Abstract
Objective
This study aims to assess the feasibility of digital perioperative behavioral pain medicine intervention in breast cancer surgery and evaluate its impact on pain catastrophizing, pain, and opioid cessation after surgery.
Design and Setting
A randomized controlled clinical trial was conducted at Stanford University (Palo Alto, CA, USA) comparing a digital behavioral pain medicine intervention (“My Surgical Success” [MSS]) with digital general health education (HE).
Participants
A convenience sample of 127 participants were randomized to treatment group. The analytic sample was 68 patients (N = 36 MSS, N = 32 HE).
Main Outcomes
The primary outcome was feasibility and acceptability of a digital behavioral pain medicine intervention (80% threshold for acceptability items). Secondary outcomes were pain catastrophizing, past seven-day average pain intensity, and time to opioid cessation after surgery for patients who initiated opioid use.
Results
The attrition rate for MSS intervention (44%) was notably higher than for HE controls (18%), but it was lower than typical attrition rates for e-health interventions (60–80%). Despite greater attrition for MSS, feasibility was demonstrated for the 56% of MSS engagers, and the 80% threshold for acceptability was met. We observed a floor effect for baseline pain catastrophizing, and no significant group differences were found for postsurgical pain catastrophizing or pain intensity. MSS was associated with 86% increased odds of opioid cessation within the 12-week study period relative to HE controls (hazard ratio = 1.86, 95% confidence interval = 1.12–3.10, P = 0.016).
Conclusions
Fifty-six percent of patients assigned to MSS engaged with the online platform and reported high satisfaction. MSS was associated with significantly accelerated opioid cessation after surgery (five-day difference) with no difference in pain report relative to controls. Perioperative digital behavioral pain medicine may be a low-cost, accessible adjunct that could promote opioid cessation after breast cancer surgery.
Keywords: Surgery, Acute pain, Digital, psychology, Behavioral medicine
Introduction
Up to 6.5% of patients prescribed opioids after surgery will transition to long-term opioid use [1,2], and up to 10% of women will continue to fill opioid prescriptions three months after breast reconstruction surgery [3]. Time to opioid cessation after surgery is predicted by an array of psychological factors such as negative pain appraisal and catastrophizing [4], anxiety [3], and depression [4, 5]. Combined, data suggest a need for interventions that address the modifiable psychological factors associated with prolonged opioid use after surgery.
Interventions that equip patients with the skills to self-manage the pain and distress related to their surgery could be integrated into multimodal perioperative pathways designed to enhance recovery after surgery [4]. Cognitive-behavioral therapy for chronic pain (pain-CBT) is effective skills-based behavioral medicine with demonstrated efficacy for improving a wide range of pain outcomes [6] including reduced opioid use [7] and misuse [8] in outpatients. Multiple barriers impede patient access to behavioral pain medicine [9], including access to skilled therapists. Brief Internet-based interventions may improve access to behavioral medicine in surgical patients and complement their medical treatments.
The goal of the current study was to test a low-cost, fully automated digital behavioral medicine intervention (“My Surgical Success” [MSS]) to address postsurgical pain, enhance surgical recovery, and potentially accelerate opioid cessation after surgery. MSS extends our prior work on brief pain interventions and is modeled after a single-session chronic pain outpatient class (“Empowered Relief”) that provides pain education and self-regulation skills, including strategies to regulate cognition, emotion, and physiological responses to pain and related distress [10,11]. MSS mirrors the instructional content and tools of “Empowered Relief” with two key adaptations: 1) the title and context are adapted to the surgical patient and 2) the mode of delivery is fully digital with no in-person contact; accordingly, the intervention is received online and individually rather than in a group classroom. The piloted version of MSS used in this study included an online 90-minute pain education and skills video and a downloadable personalized plan and digital relaxation audiofile. The primary aim was to determine the feasibility and acceptability of the MSS intervention in a breast cancer surgery sample. The secondary aims were to evaluate the impact of MSS on pain catastrophizing, past seven-day average pain intensity, and postsurgical opioid cessation for patients who initiated opioid use after surgery. The tertiary aim (exploratory) was to examine group changes in pain interference, anxiety, depression, and physical function.
Methods
The study was approved by the Stanford University Institutional Review Board and registered with ClinicalTrials.gov (NCT03076190). Surgeons informed all patients of the study and provided the names of all patients scheduled for upcoming breast cancer surgery to study staff. English-speaking women over 18 years of age who were scheduled for lumpectomy or mastectomy were assessed for eligibility. Exclusion criteria included inability to complete study procedures (e.g., due to cognitive impairment or medical disability), lack of access to Internet and phone, pregnancy, or an ongoing pain- or disability-related legal claim.
Enrollment, Randomization, and Allocation to Digital Study Groups
Recruitment occurred between August 2015 and June 2017, and all follow-up assessments were completed by 2017. Study staff contacted patients who met the inclusion criteria by phone and invited them to enroll in the study; no compensation was offered. Informed consent was obtained online, and following enrollment, patients completed their baseline questionnaires online (see Assessments). Patients were then randomly assigned to receive one of two digital treatments: MSS or health education material (HE control). The randomization scheme was computer generated with a 1:1 ratio to ensure an equal number of patients in each group. When groups became unbalanced, an 8:2 ratio was applied to rebalance group assignment. Data were de-identified for analyses, and outcome assessors were blinded to study group assignment. The intervention was deployed online, and data were collected online and by phone. There was no in-person contact with a study therapist or research staff.
Health Education
Before surgery, patients received digital text education about health and nutrition that was framed in terms of their importance in enhancing recovery after surgery (this group received no 90-minute video).
“My Surgical Success”
An MSS website contained a 90-minute pain psychoeducational video, a downloadable Personalized Plan for Surgical Success, and a downloadable relaxation audiofile. Video content included information and skills to regulate cognition, emotion, and physiologic hyperarousal related to pain, including relaxation, thought reframing, and behaviors that modulate attention and counteract helplessness about pain. During the video, learners were guided to self-tailor and apply the information by completing their Personalized Plan for Surgical Success. Thus, participant burden was substantially greater for patients assigned to the MSS intervention compared with the HE control group. Participants were required to view the 90-minute video in a single sitting in order to trigger deployment of the postvideo survey, with completion immediately following video viewing.
Primary Outcome: Feasibility and acceptability of the “My Surgical Success” intervention.
Secondary Outcomes: Group differences in 1) pain catastrophizing, 2) past seven-day average pain intensity, and 3) time to opioid cessation after surgery.
Tertiary Outcomes: Group changes in pain interference, anxiety, depression, and physical function.
Assessments
Questionnaires
After electronic informed consent, enrolled patients completed demographic items at baseline. In addition, a battery of patient-reported outcomes (described below) was administered both at baseline and at weeks 2, 4, 8, and 12 after surgery.
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) [12] is the most widely used empirical and clinical measure of pain catastrophizing (13 items), a pattern of negative cognition and emotion related to actual or anticipated pain. This measure has been used in surgical research [13], specifically to examine predictors of postsurgical opioid cessation vs prolonged use (e.g., [4]).
Patient-Reported Outcomes Measurement Information System
The Patient-Reported Outcomes Measurement Information System (PROMIS) has been successfully applied in pain [14–17] and surgical research [18, 19]. Higher scores on average pain interference, anxiety, and depression signify greater severity of these symptoms, whereas higher scores on physical function signify greater function. Questions were framed according the experience of symptoms or functioning over the past seven days.
PROMIS Pain Intensity
Pain intensity was assessed on a numerical rating scale using an abbreviated PROMIS Pain Intensity measure [20]; respondents were asked to rate their average pain intensity over the previous seven days on a scale of 0–5.
Feasibility and Acceptability
Feasibility of the MSS digital treatment was determined by the engagement rate, that is, proportion of participants who watched the whole video for participants assigned to this group. Acceptability was assessed using a set of questions asked immediately following the intervention. Patients in the MSS group were asked: 1) Was the video easy to understand? 2) How relevant was the video to you? 3) How useful was the information presented in the video? 4) Please rate your overall satisfaction with the video. 5) How likely are you to use the skills and information you learned? Likewise, participants in the HE control group were asked to rate the understandability of the digital health information, its relevance to them, perceived usefulness, likelihood they would use the information learned, and satisfaction with the intervention. The rating scale ranged from 0 to 6; thus, a score of 4.8 or greater on each of these items would indicate exceeding the 80% threshold of acceptability.
Daily Measures
After surgery, daily surveys were administered through REDCap or by phone to assess the following variables: Current Pain and Average Pain over the last 24 hours (on a Likert-type scale where 0 = no pain and 10 = worst pain imaginable), Average Distress (on a Likert-type scale where 0 = no distress and 10 = extreme distress), and Opioid Use and Opioid Stoppage items. Similar methods have been used in surgical research to calculate time to opioid cessation [21, 22].
Opioid Stop Date
Postsurgical opioid stop date was self-reported by patients and verified through the daily surveys. The opioid stop date was collected through the two-, four-, eight-, and 12-week follow-up surveys with the question “On what date did you stop taking your opioid medication?” Several patients reported more than one opioid stop date (MSS 22/36, HE 22/32), and the last reported date was utilized for analysis. The number of postsurgical days using opioids was calculated by subtracting the opioid stop date from the surgery date obtained in the medical chart. Of note, pharmacy and medical chart records were not used owing to their imprecision in indexing daily consumption of medication and cessation of use.
Surgical Date and Complexity
Medical chart review was used to determine breast cancer surgery date and type. The sample was stratified into three surgical complexity groups: 1) minor surgical complexity (lumpectomy), 2) moderate surgical complexity (lumpectomy with sentinel node dissection), and 3) major surgical complexity (mastectomy with reconstruction).
Surgical Anesthesia
In all surgical cases, study patients routinely received the maximum recommended dose of local anesthetic as a nerve and field block administered by the surgeon (cc/kg of 0.25% Marcaine). No perioperative plan was discussed before surgery; patients received Tylenol either orally or intravenously unless contraindicated. Patients in the major surgical complexity group (N = 4) were potential candidates for a regional block; chart review for these patients revealed that three (75%) received a regional nerve block.
Statistical Analysis
All analyses were conducted using SAS9.4. For acceptability and patient-reported outcomes, group differences were tested using analyses of varaiance (ANOVAs) for numerical covariates and chi-square tests for categorical covariates. The effect of the MSS vs HE control on opioid cessation in the 12-week postsurgical follow-up period was assessed using survival analyses. The opioid stop event was considered to have occurred if the patient reported an opioid stop date and was censored otherwise. Duration to opioid cessation was computed as days from surgery to the last patient-reported date of opioid stoppage or last known patient contact (for censored observations). Product-limit survival estimates (Kaplan-Meier curves) and the log-rank test of equality of survival curves between the MSS and HE control groups were generated using PROC LIFETEST. Time to opioid cessation event was assessed using a Cox proportional hazard regression model with the experimental condition as the predictor following a supremum test of the validity of the proportionality assumption (PROC PHREG). Two follow-up analyses were conducted. First, we tested whether MSS and HE control differed in the odds of postsurgical opioid cessation (independent of duration) using a logistic regression model (PROC LOGISTIC). Second, we tested whether the median time to opioid cessation was different between the two groups using a nonparametric test of medians (PROC NPAR1WAY with the median option).
For each of the psychological covariates (pain catastrophizing, pain intensity, pain interference, anxiety, depression, and physical function), we assessed the treatment effect (MSS vs HE control) as follows. We first assessed whether there was evidence of a treatment × time effect in the postsurgical period (two-, four-, eight-, and 12-week follow-up), and finding none, we averaged the postsurgical measures to create a composite postsurgical measure. For each of the covariates, we then specified a mixed-design ANOVA with treatment (MSS vs HE control, between-subjects), time (pre vs post, within-subjects), and the treatment × time interaction as predictors.
Power Analysis
The study was designed to detect a hazard ratio of 1.9, the equivalent of a “medium” effect in terms of Cohen’s d [23, 24], for the effect of the MSS treatment vs HE control in the 12-week postsurgical follow-up after surgery using Cox proportional hazards regression (PROC POWER). The analysis indicated that a total sample of 82 patients would be needed to yield a power of 0.8 and a two-tailed alpha of 0.05.
Results
Patient Characteristics and Preliminary Analyses
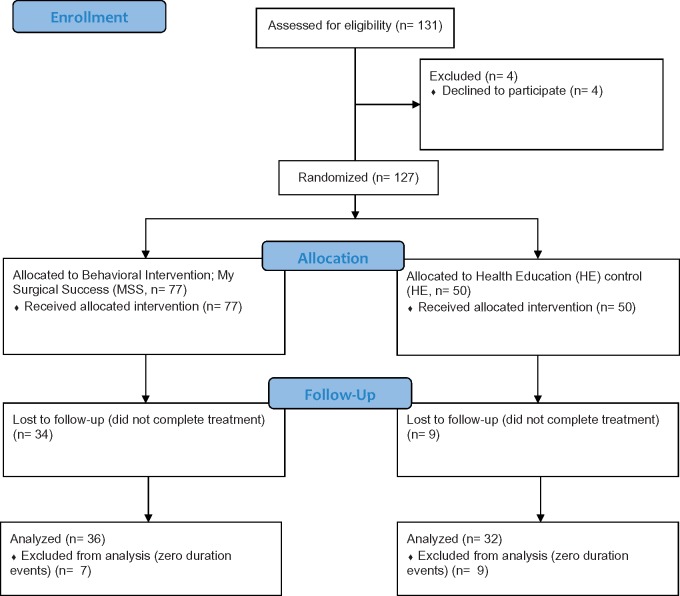
Figure 1 depicts the consort diagram [24] for the study. A total of 131 patients were referred, screened, and enrolled into the study. Four patients (3%) dropped out after providing consent but before surgery. Initially, 127 patients were randomly assigned to one of the two treatment groups, 77 to the MSS group and 50 to the HE control group. A total of 43 (MSS, N = 34 [44%]; HE control, N = 9 [18%]) either withdrew from the study or did not complete study procedures, likely reflecting the greater time and effort of the treatment burden in the MSS condition (90-minute video) compared with the online text HE control noted earlier; as such, the overall engagement rate for MSS was 56%, vs 82% for the HE control. The demographic variables (Table 1) and baseline measures (Table 2) describing the enrolled sample (N = 84) did not differ from those of the unenrolled sample (N = 43); this suggests that the attrition might be due to the perceived intervention burden rather than to a systematic association with the observed attributes of the patient population.
Figure 1.
Consort flow diagram.
Table 1.
Demographics* by study group
| Demographics | MSS N = 36 | HE Control N = 32 | P Value |
|---|---|---|---|
| Race, No. (%) | |||
| African American | 0 (0) | 1 (3.33) | 0.0728 |
| American Indian/Alaska Native | 0 (0) | 1 (3.33) | |
| Asian | 10 (27.78) | 3 (10) | |
| Caucasian | 20 (55.56) | 23 (76.67) | |
| Other/missing | 6 (16.67) | 1 (3.33) | |
| Pacific Islander | 0 (0) | 1 (3.33) | |
| Marital status, No. (%) | |||
| Married/partnered | 28 (77.78) | 23 (74.19) | 0.3124 |
| Never married | 4 (11.11) | 3 (9.68) | |
| Separated/divorced | 2 (5.56) | 5 (16.13) | |
| Widowed | 2 (5.56) | 0 (0) | |
| Income, No. (%) | |||
| <$10,000 | 2 (5.88) | 2 (6.9) | 0.8499 |
| $10,000–$30,000 | 1 (2.94) | 1 (3.45) | |
| $30,000–$50,000 | 2 (5.88) | 4 (13.79) | |
| $50,000–$70,000 | 3 (8.82) | 3 (10.34) | |
| >$70,000 | 26 (76.47) | 19 (65.52) | |
| Employment, No. (%) | 0.3178 | ||
| Full-time | 15 (41.67) | 15 (48.39) | |
| Other | 12 (33.33) | 4 (12.9) | |
| Part-time | 5 (13.89) | 6 (19.35) | |
| Retired | 4 (11.11) | 6 (19.35) | |
| Unemployed | 2 (5.56) | 0 (0) | |
| Surgical complexity, No. (%) | 14 (38.89) | 12 (37.5) | 0.834 |
| Moderate | 20 (55.56) | 17 (53.13) | |
| Severe | 2 (5.56) | 3 (9.38) | |
| Age | 0.9711 | ||
| Mean, y | 51.27 | 51.16 | |
| Median, y | 52 | 49 | |
None of the P values were significant at P < 0.05.
HE = health education material control; MSS = “My Surgical Success.”
Total N = 68.
Table 2.
Baseline presurgical measures comparison between treatment groups (analytic sample*)
| Variable | MSS N = 36 | HE Control N = 32 | P Value |
|---|---|---|---|
| Pain Intensity | 1.47 (0.12) | 1.74 (0.13) | 0.138 |
| Pain Catastrophizing | 7.33 (1.3) | 10.06 (1.4) | 0.158 |
| PROMIS Physical Function | 37.52 (5.42) | 40.49 (6.94) | 0.054 |
| PROMIS Pain Interference | 47.84 (1.59) | 50.35 (1.72) | 0.287 |
| PROMIS Anxiety | 54.49 (1.48) | 57.5 (1.6) | 0.172 |
| PROMIS Depression | 47.15 (1.29) | 50.28 (1.39) | 0.103 |
Baseline variables of physical function and depression that were different at P < 0.1 were specified as univariate predictors in the core survival model, were found to be not significant (P < 0.5906 and P < 0.2580), and therefore were excluded from the final model.
HE = health education material control; MSS = “My Surgical Success”; PROMIS = Patient-Reported Outcomes Measurement Information Systems.
N = 68 (analytic sample). PROMIS assessments are based on a mean of 50 with an SD of 10.
Of the enrolled patients, 16 (MSS, N = 9; HE control, N = 7), could not experience postsurgical opioid cessation because they were either not prescribed opioids (N = 10) or did not take opioids beyond their procedure day (N = 6). This resulted in a final analytic sample of 68 patients (MSS, N = 36; HE control, N = 32). There were no differences in demographic variables (Table 1) or baseline presurgical variables (Table 2) by group assignment, suggesting that randomization created equivalent study groups.
Primary Outcome
Feasibility and Acceptability
As noted earlier, the feasibility of the MSS treatment (56% completing the treatment) was lower than HE control (82% completing treatment). The results revealed that 97% (N = 35) of the 36 patients assigned to the MSS treatment group completed postvideo questions, and 94% assigned to the HE control completed post-treatment questions. For MSS, mean scores met or exceeded an 80% (4.8) threshold for items related to “easy to understand” (mean score = 5.9, SD = 0.2), “relevant” (mean score = 5.0, SD = 1.6), “usefulness” (mean score = 5.1, SD = 1.3), “satisfied” (mean score = 5.2, SD = 1.2), and “likelihood of use” (mean score = 5.3, SD = 1.2). In the HE control group, only “easy to understand” and “likelihood of use” met the 80% threshold. Notably, the two groups did not differ significantly on patient experience variables (P > 0.05).
Secondary Outcomes
Pain Catastrophizing
We observed an overall floor effect for pain catastrophizing in our study sample that precluded meaningful testing of pain catastrophizing treatment effects. The mean PCS scores were 7.33 (SEM = 1.3) for the MSS group and 10.06 (SEM = 1.4) for the HE control group, with no significant group differences. These PCS scores were far below subclinical range and can best be described as being nonsymptomatic of pain catastrophizing. We observed a significant main effect of time (P = 0.03); catastrophizing reduced from baseline (M = 8.69, SEM = 0.96) to postsurgery (M = 6.18, SEM = 1.03). No main effect of treatment was observed (MSS M = 6.62, SEM = 1.12, vs HE control M = 8.24, SEM = 1.19, P = 0.32). We did not observe a treatment × time interaction effect (P = 0.34).
For average pain intensity, we did not observe a significant main effect of group (MSS M = 1.73, SEM = 0.09, vs HE control M = 1.81, SEM = 0.09, F(1, 66) < 1, P = 0.53). We observed a significant main effect of time (P = 0.0016); average pain was lower at baseline (M = 1.61, SEM = 0.09) compared with postsurgery (M = 1.92, SEM = 0.07). We observed a significant group × time interaction (P = 0.0394); for the MSS group, average pain increased from baseline (M = 1.47, SEM =0.12) to postsurgery (M = 1.98, SEM = 0.10, P = 0.0002), but for the HE control group, there was no such increase from baseline (M = 1.75, SEM = 0.13) to postsurgery (M = 1.86, SEM = 0.10, P = 0.4191).
Time to Opioid Cessation After Surgery
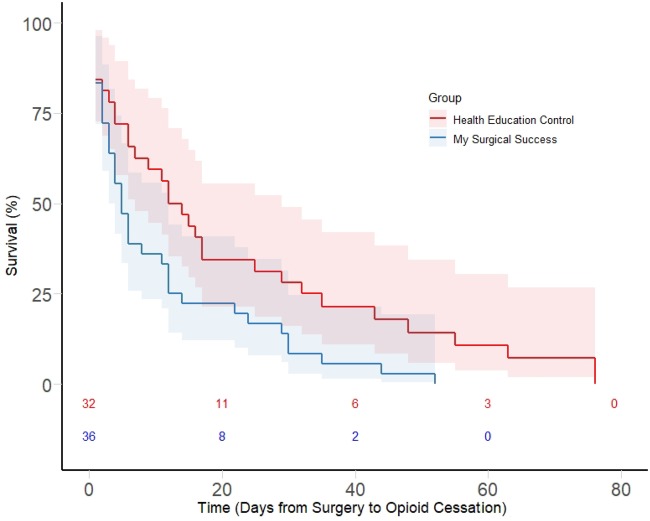
The Kaplan-Meier product-limit survival estimates are presented in Figure 2. MSS had a higher instantaneous hazard of opioid stoppage (or more rapid opioid cessation) than HE control. The nonparametric log-rank test indicated a significant difference in the two survival curves (χ2(1 df) = 5.93, P = 0.014). Both the Wilcoxon test and the likelihood ratio test yielded consistent results (P = 0.045 and P = 0.005).
Figure 2.
Kaplan-Meier survival curve for opioid cessation under “My Surgical Success” vs health education material control.
Following a supremum test of proportionality (not rejected, P = 0.80), we specified three proportional hazards models (Table 3). In the unadjusted model, only MSS group was specified as a predictor; we observed a significant effect of MSS vs HE control (hazard ratio [HR] = 1.862, 95% confidence interval [CI] = 1.12–3.10, P = 0.016). The MSS group had 86% increased odds of opioid cessation at any given time, relative to the HE control group. In the second model, we included the six patients who had been originally excluded for zero duration of opioid use and observed that the effect of MSS on accelerated opioid cessation remained intact (HR = 1.647, 95% CI = 1.02–3.67, P = 0.043). In the third model, we excluded patients with severe surgical complexity (N = 5) and continued to observe accelerated opioid cessation in the MSS group compared with HE control (HR = 1.989, 95% CI = 1.17–3.37, P = 0.0104).
Table 3.
Effects of MSS vs HE control on postsurgical opioid cessation*
| Model | Effect | HR Estimate | Lower 95% CI | Upper 95% CI | z Value | P Value |
|---|---|---|---|---|---|---|
| Unadjusted model | ||||||
| MSS vs HE control | 1.9 | 1.1 | 3.1 | 2.4 | 0.016 | |
| Unadjusted model with zero duration | ||||||
| MSS vs HE control | 1.6 | 1.1 | 2.7 | 2.0 | 0.043 | |
| Unadjusted model w/o complex surgery | ||||||
| MSS vs HE control | 2.0 | 1.2 | 3.3 | 2.6 | 0.011 | |
The hazard ratio refers to the event of opioid cessation for MSS relative to HE control during the 12-week postsurgical follow-up period. Thus, HR of 1.9 for the “Unadjusted Model”, indicates that MSS is associated with an 86% increase in the odds of opioid stoppage relative to HE control. The two models “Unadjusted Model” and “Unadjusted Model with Zero Duration” above describe the effects in two subsamples that exclude vs include zero duration events.
CI = confidence interval; HE = health education material control; HR = hazard ratio; MSS = “My Surgical Success.”
N = 68 (MSS, N = 36, HE, N = 32) for the unadjusted model without zero duration, N = 74 (MSS, N = 38, HE, N = 36), and N = 63 (MSS, N = 34, HE, N = 29) for the unadjusted model without complex surgery patients.
In the first follow-up analyses, we examined the effect of treatment (MSS vs HE control) on opioid cessation using logistic regression. We included the Firth correction to invoke the penalized likelihood estimation because one of the cells had 100% opioid cessation. We did not observe a treatment effect (χ2 (1 df) = 1.2651, P = 0.2607); both MSS and HE control were equally likely to experience opioid cessation (100% vs 93.75%). When data from patients with zero duration cessation were included, the effect remained nonsignificant (100% vs 94.44%, χ2 (1 df) = 1.1727, P = 0.2788). Clearly, the treatment is not altering the odds of opioid cessation in the postsurgical period.
In the second follow-up test, we assessed whether the median time to opioid cessation was different for the two groups using a nonparametric median (PROC NPAR1WAY) test. The median duration to opioid cessation was five days for the study group and 13 days for the control group (z = 1.93, P = 0.05). When data from patients with zero duration cessation were included, the difference remained marginally significant, at five days vs 11.5 days (z = 1.82, P = 0.07).
Examining the association between surgical complexity and opioid cessation, we did not observe a significant association (χ2 (2 df) = 3.3287, P = 0.1893). Ninety-three percent of patients in the mild (24/26), 100% of patients in the moderate (37/37), and 100% of patients in the severe complexity (5/5) conditions experienced opioid cessation during the postsurgical follow-up period.
Tertiary Outcomes
For average physical function, pain interference, and anxiety and depression, neither the main effects of time/group or the time × group interaction was significant using a P value threshold of 0.008 (=0.05/6) that corrects for multiple comparisons.
Discussion
The main finding of this randomized controlled clinical trial is that a digital behavioral pain medicine intervention was associated with five fewer days of postsurgical opioid use in women who initiated opioid use after breast cancer surgery compared with patients who received digital health education without an increase in pain report. Though we hypothesized a decrease in pain intensity in our secondary aim, the absence of an increase in pain within the context of opioid reduction is a clinically meaningful finding. Given the expected median duration of opioid use in this sample, the difference was not in likelihood of experiencing opioid cessation during the 12-week follow-up period but in the median time to opioid cessation, which was a clinically significantly shorter time frame for the MSS group. Surprisingly, surgical complexity did not impact time to opioid cessation, even when patients who underwent multiple or highly complex surgeries were excluded from analyses.
There is increasing interest in reducing postoperative opioid exposure by utilizing multimodal analgesia and nonpharmacological approaches to enhance recovery after surgery (ERAS) [25]. The importance of opioid-sparing strategies is underscored by the fact that several US states have enacted opioid prescribing limits for postsurgical analgesia [26]. Practical and accessible alternatives are needed.
Our pilot study revealed good feasibility and acceptability for a digital behavioral pain medicine intervention (MSS) in women undergoing breast cancer surgery. The overall engagement rate for patients randomly assigned to MSS was 56%, meaning that 44% of women did not engage with the digital treatment. For context, the e-health literature suggests that standard attrition rates are as high as 60–80% across various online interventions, including those that offer varying degrees of online support for enhanced engagement [27, 28]. Accordingly, a 44% “failure to engage” or attrition rate for a virtually no-cost, no-therapist contact, no social support, specialized intervention is highlighted as being quite good. Another notable feature of this attrition was that it was unrelated to the observed sample characteristics. This suggests (though not definitively) that the attrition is likely due to the perceived intervention burden and that the observed opioid cessation effects are likely not due to systematic differences in the observed sample characteristics.
Despite broad report of digital interventions evidencing lower engagement rates than control or in-person interventions, digital interventions are noteworthy and promising because of their sheer scalability and reach. Patients who would never otherwise receive specialized treatment are given the opportunity to do so [28], with costs being nominal to none. Indeed, MSS is fully automated, requires no therapist contact, and may be accessed at any time during the perioperative pathway, including in the hospital after surgery. The advantages of the remote intervention delivery system are increased access to behavioral pain medicine with low/no risks and low/no implementation costs.
Additional work is needed to optimize patient engagement with digital perioperative behavioral pain medicine. For instance, we are currently conducting a second phase study in orthopedic trauma surgery patients and are using an enhanced version of MSS that requires 45 minutes of total viewing time (treatment time burden is reduced by 50%) with videos that are modularized into three separate segments. This modular format allows viewers the convenience of engaging briefly (15 minutes at a time) and may be particularly well suited to the inpatient setting when patients are recovering from surgery. We also integrate a welcome message from the chief of surgery into the first video module, thereby enhancing the medical rationale and team-based philosophy to the patient audience. Pragmatic research is required to determine whether patient engagement rates are enhanced by having hospital staff offer patients MSS as a component of their perioperative care rather than as part of a research study, as was the case in the current study.
Our secondary aim was to investigate impacts of the MSS on pain catastrophizing and pain intensity. Our negative effects for both of these variables should be viewed within the following contexts. First, study patients were not receiving surgery for an initially painful condition. Rather, presurgical and postsurgical pain intensity and pain-specific psychological experience (pain catastrophizing) were strikingly low relative to other surgeries, such as orthopedic or spine surgeries [4, 29]. Indeed, pain catastrophizing has been shown to predict poorer postsurgical outcomes, including greater and persistent pain and opioid use [4, 30–33], and this established literature informed our secondary aim to investigate the impact of the MSS intervention on pain catastrophizing. However, we observed a floor effect for pain catastrophizing in the study sample; the average baseline PCS scores were well below the published ranges for clinically moderate or high levels in surgical populations [12, 34, 35] and could be best characterized as being in the “nonsymptomatic” range that does not require treatment. As such, we cannot rule out that our lack of group differences was due to this floor effect and our inability to meaningfully test our secondary study aim. Nevertheless, the lack of group differences in PCS change suggests that, in the context of the present study, the MSS effect on duration of opioid use did not operate through catastrophizing, and the underlying mechanisms remain uncharacterized. Additional studies are needed in populations with preexisting pain and clinical levels of pain catastrophizing.
Second, in terms of negative findings for post-treatment reductions in pain intensity for MSS relative to HE controls, we recognize that accelerated opioid cessation in the MSS group co-occurs with a lack of effects on pain intensity. In this context, it worth recalling that all the psychological correlates including pain intensity were averaged over all the measurement waves in the postsurgical period, including the postopioid tenure, and therefore are disconnected from opioid cessation. If we were to assess the impact of the psychological correlates on opioid cessation, we would need a postsurgical measurement regime that assesses the daily values of the correlates thereof, analyzed under an analytical framework that involves a survival model with time-varying covariates. Further study is needed and in different and more symptomatic patient populations.
Our study had several limitations. First, the final realized analytic sample was lower than the threshold suggested in our power calculations for detecting medium effects for opioid cessation. Our post hoc realized power for opioid cessation was 0.723, against a target of 0.8 for a two-tailed test of detecting a moderate-sized effect. This was in part due to some of the patients not initiating opioids, and thus becoming ineligible for the opioid cessation analysis. Despite reduced power, we detected a significant effect for this secondary outcome. Replication of these findings in a larger sample is warranted as a larger study may reveal effects undetected here. Second, we underscore that the current sample had no preexisting pain, no current pain at the time the intervention was administered, and nonsymptomatic levels of pain catastrophizing. Populations with preexisting pain and pain-related distress may better engage in a treatment to address a current and relevant problem rather than one that addresses a potential future problem. Third, the study was conducted in women undergoing surgery for breast cancer, which limits the findings to this surgical population. Fourth, as noted earlier, attrition in the MSS group was at least partially attributable to the greater time burden of MSS, which required viewing a 90-minute video in one sitting and completing postvideo questions. We have since abbreviated the MSS to 45 minutes of total viewing time, delivered in three video modules that allow the learner to access the information at their desired pace. Continued refinement of the digital intervention may yield superior engagement.
The existing literature on the effectiveness of perioperative psychological interventions is mixed. For instance, a Cochrane review and meta-analysis on perioperative psychological interventions in heart surgery patients revealed no impacts on postsurgical pain [36]. Only two studies measured analgesic use, as measured via PCA use in the hospital, and no effects were found for psychological interventions at this early stage of surgical recovery. Another recent Cochrane review conducted by Powell et al. examined psychological preparation and postoperative outcomes for adults undergoing surgery under general anesthesia [37]. This meta-analysis was a mixture of studies that included chronic pain patient populations (e.g., total knee replacement and total hip replacement) and others. The authors reported that out of 61 studies that measured the outcome of pain intensity, pooled results across all types of interventions revealed low-quality evidence that psychological intervention was associated with lower postoperative pain. Finally, one recent perioperative study of a perioperative mindfulness intervention delivered in patients with lumbar spine degenerative disease before surgery is noteworthy because it assessed postsurgical opioid use [38]. The patients in the mindfulness group were compared with a retrospectively constructed usual care group that involved matching to the mindfulness patients using a 1:1 ratio by age, sex, type of surgery, and preoperative opioid use. The authors reported positive impacts for the mindfulness intervention on postsurgical pain but no reduction in postsurgical opioid use or any of the other patient-reported outcomes tested. The literature on digital behavioral pain medicine is noted to be nascent, though utilization of technological solutions is expected to increase to efficiently meet the growing demand for accessible, comprehensive perioperative pain care and nonopioid approaches, particularly in light of the low/no cost incurred by all key stakeholders.
In conclusion, although further study is needed to confirm and extend the results reported here, we found that a virtually no-cost, fully automated behavioral pain medicine treatment may be useful for motivated breast cancer surgery patients and may reduce their duration of opioid exposure after surgery.
References
- 1. Sun EC, Darnall BD, Baker LC, Mackey S.. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;1769:1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;1526:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcusa DP, Mann RA, Cron DC, et al. Prescription opioid use among opioid-naive women undergoing immediate breast reconstruction. Plast Reconstr Surg 2017;1406:1081–90. [DOI] [PubMed] [Google Scholar]
- 4. Helmerhorst GT, Vranceanu A-M, Vrahas M, Smith M, Ring D.. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. JBJS 2014;966:495–9. [DOI] [PubMed] [Google Scholar]
- 5. Komatsu R, Carvalho B, Flood P.. Prediction of outliers in pain, analgesia requirement, and recovery of function after childbirth: A prospective observational cohort study. Br J Anaesth 2018;1212:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehde DM, Dillworth TM, Turner JA.. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153–66. [DOI] [PubMed] [Google Scholar]
- 7. Naylor MR, Naud S, Keefe FJ, Helzer JE.. Therapeutic Interactive Voice Response (TIVR) to reduce analgesic medication use for chronic pain management. J Pain 2010;1112:1410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamison RN, Ross EL, Michna E, et al. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain 2010;1503:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Darnall BD, Scheman J, Davin S, et al. Pain psychology: A global needs assessment and national call to action. Pain Med 2016;172:250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC.. From catastrophizing to recovery: A pilot study of a single-session treatment for pain catastrophizing. J Pain Res 2014;7:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darnall BD, Ziadni MS, Roy A, et al. Comparative efficacy and mechanisms of a single-session pain psychology class in chronic low back pain: Study protocol for a randomized controlled trial. Trials 2018;191:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sullivan MJ, Bishop SR, Pivik J.. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;74:524–32. [Google Scholar]
- 13. Granot M, Ferber SG.. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: A prospective study. Clin J Pain 2005;215:439–45. [DOI] [PubMed] [Google Scholar]
- 14. Hung M, Hon SD, Franklin JD, et al. Psychometric properties of the PROMIS physical function item bank in patients with spinal disorders. Spine (Phila Pa 1976). 2014;39(2):158–63. [DOI] [PubMed] [Google Scholar]
- 15. Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: A PROMIS fatigue item bank example. Arch Phys Med Rehabil 2011;92(10 Suppl):S20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Revicki DA, Chen WH, Harnam N, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain 2009;146(1–2):158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revicki DA, Cook KF, Amtmann D, Harnam N, Chen WH, Keefe FJ. Exploratory and confirmatory factor analysis of the PROMIS pain quality item bank. Qual Life Res. 2014;23(1):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hedrick TL, Harrigan AM, Thiele RH, et al. A pilot study of patient-centered outcome assessment using PROMIS for patients undergoing colorectal surgery. Support Care Cancer 2017;2510:3103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan SJ, Friedly JL, Amtmann D, Salem R, Hafner BJ.. Cross-sectional assessment of factors related to pain intensity and pain interference in lower limb prosthesis users. Arch Phys Med Rehabil 2017;981:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;942:149–58. [DOI] [PubMed] [Google Scholar]
- 21. Carroll IR, Hah JM, Barelka PL, et al. Pain duration and resolution following surgery: An inception cohort study. Pain Med 2015;1612:2386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: A randomized clinical trial. JAMA Surg 2018;1534:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olivier J, May WL, Bell ML.. Relative effect sizes for measures of risk. Commun Stat Theory Methods 2017;4614:6774–81. [Google Scholar]
- 24. Azuero A. A note on the magnitude of hazard ratios. Cancer 2016;1228:1298–9. [DOI] [PubMed] [Google Scholar]
- 25. Ljungqvist O, Scott M, Fearon KC.. Enhanced recovery after surgery: A review. JAMA Surg 2017;1523:292–8. [DOI] [PubMed] [Google Scholar]
- 26. Reid DB, Shah KN, Ruddell JH, et al. Effect of narcotic prescription limiting legislation on opioid utilization following lumbar spine surgery. Spine J 2019;19(4):717–25. [DOI] [PubMed] [Google Scholar]
- 27. Geraghty AW, Torres LD, Leykin Y, Perez-Stable EJ, Munoz RF.. Understanding attrition from international Internet health interventions: A step towards global eHealth. Health Promot Int 2013;283:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eysenbach G. The law of attrition. J Med Internet Res 2005;71:e11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coronado RA, George SZ, Devin CJ, Wegener ST, Archer KR.. Pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery. Arch Phys Med Rehabil 2015;9610:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: A predictive factor for postoperative pain. Am J Surg 2011;2011:122–31. [DOI] [PubMed] [Google Scholar]
- 31. Papaioannou M, Skapinakis P, Damigos D, et al. The role of catastrophizing in the prediction of postoperative pain. Pain Med 2009;108:1452–9. [DOI] [PubMed] [Google Scholar]
- 32. Pavlin DJ, Sullivan MJ, Freund PR, Roesen K.. Catastrophizing: A risk factor for postsurgical pain. Clin J Pain 2005;211:83–90. [DOI] [PubMed] [Google Scholar]
- 33. Picavet HSJ, Vlaeyen JW, Schouten JS.. Pain catastrophizing and kinesiophobia: Predictors of chronic low back pain. Am J Epidemiol 2002;15611:1028–34. [DOI] [PubMed] [Google Scholar]
- 34. Riddle DL, Wade JB, Jiranek WA, Kong X.. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010;4683:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roh YH, Lee BK, Noh JH, et al. Effect of anxiety and catastrophic pain ideation on early recovery after surgery for distal radius fractures. J Hand Surg 2014;3911:2258–64.e2252. [DOI] [PubMed] [Google Scholar]
- 36. Ziehm S, Rosendahl J, Barth J, et al. Psychological interventions for acute pain after open heart surgery. Cochrane Database Syst Rev 2017;7:CD009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powell R, Scott NW, Manyande A, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev 2016;5:CD008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yi JL, Porucznik CA, Gren LH, et al. The impact of preoperative mindfulness-based stress reduction on postoperative patient-reported pain, disability, quality of life, and prescription opioid use in lumbar spine degenerative disease: A pilot study. World Neurosurg 2019;121:e786–91. [DOI] [PubMed] [Google Scholar]