 165 |
| Primidone | 54 |
| Oxcarbazepine | 46 |
| Tiagabine | 13 |
| Zonisamide | 12 |
| Lacosamide | 8 |
| Fosphenytoin | 2 |
| Clobazam | 1 |
Acknowledgements
The contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government.
Disclaimer statements
Contributors FMW, BL, CR, LC were responsible for writing the protocol and report, conducting the research and drafting the manuscript. SM and BG conducted the data analysis and drafted the statistical analysis section of the manuscript. All authors gave final approval for the manuscript.
Funding This work was funded by the U.S. Department of Defense (DOD) [grant number #SC150092].
Conflicts of interest None.
ORCID
Cara Ray http://orcid.org/0000-0002-1383-8801
Laura D. Carbone http://orcid.org/0000-0003-3370-4680
References
- 1.Spinal Cord Injury Facts and Figures at a Glance. 2012; https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf. Accessed May 3, 2017.
- 2.Abderhalden L, Weaver FM, Bethel M, Demirtas H, Burns S, Svircev J, et al. . Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders. Osteoporos Int. 2017;28(3):925–34. doi: 10.1007/s00198-016-3841-y [DOI] [PubMed] [Google Scholar]
- 3.Carbone LD, Chin AS, Burns SP, Svircev JN, Hoenig H, Heggeness M, et al. . Mortality after lower extremity fractures in men with spinal cord injury. J Bone Miner Res. 2014;29(2):432–9. doi: 10.1002/jbmr.2050 [DOI] [PubMed] [Google Scholar]
- 4.Carbone L, et al. . The association of anticonvulsant use with fractures in spinal cord injury. Am J Phys Med Rehab. 2013;24:2261–7. [DOI] [PubMed] [Google Scholar]
- 5.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. . Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. vertebral efficacy with risedronate therapy (VERT) study group. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344 [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. . Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. fracture Intervention Trial research group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/S0140-6736(96)07088-2 [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. . Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312 [DOI] [PubMed] [Google Scholar]
- 8.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. . Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morse LR, Giangregorio L, Battaglino RA, Holland R, Craven BC, Stolzmann KL, et al. . VA-based survey of osteoporosis management in spinal cord injury. Pm r. 2009;1(3):240–4. doi: 10.1016/j.pmrj.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BM, Evans CT, Ullrich P, Burns S, Guihan M, Miskevics S, et al. . Using VA data for research in persons with spinal cord injuries and disorders: lessons from SCI QUERI. J Rehabil Res Dev. 2010;47(8):679–88. doi: 10.1682/JRRD.2009.08.0117 [DOI] [PubMed] [Google Scholar]
- 11.(CDW) CDW. VA Informatics and computing infrastructure: corporate data warehouse (CDW). 2009; http://www.virec.research.va.gov/DSS-NNDEs.htm. Accessed April 2, 2018.
- 12.Bethel M, Weaver FM, Bailey L, Miskevics S, Svircev JN, Burns SP, et al. . Risk factors for osteoporotic fractures in persons with spinal cord injuries and disorders. Osteoporos Int. 2016;27(10):3011–21. doi: 10.1007/s00198-016-3627-2 [DOI] [PubMed] [Google Scholar]
- 13.Carbone LD, Chin AS, Lee TA, Burns SP, Svircev JN, Hoenig HM, et al. . The association of opioid use with incident lower extremity fractures in spinal cord injury. J Spinal Cord Med. 2013;36(2):91–6. doi: 10.1179/2045772312Y.0000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone L, Chin AS, Lee TA, Burns SP, Svircev JN, Hoenig H, et al. . The association of anticonvulsant use with fractures in spinal cord injury. Am J Phys Med Rehabil. 2013;92(12):1037–46. quiz 47-50. doi: 10.1097/PHM.0000000000000014 [DOI] [PubMed] [Google Scholar]
- 15.Qin W, Bauman WA, Cardozo C.. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x [DOI] [PubMed] [Google Scholar]
- 16.Yun H, Curtis JR, Guo L, Kilgore M, Muntner P, Saag K, et al. . Patterns and predictors of osteoporosis medication discontinuation and switching among Medicare beneficiaries. BMC Musculoskelet Disord. 2014;15:112. doi: 10.1186/1471-2474-15-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkard D, Beckett T, Kourtjian E, Messingschlager C, Sipahi R, Padley M, et al. . Effects of bone remodeling agents following teriparatide treatment. Osteoporos Int. 2018;29(16):1351–57. doi: 10.1007/s00198-018-4434-8 [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Forciea MA, McLean RM, Denberg TD.. Clinical guidelines committee of the American college of P. treatment of Low bone density or osteoporosis to prevent fractures in Men and women: a clinical practice guideline update from the American college of physicians. Ann Intern Med. 2017;166(11):818–39. doi: 10.7326/M15-1361 [DOI] [PubMed] [Google Scholar]
- 19.Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, Van K, Hyun D.. Osteoporosis: a review of treatment options. Pharm Ther. 2018;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD.. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–37. doi: 10.1002/jbmr.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes BL, Curtis JR, Laster A, Saag K, Tanner SB, Liu C, et al. . Osteoporosis care in the United States after declines in reimbursements for DXA. J Clin Densitom. 2010;13(4):352–60. doi: 10.1016/j.jocd.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai RJ, Mahesri M, Abdia Y, Barberio J, Tong A, Zhang D, et al. . Association of osteoporosis medication use after hip fracture with prevention of subsequent nonvetebral fractures: An instrumental variable analysis. JAMA Open. 2018;1(3):e180826. doi: 10.1001/jamanetworkopen.2018.0826 doi: 10.1001/jamanetworkopen.2018.0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. . HORIZON recurrent fracture trial. Zolendronic acid and clinical fractures and morality after hip fracture. N Engl JMed. 2007;357(18):1799–809. doi: 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha S, Wang Z, Laucis N, Bhattacharyya T.. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996-2012: An ecological analysis. J Bone Miner Res. 2015;30(12):2179–87. doi: 10.1002/jbmr.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garland DE, Adkins RH, Kushwaha V, Stewart C.. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27(3):202–6. doi: 10.1080/10790268.2004.11753748 [DOI] [PubMed] [Google Scholar]
- 26.Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, et al. . Imminent risk of fracture after fracture. Osteoporos Int. 2017;28(3):775–80. doi: 10.1007/s00198-016-3868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C.. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993 [DOI] [PubMed] [Google Scholar]
- 28.Hatch MN, Raad J, Suda K, Stroupe KT, Hon AJ, Smith BM.. Evaluating the use of medicare Part D in the spinal cord injury/disorder Veteran population. Arch Phys Med Rehabil. 2018;99(6):1099–107. doi: 10.1016/j.apmr.2017.12.036 [DOI] [PubMed] [Google Scholar]
- 29.Viana R, Payne MW.. Use of calcitonin in recalcitrant phantom limb pain complicated by heterotopic ossification. Pain Res Manag. 2015;20(5):229–33. doi: 10.1155/2015/782948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh MW, Zhou H, Adams AL, Ituarte PH, Li N, Liu IL, et al. . The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715–23. doi: 10.7326/M15-1232 [DOI] [PubMed] [Google Scholar]