Abstract
Objective
To evaluate the short-term safety and effectiveness of amniotic membrane/umbilical cord particulate (AMUC) in managing pain in patients with various severities of knee osteoarthritis (OA).
Design
Single-center, prospective, investigator-initiated pilot study.
Setting
Private practice.
Subjects
A total of 20 knee OA patients aged ≥18 years were enrolled with pain >40 mm, as determined by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)–A.
Methods
Patients received an ultrasound-guided, intra-articular injection of 50 mg of AMUC particulate reconstituted in 2 mL of preservative-free saline. All patients were then monitored at six weeks, 12 weeks, and 24 weeks postinjection. Patients who did not show >30% reduction in pain received a second injection of AMUC at six weeks. WOMAC, Patient Global Assessment, medication usage, and magnetic resonance imaging (MRI) were assessed.
Results
Knee OA pain significantly decreased from 74.3 ± 17.2 at baseline to 45.0 ± 25.4 at six weeks (P < 0.01), 35.4 ± 26.6 at 12 weeks (P < 0.001), and 37.4 ± 26.7 at 24 weeks (P < 0.001). This pain reduction was associated with a significant improvement in physical function (WOMAC-C) at all time points (P < 0.05) and stiffness (WOMAC-B) at 12 weeks (P = 0.01). Eleven patients received a second injection, which was significantly correlated with body mass index >30 kg/m2 (P = 0.025). MRI evaluation of the overall population revealed an improvement in the severity of bone marrow lesions in seven patients. No adverse events were observed.
Conclusions
AMUC particulate injection relieved pain and improved physical function in patients with symptomatic knee OA.
Keywords: Amniotic Membrane, Knee Joint Pain, Orthopedics, Osteoarthritis, Treatment Outcome, Umbilical Cord, WOMAC
Introduction
Knee osteoarthritis (OA) is a degenerative joint disease that causes pain and decreased function, dramatically reducing the quality of life in those affected [1]. The prevalence of symptomatic knee OA in the United States is estimated at 15.6 million, with advanced knee OA comprising the majority of individuals [2]. The population with knee OA is expected to continually rise, with recent estimates projecting a nearly 40% increase in the prevalence of all forms of arthritis over the period 2005–2030. Notable risk factors associated with the onset of knee OA include age, female gender, increased body mass index (BMI), and previous knee injury [3].
Although the sources of pain in OA have yet to be fully elucidated, bone marrow lesions (BMLs), a common feature of painful knee OA detected by magnetic resonance imaging (MRI) [4], have been shown to play an important role and have been associated with other clinical manifestations of knee OA such as progression of articular cartilage loss [4–17]. BMLs can be graded by scoring systems using sensitive MRI sequences [9], and their severity strongly correlates with Kellgren-Lawrence grades [18]. Furthermore, reduction of the BML size has been shown to be correlated with knee OA pain relief, supporting the notion that BML is the origin of knee OA pain [19].
Among the pharmacologic therapies for knee OA, nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics are commonly used [20]. Although these treatments can effectively relieve pain, long-term use is not recommended as they can cause adverse cardiovascular and gastrointestinal effects [21,22]. Further, there is widespread recognition of the opioid abuse crisis, highlighting the need for safer but effective options [23–26]. Intra-articular injection of steroids or hyaluronic acid (HA) has become an appealing alternative treatment approach given their ability to reduce pain with fewer side effects than some oral medications [27]. Although corticosteroids have been shown to reduce pain in the short term, HA appears to be superior in safety and efficacy [27–29]. However, the true effectiveness of HA is questionable given controversial findings in many studies [27–29]. Thus, there is a need for a safe “nonopioid” OA treatment that not only alleviates symptomatic joint pain and improves function, but also slows progression of the disease.
Cryopreserved human umbilical cord (UC) and amniotic membrane (AM) are both commercially available and have a long history of safe use in a variety of clinical applications due to their anti-inflammatory, antiscarring, and proregenerative properties [30–33]. Injection of AMUC particulate matrix has been shown to attenuate progressive cartilage degeneration in a small animal model of knee OA [34]. In that study, EPIC-μCT analysis demonstrated that animals injected with AMUC had a significant increase in both cartilage thickness and volume, as well as a significant decrease in total lesions when compared with saline-injected control animals. Osteoarthritis Research Society International (OARSI) histology scores of tibial sections further verified EPIC-μCT results. Therefore, this pilot study was initiated to assess the safety and effectiveness of intra-articular injection of AMUC particulate in relieving pain and improving function in patients presenting with knee OA and to assess the correlation of these outcomes with resolution of BML.
Methods
After approval from the Western Institutional Review Board (Puyallup, WA, USA), this prospective, single-center, investigator-initiated pilot study was conducted to evaluate the safety and effectiveness of intra-articular injection of AMUC particulate in patients presenting with refractory knee OA pain. The study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from all subjects before participation. A total of 20 consecutive patients with knee OA who met the inclusion/exclusion criteria were enrolled in the study. The inclusion criteria included patients (aged ≥ 18 years old) clinically diagnosed with symptomatic knee OA who had not received another intra-articular injection in the affected knee within 30 days. The exclusion criteria included 1) patients with acute fractures, avascular necrosis, and/or severe deformity in the affected knee; 2) patients with inflammatory disease of the knee other than OA (e.g., rheumatoid arthritis, chronic hemochromatosis, sickle cell anemia, and/or arthropathies of systemic diseases, such as chondrocalcinosis, gout, hemophilia, and infectious diseases of the joints); and 3) patients who had surgery on the affected knee within the previous six months, had a planned surgery to the knee within the study period, or had participated in another interventional study within 30 days of screening.
At screening, patients were considered to have symptomatic knee OA if they had knee pain >40 mm, as determined by averaging the five scores of the modified 100-mm visual analog scale Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale. The WOMAC is a self-administered questionnaire that has three subscales that measure pain (WOMAC-A, five questions), stiffness (WOMAC-B, two questions), and physical function (WOMAC-C, 17 questions), with higher scores indicating worse status; it has been validated for knee OA [35]. Patients with bilateral knee OA designated the more painful knee as the study index knee at screening. In addition, diagnosis of knee OA was confirmed at baseline screening with MRI.
Patients who met the eligibility criteria received subcutaneous injection of 5 cc of lidocaine using a 25-gauge needle to numb the skin, followed by an ultrasound-guided aspiration of all persistent intra-articular synovial fluid using an 18-gauge needle and intra-articular injection of 50 mg of AMUC particulate (CLARIX FLO, Amniox Medical, Inc., Miami, FL, USA) reconstituted in 2 mL of preservative-free saline. The injection was performed with the knee in the bent position and approached laterally. Injection of intra-articular anesthetics was not performed. Patients were then monitored at six weeks, 12 weeks, and 24 weeks postinjection. Patients who did not show a >30% reduction in pain (as measured by averaging the 5 scores of the WOMAC-A) received a second injection at six weeks, a duration that was determined in a previously reported study for plantar fasciitis [36]. A 30% cutoff was used because placebo injections have been shown to provide pain relief of up to 30% [37], and WOMAC-A minimal clinically important differences have been reported to be <26% [38, 39]. The follow-up visit schedule was chosen based on the Food and Drug Administration Guidance for Industry, stating that the trial duration for demonstration of OA symptom improvement should be at least three months [40]. After injection of AMUC, all patients followed the same standardized rehabilitation protocol, which included ice application to the affected area three times a day for no more than 15 minutes for three days postinjection. All patients were asked to stop use of pain medications 24 hours before all study visits to reduce a potential confounding effect on pain outcome measures. Patients were also asked not to use NSAIDs, including but not limited to ibuprofen, naproxen, aspirin (>80 mg), and diclofenac for a duration of six weeks postinjection. Patients were asked not to perform sports activities for five days postinjection and were allowed to perform usual daily activities 24 hours postinjection. Assistive devices (crutches, canes, walkers, and braces) were not prescribed, but the patients were allowed to use them if needed.
High-field (HF) MRI with a field strength of 1.5 Tesla was performed at baseline and 24 weeks. Images were captured using sagittal and coronal T2-weighted, fat-saturated, fast spin-echo images. Aside from baseline images being reviewed by the investigator to confirm eligibility criteria, both baseline and 24-week images were graded by a blinded radiologist using the Whole Organ Magnetic Resonance Imaging Score (WORMS) [41] to determine the presence and degree of severity of BML in the index knee. The WORMS grading was as follows: Grade 0: no BML; Grade 1: <25% of the region; Grade 2: 25–50% of the region; Grade 3: >50% of the region. The BML was scored individually per subregion, and the worst score out of all subregions was determined to be the final BML score for each knee. An independent radiologist was blinded to the information of the study trial design and what treatment (if any) was administered, but not to subject and time points of image acquisition to score in longitudinal fashion, of which this information has been shown to increase sensitivity in the detection of clinically relevant changes [42].
The primary efficacy end point was determined by the change in knee OA pain (as measured by the average of five WOMAC-A pain questions) between 12 weeks and baseline. Secondary outcomes measures included the change from baseline in average WOMAC-B scores, average WOMAC-C scores, average WOMAC global score, opioid and nonopioid medication use related to knee pain, and change in severity of BML. Another efficacy measure was Patient Global Assessment (PGA), which was determined using a seven-point Likert scale at six weeks, 12 weeks, and 24 weeks postinjection. Patients were asked: “Considering all the ways your osteoarthritis affects you, how have you been since your last visit?” They provided a response of “very much worse,” “much worse,” “a little worse,” “no change,” “a little better,” “better,” or “much better.”
Safety was assessed by the investigator at 30 minutes postinjection and at each follow-up visit by conducting routine physical evaluations and index knee assessments for tenderness, heat, redness, and swelling. Safety was also evaluated via adverse events (AEs), that is, any unfavorable and unintended sign, symptom, or disease reported or discovered by the investigator at each visit. Subjects were also instructed to call the investigator if any side effects occurred between visits.
The AMUC particulate product is commercially available and derived from donated human placental tissue following healthy, live, caesarian section, full-term births. Under aseptic conditions, the placenta was first cleaned of blood with PBS before separation of the AM and UC by blunt dissection. The AM and UC were then gently rinsed in PBS until all blood coloration was removed, followed by morselization, lyophilization, and terminal sterilization. The manufacturing utilized a cryopreservation process at low temperatures, which devitalizes the living cells but retains the natural biological characteristics relevant to this tissue, including anti-inflammatory actions to upregulate IL-10 and downregulate IL-12 [33]. The final product was stored at room temperature, and the dosage was chosen based on prior preclinical studies [34].
Statistical Analysis
All statistical analyses were carried out using IBM SPSS, version 20.0. Categorical variables were described by percentages and frequencies, whereas continuous variables were described by means and standard deviations. Continuous outcome measures were evaluated using the Kruskal-Wallis test. In addition, the related-samples Friedman’s two-way analysis of variance (ANOVA) by ranks was utilized to test whether the distributions of WOMAC pain, function, and stiffness scores significantly differed across time points. This test is the nonparametric alternative to the one-way ANOVA with repeated measures. The proportion of patients who achieved ≥50% improvement in pain from their baseline WOMAC-A score was compared across time points using the related-samples Cochran’s Q test, which is a nonparametric test for differences on a dichotomous dependent variable across three or more groups. Chi-square and Fisher exact tests were used to compare categorical outcome variables. In addition, regression analyses were conducted to detect any covariates or predictor variables of various outcome measures. Additionally, a logistic regression model was used to control for potential confounding variables. A P value of less than 0.05 was considered statistically significant.
Results
The baseline characteristics of the 20 enrolled patients are summarized in Table 1. There were five males and 15 females, with an average age of 71.0 ± 6.4 years and a mean BMI of 29.7 ± 4.3 kg/m2, reflecting the general knee osteoarthritis population [3]. Their knee OA pain persisted despite prior intra-articular injection of steroid, HA, stem cells, and Platelet-Rich Plasma in a period of one month to five years.
Table 1.
Baseline patient clinical characteristics
| AMUC Treatment Group | |
|---|---|
| Sex (female/male), No. (%) | 15/5 (75/25) |
| Age, mean ± SD, y | 71.0 ± 6.4 |
| BMI, mean ± SD, kg/m2 | 29.7 ± 4.3 |
| Normal (18.5–<25 kg/m2), No. (%) | 2 (10) |
| Overweight (25–<30 kg/m2), No. (%) | 10 (50) |
| Obese (≥30 kg/m2), No. (%) | 8 (40) |
| Side (right/left), No. (%) | 14/6 (70/30) |
| Use of pain medication, No. (%) | |
| NSAIDs | 10 (50) |
| Opiates | 9 (45) |
| Risk factors, No. (%) | |
| Diabetes | 7 (35) |
| Hypertension | 11 (55) |
| Prior knee intervention within 5 y, No. (%) | |
| Corticosteroid injection | 8 (40) |
| Hyaluronic acid injection | 2 (10) |
| Stem cell | 3 (15) |
| Platelet-rich plasma | 3 (15) |
| AMUC injection | 1 (5) |
| Surgery | 4 (20) |
AMUC = amniotic membrane and umbilical cord; BMI = body mass index; BME = bone marrow edema; NSAID = nonsteroidal anti-inflammatory drug; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
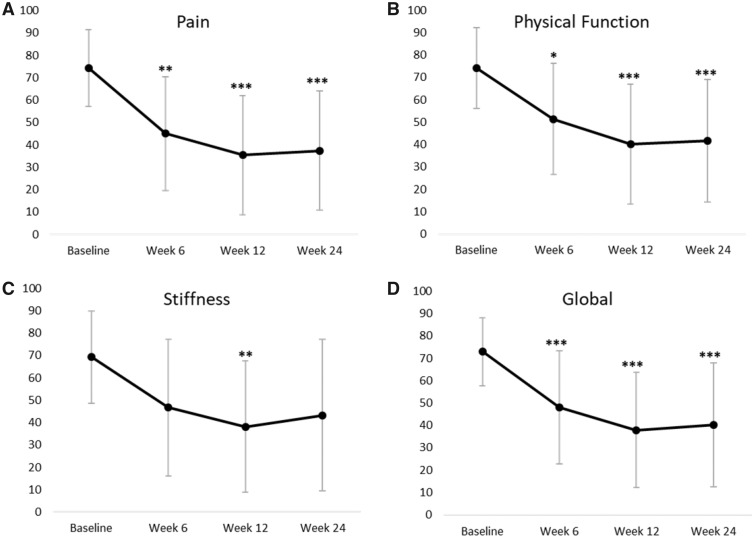
After injection of 50 mg of AMUC particulate, pain and physical function scores significantly improved from baseline at all follow-up time points (Figure 1). The average pain score significantly reduced from 74.3 ± 17.2 mm at baseline to 45.0 ± 25.4 mm at six weeks (P < 0.0001), 35.4 ± 26.6 mm at 12 weeks (P < 0.0001), and 37.4 ± 26.7 mm at 24 weeks (P < 0.0001). This change from baseline equates to an average pain reduction of 37.6%, 55.1%, and 51.7% at six weeks, 12 weeks, and 24 weeks, respectively. The proportion of patients achieving ≥50% improvement in pain from baseline significantly differed across time (P = 0.03) (Table 2): 25% (5/20) at six weeks, 45% (9/20) at 12 weeks, and 50% (10/20) by 24 weeks. To adjust for factors other than treatment that could influence improvement in pain at 12 and 24 weeks, a logistic regression model was used. The covariates entered included gender, BMI, diabetes, hypertension, prior treatment, and number of AMUC injections administered. None of the factors were statistically significant predictors of pain improvement.
Figure 1.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscale scores over 24 weeks following intra-articular injection of amniotic membrane/umbilical cord particulate. WOMAC-A pain scores (expressed as mean ± SD in mm) (A), WOMAC-C physical function (B), WOMAC-B stiffness (C), and WOMAC global (D) are plotted against time. All data points were compared with baseline. *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Table 2.
Proportion of patient improvement in WOMAC pain subscale compared with baseline
| Week 6, No. (%) | Week 12, No. (%) |
Week 24, No. (%) |
|||
|---|---|---|---|---|---|
| 1 Injection (N = 9) | 2 Injections (N = 11) | 1 Injection (N = 9) | 2 Injections (N = 11) | ||
| ≥20% improvement | 11 (55) | 8 (89) | 10 (91) | 8 (89) | 9 (82) |
| ≥30% improvement | 10 (50) | 7 (78) | 9 (82) | 7 (78) | 7 (64) |
| ≥40% improvement | 6 (30) | 6 (67) | 6 (55) | 6 (67) | 7 (64) |
| ≥50% improvement | 5 (25) | 5 (56) | 4 (36) | 5 (56) | 5 (46) |
WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
This reduction in pain was accompanied by improvement in patients’ physical function scores, which significantly reduced from 74.2 ± 18.0 mm at baseline to 51.4 ± 24.9 mm at six weeks (P = 0.016), 40.3 ± 26.8 mm at 12 weeks (P < 0.0001), and 41.7 ± 27.3 mm at 24 weeks (P < 0.0001) (Figure 1). This change equates to an average physical function improvement of 25.3%, 42.3%, and 44.5% at six weeks, 12 weeks, and 24 weeks, respectively. Stiffness scores only differed significantly from baseline at the 12-week follow-up (P = 0.018) and showed a nonsignificant improvement by 24 weeks (34% decrease, P = 0.18). Covariate analysis showed hypertension to be a significant factor regarding stiffness (P = 0.02). No patient had adverse effects throughout the duration of the trial.
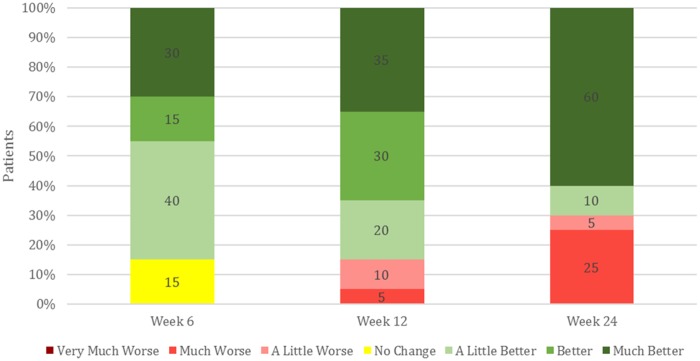
Patient Global Assessment (PGA) was performed at each visit by asking the patient how their knee OA has affected them as compared with the last visit. Seventeen of 20 patients reported positive improvement at six and 12 weeks (Figure 2). By 24 weeks, this number decreased to 14 patients, with hypertension again being a significant covariate (P = 0.01). Overall the PGA score correlated to improvement in pain and function. Along with the improvement in pain, the usage of NSAIDs for pain management significantly decreased from 10 patients at baseline to two patients at 24 weeks (P = 0.006). Five of nine patients who reported taking opiates at baseline ceased usage by 24 weeks, whereas one patient who only took NSAIDs at baseline reported taking opiates “once in a while” at follow-up. Neither of these differences were statistically significant.
Figure 2.
Patient Global Assessment. Patient’s evaluated how osteoarthritis affected them since their last follow-up visit. Seventeen of 20 patients reported positive improvement at six and 12 weeks. By 24 weeks, this number decreased to 14 patients, with hypertension being a significant covariate (P = 0.01).
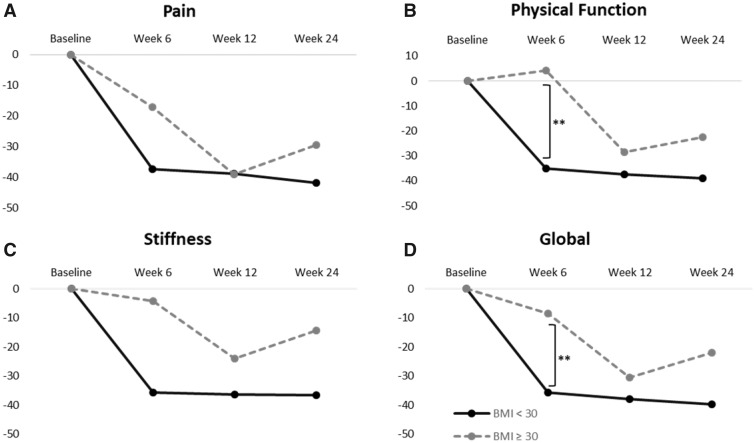
Ten of the 20 patients (50%) in this study did not show >30% reduction in pain from baseline and received a second injection at the six-week follow-up visit. One additional patient who had 38% improvement received a second injection per the investigator’s discretion. Interestingly, seven of the eight (87.5%) patients with BMI ≥30 kg/m2 (i.e., clinically obese) received a second injection compared with only four of 12 patients (33.3%) with a BMI <30 kg/m2 (i.e., clinically nonobese), suggesting that obesity was significantly correlated with the need of a second injection (P = 0.025). We then analyzed all WOMAC subscale scores by comparing those with BMI <30 kg/m2 to those with BMI ≥30 kg/m2. The results showed significant improvement from baseline for nonobese patients at all time points, whereas obese patients only showed a significant difference in pain scores at 12 and 24 weeks (Figure 3). At six weeks, physical function scores improved by 44.3% in nonobese patients, whereas scores for obese patients got worse by 3.1% (P = 0.012). Physical function scores continued to improve by 12 weeks in both groups, but all subscale scores regressed at week 24 in obese patients (which corresponded with PGA scores) (Figure 2).
Figure 3.
Change in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores from baseline according to body mass index (BMI). A) WOMAC-A pain scores, (B) WOMAC-C physical function scores, (C) WOMAC-B stiffness scores, and (D) WOMAC global scores are plotted against time for patients with BMI <30 kg/m2 (solid line, N = 12) and those with BMI ≥30 kg/m2 (dashed line, N = 8). **P ≤ 0.01.
MRIs were reviewed by a blinded radiologist to determine the BML severity before and after treatment. At baseline, 19 patients presented with a BML with a severity grade of ≥1. Patients with more severe BML had more severe pain scores; however, this trend did not reach statistical significance. The majority (50%) of patients had a BML score of 1, indicating that they presented with mild severity, whereas 35% had Grade 2 and 10% had Grade 3. By 24 weeks, seven (37%) patients had an improvement in BML scores and nine (47%) patients had no change. The proportion of patients with each BML severity was 10% Grade 0, 53% Grade 1, 37% Grade 2, and 0% Grade 3. One patient declined to receive a follow-up MRI, so their data were omitted from the comparison. Kendall’s tau-b correlation showed that higher baseline BML scores were more likely to improve in BML severity by 24 weeks, whereas less severe BMLs were more likely to get worse (τb = 0.55, P = 0.01). However, there was no significant correlation between baseline BML and improvement in pain or function at any time point. There was also a significant negative correlation between age and BML score improvement (τb = 0.40, P = 0.038), as younger patients tended to improve more than older patients.
Discussion
This prospective pilot study showed that intra-articular injection of AMUC particulate may be a safe and effective treatment by significantly reducing pain and improving physical function and stiffness at 12 weeks postinjection in patients with symptomatic knee OA. Such improvement in pain and physical function also extended to 24 weeks, when patients reported a 52% improvement in pain scores and a 45% improvement in physical function. Both outcomes exceed the minimal clinically important difference (MCID), or the smallest outcome change that is meaningful to patients, which is reported to be 12–26% for the WOMAC index [38, 39]. In fact, the majority of patients (65%) showed an MCID with ≥30% improvement in both WOMAC pain and physical function scores. Additionally, no adverse events or complications were noted during the study period. Despite these findings, future randomized controlled studies are needed to further resolve whether there is a dose-dependent lasting benefit beyond 24 weeks.
For the 12 nonobese patients with a BMI <30 kg/m2, a single injection of AMUC was sufficient to achieve a significant reduction of pain (47.6%) and improvement in function (44.3%) as early as six weeks; they continued to show progressive improvement at 12 and 24 weeks (Figure 3). In contrast, for seven of the eight obese patients with a BMI ≥30 kg/m2, a single injection was not sufficient to achieve pain reduction >30% at six weeks. These patients received a second injection, which resulted in significant reduction of pain and improvement of function at 12 weeks. However, by 24 weeks, all WOMAC subscale scores regressed, suggesting that the treatment might not have a lasting effect in obese patients (Figure 3). Collectively, this result resembled what has been reported in obese patients with symptomatic knee OA who tend to present failure in the treatment of viscosupplementation [43, 44]. It should be noted, however, that use of a second injection for patients not showing relief introduced a potential bias that may give a negative case a second chance to be positive. Future randomized controlled trials with AMUC need to consider stratification according to BMI in knee OA patients to assess the dose response and the lasting benefit.
In this study, the majority (95%) of patients at baseline presented with a BML, which has been described as an origin of knee OA pain [19]. The natural history suggests that BMLs tend to get worse in knee OA patients as time goes on [6, 8, 45–47]. However, AMUC treatment resulted in a decrease of BML severity in seven (37%) of 19 patients, which is comparable to the 50% proportion of patients who had ≥50% improvement in pain at 24 weeks. Interestingly, patients with more severe baseline BML scores were more likely to show BML improvement after AMUC injection than those with lower BML scores. This finding is different from injection of HA, which is less responsive in patients with higher BML severity [48]. Nonetheless, we acknowledge that our pilot study has the limitations of a small sample size and a lack of a control group for comparison. The small sample size could have also been a primary reason why BML scores did not significantly correlate to WOMAC pain at baseline or 24 weeks. Other studies have found no relationship between WOMAC scores and BML severity [12, 46, 47, 49], whereas some studies have [15, 50, 51]. Hence, it is important to conduct randomized controlled studies to verify whether there is a potential benefit of AMUC to modify OA by reducing BML, which might then lead to reduction of progressive cartilage damage, as suggested by preclinical animal models [34].
If the aforementioned efficacy were verified, the underlying mechanism may be related to the anti-inflammatory actions of AMUC, which has been shown to promote apoptosis of inflammatory cells and reduce secretion of inflammatory mediators known to be associated with knee OA [52, 53]. Additionally, the mechanism of action may stem from suppression of TGF-β signaling, which prevails in inflammation. This notion is derived from in vitro and in vivo studies that disclose a pathogenic role of TGF-β in the development of knee OA [54]. Furthermore, animal studies have shown that blockade of TGF-β signaling in the subchondral bone attenuates BML and other pathological OA changes including articular cartilage degeneration [55, 56]. In this regard, it is relevant to appreciate that AMUC is known to suppress TGF-β signaling [57, 58] and AMUC’s innate major biochemical component, that is, HC-HA/PTX3, has been shown to be responsible in part for anti-inflammatory, antiscarring, and proregenerative effects [59]. Unlike high–molecular weight HA, which is conventionally used for treatment of knee OA, HC-HA/PTX3 has been shown to significantly promote apoptosis of activated neutrophils [52, 53] and promote phagocytosis by macrophages [53]. HC-HA/PTX3 also promotes M2 macrophage polarization [53], whereas HA alone does not [60]. Overall, these AMUC properties may facilitate suppression of TGF-β, resolution of inflammation, and halting of cartilage degradation, thus leading to a better healing response.
Conclusions
This study suggests that intra-articular injection of AMUC particulate shows preliminary safety and effectiveness in relieving pain and improving function in patients with symptomatic knee OA presenting various severities with BML Grades 0–3. A single injection on average yields benefit in nonobese patients, whereas a second injection may be warranted in obese patients. Further randomized controlled studies are needed to see if this might be developed into a nonopioid alternative to manage knee OA pain.
Acknowledgments
The authors would like to thank Rodrigo Santos, RN, for assistance in data acquisition, Scheffer Tseng, MD, PhD, for assistance in the study design, Olivia Mead for assistance in the statistical analysis, and the subjects for participating in the study.
References
- 1. Nuki G. Osteoarthritis: A problem of joint failure. Z Rheumatol 1999;583:142–7. [DOI] [PubMed] [Google Scholar]
- 2. Deshpande B, Katz JN, Solomon DH, Yelin EH, Losina E.. Burden of symptomatic knee osteoarthritis in the United States: Impact of race/ethnicity, age and sex. Arthritis Rheumatol 2015;6710; https://acrabstracts.org/abstract/burden-of-symptomatic-knee-osteoarthritis-in-the-united-states-impact-of-raceethnicity-age-and-sex/. (accessed June 10, 2019). [Google Scholar]
- 3. Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP.. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthritis Cartilage 2015;234:507–15. [DOI] [PubMed] [Google Scholar]
- 4. Wilson AJ, Murphy WA, Hardy DC, Totty WG.. Transient osteoporosis: Transient bone marrow edema? Radiology 1988;1673:757–60. [DOI] [PubMed] [Google Scholar]
- 5. Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001;1347:541–9. [DOI] [PubMed] [Google Scholar]
- 6. Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum 2006;545:1529–35. [DOI] [PubMed] [Google Scholar]
- 7. Kubota M, Ishijima M, Kurosawa H, et al. A longitudinal study of the relationship between the status of bone marrow abnormalities and progression of knee osteoarthritis. J Orthop Sci 2010;155:641–6. [DOI] [PubMed] [Google Scholar]
- 8. Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: The MOST study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis 2009;689:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu L, Hayashi D, Roemer FW, Felson DT, Guermazi A.. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin Arthritis Rheum 2012;422:105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Nevitt M, Niu J, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum 2011;633:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ 2012;345:e5339.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ip S, Sayre EC, Guermazi A, et al. Frequency of bone marrow lesions and association with pain severity: Results from a population-based symptomatic knee cohort. J Rheumatol 2011;386:1079–85. [DOI] [PubMed] [Google Scholar]
- 13. Javaid MK, Kiran A, Guermazi A, et al. Individual magnetic resonance imaging and radiographic features of knee osteoarthritis in subjects with unilateral knee pain: The Health, Aging, and Body Composition Study. Arthritis Rheum 2012;6410:3246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javaid MK, Lynch JA, Tolstykh I, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: The Most Study. Osteoarthritis Cartilage 2010;183:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim IJ, Kim DH, Jung JY, et al. Association between bone marrow lesions detected by magnetic resonance imaging and knee pain in community residents in Korea. Osteoarthritis Cartilage 2013;219:1207–13. [DOI] [PubMed] [Google Scholar]
- 16. Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M.. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011;933:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefanik JJ, Gross KD, Guermazi A, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: The Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis Cartilage 2015;234:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park HJ, Kim SS, Lee SY, et al. A practical MRI grading system for osteoarthritis of the knee: Association with Kellgren-Lawrence radiographic scores. Eur J Radiol 2013;821:112–7. [DOI] [PubMed] [Google Scholar]
- 19. Sharkey PF, Cohen SB, Leinberry CF, Parvizi J.. Subchondral bone marrow lesions associated with knee osteoarthritis. Am J Orthop (Belle Mead, NJ) 2012;419:413–7. [PubMed] [Google Scholar]
- 20. Ringdahl E, Pandit S.. Treatment of knee osteoarthritis. Am Fam Physician 2011;8311:1287–92. [PubMed] [Google Scholar]
- 21. Harirforoosh S, Asghar W, Jamali F.. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 2013;165:821–47. [DOI] [PubMed] [Google Scholar]
- 22. O'Neil CK, Hanlon JT, Marcum ZA.. Adverse effects of analgesics commonly used by older adults with osteoarthritis: Focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother 2012;106:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Power PD, Perruccio AV, Gandhi R, et al. (2019), Factors Associated With Opioid Use in Pre · surgical Knee, Hip and Spine Osteoarthritis Patients. Arthritis Care Res. (doi:10.1002/acr.23831). [DOI] [PubMed] [Google Scholar]
- 24. Wright EA, Katz JN, Abrams S, Solomon DH, Losina E.. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res 2014;6610:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Prescription Opioid Overdose Data. Atlanta: Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/drugoverdose/data/overdose.html (accessed January 8, 2019).
- 26.Centers for Disease Control and Prevention. Understanding the Epidemic. Atlanta: Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/drugoverdose/epidemic/index.html (accessed January 8, 2019).
- 27. Fibel KH, Hillstrom HJ, Halpern BC.. State-of-the-art management of knee osteoarthritis. World J Clin Cases 2015;32:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ammar TY, Pereira TA, Mistura SL, Kuhn A, Saggin JI, Lopes JO.. Viscosupplementation for treating knee osteoarthrosis: Review of the literature. Rev Bras Ortop 2015;505:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jevsevar D, Donnelly P, Brown GA, Cummins DS.. Viscosupplementation for osteoarthritis of the knee: A systematic review of the evidence. J Bone Joint Surg Am 2015;9724:2047–60. [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Sheha H, Fu Y, Liang L, Tseng SC.. Update on amniotic membrane transplantation. Expert Rev Ophthalmol 2010;55:645–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dua HS, Gomes JA, King AJ, Maharajan VS.. The amniotic membrane in ophthalmology. Surv Ophthalmol 2004;491:51–77. [DOI] [PubMed] [Google Scholar]
- 32. Bouchard CS, John T.. Amniotic membrane transplantation in the management of severe ocular surface disease: Indications and outcomes. Ocul Surf 2004;23:201–11. [DOI] [PubMed] [Google Scholar]
- 33. Cooke M, Tan EK, Mandrycky C, He H, O'Connell J, Tseng SC.. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care 2014;2310:465–76. [DOI] [PubMed] [Google Scholar]
- 34. Raines AL, Shih MS, Chua L, Su CW, Tseng SC, O'Connell J.. Efficacy of particulate amniotic membrane and umbilical cord tissues in attenuating cartilage destruction in an osteoarthritis model. Tissue Eng Part A 2017;23(1–2):12–9. [DOI] [PubMed] [Google Scholar]
- 35. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW.. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;1512:1833–40. [PubMed] [Google Scholar]
- 36. Garras D, Scott R. Plantar fasciitis treatment with particulated human amniotic membrane. Paper presented at: AOFAS Annual Meeting, Seattle, Washington July 12–15, 2017; 2016.
- 37. Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H.. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2012;205:350–6. [DOI] [PubMed] [Google Scholar]
- 38. Angst F, Aeschlimann A, Stucki G.. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum 2001;454:384–91. [DOI] [PubMed] [Google Scholar]
- 39. Tubach F, Wells GA, Ravaud P, Dougados M.. Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: Methodological issues. J Rheumatol 2005;3210:2025–9. [PubMed] [Google Scholar]
- 40. FDA. Guidance for Industry Clinical Development Programs for Drugs, Devices, and Biological Products Intended for the Treatment of Osteoarthritis (OA). Rockville, MD: US Department of Health and Human Services; 1999.
- 41. Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;123:177–90. [DOI] [PubMed] [Google Scholar]
- 42. Roemer FW, Hunter DJ, Crema MD, Kwoh CK, Ochoa-Albiztegui E, Guermazi A.. An illustrative overview of semi-quantitative MRI scoring of knee osteoarthritis: Lessons learned from longitudinal observational studies. Osteoarthritis Cartilage 2016;242:274–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eymard F, Chevalier X, Conrozier T.. Obesity and radiological severity are associated with viscosupplementation failure in patients with knee osteoarthritis. J Orthop Res 2017;3510:2269–74. [DOI] [PubMed] [Google Scholar]
- 44. Conrozier T, Monfort J, Chevalier X, et al. EUROVISCO recommendations for optimizing the clinical results of viscosupplementation in osteoarthritis. Cartilage. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mclure SW, Bowes MA, Wolstenholme CB, Vincent GR, Williams S, Conaghan PG. 416 The natural history of OA associated BMLs in the OAI progressor cohort. Osteoarthritis Cartilage 2010;18:S184–S5. [Google Scholar]
- 46. Phan CM, Link TM, Blumenkrantz G, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol 2006;163:608–18. [DOI] [PubMed] [Google Scholar]
- 47. Kornaat PR, Kloppenburg M, Sharma R, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol 2007;1712:3073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Altman RD, Farrokhyar F, Fierlinger A, Niazi F, Rosen J.. Analysis for prognostic factors from a database for the intra-articular hyaluronic acid (Euflexxa) treatment for osteoarthritis of the knee. Cartilage 2016;73:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wildi LM, Raynauld JP, Martel-Pelletier J, Abram F, Dorais M, Pelletier JP.. Relationship between bone marrow lesions, cartilage loss and pain in knee osteoarthritis: Results from a randomised controlled clinical trial using MRI. Ann Rheum Dis 2010;6912:2118–24. [DOI] [PubMed] [Google Scholar]
- 50. Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007;569:2986–92. [DOI] [PubMed] [Google Scholar]
- 51. Sansone V, Maiorano E, Pascale V, Romeo P.. Bone marrow lesions of the knee: Longitudinal correlation between lesion size changes and pain before and after conservative treatment by extracorporeal shockwave therapy. Eur J Phys Rehabil Med 2019;552:225–30. [DOI] [PubMed] [Google Scholar]
- 52. He H, Li W, Chen SY, et al. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci 2008;4910:4468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He H, Zhang S, Tighe S, Son J, Tseng SC.. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem 2013;28836:25792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen J, Li S, Chen D.. TGF-beta signaling and the development of osteoarthritis. Bone Res 2014;2:pii: 14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cui Z, Crane J, Xie H, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis 2016;759:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhen G, Wen C, Jia X, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 2013;196:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC.. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 2000;204:325–34. [PubMed] [Google Scholar]
- 58. Tseng SC, Li DQ, Ma X.. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999;1793:325–35. [DOI] [PubMed] [Google Scholar]
- 59. Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci 2016;575:ORSFh1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-a-inhibitor (HC•HA) purified from extracts of human amniotic membrane. J Biol Chem 2009;28430:20136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]