ABSTRACT
Influenza A virus infections occur in different species, causing mild to severe respiratory symptoms that lead to a heavy disease burden. Eurasian avian-like swine influenza A(H1N1) viruses (EAS-H1N1) are predominant in pigs and occasionally infect humans. An influenza A(H1N1) virus was isolated from a boy who was suffering from fever and headache and designated as A/Tianjin-baodi/1606/2018(H1N1). Full-genome sequencing and phylogenetic analysis revealed that A/Tianjin-baodi/1606/2018(H1N1) is a novel reassortant EAS-H1N1 containing gene segments from EAS-H1N1 (HA and NA), classical swine H1N1(NS) and A(H1N1)pdm09(PB2, PB2, PA, NP and M) viruses. The isolation and analysis of A/Tianjin-baodi/1606/2018(H1) provide further evidence that EAS-H1N1 poses a threat to human health and greater attention should be paid to surveillance of influenza virus infection in pigs and humans.
KEYWORDS: Influenza A virus, EAS-H1N1, triple-reassortant, molecular characteristics, Phylogenetic analysis
Introduction
Influenza A virus can be classified into different subtypes based on antigenic variation in two surface glycoproteins, haemagglutinin (HA) and neuraminidase (NA) [1]. Genetic mutations that encode a range of amino acid substitutions result in antigenic changes in the surface glycoproteins and lead to immunologic escape, which is known as antigenic drift [2–4]. In addition, gene segment exchange between two or more viruses, known as genetic reassortment, can also play an important role in the evolution of new influenza viruses [5–6].
Influenza A virus infection occurs in different animals, including pigs. Both α-2,3-linked sialic acids (Sias) (avian influenza virus receptor) and α-2,6-linked Sias (human influenza virus receptor) are found in pigs; hence, they are recognized as genetic mixing vessels for humans and avian influenza viruses [7–8]. Classical H1N1 swine influenza viruses (SIVs) cause sporadic zoonotic infections and a reassortant, swine-origin influenza A H1N1 virus (A(H1N1)pdm09) caused an influenza pandemic among humans in 2009 [9].
The first EAS-H1N1 human infection was reported in Switzerland in 1986 [10]. Since then, several human infections with EAS-H1N1 have occurred in European countries [11–12]. China is the largest pork-producing country in the world and it is known that EAS-H1N1 circulates in swine [13–17]. Furthermore, four zoonotic cases related to EAS-H1N1 infection have been reported in China, indicating the potential to cause a human influenza pandemic [18–21]. In this study, we isolated and characterized a novel triple-reassortant EAS-H1N1 from a nine-year-old boy.
Materials and methods
Epidemiologic information, sample collection and virus identification
A nine-year-old boy, who lived in the countryside with his family, presented with fever of 38.7°C and started coughing with pharyngeal pain and headache on 10 December 2018. The following day, the boy was sent directly to Baodi People’s Hospital (an influenza surveillance network hospital) and a throat-swab specimen was collected and transferred to Jizhou District Center for Disease Control and Prevention (Jizhou CDC). A second throat-swab sample was collected on 14 December. Throat swabs from all his close contacts and 28 environmental specimens (including cage surface wipes, poultry and swine excrement and drinking water) were collected for influenza virus detection using real-time reverse transcription polymerase chain reaction (real-time RT–PCR). Nucleic acid was extracted from 500 µl of the clinical specimen using NucliSENS easyMAG (BioMetrix, France) and real-time RT–PCR was carried out using FluA real-time PCR kit (Bojie, Shanghai, China) in accordance with the manufacturer’s instructions.
Virus isolation and antigenic characteristics
Clinical specimen was inoculated onto Madin–Darby Canine Kidney cells (MDCK) and cultured with serum-free minimum essential medium (MEM; Gibcol, USA) in the presence of 2.0 µg/ml of trypsin (Sigma, USA) at 34°C [22]. Checking for cytopathic effect (CPE) was conducted every day. Medium was collected when the CPE was up to 75–100% cells. Virus haemagglutination titre (HAT) and haemagglutination inhibition (HI) titres using antisera raised against specific A(H1N1)pdm09 reference viruses, distributed by the Chinese National Influenza Center (CNIC), were determined with 1.5% human-type “O” erythrocytes. If the HI titre of test viruses with the reference antiserum was less than eight-fold different to that of the reference virus, this result meant the test viruses were antigenically similar.
Antiviral susceptibility
The susceptibility of A/Tianjin-baodi/1606/2018(H1N1) to the NA inhibitor oseltamivir carboxylate (Roche Diagnostics GmbH) was evaluated using an NA-Fluor Influenza Neuraminidase Assay Kit (Applied Biosystems). Oseltamivir-resistant virus (A/Texas/23/2012(H1)Y275) and oseltamivir-sensitive virus (A/Texas/23/2012(H1)H275), provided by CNIC, were used as controls. The half maximal inhibitory concentration (IC50), which represented the concentration of oseltamivir that could inhibit 50% of NA activity, was used to evaluate the antiviral susceptibility of the virus. Viruses are considered to show normal inhibition (NI) if IC50 is increased no more than 10-fold compared to the oseltamivir-sensitive control virus, reduced inhibition (RI) if the IC50 is increased 10–100-fold and highly reduced inhibition (HRI) if IC50 is increased >100-fold (see the WHO web site https://www.who.int/influenza/gisrs_laboratory/antiviral_susceptibility/nai_overview/en/).
Genome sequencing and phylogenetic analysis
Viral RNA was extracted from 140 μl of virus stock using an RNeasy Mini Kit (QiaGen, Germany) as described by the manufacturer. Primers specific for each gene segment of influenza A virus were used for RT–PCR [23] and the products were purified using a QIAamp Gel Extraction Kit (QiaGen, Germany). Whole genome sequencing was carried out using an Illumina MiniSeq platform (Illumina, USA). Sequences were edited using the Lasergene sequence analysis software package (DNAStar, Madison, WI, USA). Sequence alignments and phylogenetic analysis were performed with MEGA X software and neighbour-joining trees were assembled with bootstrap values determined from 1000 replicates. All reference sequences were downloaded from the EpiFlu database of the Global Initiative on Sharing All Influenza Data (GISAID). The key molecular features were observed and analysed through the alignment with other reference viruses.
Results
The patient and epidemiology survey
A previously healthy nine-year-old boy, with no history of travel, presented with influenza-like illness (ILI) symptoms on 10 December 2018, having a fever of 38.7°C, pharyngeal pain and headache. He recovered within a week with neither hospitalization nor oseltamivir treatment. A retrospective investigation was conducted to identify the potential source of infection and any other possible cases. The patient had no contact with individuals showing ILI symptoms within 10 days before onset of his symptoms and his family raised eight chickens in captivity while their neighbours raised pigs in captivity. He lived with his parents and grandparents. None of his close contacts developed ILI symptoms during the period of the investigation.
Sample identification and viral isolation
The first throat-swab specimen was sent to the Jizhou CDC influenza surveillance network laboratory. By real-time RT–PCR, the sample was positive for influenza A virus, but negative for H3N2, H1N1pdm09, H5, H7 and H9 influenza viruses. The second specimen was negative for influenza virus. The influenza-positive specimen was subsequently transferred to Tianjin CDC for further investigation. Influenza A-positivity was confirmed and shown to be caused by an H1N1 virus which was different from H1N1pdm09. The virus, designated as A/Tianjin-baodi/1606/2018(H1N1) (TJ/1606/18), was isolated using MDCK cells. All specimens taken from his close contacts and the environment (including cage surface wipe swabs, poultry and swine excrement and drinking water of the animals) were negative for influenza virus.
Antigenic characteristics
Results from HI revealed that TJ/1606/18 was antigenically similar to A(H1N1)pdm09 virus vaccine strains A/California/07/2009(H1N1) and A/Michigan/45/2015(H1N1) (Table 1).
Table 1. Antigenic analysis of TJ/1606/18 using HI assay.
| Virus | HI antibody titres of ferret antiserum againsta | ||
|---|---|---|---|
| A/California/7/2009C | A/Michigan/45/2015E | A/Michigan/45/2015C | |
| A/California/7/2009(H1N1)Cb | 2560 | 160 | 640 |
| A/Michigan/45/2015(H1N1)Ec | 1280 | 1280 | 2560 |
| A/Michigan/45/2015(H1N1)Cb | 2560 | 2560 | 2560 |
| A/Tianjin-baodi/1606/2018(H1N1) | 640 | 640 | 1280 |
aHomologous titres are shown in boldface. Antiserum was obtained from ferret after immunized with virus stock once.
bViruses were isolated using MDCK cells.
cViruses were isolated using embryonated hens’ eggs.
Antiviral susceptibility
TJ/1606/18 displayed an NI phenotype, 1.2-fold increase in IC50 compared to the oseltamivir-sensitive control virus, with the NA inhibitor oseltamivir (Table 2).
Table 2. Antiviral susceptibility.
| Virusa | IC50 (95% confidence interval) |
|---|---|
| A/Tianjin-baodi/1606/2018(H1N1) | 0.46 (0.30–0.62) |
| A/Texas/23/2012(H1)H275 (NAI sensitive) | 0.37 (0.27–0.47) |
| A/Texas/23/2012(H1)Y275 (NAI resistance) | 55.50 (45.70–65.31) |
aNAI: neuraminidase inhibitor.
Molecular characteristics
Full-length sequences of the 8 gene segments of TJ/1606/18 (PB2, PB1, PA, HA, NP, NA, M and NS) were obtained, consisting of 2280, 2274, 2151, 1701, 1515, 1410, 982 and 838 nucleotides (only the nucleotides in the Open Reading Frame were calculated), respectively. Sequences of all eight segments have been submitted to GISAID, accession numbers: EPI1431720-EPI1431727.
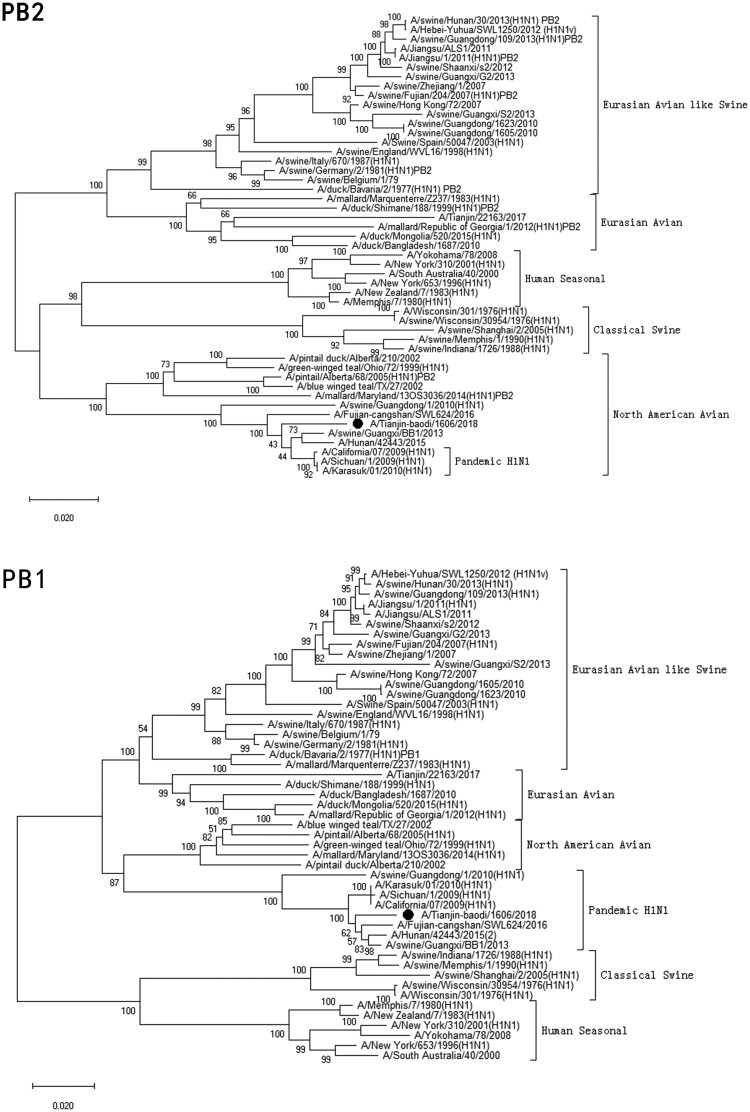
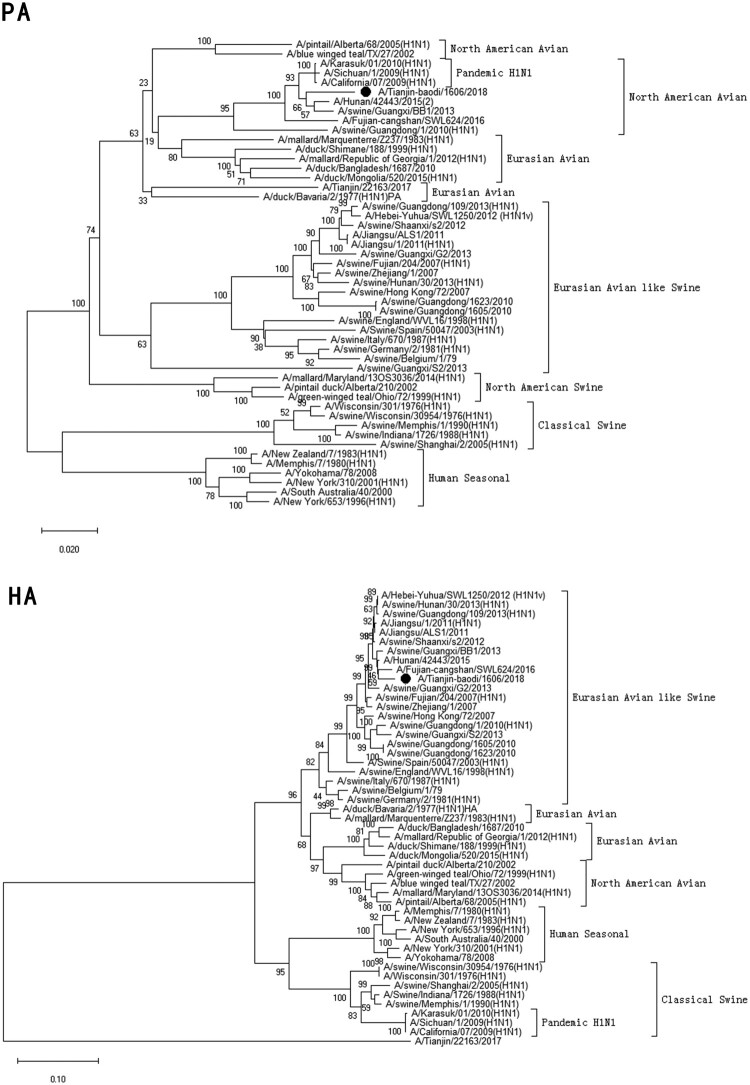
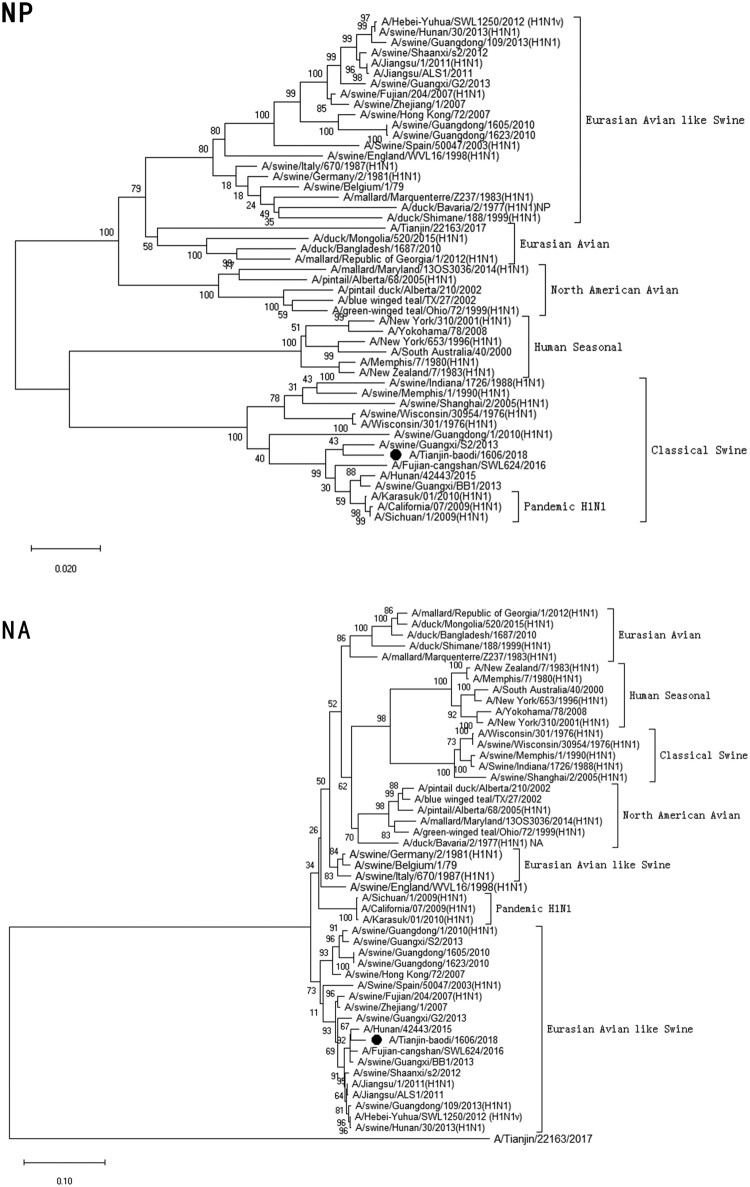
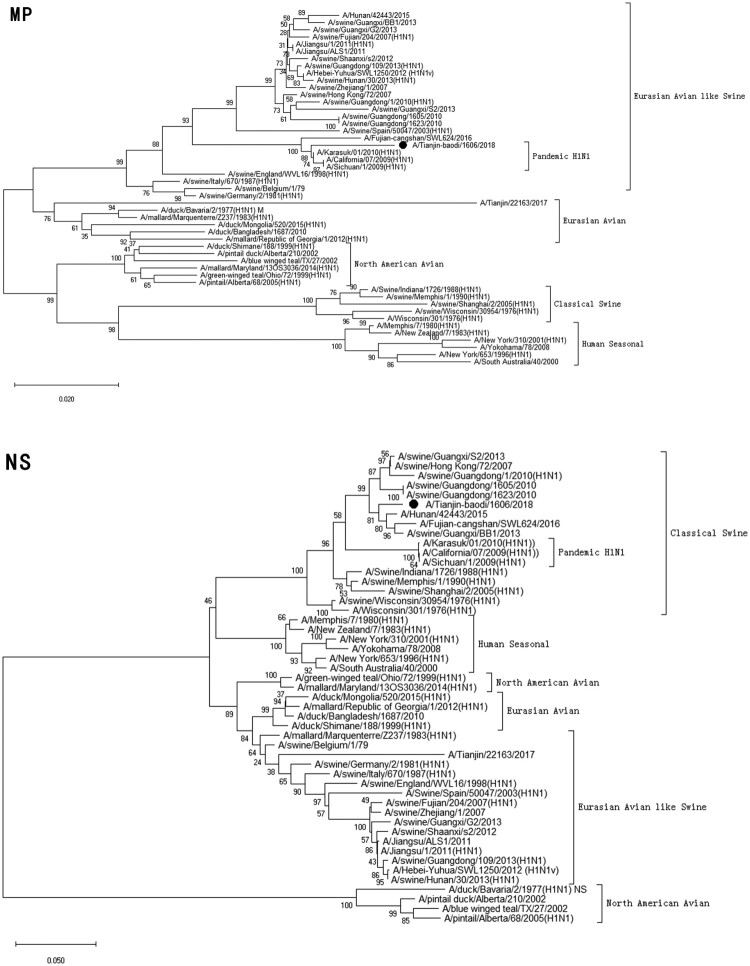
Phylogenetic analysis revealed that TJ/1606/18 was a novel EAS-H1N1 containing genes from Eurasian avian-like swine H1N1 (HA and NA), A(H1N1)pdm09 (PB2, PB1, PA, NP and M), and classical swine H1N1 (NS) (Figure 1 and Table 3). All eight segments shared 93.6–97.9% and 94.9–97.1% nucleotide identity with A/Hunan/42443/2015 and A/Fujian-cangshan/SWL624/2016, respectively (Table 4), with94.5–98.8% and 94.0–99.6% identities in deduced amino acids (Table 5). However, the gene segments PA, NP and M of TJ/1606/18 showed the highest nucleotide homologies, 97%, 97.1%, and 98.1% respectively, with A/California/07/2009 virus (Table 4) and corresponding amino acid homologies of 98.5%, 98.6% and 99–100% (Table 5).
Figure 1.
Phylogenetic analysis of eight gene segments on A/Tianjin-baodi/1606/2018(H1). The reliability of the trees was assessed via bootstrap analysis with 1000 replications. Pandemic H1N1 means A(H1N1)pdm09.
Table 3. Genetic origin of A/TJ/1606/18 based on phylogenetic analyses.
| Lineage assigned to gene segment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Isolates | PB2 | PB1 | PA | HA | NP | NA | M | NS |
| A/California/07/2009(H1N1) | PDM | PDM | PDM | PDM | PDM | PDM | PDM | PDM |
| A/Jiangsu/1/2011(H1N1)a | EAS | EAS | EAS | EAS | EAS | EAS | EAS | EAS |
| A/Fujian-cangshan/SWL624/2016(H1N1)a | PDM | PDM | PDM | EAS | PDM | EAS | PDM | CS |
| A/Hebei-yunhua/SWL1250/2012(H1N1)a | EAS | EAS | EAS | EAS | EAS | EAS | EAS | EAS |
| A/Hunan/42443/2015(H1N1)a | PDM | PDM | PDM | EAS | PDM | EAS | EAS | CS |
| A/Tianjin/22163/2017(H7N9)b | EAS | EAS | EAS | AIV | EAS | AIV | EAS | EAS |
| A/swine/Guangdong/1/2010(H1N1) | PDM | PDM | PDM | EAS | PDM | EAS | EAS | CS |
| A/swine/Tianjin/9/2013(H1N1)c | PDM | PDM | PDM | EAS | PDM | EAS | EAS | CS |
| A/Tianjin-baodi/1606/2018(H1N1) | PDM | PDM | PDM | EAS | PDM | EAS | PDM | CS |
Notes: PDM, genes closest homology to A(H1N1)pdm09 viruses; EAS, genes with closest homology to Eurasianavian-like swine influenza viruses; CS, genes with closest homology to classical swine influenza viruses; AIV, avian influenza viruses.
aHuman infection with Eurasian avian-like swine influenza virus.
bHuman infection with avian influenza virus H7N9 in Tianjin.
cNoveltriple-reassortant H1N1 swine influenza viruses in pigs in Tianjin, China [35].
Table 4. Nucleotide homology analysis of the eight gene segments of A/TJ/1606/18.
| Isolates | A/Tianjin-baodi/1606/2018(H1) (nucleotide identities %) | |||||||
|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PA | HA | NP | NA | M | NS | |
| A/California/07/2009(H1N1) | 96.6 | 97.4 | 97.0 | 66.6 | 97.1 | 88.2 | 98.1 | 91.2 |
| A/Jiangsu/1/2011(H1N1)a | 80.6 | 81.2 | 81.2 | 96.6 | 79.8 | 96.9 | 94.1 | 79.6 |
| A/Fujian-cangshan/SWL624/2016(H1N1)a | 95.2 | 97.1 | 94.9 | 96.1 | 95.6 | 97.1 | 97.1 | 96.4 |
| A/Hebei-yunhua/SWL1250/2012(H1N1)a | 80.1 | 81.5 | 80.8 | 96.4 | 80.0 | 96.7 | 93.6 | 78.6 |
| A/Hunan/42443/2015(H1N1)a | 97.0 | 97.9 | 96.9 | 97.5 | 96.4 | 97.2 | 93.6 | 97.0 |
| A/Tianjin/22163/2017(H7N9)b | 81.2 | 81.8 | 86.2 | 24.3 | 79.8 | 29.9 | 84.4 | 75.6 |
| A/swine/Guangdong/1/2010(H1N1) | 92.7 | 92.8 | 92.0 | 91.7 | 92.3 | 85.1 | 93.4 | 94.7 |
aHuman infection with Eurasian avian-like swine influenza virus.
bHuman infection with avian influenza virus H7N9 in Tianjin.
Table 5. Amino acid homology analysis of A/TJ/1606/18 proteins.
| Isolates | A/Tianjin-baodi/1606/2018(H1) (identities %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PB1-F2 | PA | HA | NP | NA | M1 | M2 | NS1 | NS2 | |
| A/California/07/2009(H1N1) | 98.3 | 98.9 | 98.0 | 98.5 | 78.9 | 98.6 | 92.1 | 100 | 99.0 | 88.6 | 89.0 |
| A/Jiangsu/1/2011(H1N1)a | 93.8 | 94.5 | 64.0 | 92.3 | 97.0 | 92.2 | 96.4 | 98.8 | 93.8 | 78.5 | 87.7 |
| A/Fujian-cangshan/SWL624/2016(H1N1)a | 97.2 | 98.7 | 94.0 | 97.1 | 97.2 | 98.4 | 97.3 | 99.6 | 97.9 | 94.7 | 94.5 |
| A/Hebei-yunhua/SWL1250/2012(H1N1)a | 93.7 | 94.3 | 62.0 | 92.5 | 97.4 | 92.0 | 96.0 | 98.8 | 93.8 | 77.2 | 84.9 |
| A/Hunan/42443/2015(H1N1)a | 98.0 | 98.8 | 98.0 | 98.7 | 97.7 | 98.0 | 97.9 | 98.4 | 94.8 | 95.2 | 94.5 |
| A/Tianjin/22163/2017(H7N9)b | 96.3 | 95.5 | 56.0 | 95.0 | 42.4 | 92.6 | 48.9 | 92.1 | 88.7 | 75.4 | 83.6 |
| A/swine/Guangdong/1/2010(H1N1) | 96.4 | 97.4 | 90.0 | 95.5 | 92.4 | 97.4 | 93.6 | 99.6 | 93.8 | 92.1 | 89.0 |
aHuman infection with Eurasian avian-like swine influenza virus.
bHuman infection with avian influenza virus H7N9 in Tianjin.
The key molecular features of TJ/1606/18 known to be associated with increased virulence in mammals, mammalian transmissibility and antiviral susceptibility were shown in Table 6. TJ/1606/18 contained the amino acid motif PSIQSR↓GL at the HA1/HA2 cleavage site, a characteristic of influenza viruses with low pathogenicity [24]. Furthermore, seven potential glycosylation sites (N-X-S/T) were found at positions 27, 28, 40, 212, 291, 498 and 557 in the HA protein of the isolated virus. TJ/1606/18 had 190D and 225E in HA, indicative of increased binding to swine or human receptors.
Table 6. Molecular analysis of A/TJ/1606/18 compared to other viruses.
| Gene product | Function | Amino acid substitution | Virusa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TJ | FJ | HN | HB | JS | GD | CA | |||
| HA | Altered receptor specificity | E190D | D | D | D | D | D | V | D |
| D225E | E | E | E | E | E | E | D | ||
| NA | Antiviral resistance (oseltamivir) | H275Y | H | H | H | H | H | H | H |
| N295S | N | N | N | N | N | N | N | ||
| PB2 | Enhanced polymerase activity | L89V | V | V | V | V | V | V | V |
| Virus replication in mammals | Q591R | R | Q | R | Q | Q | R | R | |
| E627K | E | E | E | E | E | E | E | ||
| D701N | D | D | D | N | N | D | D | ||
| PB1 | Between species transmission | X99H | H | H | H | H | H | H | H |
| I368V | I | I | I | I | I | I | I | ||
| PA | Increased polymerase activity in mice | L336M | M | M | M | L | L | L | M |
| Species-associated signature positions | K356R | R | R | R | K | K | K | R | |
| S409N | N | N | N | N | N | N | N | ||
| M1 | Increased virulence in mice | T215A | A | A | A | A | A | A | A |
| M2 | Antiviral resistance (amantadine) | S31N | N | N | N | N | N | N | N |
| NS1 | Increased virulence in mice | P42S | S | S | S | S | S | S | S |
| NP | Mammalian-adaptive and increased virulence in mice | Q357K | K | K | K | Q | Q | K | K |
aTJ, A/Tianjin-baodi/1606/2018(H1N1);FJ, A/Fujian-cangshan/SWL624/2016(H1N1); HN, A/Hunan/42443/2015(H1N1);HB, A/Hebei-yunhua/SWL1250/2012(H1N1);JS, A/Jiangsu/1/2011(H1N1); GD, A/swine/Guangdong/1/2010(H1N1);CA, A/California/07/2009(H1N1)pdm09. For each virus, amino acid positions carrying the substitution are highlighted.
The amino acid substitutions (H275Y and N295S) associated with reduced susceptibility to NA inhibitors were not observed in TJ/1606/18 NA, suggesting that the isolated virus was sensitive to antiviral drugs oseltamivir and zanamivir. This was consistent with the results of antiviral susceptibility test. However, the M2 protein had S37N amino acid substitution like A(H1N1)pdm09 viruses, indicative of resistance to the antiviral drugs amantadine and rimantadine [25–26].
In PB1 polymerase, TJ/1606/18 owned 99H and 368I. The TJ/1606/18 PB1-F2 protein is unlikely to function as the reading frame was interrupted by stop codons at positions equivalent to amino acid residues 12 and 86.
In addition, several amino acid substitutions related to virus virulence or host adaption have been reported, including L89V, Q591R, E627K, and D701N in PB2 polymerase, L336M, K256R and S409N in PA, T215A in M1 protein, P42S in NS1and Q357K in NP protein [27–33]. TJ/1606/18gene sequences encoded all but two, E627K and D701N in PB2 polymerase, of these amino acid substitutions (Table 6).
Discussion
Influenza virus infection usually causes substantial mortality and morbidity. Pigs play an important role in the generation of novel influenza viruses with pandemic potential because they may be infected with both humans and avian influenza virus [34]. Currently, influenza viruses of subtypes H1N1, H3N2 and H1N2 are known to co-circulate in pigs [14]. Since 2005, the EAS-H1N1 viruses have become dominant [13–14] after their long-term evolution, the EAS-H1N1 viruses in China have been reported to preferentially bind to human-type receptors, and some of the viruses tested were transmitted in ferrets by respiratory droplets. This suggests that among the influenza viruses currently circulating in animals, the EAS-H1N1 SIVs pose the greatest pandemic threat [13].
Sun et al. reported that novel triple-reassortant EAS-H1N1 SIVs, containing gene segments from A(H1N1)pdm09 (PB1, PB2, PA and NP), EAS-H1N1 (HA, NA and M) and Classical SIVs (NS),were isolated from pigs in Tianjin, together with human-like H1N1, classical swine H1N1 and Eurasian swine H1N1 viruses [35], which indicated that multiple genetic lineages of swine H1N1 viruses were co-circulating in the swine population in Tianjin, China. Here, we show that TJ/1606/18 had close antigenic and genetic relationships with EAS-H1N1 viruses that were circulating in pigs in China. As the gene sequences can’t be available from GenBank or GISAID, we couldn’t analyse the detailed molecular characteristics between TJ/1606/18 and the novel triple-reassortant EAS-H1N1 isolated in pigs in Tianjin (mentioned above), and based on the phylogenetic trees published by Sun et al. in 2013 [35], it can be recognized that the novel triple-reassortant EAS-H1N1 viruses were closely related to TJ/1606/18, with only a difference that M gene derived from EAS-H1N1. This means EAS-H1N1 viruses have continued to circulate in pigs in Tianjin, with mutation and recombination occurring, yielding reassortant viruses that occasionally infect humans.
At the time of manuscript preparation, TJ/1606/18 was isolated from the fifth case of human infection with an EAS-H1N1 reported in China; the other four yielding A/Jiangsu/1/2011(H1N1) (JS), A/Heibei-yuhua/SWL1250/2012(H1N1) (HB), A/Hunan/42443/2015(H1N1) (HN) and A/Fujian-cangshan/SWL624/2016(H1N1) (FJ) [18–21]. The first case, which began in late December 2010 in a three-year-old boy in Jiangsu, resulted in death; however, the child had a history of renal disease [18,36]. The second case, which began in December 2012 in a three-year-old boy in Hebei Province, caused mild influenza-like illness [19]. The third case was a 30-month-old boy in late June 2015 in Hunan Province, who developed severe pneumonia and recovered after hospitalization treatment for 38 days. The fourth case was a 46-year-old man with severe pneumonia in October 2016 in Fujian province, and the patient died due to multi-organ failure. The fifth case reported here was a nine-year-old boy with mild ILI symptoms in December 2018, and recovered in a week without hospitalization and oseltamivir treatment. This is the first human infected with novel EAS-H1N1 in northern China, and the second case in China (the case from Fujian is the first one).
Of the four previous EAS-H1N1 virus causing human infections (Tables 3 and 4), a full-genome analysis of these viruses showed that they can be divided into two main genotypes, represented by the JS and HN viruses [33]. All eight gene segments of JS and HB belonged to EAS-H1N1 (JS-like viruses), while those of HN and FJ were derived from EAS-H1N1, A(H1N1)pdm09 and classical swine H1N1 viruses, as was the case for TJ/1606/18 (HN-like viruses). And research has indicated than HN virus showed higher infectivity, virulence, and substantially higher replication in respiratory tract of mice than JS virus [20]. TJ/1606/18 belonged to HN-like virus, but caused mild respiratory infection in human. More research about its pathogenesis in mice should be evaluated in future.
Based on previous studies, amino acid substitutions E190D and G225D/E in HA could lead to a shift in receptor-binding specificity from avian α-2,3-linked sialic acid (Sias) to human α-2,6-linked Sias, thereby increasing the binding of H1N1 virus to cells in the human upper respiratory tract [37–38]; TJ/1606/18 had 190D and 225E, as was the case in other EAS-H1N1 viruses isolated from humans (Table 6), indicating the potential risk of transmission among humans. However, the amino acid motif PSIQSR↓GL was found at the HA1/HA2 cleavage site, a characteristic of influenza viruses with low pathogenicity.
The viral polymerase is a major determinant of interspecies transmission and pathogenesis. It has been reported that L89V in PB2 [21] can enhance the polymerase activity, Q591R and E627K [21] can increase virus replication in mammals, and most recently published D701N in PB2 can indeed enhance the viral polymerase activity, viral replication, and pathogenicity in mice [17]. Here, TJ/1606/18 had L89V substitution and 627E, which exist in all five EAS-H1N1 viruses, but owned Q591R substitution and 701D, which are specific to HN-like viruses (Table 6).
Increased virus transmission between hosts has been reported for viruses carrying X99H and I368V amino acid substitutions in their PB1 polymerase [39]; TJ/1606/18 possessed 99H and 368I, which are the same as in all five EAS-H1N1 (Table 6). Furthermore, studies have shown that the influenza virus PB1-F2, encoded by a second reading frame, protein can play a key role in viral infection and virulence [24,40–41] with the protein locating in the mitochondria and leading to apoptosis [42]. However, truncated PB1-F2 with fewer than 87 amino acid residues lacks the mitochondria translocation signal and cannot function [42]. Otherwise, PB1-F2 plays critical roles in viral pathogenesis by interfering with the host immune response and influencing inflammatory responses [43]. However, PB1-F2 has dissimilar functions in different virus types/subtypes and hosts, which are determined by its length. Usually, avian influenza viruses have full-length PB1-F2 protein, enabling all functions [44], while classical swine H1N1 and A(H1N1)pdm09 viruses frequently contain truncated PB1-F2 with associated loss of function as seen in TJ/1606/18.
Recently research showed that Q357K substitution in NP protein, which is a typical human signature marker, is an adaptive signature of the influenza A viruses, allowing them to cross species barriers to circulate in humans and induces a fatal infection in mice [33]. TJ/1606/18 possessed 357K, the same as the other two HN-like viruses (HN and FJ) (Table 6), this may indicate that EAS-H1N1 already acquired the trait necessary to cause a human influenza pandemic.
Alternatively, amino acid substitutions related to increased virulence were found in PA (336M and 356R, unique in HN-like viruses), M1 and NS1 proteins of TJ/1606/18. This complex pattern of amino acid substitutions and their interaction in determining virus virulence, notably in relation to infection of humans, requires further investigation.
TJ/1606/18, like other EAS-H1N1 viruses isolated from humans, showed antiviral resistance to amantadine and rimantadine due to S31N amino acid substitution in M2 protein, but remained sensitive to neuraminidase inhibitors. Hence, at the early stage of infection by EAS-H1N1-like viruses, administration of oseltamivir or zanamivir may reduce the severity of infection [1].
In conclusion, human infection with EAS-H1N1 or a reassortant EAS-H1N1virus could result in mild or severe clinical symptoms. Whole genome sequencing has revealed a number of gene mutations encoding amino acid substitutions in a number of the virus proteins, coming together by mean of gene reassortment, which have been associated with increased virulence and likelihood of transmission to other mammals and humans. It is imperative to continue and enhance surveillance of influenza in swine as they represent a host species that can produce novel virus reasortants that could lead to another human pandemic, as seen with A(H1N1)pdm09 viruses.
Acknowledgements
We thank the data submitters from the GISAID EpiFlu and GenBank databases for the A(H1N1) gene sequences used in the course of this study. And we also sincerely thank Daniels R.S. from WHO Collaborating Center for Reference and Research on Influenza, The Francis Crick Institute for the English language editing of the manuscript.
Funding Statement
This research was funded by the Tianjin Science and Technology Commission [grant number 15JCYBJC24700], a National Science and Technology Major Project [grant number 2017ZX10103007-002], and the APC was funded by the Tianjin Municipal Health and Health Committee.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Webster RG, Bean WJ, et al. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster RG, Laver WG, Air GM, et al. Molecular mechanisms of variation in influenza viruses. Nature. 1982;296:115–121. doi: 10.1038/296115a0 [DOI] [PubMed] [Google Scholar]
- 3.Drake J. Rates of spontaneous mutation among RNA viruses. Proc Natl Acad Sci USA. 1993;90:4171–4175. doi: 10.1073/pnas.90.9.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley SE, Das SR, Gibbs JS, et al. Influenza A virus hemagglutinin antibody escape promotes neuraminidase antigenic variation and drug resistance. PLoS One. 2011;6:e15190. doi: 10.1371/journal.pone.0015190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedford T, Suchard MA, Lemey P, et al. Integrating influenza antigenic dynamics with molecular evolution. Elife. 2014;3:e01914. doi: 10.7554/eLife.01914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambaut A, Pybus OG, Nelson MI, et al. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453:615–619. doi: 10.1038/nature06945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrucci MR, Donatelli I, Sidoli L, et al. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193:503–506. doi: 10.1006/viro.1993.1155 [DOI] [PubMed] [Google Scholar]
- 8.Ma W, Kahn RE, Richt JA.. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J Mol Genet Med. 2008;3:158–166. [PMC free article] [PubMed] [Google Scholar]
- 9.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;25:2605–2615. doi: 10.1056/NEJMoa0903810 [DOI] [PubMed] [Google Scholar]
- 10.de Jong JC, Claas EC, Osterhaus AD, et al. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong JC, Paccaud MF, de Ronde-Verloop FM, et al. Isolation of swine-like influenza A(H1N1) viruses from man in Switzerland and The Netherlands. Ann Inst Pasteur Virol. 1988;139:429–437. doi: 10.1016/S0769-2617(88)80078-9 [DOI] [PubMed] [Google Scholar]
- 12.Myers KP, Olsen CW, Gray GC.. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44:1084–1088. doi: 10.1086/512813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Chen Y, Qiao C, et al. Prevalence, genetics, and transmissibility in ferrets of Eurasian avian-like H1N1 swine influenza viruses. Proc Natl Acad Sci USA. 2016;113:392–397. doi: 10.1073/pnas.1522643113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijaykrishna D, Smith GJ, Pybus OG, et al. Long-term evolution and transmission dynamics of swine influenza A virus. Nature. 2011;473:519–522. doi: 10.1038/nature10004 [DOI] [PubMed] [Google Scholar]
- 15.Liang H, Lam TT, Fan X, et al. Expansion of genotypic diversity and establishment of 2009 H1N1 pandemic-origin internal genes in pigs in China. J Virol. 2014;88:10864–10874. doi: 10.1128/JVI.01327-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijaykrishna D, Poon LL, Zhu HC, et al. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science. 2010;328:1529. doi: 10.1126/science.1189132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Zhu W, Feng Z, et al. Substitution of D701N in the PB2 protein could enhance the viral replication and pathogenicity of Eurasian avian-like H1N1 swine influenza viruses. Emerg Microbes Infect. 2018;7(1):75. doi: 10.1038/s41426-018-0073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi X, Cui L, Jiao Y, et al. Antigenic and genetic characterization of a European avian-like H1N1 swine influenza virus from a boy in China in 2011. Arch Virol. 2013;158:39–53. doi: 10.1007/s00705-012-1423-7 [DOI] [PubMed] [Google Scholar]
- 19.Wang DY, Qi SX, Li XY, et al. Human infection with Eurasian avian-like influenza A(H1N1) virus, China. Emerg Infect Dis. 2013;19:1709–1711. doi: 10.3201/eid1910.130420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W, Zhang H, Xiang X, et al. Reassortant Eurasian avian-like influenza A(H1N1) virus from a severely ill child, Hunan Province, China, 2015. Emerg Infect Dis. 2016;22:1930–1936. doi: 10.3201/eid2211.160181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie JF, Zhang YH, Zhao L, et al. Emergence of Eurasian avian-like swine influenza A (H1N1) virus from an adult case in Fujian Province, China. Virol Sin. 2018;33:282–286. doi: 10.1007/s12250-018-0034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham M, Liang B, Van Domselaar G, et al. Nationwide molecular surveillance of pandemic H1N1 influenza A virus genomes: Canada, 2009. PLoS One. 2011;6:e16087. doi: 10.1371/journal.pone.0016087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue E, Wang X, Osawa Y, et al. Full genomic amplification and subtyping of influenza A virus using a single set of universal primers. Microbiol Immunol. 2010;54:129–134. doi: 10.1111/j.1348-0421.2009.00193.x [DOI] [PubMed] [Google Scholar]
- 24.Korteweg C, Gu J.. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumbholz A, Schmidtke M, Bergmann S, et al. High prevalence of amantadine resistance among circulating European porcine influenza A viruses. J Gen Virol. 2009;90(Pt 4):900–908. doi: 10.1099/vir.2008.007260-0 [DOI] [PubMed] [Google Scholar]
- 26.Gregory V, Lim W, Cameron K, et al. Infection of a child in Hong Kong by an influenza A H3N2 virus closely related to viruses circulating in European pigs. J Gen Virol. 2001;82(Pt 6):1397–1406. doi: 10.1099/0022-1317-82-6-1397 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen HT, Fry AM, Gubareva LV.. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther. 2012;17(Pt B):159–173. doi: 10.3851/IMP2067 [DOI] [PubMed] [Google Scholar]
- 28.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459 [DOI] [PubMed] [Google Scholar]
- 29.Yamada S, Hatta M, Staker BL, et al. Biological and structural characterization of a host-adapting amino acid in influenza virus. PLoS Pathog. 2010;6:e1001034. doi: 10.1371/journal.ppat.1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Yang L, Zhu W, et al. Two outbreak sources of influenza A (H7N9) viruses have been established in China. J Virol. 2016;90:5561–5573. doi: 10.1128/JVI.03173-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan S, Deng G, Song J, et al. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology. 2009;384:28–32. doi: 10.1016/j.virol.2008.11.044 [DOI] [PubMed] [Google Scholar]
- 32.Jiao P, Tian G, Li Y, et al. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol. 2008;82:1146–1154. doi: 10.1128/JVI.01698-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu W, Feng Z, Chen Y, et al. Mammalian-adaptive mutation NP-Q357K in Eurasian H1N1 swine influenza viruses determines the virulence phenotype in mice. Emerg Microbes Infect. 2019;8:989–999. doi: 10.1080/22221751.2019.1635873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Couceiro JN, Kelm S, et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun YF, Wang XH, Li XL, et al. Novel triple-reassortant H1N1 swine influenza viruses in pigs in Tianjin, northern China. Vet Microbiol. 2016;183:85–91. doi: 10.1016/j.vetmic.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 36.Zu R, Dong L, Qi X, et al. Virological and serological study of human infection with swine influenza A H1N1 virus in China. Virology. 2013;446:49–55. doi: 10.1016/j.virol.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 37.Matrosovich M, Tuzikov A, Bovin N, et al. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J Virol. 2000;74:8502–8512. doi: 10.1128/JVI.74.18.8502-8512.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumpey TM, Maines TR, Van Hoeven N, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212 [DOI] [PubMed] [Google Scholar]
- 39.Welkers MRA, Pawestri HA, Fonville JM, et al. Genetic diversity and host adaptation of avian H5N1 influenza viruses during human infection. Emerg Microbes Infect. 2019;8:262–271. doi: 10.1080/22221751.2019.1575700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basler CF, Aguilar PV.. Progress in identifying virulence determinants of the 1918 H1N1 and the Southeast Asian H5N1 influenza A viruses. Antiviral Res. 2008;79:166–178. doi: 10.1016/j.antiviral.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park ES, Byun YH, Park S, et al. Co-degradation of interferon signaling factor DDX3 by PB1-F2 as a basis for high virulence of 1918 pandemic influenza. EMBO J. 2019;38. doi: 10.15252/embj.201899475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamarin D, Ortigoza MB, Pales P.. Influenza A virus PB1-F2 protein contributes to viral pathogenesis in mice. J Virol. 2006;80:7976–7983. doi: 10.1128/JVI.00415-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Calvo PA, Malide D, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–1312. doi: 10.1038/nm1201-1306 [DOI] [PubMed] [Google Scholar]
- 44.Alymova IV, McCullers JA, Kamal RP, et al. Virulent PB1-F2 residues: effects on fitness of H1N1 influenza A virus in mice and changes during evolution of human influenza A viruses. Sci Rep. 2018;8:7474. doi: 10.1038/s41598-018-25707-y [DOI] [PMC free article] [PubMed] [Google Scholar]