Abstract
The complex nature and structure of the human immunodeficiency virus has rendered the cure for HIV infections elusive. The advances in antiretroviral treatment regimes and the development of highly advanced anti-retroviral therapy, which primarily targets the HIV enzymes, have dramatically changed the face of the HIV epidemic worldwide. Despite this remarkable progress, patients treated with these drugs often witness inadequate efficacy, compound toxicity and non-HIV complications. Considering the limited inventory of druggable HIV proteins and their susceptibility to develop drug resistance, recent attempts are focussed on targeting HIV-host interactomes that are essential for viral reproduction. Noticeably, unlike other viruses, HIV subverts the host nuclear pore complex to enter into and exit through the nucleus. Emerging evidence suggests a crucial role of interactions between HIV-1 proteins and host nucleoporins that underlie the import of the pre-integration complex into the nucleus and export of viral RNAs into the cytoplasm during viral replication. Nevertheless, the interaction of HIV-1 with nucleoporins has been poorly described and the role of nucleoporins during nucleocytoplasmic transport of HIV-1 still remains unclear. In this review, we highlight the advances and challenges in developing a more effective antiviral arsenal by exploring critical host-HIV interactions with a special focus on nuclear pore complex (NPC) and nucleoporins.
Keywords: anti-retroviral therapy, drug resistance, protein-protein interactions, host factors, nuclear pore complex
1. Introduction
HIV (Human Immunodeficiency Virus), a major killer worldwide was first recognised in the early 1980s in the United States as a strange retrovirus causing an entirely new disease called AIDS (Acquired Immunodeficiency Syndrome) [1]. To date, we do not have any cure for AIDS. The current anti-HIV treatment (Anti-Retroviral Therapy, or ART) has certainly resulted in a dramatic decrease in AIDS and AIDS-related complications in treated patients; however, the emergence of drug resistance and side effects posed by conventional medicines are currently restraining the clinical use of many of these antiviral compounds. In this review, we emphasize on the significance of host-viral interactions, particularly HIV-1 and human interactomes, which are now being revisited for exploring novel drug targets with higher efficacy. Furthermore, focus has been laid on the HIV-1 dependence on different host factors in the nuclear import and export of viral nucleic acids and proteins. While proper nuclear entry and exit is essential for viral replication, the nuclear pore complex can be a key target for virus subversion and may provide the basis for developing antiviral compounds for therapeutic intervention that not only target viral proteins alone but their interactions with nuclear membrane partners like nucleoporins. We also highlight the current challenges and further scope in the field. The lack of complete molecular understanding and structural knowledge of the host-viral protein complexes is the most significant limiting factor in the area.
Global Burden and Geographic Distribution of HIV
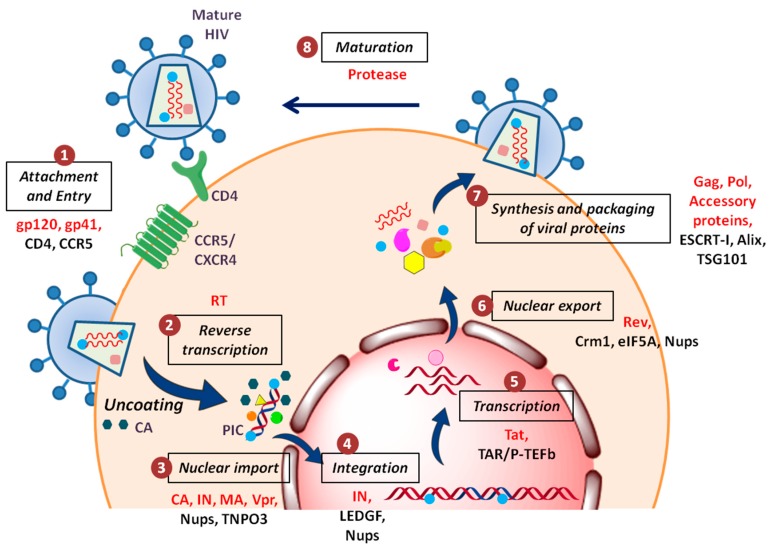
HIV arose through zoonotic transmissions of simian immunodeficiency viruses (SIV) during the past century in East and Central Africa [2,3]. Today, HIV-1, along with its less widespread cousin HIV-2, is responsible for a worldwide pandemic, infecting more than 36 million people. On the other hand, HIV-2 is relatively less prevalent and restricted to West Africa and some parts of Asia. Moreover, the infection with HIV-2 seems to be slowly progressing and less efficiently transmissible. These retroviruses are divided into different groups based on their genetic diversity. While HIV-2 has five groups and no subtypes/clades, three groups of HIV-1 have been identified, viz. M, N, and O, based on their genome differences. Most of the HIV-1 infections are caused by group M viruses which are further classified into nine clades (A–D, F–H, J and K) [4,5]. The DNA sequences of viruses belonging to distinct clades differ by 15–20%. Apparently, the extent of genetic divergence among the subtypes account for the different levels of their pathogenic efficiency and potential. Therefore, the evolutionary success of these viruses lies in their ‘deceptive simplicity’, i.e., even though these viruses possess a small genome and encodes few similar proteins, they are difficult to target due to their enormous genetic variations and capability of mutation. Despite encoding only 15 mature proteins (Table 1), HIV-1 can persistently attack and compromise the innate and adaptive immune systems of an individual [6]. Furthermore, for a successful viral replication, it employs a series of well-planned steps which engage complex relationship between cellular and viral factors (Figure 1).
Table 1.
HIV-encoded proteins and their functions.
| Class | Protein | Function |
|---|---|---|
| Viral enzymes (encoded by pol) | Reverse transcriptase (RT) | builds a DNA copy of the viral RNA genome; reverse transcription and RNAse H activity [9] |
| Integrase (IN) | takes the DNA copy of the viral genome inside nucleus and inserts it into the infected cellular genome [10] | |
| Protease (PR) | essential for cleavage of polypeptides and maturation of HIV particles [11] | |
| Structural proteins (encoded by gag and env) | Matrix (MA) | forms a coat on the inner surface of the viral membrane [12] |
| Capsid (CA) | forms a cone-shaped coat around the viral RNA, delivering it into the cell during infection [13] | |
| Nucleocapsid (NC) | forms a stable complex with the viral RNA to protect it [14] | |
| Gp120 (SU envelope protein) | bind to receptors on the surface of host cell and then penetrate the cell surface making way for fusion [15,16] | |
| Gp41 (TM envelope protein) | ||
| Accessory proteins | Tat | transactivator of transmembrane, enhances transcription of viral mRNA [17] |
| Rev (regulator of virion expression) | regulates the splicing and nuclear export of viral RNA [18] | |
| Nef (negative regulatory factor) | inhibits host’s defences; pleiotropic effects, can increase or decrease virus replication [19] | |
| P6 | incorporation of Vpr into new viruses; virion budding [20] | |
| Vif (viral infectivity factor) | increases virus infectivity by inhibiting host’s defence proteins; helps in virion assembly [21] | |
| Vpr (viral protein r) | nuclear import of viral DNA [22,23] | |
| Vpu (viral protein u) | Helps in virus budding and release [24] |
Figure 1.
Schematic overview of the HIV-1 replication cycle: (1) The HIV-1 particle attaches and fuses itself to the host cell membrane via its surface glycoproteins and enters the cell cytoplasm; (2) viral genomic RNA is reverse transcribed into DNA, accompanied by uncoating of the viral capsid; (3) nuclear entry of pre-integration complex occurs with the help of various host nuclear proteins; (4) integration of vDNA into the host chromatin; (5) during productive infection, viral transcription takes place; (6) RNA splicing and nuclear export of viral RNA occurs; (7) production and assembly of new virus particles, which bud from the plasma membrane; and (8) virions become infectious after maturation (action of protease). The viral and host cellular proteins involved in the replication cycle of HIV-1 are highlighted at each step in red and black, respectively.
One of the surprising facets of the HIV/AIDS pandemic is the unequal distribution of HIV. In the year 2018, an estimated 37.9 million individuals were living with HIV worldwide with highest burden witnessed in southern and eastern Africa. In the same year, an estimated 1.7 million new HIV cases were reported [7]. Every year, the number of deaths occurring globally from HIV-related causes reaches 1.0 million. Even though no population or continent has been spared by HIV, some regions and populations have been affected far more than the others [8]. This indicates that a varied combination of biological and social factors governs the spread of HIV. Moreover, human susceptibility to HIV infection also differs. Vulnerability to infection depends on multiple aspects like genetic factors, innate resistance and psychosocial determinants of health, such as poverty, stigma, gender-based violence, mental health, etc.
2. HIV-1 Antiretroviral Therapy: Current Status and Challenges
ART mainly targets the enzymatic processes of the HIV-1 replication cycle. The current conventional antiretroviral drugs are classified into six classes of therapeutic mechanisms (Table 2) [25,26,27]. However, soon after the success of the first drug, Azidothymidine or Zidovudine (nucleoside reverse transcriptase inhibitors; NRTI), it became apparent that the virus is able to generate drug-resistant mutants [28,29]. Thus, looking at the high rates of virus production and mutation, ART regime now includes administration of two or more pharmacological agents in combination, referred to as highly active antiretroviral therapy (HAART) [27]. At present, more than 30 drugs have been approved by the United States Food and Drug Administration (FDA) for the treatment of HIV infection [30]. Currently, over 23.3 million HIV patients are receiving antiretroviral therapy across the globe [7].
Table 2.
Types of antiviral drugs for HIV treatments.
| Drug Classes | Mode of Action |
|---|---|
| Receptor/co-receptor antagonist | Inhibits HIV fusion to host cells (gp120/CCR5/CXCR4) [31] |
| Fusion Inhibitors | Blocks virus penetration through host cell membrane [32] |
| NNRTI | Non-nucleoside reverse transcriptase inhibitors; binds at position distant from active sites of RT [33] |
| NRTI | Nucleoside reverse transcriptase inhibitors; competitive inhibitor [34] |
| PR inhibitors | Inhibits protease; prevents virion maturation [35] |
| INSTI | Integrase strand transfer inhibitor; prevents vDNA integration into the host genome [36] |
* Table modified from reference [10].
2.1. Constraints to Current ART/HAART Regime
Antiretroviral drugs are suppressive, but cannot cure or eradicate the HIV-1 infection completely, i.e., the patients still carry HIV in their bodies in latent viral reservoirs, ready to rebound in case of HAART interruption [26,37,38]. The major drawbacks in the current antiretroviral medication are inadequate efficacy, incomplete coverage, unaffordability and compound toxicity. The complications arising from the emergence of resistant strains, further defies the therapeutic potential of these drugs. Moreover, along with various side effects, patients are at an increased risk of developing non-AIDS comorbidities, such as cardiovascular diseases, tuberculosis and cancers [39,40,41,42,43].
To control, and eventually eliminate, HIV globally, there is a need of widely accessible and efficient HIV prevention tools like vaccines. However, in the case of HIV, various attempts to develop a protective vaccine (for prevention) as well as a therapeutic vaccine (for treatment) have been severely compromised by the complex structure and nature of the virus. Additionally, the inability of the host to induce a sufficiently potent immune response against HIV is poorly understood [44]. Thus, there is currently no vaccine available that can prevent HIV infection or treat those who have it [45].
2.2. Preventive Measures
Advances are being made to develop prevention tools to protect people who are at substantial risk for HIV infection [46,47]. These are termed “pre-exposure prophylaxis (PrEP)” and “post-exposure prophylaxis (PEP)”, wherein taking a pill daily could provide protection against HIV. These include microbicides in the form of intravaginal rings and antiviral oral medications, etc. Moreover, after recognizing that taking a pill on a daily basis is uncomfortable and unachievable for some people, researchers are working to create new forms of PrEP that do not require a daily dose of HIV prevention medicines. There are different forms of long-acting PrEP that are being tested, such as implants releasing a fixed dose of antiretroviral drug over time, injectable long-acting antiviral agents and broadly neutralizing antibodies (bNAbs) [48]. On similar grounds, UNAIDS (Joint United Nations Programme on HIV/AIDS) has initiated LATITUDE (Long Acting Therapy to Improve Treatment sUccess in Daily lifE), a study which is comparing monthly injectable ART to daily oral drugs [49]. Whilst the search for an effective cure continues, the attempts to develop new drugs remain an important priority due to the development of resistance against existing drugs and the unwanted side effects associated with the current drugs.
3. Host–Viral Interactomes Offering New Drug Targets
Viruses are obligate intracellular parasites relying on host machinery for their survival. They are capable of engaging with numerous cellular proteins for successful viral replication. To achieve this, one of the ways virus use is the direct interaction of viral proteins with the host factors via binding sites. Alternatively, they have evolved intrinsically disordered, short protein regions called as eukaryotic linear motifs (ELMs) or short linear motifs (SLiMs) which mediate interactions with their host [50]. The mechanism often used by viruses is molecular mimicry, where ELMs acts as docking sites for several protein domains (e.g., SH3 and WW domains), as nuclear-localizing signal or for posttranslational modifications [51]. Although the set of identified ELMs is limited and studied only for a few viruses, it will be useful to comprehensively assess the contribution of ELMs in shaping the interaction network with the host. Undoubtedly, viruses have evolved a variety of strategies to manipulate the cellular machinery as well as to counteract or evade host immune defences.
3.1. Advances in Viral-Host Interactomics
Apparently, majority of viral replication steps are supported by interacting host proteins leading to the manipulation of cellular processes by viruses. Therefore, the interpretation of these physical interactions between viral and host proteins (V-H interactome) allows the identification of cellular proteins/functions that are essential in the virus life-cycle and thereby can be considered as new antiviral targets [52]. The study of V-H protein-protein interactions (PPI) data has been accelerated with time by the high-throughput screening techniques like Y2H (Yeast 2 Hybrid) system, protein array, protein complementation assays and co-affinity purification/MS [53]. We now have access to nearly complete interactomes for various viruses like influenza virus, hepatitis C virus (HCV) and dengue virus [54,55,56]. Furthermore, combining the evolving VH PPI information with the druggable interactions revealed a very large potential of drug repurposing for the discovery of molecules with antiviral activity [57].
3.2. Drug Discovery Now Targets Host Factors
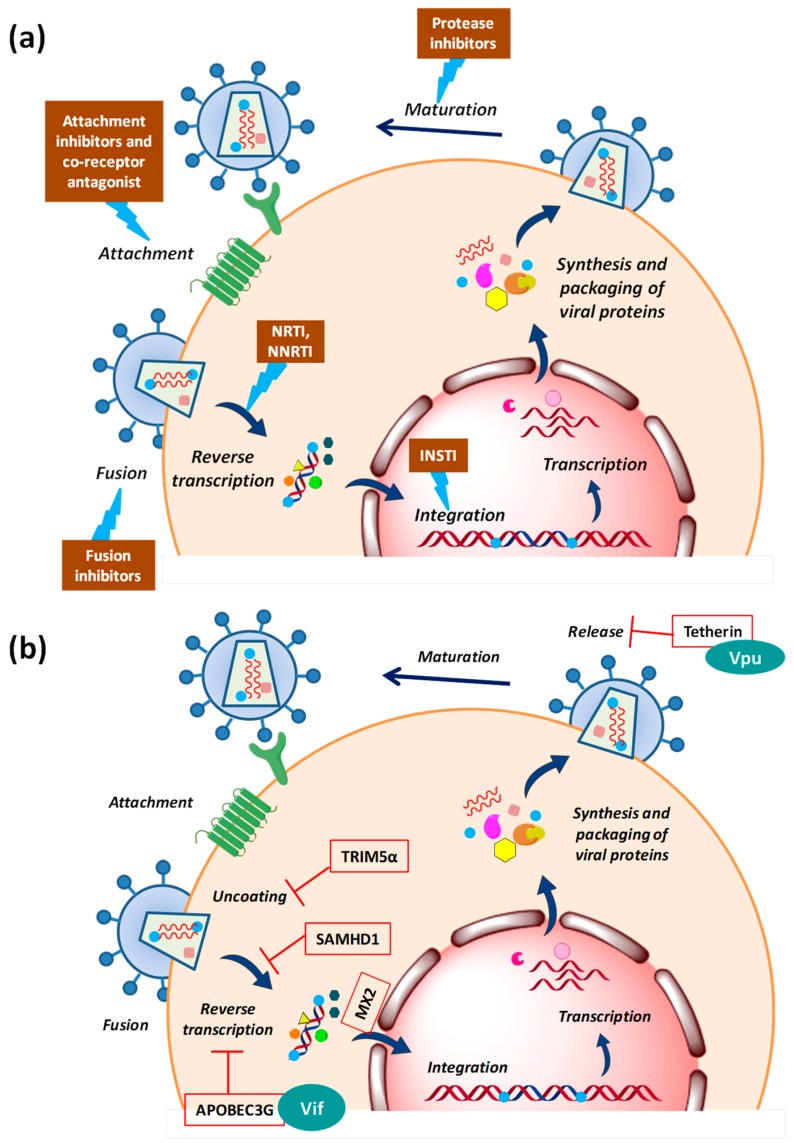
The conventional antiviral (anti-HIV) drugs mainly target the three viral enzymes: Reverse transcriptase, integrase and protease, as shown in Figure 2a. Since the inventory of viral proteins which can be targeted for drug discovery is quite limited, a major drawback in the use of these direct-acting drugs is the emergence of resistance. This limitation has opened a new avenue in the antiviral drug discovery which now focuses exploring host-oriented molecules that are essential for viruses to replicate [58]. Promising results have been obtained in case of LASAG (d,l-lysine-acetylsalicylate glycine) against the Influenza virus. This compound with new antiviral mode of action inhibits signal transduction module which is essential for viral replication, has successfully completed Phase II clinical trials [59]. Another successful example is the development of co-receptor inhibitor maraviroc which blocks the cellular entry of HIV [60]. This is the first and only approved drug which targets the host protein. These encouraging results demonstrated the potential of cellular factors as drug targets, widening the repertoire of druggable molecules and also offer a greater barrier to the emergence of resistance.
Figure 2.
Diagrammatic representation of the sites of action of (a) clinical inhibitors of HIV-1 (ART) and (b) host restriction factors which are counteracted by HIV-1 accessory proteins. Abbreviations: APOBEC3G—apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G, TRIM5α—tripartite motif-containing protein 5α-isoform, SAMHD1—SAM domain and HD domain-containing protein 1, MX2—human myxovirus resistance 2, Vif—viral infectivity factor, Vpu—viral protein U.
4. HIV-1-Host Interactors: Evaluating the Therapeutic Potential
4.1. Host Restriction Factors
During an HIV attack, the host cell does not offer a friendly environment to the virus. HIV faces several host defence factors which interfere with retroviral replication at different steps [61,62,63]. These restriction factors are the part of host’s innate anti-viral immunity that has possibly evolved under positive selective pressure due to past encounters with ancient viruses (Figure 2b). The first factor identified as the natural inhibitor of HIV-1 infection was a cytidine deaminase, APOBEC3G (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G) which induces lethal hypermutations (deamination of C to U) in the HIV-1 genome, which are detrimental to viral replication [64,65]. Another restriction factor is Fv1/TRIM5α (tripartite motif-containing protein 5α-isoform), the C-terminus of which interacts directly with the incoming viral capsid causing premature uncoating of HIV-1 nucleocapsid, which is ultimately targeted to proteasomal degradation [66]. Tetherin/CD317 is another potent barrier to HIV-1 release that tethers the budding virions to the cell surface in order to prevent their efficient release and further triggers the proinflammatory signalling upon sensing the virion assembly [67]. HIV-1 replication is very inefficient in cells of the myeloid lineage, like dendritic cells. This could be because the host restriction factor SAMHD1 (SAM domain and HD domain-containing protein 1) regulates the dNTP pool in myeloid cells, thus blocking HIV reverse transcription by controlling the dNTP supply [68]. The most recently identified restriction factor is MX2/MXB (human myxovirus resistance 2) which is an interferon-induced protein. The N-terminal of MX2 containing a triple-arginine motif binds to HIV-1 capsid and blocks the nuclear import of viral cDNA [69].
These antiviral defence mechanisms would have been quite successful in the past encounters; however, HIV-1 has evolved the ability to counteract these by several accessory viral proteins (Figure 2b) like viral infectivity factor (Vif), viral protein U (Vpu)) or can evade them by its high variability and thus is capable to replicate efficiently even in the hostile environment of the host cell [70,71,72].
4.2. Druggable Host Factors Involved during HIV-1 Entry
The initial steps of HIV-1 infection have been studied in substantial detail with a large number of attempts made to block the well-defined interactions occurring during the HIV-1 entry. The first step of HIV-1 infection is the entry of virus which takes place in three sub-steps. First being the ‘attachment’ to the cell surface when gp120 (viral envelope protein) engages with a principal cell surface CD4 receptor. This interaction results in exposure of a ‘co-receptor binding’ site which interacts with an auxiliary co-receptor CCR5 or CXCR4. Subsequently ‘fusion’ occurs where gp41 exposes the fusion peptide to destabilize the host cell membrane and drives membrane fusion [73]. HIV-1 entry inhibitors, therefore, fall under three categories: attachment inhibitors, co-receptor binding inhibitors and fusion inhibitors.
A variety of strategies have been pursued to develop entry inhibitors [73,74]. Attachment inhibitors include CD4 mimics, small molecule inhibitors which target CD4-gp120 binding site as well as CD4 antibodies (TNX-355). However, the oral bioavailability of the promising candidate was poor. To target the fusion step, peptide fusion inhibitors were designed which soon showed resistance compromising the drug efficiency. For inhibiting co-receptor binding, several modified forms of the natural ligand (CCL5/RANTES) of CCR5 were developed which were highly effective in vitro though failed in clinical trials [74,75]. Currently, there are no FDA approved attachment and fusion inhibitors in clinic.
Another strategy for co-receptor binding inhibition which worked well was the development of small molecules that bind to a hydrophobic pocket in CCR5 inducing conformational changes rather than directly occupying the gp120 binding site [74]. One of these inhibitors, maraviroc, has been approved by the US FDA and is currently the only antiretroviral drug targeting a cellular factor used in the clinic [60]. Several other related agents and anti-CCR5 antibodies are currently being evaluated in clinical trials [73,76] (see Box 1).
Box 1. The Berlin patient (2007) [83,84] and The London patient (2016) [85]: the only individuals ever to be cured of HIV/AIDS.
Cure for HIV means elimination of all the infected cells from body. Standard ART is effective in reducing the viral load but cannot eradicate the viral reservoir from the patient’s body. The Berlin patient (Timothy Ray Brown) and the London patients shared similar medical circumstances. They both were HIV positive and receiving ART. Later, both developed cancer (acute myeloid leukemia and Hodgkin’s lymphoma, respectively) and required bone marrow transplants to replenish the stem cells which were destroyed by chemotherapy. Doctors in both instances used bone marrow cells from a CCR5 (Δ32/Δ32) donor. A homozygous mutation (Δ32/Δ32) in the CCR5 gene encoding the defective HIV co-receptor CCR5 confers resistance to HIV-1 infection. This Δ32/Δ32 allogenic stem cell transplantation in both the patients cured them with no viral detection for years and months. While these two cases have provided important insight for HIV research, bone marrow transplantation is a very risky procedure with a 50% chance of success and, hence, not a feasible strategy to cure HIV infection. The impetus obtained from these two cured patients led to the development of potent inhibitors of HIV co-receptors, but their clinical development was halted due to undesired side effects. Therefore, though the inhibition or knock-down of CCR5 or CXCR4 using small inhibitor molecules or gene therapy techniques is promising, there are some major concerns which need to be addressed carefully.
4.3. Other HIV-1-Cellular Interactors as Possible Targets
HIV-1 can be tackled and/or inhibited at each step of its lifecycle in order to block its spread. Post-entry steps like uncoating, reverse transcription, nuclear entry, integration, transcription, nuclear exit, maturation, assembly and release from the cell membrane, all represent valid targets and are currently being investigated. For instance, during integration, disruption of the interaction between HIV-1 IN (integrase) and LEDGF/p75 (lens epithelium-derived growth factor) leads to impaired viral replication. Targeting the interaction interface, thus, offers hope for a new target. Moreover, post-integration, different viral accessory proteins interact with cellular factors to achieve successful replication. For instance, during elongation of viral transcripts, HIV-1 Tat binds to transactivation response element (TAR, located in the HIV 5′-LTR) which in turn interacts with host factor p-TEFb (positive transcription elongation factor b). So, Tat/TAR/P-TEFb interaction seems to be a promising target [77,78]. Similarly, targeting of DEAD-box helicases may inhibit HIV replication by suppressing function of Rev [79]. Furthermore, during virion release HIV-1 requires cellular ESCRT-I (endosomal sorting complex required for transport I) [80] Interactions between Gag and the endosomal sorting proteins Alix and TSG101 (tumor susceptibility gene 101) also suggest potential targets [81,82]. Based on these observations, various small compounds that disrupt such kind of host-virus interactions have been proposed and being tested. Moreover, since host factors involved are also necessary for the host cellular functions, designing an inhibitor against these has proven difficult and challenging.
Diverse independent genome-wide RNA interference-based screens evaluated more than 842 genes that reduce HIV-1 infection when knocked-down [86,87,88]. Additionally, the National Library of Medicine has a comprehensive list of all cellular proteins shown to interact physically or functionally with HIV-1 available in Human Protein Interaction Database [89,90,91]. These screens provided a good starting point for the identification of cellular targets for HIV therapy. However, the cellular factors that overlapped in more than one screen were quite few. The important ones included host factors involved in HIV infection like surface/receptor molecules, cytoskeletal and signalling proteins, transcription and DNA binding proteins, translation and RNA binding proteins, etc. Some of the most interesting candidates belong to nucleoporins (Nups) and other NE (nuclear envelope)-associated proteins which are indispensable for the nuclear import and export of HIV.
5. Nuclear Entry and Egress of HIV-1
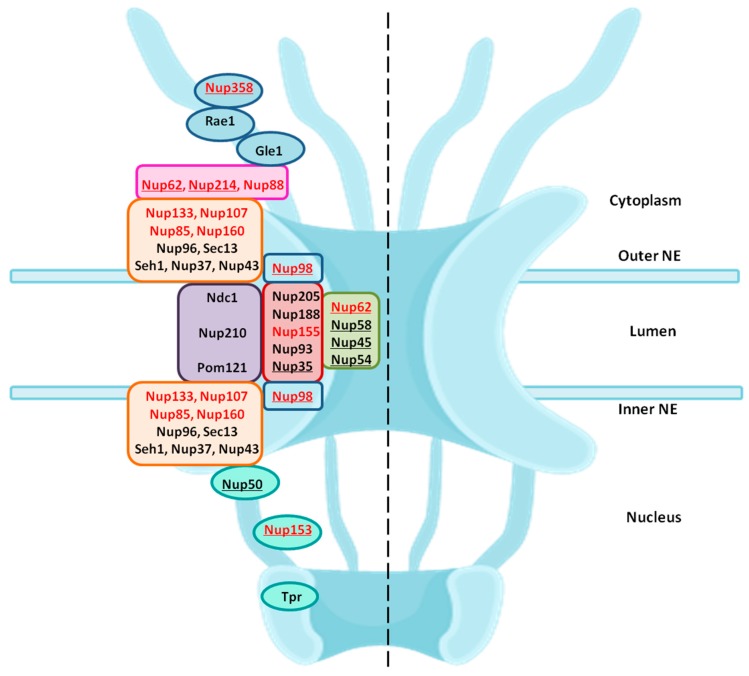
HIV is capable of infecting terminally differentiated and non-dividing cells [92]. The long interphase stage provides a very large benefit to HIV-1 and may account for its high replication rate observed. Entrance into and exit from the nucleus occurs via the nuclear pore complexes (NPCs) which are embedded in the double membrane of the nuclear envelope and mediate both passive (small molecules) and highly regulated active transport (cargoes larger than ~40 kDa) through the nuclear envelope. NPCs are macromolecular structures built from multiple copies of 30 different nucleoporins. These Nups are organized into various subcomplexes that are biochemically defined by their affinity to each other (Figure 3) [93,94,95,96]. One-third of all Nups are FG-Nups (Nup58, Nup54, Nup62, Nup45, Nup50, Nup98, Nup35, Nup214, and Nup358), containing phenylalanine-glycine repeats (FG-repeats). The FG Nups present at the central channel form a dense network of unstructured FG repeat regions which form the permeability barrier of the NPC and can transiently interact with soluble nuclear transport receptors (NTRs) conferring dynamic selectivity to the pore. NTRs like karyopherins (importins and exportins) recognize different types of nuclear localization signals (NLSs) or nuclear export signals (NESs) on cargo molecules and facilitate their import into or export from the nucleus, respectively [97,98,99].
Figure 3.
Structural organisation of nucleoporins in the NPC: Nups are organised into various subcomplexes, which here are shown in different coloured boxes. The location of Nups is marked on the left half of the NPC framework—the central transport channel/Nup62 subcomplex is shown in green; adapter ring/inner ring complex is in brick red; transmembrane Nups are in purple; Y-complexes are shown in orange; cytoplasmic ring Nups are in pink; Nups forming the cytoplasmic filaments are in light blue; nuclear basket is shown in teal color. The Nups highlighted in red are the ones identified as essential during the HIV-1 infection. The FG-Nups are underlined.
During its whole lifecycle, HIV-1 encounters the nuclear membrane via NPC twice: (1) entry of HIV-1 PIC into the nucleus and (2) exit of viral RNAs after transcription into the cytoplasm. The dependency of HIV-1 on the nuclear envelope is reflected by the changes in the expression of nucleocytoplasmic shuttling proteins [100]. Various genome wide RNA interference (RNAi) screens and LC-MS/MS studies identified a list of NPC components and NE-associated proteins as essential host co-factors for HIV-1 infection. These include Nup98, Nup85, Nup133, Nup107, Nup160, Nup153, Nup214, Nup358, Nup155 and TNPO3 (tansportin 3) [86,87,100,101]. These Nups found in different screens belong to different subcomplexes in the NPC architecture. Some of these are FG-Nups which participate in nuclear transport. Figure 3 highlights the location of these Nups in their subcomplexes within the NPC.
5.1. Nuclear Import of PIC
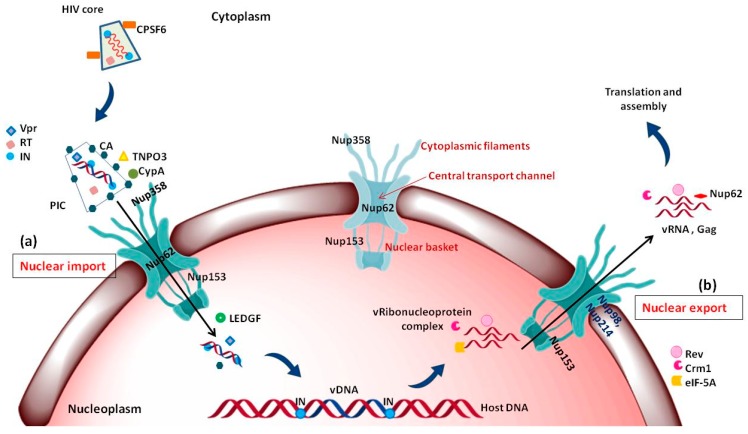
Reverse transcription of the viral RNA genome into viral DNA takes place in the cytoplasm followed by translocation to the nucleus. The newly synthesized viral DNA remains associated with viral and cellular proteins in a large nucleoprotein complex called the pre-integration complex (PIC) [102,103,104]. HIV-1 PICs were shown to contain several NLS containing viral proteins such as matrix (MA), Integrase (IN) and viral protein R (Vpr) [105,106,107,108,109,110]. There are different Nups and nuclear factors that have the ability to bind to HIV-1 cores and to participate in the early steps of HIV-1 infection: TNPO3, CPSF6, Nup358/RanBP2, Nup98, Nup214 and Nup153 (Figure 4a) [111,112]. Upon depletion, all four Nups induced defects in HIV-1 infectivity. Nup153 was found to participate in nuclear import of HIV-1 PIC; while, Nup358 interacts with the HIV-1 core and mediate its docking at the nuclear pore [101]. Interestingly, knockdown of other two Nups: Nup98 and Nup214 lead to decreased HIV-1 infectivity but are not directly involved in nuclear import of HIV-1 PIC [101]. Another study further suggested direct interaction of Nup62 with the HIV-1, which might play a role in import and chromosome tethering [113]. Following are few examples highlighting the involvement of Nups in HIV-1 docking at the NPC and nuclear entry.
Figure 4.
Major events occurring during nuclear import and export of HIV-1: (a) Nuclear import of HIV-1 PIC: CPSF6 interacts with intact HIV core which upon uncoating and reverse transcription forms the PIC. TNPO3, CypA and CypA like domain of Nup358 binds to the left over CA and helps in docking of PIC on the cytoplasmic face of NPC; while Vpr and IN having NLS, interact with Nups like Nup153 which help in import of the PIC into the nucleus. In nucleoplasm, LEDGF is involved in release of PIC from Nup153 and helps in integration of vDNA into the host DNA; (b) nuclear export of vRNA-protein complex: The unspliced and partially spliced mRNA need viral accessory protein Rev which interacts with various cellular factors like Crm1, eIF-5A and Nups, such as Nup62, Nup214, Nup98 and facilitate the nuclear export of this viral ribonucleoprotein complex. Abbreviations: NPC—nuclear pore complex, CPSF6—cleavage and polyadenylation specific factor 6, TNPO3—transportin 3, CypA—cyclophillin, CA—capsid, Vpr—viral protein r, IN—integrase, RT—reverse transcriptase, PIC—pre initiation complex, LEDGF—lens epithelium-derived growth factor, IN—integrase, Crm1—chromosome region maintenance- 1, Rev—regulator of virion expression, eIF-5A—eukaryotic translation initiation factor-5A.
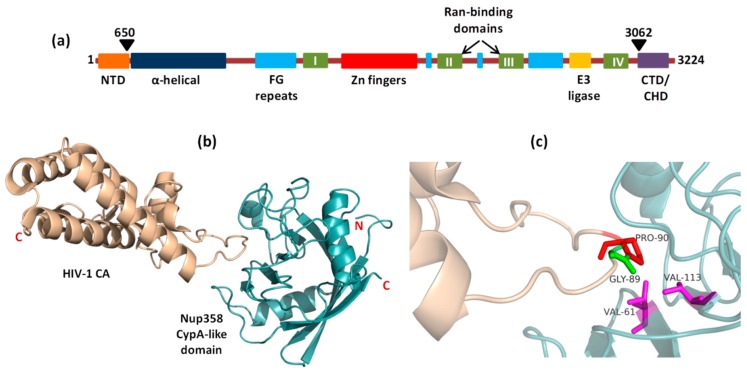
5.1.1. Interaction of Nup358 with HIV-1 CA
Nup358 assist HIV-1 core to dock at the NPC via interaction with viral CA protein. Nup358/RanBP2 is the largest Nup situated in the cytoplasmic fibrils of NPC (Figure 3 and Figure 4a). It is a multidomain assembly (Figure 5a) containing a N-terminal α-helical region, four Ran-binding domains, eight zinc-finger motifs, E3 ligase domain and a C-terminal cyclophilin A (CypA) - homologous domain (CHD) and multiple FG-repeat domains which are involved in the binding and transport of cargo-receptor complexes through the NPC [114,115]. When the HIV-1 core enters the cell upon fusion of viral envelope with cellular membrane, disassembly of the core is initiated, releasing the RTC (reverse transcription complex) where reverse transcription of viral RNA takes place into cDNA. However, the precise location and mechanism of CA core uncoating remains controversial [12,116]. While the initial steps of core uncoating are linked to reverse transcription, subsequent events may involve binding to host proteins like CPSF6 and Cyp A (cyclophilin A) and a little leftover CA also remains associated with the HIV-1 PIC (Figure 4a) [117]. The CypA and the Nup358’s cyclophilin-homologous domain (CHD), both bind to the cyclophilin binding loop (with GP residues) exposed on the top of CA N- terminal domain (Figure 5b,c). When the structures of Nup358 CHD and CypA with CA were overlapped, the binding of both Nup358 CHD and CypA in the proline-rich loop of HIV-1 CA appeared similar [118]. Reports suggest that the peptidyl-propyl isomerase activity exhibited by Nup358 could destabilize the viral core by causing cis-to-trans isomerization which lead to conformational changes in the capsid and thereby promote uncoating [118,119,120,121]. It was further hypothesized that the interaction between the CHD of Nup358 and the capsid molecules associated with the HIV-1 PIC could dock the PIC to the cytoplasmic side of the NPC for translocation into the nucleus [101]. However, the lack of structural information about the full length Nup358 limits our knowledge regarding its role in regulating the cellular functions as well as during interaction with capsid protein.
Figure 5.
Complex of HIV-1 CA (wheat) and Nup358CypA (Cyan) (PDB code 4LQW): (a) Domain architecture of human Nup358 (b) Loop of HIV-1 CA bound in cleft of Nup358CypA; (c) Val61 and 113 which form the hydrophobic pocket in Nup358 CHD, interact with Gly89 and Pro90 of Cyp binding loop in HIV-1 CA.
5.1.2. Interaction of Nup153 with Different HIV-1 Proteins
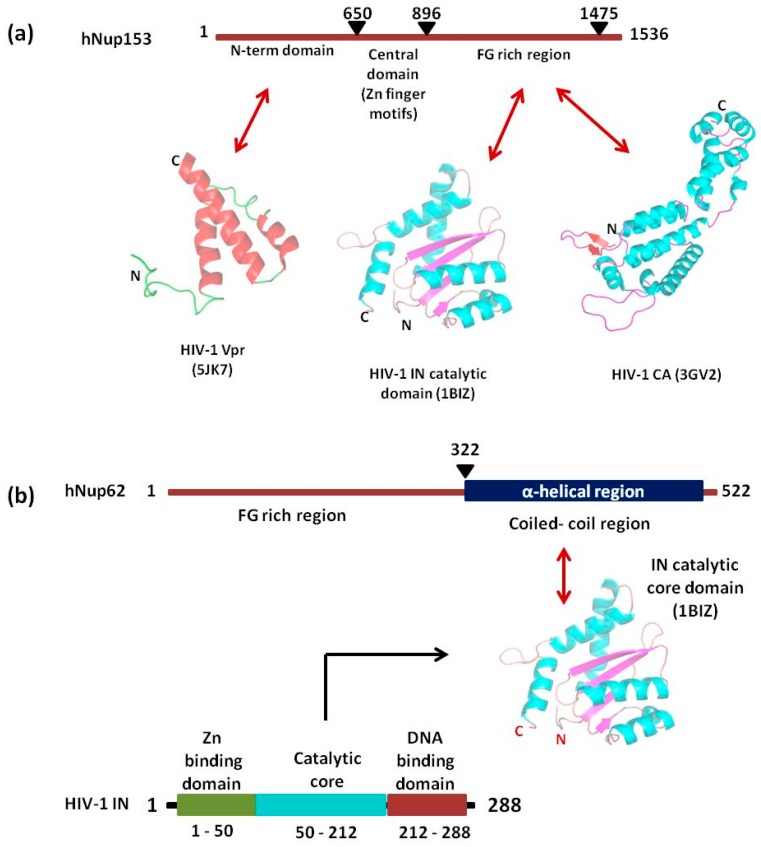
Nup153 is a nucleoplasmically oriented Nup that predominantly located to the nuclear side of the NPC as a member of nuclear basket (Figure 3). While the N-terminal domain of Nup153 is anchored to the nuclear rim of the NPC, its C-terminal FG rich domain is natively unfolded with no appreciable secondary structure. It has been shown that the 200 nm long and flexible Nup153 C-terminal can reach to the cytoplasmic side of the NPC channel. Human Nup153 C-terminal contains 29 FG motifs (FxF, FG and FxFG patterns), which play a vital role in Nup153-mediated nucleocytoplasmic transport [122,123,124,125,126]. The dynamic Nup153 located in the nuclear basket of NPC plays a significant role in HIV-1 PIC import. It binds directly with HIV-1 IN, CA, and Vpr in an α-importin-dependent and (or) β-importin-independent manner for import. The contact between the C-terminal domain of Nup153 and the PIC is made at the cytoplasmic face of the NPC. One of the key event in the nuclear import of the HIV-1 PIC is the direct interaction between the C-terminal domain (amino acid residues 896–1475) of NUP153 and PIC-associated IN (Figure 6a) [127]. The same FG domain also binds to the PIC-associated CA molecules [128]. These interactions mediated by Nup153 help in drawing the PIC into the nuclear side through the NPC. Moreover, the viral protein Vpr has also been shown to interact with a region of Nup153 in the N-terminal domain (amino acids 447–634), but the mechanism and significance of this interaction is still not clear (Figure 6a) [129].
Figure 6.
Schematic representation of interacting regions of Nup153 (a) and Nup62 (b) with different HIV-1 proteins.
5.1.3. Role of Nup62
Nup62, a glycosylated FG-Nup is a component of the central transport channel (CTC) of the NPC (Figure 3). This Nup62 sub complex (composed of Nup62 along with Nup58, Nup45 and Nup54) forms a dense network of unstructured FG repeat regions to regulate the nucleocytoplasmic transport via interactions between their FG domains and the karyopherins [95,97]. Nup62 consists of two distinct domains, the N-terminal FG-rich region and the C-terminal α-helical coiled-coil domain (Figure 6b). The C-terminal (amino acids 328–522) domain of Nup62 facilitates nucleocytoplasmic transport interacting with nuclear transport receptors and/or cargoes [95]. The coiled-coil domains present in the α-helical regions of Nup62 enables it to form homotrimer and heterotrimer either with Nup54 or Nup54-Nup58 within the CTC. This coiled coil motif is also responsible for various protein-protein interactions like with that of Exo70 (a member of the exocyst complex in the cytosol) [130]. Furthermore, in the context of evolution of the CTC nups, Nup62 is comparatively conserved from fungi to metazoan [131]. Nup62 not only contributes in nuclear transport but is also involved at several steps during HIV-1 replication. The coiled-coil domain of Nup62 is shown to interact directly with HIV-1 IN and aid in viral integration (Figure 6b) [113]. The level of viral-integrated DNA in Nup62-knockdown cells was drastically decreased indicating a major effect of Nup62 on integration step [113]. Possibly, by interacting with HIV-IN, Nup62 offers the HIV PIC a direct entry way from the NPC to the chromosomal DNA, thus enabling efficient integration. Additionally, immunofluorescence and co-localization studies suggested that Nup62 is essential for viral genomic RNA (vRNA) export and revealed that Nup62 is exported from the nucleus along with Rev, vRNA and Gag into the cytoplasm (Figure 4b) [100]. In normal cells, Nup62 resides mostly in the nuclear envelope; while, during HIV-1 infection, it is re-distributed to the nucleoplasm and then to cytoplasm, where it resides with the viral ribonucleoprotein complex [100].
It is noteworthy that NPC components not only facilitate nuclear import of the PIC but also plays a role during the integration of the proviral cDNA into the host chromosome. Few Nups, particularly Nup62, Nup153 and Nup98, can be found in the nucleoplasm and regulate the expression of certain cellular genes [132]. These Nups might be contributing in HIV-1 infection by interacting with HIV-IN and cellular factor LEDGF in the nucleoplasm and locating the integration site in the host chromosome followed by successful proviral integration [113,132,133]. Thus, various Nups are found to be involved directly or indirectly in HIV-1 replication at different steps.
5.2. Nuclear Export of Viral mRNAs
Upon integration of the vDNA into the host chromosome, the viral 5′-LTR acts as a weak promoter for the transcription of viral mRNA. The viral mRNA is spliced using host splicing mechanisms and results in three different mature RNAs, viz. completely spliced, partially spliced and unspliced RNAs. The latter two are too big to be exported into the cytoplasm. Hence, only the spliced vRNA expressing the viral proteins Rev, Tat and Nef are readily translocated to cytoplasm [134,135]. The expressed Tat and Rev proteins possess arginine-rich NLS which helps in translocating them to the nucleus via interaction with importin β [136]. In the nucleus, Tat transactivates the 5′-LTR, increasing the level of viral mRNA transcription and thereby protein expression [137]. The Rev protein promotes the nuclear export of unspliced (act as viral genome) and partially spliced (encoding gag proteins) forms of viral mRNA to the cytoplasm [17,138]. The import of Rev is also aided by another host protein namely, nucleolar phosphoprotein B23 [139]. Rev also has a leucine-rich NES and interacts with a number of cellular proteins like Crm1, eIF-5A and Nups in order to facilitate this export of Rev-RRE RNA complex. eIF-5A localized at the nucleoplasmic face of the NPC interacts with Nup214, Nup153, Nup98 and Nup62, which are involved in nuclear export [140,141,142,143,144]. eIF-5A may act as an adapter that targets the Rev-NES to the nucleoplasmic face of the NPC and mediates efficient binding to Crm1. Interestingly, Nup62 translocates to cytoplasm along with viral Gag, RNAs and Rev (Figure 4b) [100]. The immunoblot analysis of purified virions identifies Nup62 as an incorporated, virion-associated protein and suggests that Nup62 may be important for HIV-1 assembly [100]. In summary, Nups also are associated with the export of viral ribonucleoprotein complex into the cytoplasm; however, the mechanism of this export is not clearly understood.
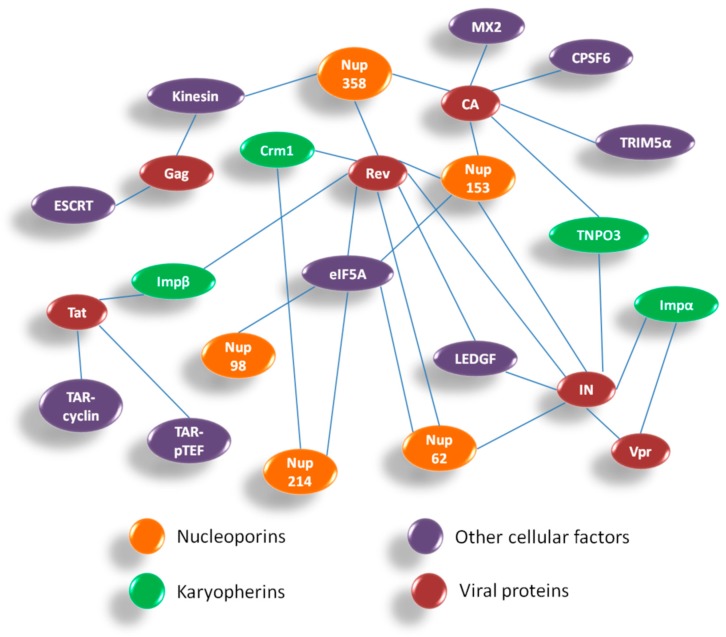
Overall, HIV-1 utilizes components of the NPC for both PIC import and Rev-vRNA RNP export. Hence, inhibiting the interactions between HIV-1 proteins and Nups might be helpful in developing novel anti-viral strategies. Figure 6 summarizes the interactions between HIV-1 and host nuclear factors including Nups and karyopherins which are important for HIV-1 replication. The interactome comprises of various host–viral interactions occurring during nuclear import and export steps in the life cycle of HIV-1. The four viral proteins playing a crucial role in these steps are CA, Vpr, IN and Rev. While the Nups and associated nuclear proteins which contribute maximally in these interactions are Nup358, Nup153, Nup62, Nup214, Nup98, TNPO3, LEDGF, Impα and Crm1.
6. Significance of Targeting Nup-HIV-1 Interactions as Drug Targets
The nuclear pore complex plays a significant role as HIV-1 host partner and helps in the nuclear translocation of viral DNA, RNA and proteins. Nups are the major components of the NPC which not only contribute to NPC assembly and overall architecture and but also maintains a strict permeability barrier by allowing selective active translocation of macromolecules through the NPC. Additionally, Nups are also involved in the regulation of cellular gene expression and interaction with chromatin in the nucleoplasm [145]. Any dysregulation of the nucleocytoplasmic transport is associated with the pathogenesis of various diseases, such as cancer, viral infections and neurodegenerative conditions [146,147,148]. Different studies have shown that the NPCs are heavily remodelled in cancer as well as in HIV-1 infection. Employing LC-MS/MS studies, Chan et al. [149,150] and Monette et al. [100] have independently highlighted the upregulation and downregulation of NPC and shuttling proteins by HIV-1. Since Nups prove to be an essential requirement for the nuclear entry of the PIC, the nuclear export of the vRNA RNP, and thereby for virus infectivity, the prospect of targeting the interacting protein domains of Nups and viral components may be targeted to bring the HIV-1 replication cycle to a halt.
A number of reports have also pointed towards the compositional variability of the NPC both within and between the cells [151,152]. Further, an altered expression under certain disease conditions has been reported [100,146]. These reports raise the fascinating opportunity that NPCs with different compositions may possess different functional properties in different cells. It is now evident that NPCs are highly dynamic complexes with many transport-independent functions and that the expression of several NPC components vary among different cell types and tissues and also during development [153,154]. These findings, together with the fact that mutations in certain Nups result in tissue-specific diseases, give an impression that NPC composition may play an important role in cellular function. The NPC variability is observed not only in HIV-1 infected and uninfected cells but also in immune cells and other body cells. This cell- and tissue-specific compositional variation of Nups presents another prospect which should be thoroughly understood in order to harness their potential.
The physiological and pathophysiological significance of the nuclear membrane and nucleocytoplasmic transport has been recently reported [155,156]. Treating NTRs as the therapeutic targets against various diseases has led to the development of nuclear transport inhibitors [156]. Leptomycin B (LMB) which covalently binds to C-528 in the central conserved binding region of Crm1 blocks its interaction with the NES of a cargo protein in an irreversible manner [157,158]. LMB was tested in phase-I clinical trials to treat advanced refractory cancer; however, the clinical trials were discontinued due to significant systemic toxicity [159]. Similarly, there were more than 20 nuclear transport inhibitors described of which very few could be monitored in a clinical setting [160]. Due to the indispensable role of nuclear import and export pathways in normal cell functioning, most of the broad-spectrum inhibitors were unsuccessful. The inhibitors which can specifically target the Nup–HIV-1 interaction interfaces can be viewed as an innovative class of therapeutic intervention. Some small molecules and short peptides have been designed which could modulate such interactions. These act either by specifically interacting with the NLS domain of a karyophilic protein and thus preventing its interaction with the appropriate importin, or they can mimic the domain which competes with the NLS carrying protein for interaction with importin [156]. Examples include peptides blocking HIV-1 IN interaction with importin α and LEDGF [161,162]. On similar grounds, host Nups - HIV-1 interactions like Nup153 with IN or CA, Nup358 with CA, etc., can be targeted to develop and test new antiviral compounds. However, one must keep in mind that some level of cytotoxicity is always associated while considering any cellular protein. Nonetheless, it can now be expected that new findings will enrich the existing knowledge about the role of Nups and NE-associated proteins in HIV-1 infection. This would provide the design of compounds targeting not only viral or cellular proteins, but their ‘interactions’ which might pose less toxicity and low chances of drug resistance (Figure 7). Therefore, specific inhibitors for HIV interactions with Nups and NTR, alone or in combination with other antiviral agents might be a promising strategy to target HIV infection.
Figure 7.
Host-HIV-1 interactome during nuclear import and export of HIV-1 at a glance. Abbreviations: ESCRT—endosomal sorting complex required for transport, TAR—transactivation response element, pTEFb—positive transcription elongation factor b, Imp—importin, Crm1—chromosome region maintenance-1, eIF5A—eukaryotic translation initiation factor-5A, CA—capsid; MX2—human myxovirus resistance 2; CPSF6—cleavage and polyadenylation specific factor 6, TRIM5α—tripartite motif-containing protein 5α-isoform, TNPO3—transportin 3, IN—integrase, Vpr—viral protein r, Tat—transactivator of transmembrane, Rev—regulator of virion expression.
7. Challenges and Perspectives
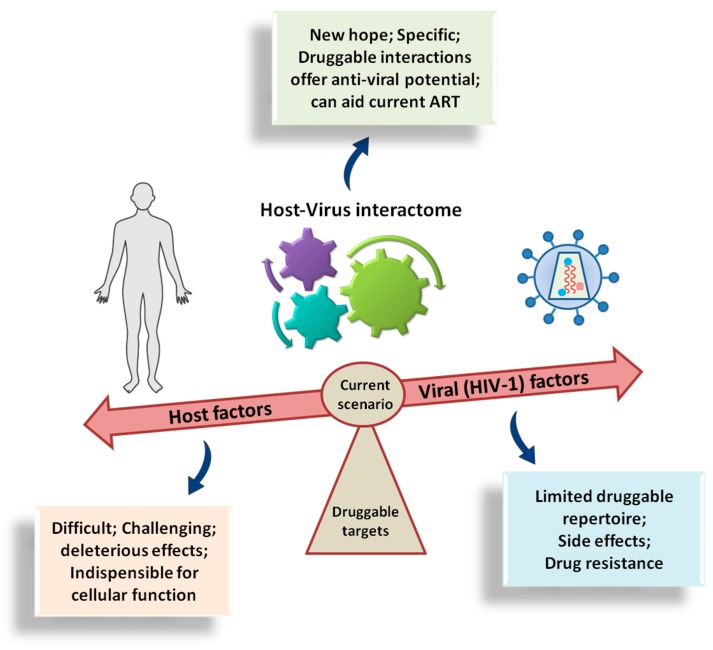
HIV still remains a major global concern. Although there is no sterilising cure or preventive vaccine at present, a functional cure for HIV-1 might be more realistic to achieve. To date, all the antiviral drugs in clinical use for HIV-1 have been designed either to inhibit one of the three viral enzymes or to block the viral entry into the cell during attachment and fusion with the cellular membrane. The ART have certainly resulted in an appreciable decrease in HIV infections. However, development of HIV-1 resistance to these drugs has become one of the greatest challenges in identifying an appropriate combination of drug regimes with the fewest side effects for the treatment of patients. Figure 8 summarises the current scenario of the drug targets for developing anti-HIV medication. Consequently, there is a clear need to identify and develop new targets and novel drugs for the treatment of HIV-1 infection. The two areas of scope are presented by targeting the HIV-1 regulatory proteins, such as Tat and Rev, and by strengthening the host restriction factors like APOBEC3G, etc. Another direction is offered by the nuclear transport factors including NTRs and Nups, both of which are important for HIV replication and are yet to be exploited for clinical treatment of HIV-1-infected patients. Development of new drugs that consider these proteins would considerably increase the number of available treatment options for HIV-1.
Figure 8.
Cartoon depiction of the current scenario showing the drug targets for anti-viral (anti-HIV) medication.
Given that the interaction between HIV-1 and its human host is far more complex than previously anticipated, a major challenge to the field will be the identification of essential interactions between viral and host factors which can be blocked without important physiological side-effects/consequences. Moreover, the drugability of the identified potential targets poses another serious challenge. In the past decades, structure-guided drug discovery has led to the development of various molecules which are being tested for clinical trials. The structural information available for HIV-1 is far more than any other virus; yet we are limited by the structural knowledge of host-viral protein complexes in both infected and uninfected conditions. The in-depth understanding of the structural and functional aspects of cellular proteins, such as Nups and other nuclear factors, which are important in HIV-1 life cycle is needed, which can then be exploited to speculate disease conditions. Even though a large number of cellular protein-protein interactions have been described, the way to the development of clinical drugs is still far. Nevertheless, significant progress is being made in the field and new targets are being discovered to keep the journey of developing novel antivirals going and as well as keep the hope to win over HIV/AIDS alive.
Acknowledgments
The authors acknowledge Priyanka Dutta (NCCS, Pune) for critical reading and suggestions.
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| APOBEC3G | Apolipoprotein B mRNA Editing Enzyme Catalytic polypeptide-like 3G |
| CA | Capsid |
| CHD | Cypa Homology Domain |
| CPSF6 | Cleavage and Polyadenylation Specific Factor 6 |
| CypA | Cyclophillin A |
| eIF-5A | Eukaryotic translation Initiation Factor-5A |
| ESCRT-I | Endosomal Sorting Complex Required for Transport I |
| FDA | Food and Drug Association |
| HAART | Highly Advanced AntiRetroviral Therapy |
| HIV | Human Immunodeficiency Virus |
| IN | Integrase |
| Crm1 | Chromosome Region Maintenance-1 |
| LEDGF | Lens Epithelium Derived Growth Factor |
| LTR | Long Terminal Repeat |
| MX2 | human Myxovirus resistance 2 |
| NPC | Nuclear Pore Complex |
| Nup | Nucleoporin |
| NTR | Nuclear Transport Receptor |
| NLS | Nuclear Localisation Signal |
| NES | Nuclear Export Signal |
| PIC | Pre Initiation Complex |
| PPI | Protein-Protein Interaction |
| pTEFb | positive Transcription Elongation Factor b |
| Rev | regulator of virion expression |
| RT | Reverse Transcriptase |
| SAMHD1 | SAM domain and HD domain-containing protein 1 |
| TNPO3 | transportin 3 |
| Tat | Transactivator of Transmembrane |
| TRIM5α | Tripartite Motif-containing protein 5α- isoform |
| TSG101 | Tumor Susceptibility Gene 101 |
| UNAIDS | joint United Nations program for Acquired Immunodeficiency Syndrome |
| V-H interactome | Viral Host Interactome |
| Vif | Viral Infectivity Factor |
| Vpr | Viral Protein R |
| Vpu | Viral Protein U |
Author Contributions
Writing—original draft preparation: E.S.; writing—review and editing: R.C.
Funding
This work is supported by Centre of Excellence in Biomolecular Structure and Function on Host-Pathogen Interactions grant (RC/PR/15450) to R.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Centers for Disease Control (CDC) Pneumocystis Pneumonia - Los Angeles. Morb. Mortal. Wkly Rep. 1981;30:250–252. [PubMed] [Google Scholar]
- 2.Hahn B.H., Shaw G.M., de Cock K.M., Sharp P.M. AIDS as a Zoonosis: Scientific and Public Health Implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 3.Lemey P., Pybus O.G., Wang B., Saksena N.K., Salemi M., Vandamme A.M. Tracing the Origin and History of the HIV-2 Epidemic. Proc. Natl. Acad. Sci. USA. 2003;100:6588–6592. doi: 10.1073/pnas.0936469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCutchan F.E. Understanding the Genetic Diversity of HIV-1. AIDS. 2000;14:S31–S44. [PubMed] [Google Scholar]
- 5.Peeters M. The Genetic Variability of HIV-1 and Its Implications. Transfus. Clin. Biol. 2001;8:222–225. doi: 10.1016/S1246-7820(01)00131-8. [DOI] [PubMed] [Google Scholar]
- 6.Levy J.A. HIV and the Pathogenesis of AIDS. 3rd ed. ASM Press; Washington, DC, USA: 2007. p. 588. [Google Scholar]
- 7.UNAIDS Global HIV and AIDS Statistics—2019 Fact Sheet. [(accessed on 10 September 2019)]; Available online: https://www.unaids.org/en/resources/fact-sheet.
- 8.UNAIDS 2018 Global AIDS Update Slides. [(accessed on August 2018)]; Available online: https://www.unaids.org/en/resources/documents/2018/2018-global-aids-update-slides-part1.
- 9.Sarafianos S.G., Marchand B., Das K., Himmel D.M., Parniak M.A., Hughes S.H., Arnold E. Structure and Function of HIV-1 Reverse Transcriptase: Molecular Mechanisms of Polymerization and Inhibition. J. Mol. Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu T.K., Davies D.R. Structure and Function of HIV-1 Integrase. Curr. Top Med. Chem. 2004;4:965–977. doi: 10.2174/1568026043388547. [DOI] [PubMed] [Google Scholar]
- 11.Brik A., Wong C.-H. HIV-1 Protease: Mechanism and Drug Discovery. Org. Biomol. Chem. 2003;1:5–14. doi: 10.1039/b208248a. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentini S., Marini E., Caracciolo S., Caruso A. Functions of the HIV-1 Matrix Protein P17. New Microbiol. 2006;29:1–10. [PubMed] [Google Scholar]
- 13.Campbell E.M., Hope T.J. HIV-1 Capsid: The Multifaceted Key Player in HIV-1 Infection. Nat. Rev. Microbiol. 2015;13:471–483. doi: 10.1038/nrmicro3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin J.G., Mitra M., Mascarenhas A., Musier-Forsyth K. Role of HIV-1 Nucleocapsid Protein in HIV-1 Reverse Transcription. RNA Biol. 2010;7:754–774. doi: 10.4161/rna.7.6.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structure of an HIV Gp120 Envelope Glycoprotein in Complex with the CD4 Receptor and a Neutralizing Human Antibody. Nature. 1998;393:648. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postler T.S., Desrosiers R.C. The Tale of the Long Tail: The Cytoplasmic Domain of HIV-1 Gp41. J. Virol. 2013;87:2–15. doi: 10.1128/JVI.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice A.P. The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies. Curr. Pharm. Des. 2017;23:4098–4102. doi: 10.2174/1381612823666170704130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malim M.H., Pollard V.W. The HIV-1 Rev Protein. Annu. Rev. Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 19.Pereira E.A., da Silva L.L.P. HIV-1 Nef: Taking Control of Protein Trafficking. Traffic. 2016;17:976–996. doi: 10.1111/tra.12412. [DOI] [PubMed] [Google Scholar]
- 20.Solbak S.M.O., Reksten T.R., Hahn F., Wray V., Henklein P., Henklein P., Halskau O., Schubert U., Fossen T. HIV-1 P6 - A Structured to Flexible Multifunctional Membrane-Interacting Protein. Biochim. Biophys. Acta-Biomembranes. 2013;1828:816–823. doi: 10.1016/j.bbamem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Rose K.M., Marin M., Kozak S.L., Kabat D. The Viral Infectivity Factor (Vif) of HIV-1 Unveiled. Trends Mol. Med. 2004;10:291–297. doi: 10.1016/j.molmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.González M. The HIV-1 Vpr Protein: A Multifaceted Target for Therapeutic Intervention. Int. J. Mol. Sci. 2017;18:126. doi: 10.3390/ijms18010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey R.C., Datta D., Mukerjee R., Srinivasan A., Mahalingam S., Sawaya B.E. HIV-1 Vpr: A Closer Look at the Multifunctional Protein from the Structural Perspective. Curr. HIV Res. 2009;7:114–128. doi: 10.2174/157016209787581508. [DOI] [PubMed] [Google Scholar]
- 24.González M. Vpu Protein: The Viroporin Encoded by HIV-1. Viruses. 2015;7:4352–4368. doi: 10.3390/v7082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomerantz R.J., Horn D.L. Twenty Years of Therapy for HIV-1 Infection. Nat. Med. 2003;9:867–873. doi: 10.1038/nm0703-867. [DOI] [PubMed] [Google Scholar]
- 26.Palella F.J., Jr., Delaney K.M., Moorman A.C., Loveless M.O., Fuhrer J., Satten G.A., Aschman D.J., Holmberg S.D. Declining Morbidity and Mortality among Patients with HIV Infection: HIV Outpatient Study Investigators. N. Engl. J. Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 27.Lu D.Y. High Active Anti-Retroviral Therapy for HIV/AIDS, Progresses and Drawback. Adv. Pharmacoepidemiol. Drug Saf. 2012;1:e115. doi: 10.4172/2167-1052.1000e115. [DOI] [Google Scholar]
- 28.Larder B.A., Darby G., Richman D.D. HIV with Reduced Sensitivity to Zidovudine (AZT) Isolated during Prolonged Therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 29.Larder B. Mechanisms of HIV-1 Drug Resistance. AIDS. 2001;15:S27–S34. doi: 10.1097/00002030-200100005-00005. [DOI] [PubMed] [Google Scholar]
- 30.USFDA HIV and AIDS Activities - Antiretroviral Drugs Used in the Treatment of HIV Infection. [(accessed on 3 July 2019)]; Available online: https://www.fda.gov/patients/hiv-treatment/antiretroviral-drugs-used-treatment-hiv-infection.
- 31.Ray N., Doms R.W. HIV-1 Coreceptors and Their Inhibitors. Curr. Top Microbiol. Imm. 2006;303:97–120. doi: 10.1007/978-3-540-33397-5_5. [DOI] [PubMed] [Google Scholar]
- 32.Eggink D., Berkhout B., Sanders R.W. Inhibition of HIV-1 by Fusion Inhibitors. Curr. Pharm. Des. 2010;16:3716–3728. doi: 10.2174/138161210794079218. [DOI] [PubMed] [Google Scholar]
- 33.Zhan P., Chen X., Li D., Fang Z., De Clercq E., Liu X. HIV-1 NNRTIs: Structural Diversity, Pharmacophore Similarity, and Impliations for Drug Design. Med. Res. Rev. 2013;33:E1–E72. doi: 10.1002/med.20241. [DOI] [PubMed] [Google Scholar]
- 34.Waller D.G., Sampson A.P. Chemotherapy of infections. Medical Pharmacology and Therapeutics. 5th ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 581–629. [Google Scholar]
- 35.Flexner C. HIV-Protease Inhibitors. N. Engl. J. Med. 1998;338:1281–1293. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 36.Hazuda D.J., Felock P., Witmer M., Wolfe A., Stillmock K., Grobler J.A., Espeseth A., Gabryelski L., Schleif W., Blau C., et al. Inhibitors of Strand Transfer That Prevent Integration and Inhibit HIV-1 Replication in Cells. Science. 2000;287:646–650. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann D.E., Lichterfeld M., Altfeld M., Addo M.M., Johnston M.N., Lee P.K., Wagner B.S., Kalife E.T., Strick D., Rosenberg E.S., et al. Limited Durability of Viral Control Following Treated Acute HIV Infection. PLoS Med. 2004:137–148. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vervoort S.C., Borleffs J.C., Hoepelman A.I., Grypdonck M.H. Adherence in Antiretroviral Therapy: A Review of Qualitative Studies. AIDS. 2007;21:271–281. doi: 10.1097/QAD.0b013e328011cb20. [DOI] [PubMed] [Google Scholar]
- 39.Brinkman K., ter Hofstede H.J.M., Burger D.M., Smeitink J.A.M., Koopmans P.P. Adverse Effects of Reverse Transcriptase Inhibitors. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Brown T., Qaqish R. Antiretroviral Therapy and the Prevalence of Osteopenia and Osteoporosis. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 41.Croxford S., Kitching A., Desai S., Kall M., Edelstein M., Skingsley A., Burns F., Copas A., Brown A.E., Sullivan A.K. Mortality and Causes of Death in People Diagnosed with HIV in the Era of Highly Active Antiretroviral Therapy Compared with The General Population: An Analysis Of A National Observational Cohort. Lancet Public Health. 2017;2:e35–e46. doi: 10.1016/S2468-2667(16)30020-2. [DOI] [PubMed] [Google Scholar]
- 42.Ganji R., Dhali S., Rizvi A., Sankati S., Vemula M.H., Mahajan G., Rapole S., Banerjee S. Proteomics Approach to Understand Reduced Clearance of Mycobacteria and High Viral Titers during HIV--Mycobacteria Co-Infection. Cell Microbiol. 2016;18:355–368. doi: 10.1111/cmi.12516. [DOI] [PubMed] [Google Scholar]
- 43.Ganji R., Dhali S., Rizvi A., Rapole S., Banerjee S. Understanding HIV-Mycobacteria Synergism through Comparative Proteomics of Intra-Phagosomal Mycobacteria during Mono-and HIV Co-Infection. Sci. Rep. 2016;6:22060. doi: 10.1038/srep22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston M.I., Fauci A.S. An HIV Vaccine - Evolving Concepts. N. Engl. J. Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 45.Escolano A., Dosenovic P., Nussenzweig M.C. Progress toward Active or Passive HIV-1 Vaccination. J. Exp. Med. 2017;214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen M.S., Hellmann N., Levy J.A., Decock K., Lange J. The Spread, Treatment, and Prevention of HIV-1: Evolution of a Global Pandemic. J. Clin. Invest. 2008;118:1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perreau M., Banga R., Pantaleo G. Targeted Immune Interventions for an HIV-1 Cure. Trends Mol. Med. 2017;23:945–961. doi: 10.1016/j.molmed.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 48.CDC CDC HIV Basics. [(accessed on 30 April 2019)]; Available online: https://www.cdc.gov/hiv/basics/
- 49.NIH News Release. [(accessed on 31 May 2019)]; Available online: https://www.nih.gov/news-events/news-releases/nih-trial-evaluates-long-acting-hiv-medication-unable-adhere-strict-daily-regimens.
- 50.Gibson T.J., Davey N.E., Trave G. How Viruses Hijack Cell Regulation. Trends Biochem. Sci. 2011;36:159–169. doi: 10.1016/j.tibs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Hagai T., Azia A., Babu M.M., Andino R. Use of Host-like Peptide Motifs in Viral Proteins Is a Prevalent Strategy in Host-Virus Interactions. Cell Rep. 2014;7:1729–1739. doi: 10.1016/j.celrep.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyniel-Schicklin L., de Chassey B., André P., Lotteau V. Viruses and Interactomes in Translation. Mol. Cell Proteom. 2012;11 doi: 10.1074/mcp.M111.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Chassey B., Meyniel-Schicklin L., Vonderscher J., André P., Lotteau V. Virus-Host Interactomics: New Insights and Opportunities for Antiviral Drug Discovery. Genome Med. 2014;6:115. doi: 10.1186/s13073-014-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolan P.T., Zhang C., Khadka S., Arumugaswami V., Vangeloff A.D., Heaton N.S., Sahasrabudhe S., Randall G., Sun R., Lacount D.J. Identification and Comparative Analysis of Hepatitis C Virus-Host Cell Protein Interactions. Mol. BioSys. 2013;9:3199–3209. doi: 10.1039/c3mb70343f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mairiang D., Zhang H., Sodja A., Murali T., Suriyaphol P., Malasit P., Limjindaporn T., Finley R.L. Identification of New Protein Interactions between Dengue Fever Virus and Its Hosts, Human and Mosquito. PLoS ONE. 2013;8:e53535. doi: 10.1371/journal.pone.0053535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao M., Wang L., Li S. Influenza A Virus-Host Protein Interactions Control Viral Pathogenesis. Int. J. Mol. Sci. 2017;18:1673. doi: 10.3390/ijms18081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pizzorno A., Padey B., Terrier O., Rosa-Calatrava M. Drug Repurposing Approaches for the Treatment of Influenza. Front Immunol. 2019;10:531. doi: 10.3389/fimmu.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Chassey B., Meyniel-Schicklin L., Aublin-Gex A., André P., Lotteau V. New Horizons for Antiviral Drug Discovery from Virus-Host Protein Interaction Networks. Curr. Opin. Virol. 2012;2:606–613. doi: 10.1016/j.coviro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Scheuch G., Canisius S., Nocker K., Hofmann T., Naumann R., Pleschka S., Ludwig S., Welte T., Planz O. Targeting Intracellular Signaling as an Antiviral Strategy: Aerosolized LASAG for the Treatment of Influenza in Hospitalized Patients. Emerg. Microbes Infect. 2018;7:21. doi: 10.1038/s41426-018-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sayana S., Khanlou H. Maraviroc: A New CCR5 Antagonist. Expert Rev. Anti Infect. Ther. 2009;7:9–19. doi: 10.1586/14787210.7.1.9. [DOI] [PubMed] [Google Scholar]
- 61.Yan N., Chen Z.J. Intrinsic Antiviral Immunity. Nat. Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris R.S., Hultquist J.F., Evans D.T. The Restriction Factors of Human Immunodeficiency Virus. J. Biol. Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malim M.H., Bieniasz P.D. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb. Perspect Med. 2012;2:a006940. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mbisa J.L., Barr R., Thomas J.A., Vandegraaff N., Dorweiler I.J., Svarovskaia E.S., Brown W.L., Mansky L.M., Gorelick R.J., Harris R.S., et al. Human Immunodeficiency Virus Type 1 CDNAs Produced in the Presence of APOBEC3G Exhibit Defects in Plus-Strand DNA Transfer and Integration. J. Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bishop K.N., Verma M., Kim E.Y., Wolinsky S.M., Malim M.H. APOBEC3G Inhibits Elongation of HIV-1 Reverse Transcripts. PLoS Pathog. 2008;4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stremlau M., Perron M., Lee M., Li Y., Song B., Javanbakht H., Diaz-Griffero F., Anderson D.J., Sundquist W.I., Sodroski J. Specific Recognition and Accelerated Uncoating of Retroviral Capsids by the TRIM5alpha Restriction Factor. Proc. Natl. Acad. Sci. USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans D.T., Serra-Moreno R., Singh R.K., Guatelli J.C. BST-2 Tetherin a New Component of the Innate Immune Response to Enveloped Viruses. Trends Microbiol. 2010;18:388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Ségéral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. SAMHD1 Is the Dendritic- and Myeloid-Cell-Specific HIV-1 Restriction Factor Counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dicks M.D.J., Betancor G., Jimenez-Guardeño J.M., Pessel-Vivares L., Apolonia L., Goujon C., Malim M.H. Multiple Components of the Nuclear Pore Complex Interact with the Amino-Terminus of MX2 to Facilitate HIV-1 Restriction. PLoS Pathog. 2018;14:e1007408. doi: 10.1371/journal.ppat.1007408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malim M.H., Emerman M. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 71.Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a Human Gene That Inhibits HIV-1 Infection and Is Suppressed by the Viral Vif Protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 72.Neil S.J.D., Zang T., Bieniasz P.D. Tetherin Inhibits Retrovirus Release and Is Antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 73.Lobritz M.A., Ratcliff A.N., Arts E.J. HIV-1 Entry, Inhibitors, and Resistance. Viruses. 2010;2:1069–1105. doi: 10.3390/v2051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tilton J.C., Doms R.W. Entry Inhibitors in the Treatment of HIV-1 Infection. Antiviral Res. 2010;85:91–100. doi: 10.1016/j.antiviral.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Arhel N., Kirchhoff F. Host Proteins Involved in HIV Infection: New Therapeutic Targets. Biochim. Biophys. Acta-Mol. Basis Dis. 2010;1802:313–321. doi: 10.1016/j.bbadis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Klibanov O.M. Vicriviroc, a CCR5 Receptor Antagonist for the Potential Treatment of HIV Infection. Curr. Opin. Investig. Drugs. 2009;10:845–859. [PubMed] [Google Scholar]
- 77.Zhou Q., Yik J.H.N. The Yin and Yang of P-TEFb Regulation: Implications for Human Immunodeficiency Virus Gene Expression and Global Control of Cell Growth and Differentiation. Microbiol. Mol. Biol. Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bieniasz P.D., Grdina T.A., Bogerd H.P., Cullen B.R. Recruitment of Cyclin T1/P-TEFb to an HIV Type 1 Long Terminal Repeat Promoter Proximal RNA Target Is Both Necessary and Sufficient for Full Activation of Transcription. Proc. Natl. Acad. Sci. USA. 1999;96:7791–7796. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishaq M., Hu J., Wu X., Fu Q., Yang Y., Liu Q., Guo D. Knockdown of Cellular RNA Helicase DDX3 by Short Hairpin RNAs Suppresses HIV-1 Viral Replication without Inducing Apoptosis. Mol. Biotechnol. 2008;39:231–238. doi: 10.1007/s12033-008-9040-0. [DOI] [PubMed] [Google Scholar]
- 80.Stuchell M.D., Garrus J.E., Müller B., Stray K.M., Ghaffarian S., McKinnon R., Kräusslich H.G., Morham S.G., Sundquist W.I. The Human Endosomal Sorting Complex Required for Transport (ESCRT-I) and Its Role in HIV-1 Budding. J. Biol. Chem. 2004;279:36059–36071. doi: 10.1074/jbc.M405226200. [DOI] [PubMed] [Google Scholar]
- 81.Garrus J.E., Von Schwedler U.K., Pornillos O.W., Morham S.G., Zavitz K.H., Wang H.E., Wettstein D.A., Stray K.M., Côté M., Rich R.L., et al. Tsg101 and the Vacuolar Protein Sorting Pathway Are Essential for HIV-1 Budding. Cell. 2001;107:55–65. doi: 10.1016/S0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 82.Fujii K., Hurley J.H., Freed E.O. Beyond Tsg101: The Role of Alix in “ESCRTing” HIV-1. Nat. Rev. Microbiol. 2007;5:912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 83.Hütter G., Nowak D., Mossner M., Ganepola S., Müssig A., Allers K., Schneider T., Hofmann J., Kücherer C., Blau O., et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. Pediatrics. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 84.Brown T.R. I Am the Berlin Patient: A Personal Reflection. AIDS Res. Hum. Retrov. 2015;31:2–3. doi: 10.1089/aid.2014.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M., Martinez-Picado J., Nijhuis M., Wensing A.M.J., Lee H., et al. HIV-1 Remission Following CCR5Δ32/Δ32 Haematopoietic Stem-Cell Transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brass A.L., Elledge S.J., Xavier R.J., Dykxhoorn D.M., Yan N., Lieberman J., Benita Y., Engelman A. Identification of Host Proteins Required for HIV Infection through a Functional Genomic Screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 87.König R., De Jesus P.D., Opaluch A.M., Chanda S.K., Elleder D., Seidel S., Young J.A., Lilley C.E., Weitzman M.D., Bandyopadhyay S., et al. Global Analysis of Host-Pathogen Interactions That Regulate Early-Stage HIV-1 Replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou H., Xu M., Huang Q., Gates A.T., Zhang X.D., Castle J.C., Stec E., Ferrer M., Strulovici B., Hazuda D.J., et al. Genome-Scale RNAi Screen for Host Factors Required for HIV Replication. Cell Host Microbe. 2008;4:495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Fu W., Sanders-Beer B.E., Katz K.S., Maglott D.R., Pruitt K.D., Ptak R.G. Human Immunodeficiency Virus Type 1, Human Protein Interaction Database at NCBI. Nucleic Acids Res. 2009;37:D417–D422. doi: 10.1093/nar/gkn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ptak R.G., Fu W., Sanders-Beer B.E., Dickerson J.E., Pinney J.W., Robertson D.L., Rozanov M.N., Katz K.S., Maglott D.R., Pruitt K.D., et al. Cataloguing the HIV-1 human protein interaction network. AIDS Res. Hum. Retrovir. 2008;24:1497–1502. doi: 10.1089/aid.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinney J.W., Dickerson J.E., Fu W., Sanders-Beer B.E., Ptak R.G., Robertson D.L. HIV-Host Interactions: A Map of Viral Perturbation of the Host System. AIDS. 2009;23:549–554. doi: 10.1097/QAD.0b013e328325a495. [DOI] [PubMed] [Google Scholar]
- 92.Fassati A. HIV Infection of Non-Dividing Cells: A Divisive Problem. Retrovirology. 2006;3:74. doi: 10.1186/1742-4690-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kabachinski G., Schwartz T.U. The Nuclear Pore Complex - Structure and Function at a Glance. J. Cell Sci. 2015;128:423–429. doi: 10.1242/jcs.083246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurt E., Beck M. Towards Understanding Nuclear Pore Complex Architecture and Dynamics in the Age of Integrative Structural Analysis. Curr. Opin. Cell Biol. 2015;34:31–38. doi: 10.1016/j.ceb.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Beck M., Hurt E. The Nuclear Pore Complex: Understanding Its Function through Structural Insight. Nat. Rev. Mol. Cell Biol. 2017;18:73–89. doi: 10.1038/nrm.2016.147. [DOI] [PubMed] [Google Scholar]
- 96.Von Appen A., Beck M. Structure Determination of the Nuclear Pore Complex with Three-Dimensional Cryo Electron Microscopy. J. Mol. Biol. 2016;428:2001–2010. doi: 10.1016/j.jmb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wälde S., Kehlenbach R.H. The Part and the Whole: Functions of Nucleoporins in Nucleocytoplasmic Transport. Trends Cell Biol. 2010;20:461–469. doi: 10.1016/j.tcb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Jamali T., Jamali Y., Mehrbod M., Mofrad M.R.K. Nuclear Pore Complex. Biochemistry and Biophysics of Nucleocytoplasmic Transport in Health and Disease. Int. Rev. Cell Mol. Biol. 2011;287:233–286. doi: 10.1016/B978-0-12-386043-9.00006-2. [DOI] [PubMed] [Google Scholar]
- 99.Hoelz A., Debler E.W., Blobel G., Fahrenkrog B., Aebi U. The Nuclear Pore Complex: Nucleocytoplasmic Transport and Beyond. Nat. Rev. Mol. Cell Biol. 2003;4:757–766. doi: 10.1038/nrm1230. [DOI] [PubMed] [Google Scholar]
- 100.Monette A., Panté N., Mouland A.J. HIV-1 Remodels the Nuclear Pore Complex. J. Cell Biol. 2011;193:619–631. doi: 10.1083/jcb.201008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nunzio F.D., Danckaert A., Fricke T., Perez P., Fernandez J., Perret E., Roux P., Shorte S., Charneau P., Diaz-Griffero F., et al. Human Nucleoporins Promote HIV-1 Docking at the Nuclear Pore, Nuclear Import and Integration. PLoS ONE. 2012;7:e46037. doi: 10.1371/journal.pone.0046037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matreyek K.A., Engelman A. Viral and Cellular Requirements for the Nuclear Entry of Retroviral Preintegration Nucleoprotein Complexes. Viruses. 2013;5:2483–2511. doi: 10.3390/v5102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jayappa K.D., Ao Z., Yao X. The HIV-1 Passage from Cytoplasm to Nucleus: The Process Involving a Complex Exchange between the Components of HIV-1 and Cellular Machinery to Access Nucleus and Successful Integration. Int. J. Biochem. Mol. Biol. 2012;3:70–85. [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki Y., Craigie R. The Road to Chromatin - Nuclear Entry of Retroviruses. Nat. Rev. Microbiol. 2007;5:187–196. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- 105.Burkinsky M.I., Haggerty S., Sharova N., Adzhubei A., Spitz L., Lewis P., Goldfarb D., Emerman M., Stevenson M. A Nuclear Localization Signal within HIV-1 Matrix Protein That Governs Infection of Non-Dividing Cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Von Schwedler U., Kornbluth R.S., Trono D. The Nuclear Localization Signal of the Matrix Protein of Human Immunodeficiency Virus Type 1 Allows the Establishment of Infection in Macrophages and Quiescent T Lymphocytes. Proc. Natl. Acad. Sci. USA. 2006:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jenkins Y., McEntee M., Weis K., Greene W.C. Characterization of HIV-1 Vpr Nuclear Import: Analysis of Signals and Pathways. J. Cell Biol. 1998;91:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karni O., Friedler A., Zakai N., Gilon C., Loyter A. A Peptide Derived from the N-Terminal Region of HIV-1 Vpr Promotes Nuclear Import in Permeabilized Cells: Elucidation of the NLS Region of the Vpr. FEBS Lett. 1998;429:421–425. doi: 10.1016/S0014-5793(98)00645-0. [DOI] [PubMed] [Google Scholar]
- 109.Gallay P., Hope T., Chin D., Trono D. HIV-1 Infection of Nondividing Cells through the Recognition of Integrase by the Importin/Karyopherin Pathway. Proc. Natl. Acad. Sci. USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Depienne C., Leh H., Le Rouzic E., Dormont D., Benichou S., Dargemont C. Characterization of the Nuclear Import Pathway for HIV-1 Integrase. J. Biol. Chem. 2001;276:18102–18107. doi: 10.1074/jbc.M009029200. [DOI] [PubMed] [Google Scholar]
- 111.Hamid F.B., Kim J., Shin C.-G. Cellular and Viral Determinants of Retroviral Nuclear Entry. Can. J. Microbiol. 2016;62:1–15. doi: 10.1139/cjm-2015-0350. [DOI] [PubMed] [Google Scholar]
- 112.Bhargava A., Lahaye X., Manel N. Let Me in: Control of HIV Nuclear Entry at the Nuclear Envelope. Cytokine Growth Factor Rev. 2018;40:59–67. doi: 10.1016/j.cytogfr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Ao Z., Jayappa K.D., Wang B., Zheng Y., Wang X., Peng J., Yao X. Contribution of Host Nucleoporin 62 in HIV-1 Integrase Chromatin Association and Viral DNA Integration. J. Biol. Chem. 2012;287:10544–10555. doi: 10.1074/jbc.M111.317057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu J., Matunis M.J., Kraemer D., Blobel G., Coutavas E. Nup358, a Cytoplasmically Exposed Nucleoporin with Peptide Repeats, Ran- GTP Binding Sites, Zinc Fingers, a Cyclophilin a Homologous Domain, and a Leucine-Rich Region. J. Biol. Chem. 1995;270:14209–14213. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- 115.Yokoyama N., Hayashi N., Seki T., Pante N., Ohba T., Nishii K., Kuma K., Hayashida T., Miyata T., Aebi U., et al. A Giant Nucleopore Protein That Binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- 116.Francis A.C., Melikyan G.B. Live-Cell Imaging of Early Steps of Single HIV-1 Infection. Viruses. 2018;10:275. doi: 10.3390/v10050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rawle D.J., Harrich D. Toward the “Unravelling” of HIV: Host Cell Factors Involved in HIV-1 Core Uncoating. PLoS Pathog. 2018;14:e1007270. doi: 10.1371/journal.ppat.1007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bichel K., Price A.J., Schaller T., Towers G.J., Freund S.M., James L.C. HIV-1capsid undergoes coupled binding and isomerization by the nuclear pore proteinNUP358. Retrovirology. 2013;10:81. doi: 10.1186/1742-4690-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Nunzio F. New Insights in the Role of Nucleoporins: A Bridge Leading to Concerted Steps from HIV-1 Nuclear Entry until Integration. Virus Res. 2013;178:187–196. doi: 10.1016/j.virusres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Schaller T., Ocwieja K.E., Rasaiyaah J., Price A.J., Brady T.L., Roth S.L., Hué S., Fletcher A.J., Lee K.E., KewalRamani V.N., et al. HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency. PLoS Pathog. 2011;7:e1002439. doi: 10.1371/journal.ppat.1002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin D.H., Zimmermann S., Stuwe T., Stuwe E., Hoelz A. Structural and Functional Analysis of the C-Terminal Domain of Nup358/RanBP2. J. Mol. Biol. 2013;425:1318–1329. doi: 10.1016/j.jmb.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakielny S. Nup153 Is an M9-Containing Mobile Nucleoporin with a Novel Ran-Binding Domain. EMBO J. 1999;18:1982–1995. doi: 10.1093/emboj/18.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Nunzio F., Souque P., Charneau P., Fricke T., Valle-Casuso J.C., Perez P., Diaz-Griffero F., Miccio A., Mavilio F., Rizzi E., et al. Nup153 and Nup98 Bind the HIV-1 Core and Contribute to the Early Steps of HIV-1 Replication. Virology. 2013;440:8–18. doi: 10.1016/j.virol.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paulillo S.M., Phillips E.M., Köser J., Sauder U., Ullman K.S., Powers M.A., Fahrenkrog B. Nucleoporin Domain Topology Is Linked to the Transport Status of the Nuclear Pore Complex. J. Mol. Biol. 2005;351:784–798. doi: 10.1016/j.jmb.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 125.Ball J.R., Ullman K.S. Versatility at the Nuclear Pore Complex: Lessons Learned from the Nucleoporin Nup153. Chromosoma. 2005;114:319–330. doi: 10.1007/s00412-005-0019-3. [DOI] [PubMed] [Google Scholar]
- 126.Bayliss R., Littlewood T., Stewart M. Structural basis for the interaction between FXFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 2000;102:99–108. doi: 10.1016/S0092-8674(00)00014-3. [DOI] [PubMed] [Google Scholar]
- 127.Woodward C.L., Prakobwanakit S., Mosessian S., Chow S.A. Integrase Interacts with Nucleoporin NUP153 To Mediate the Nuclear Import of Human Immunodeficiency Virus Type 1. J. Virol. 2009;83:6522–6533. doi: 10.1128/JVI.02061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matreyek K.A., Engelman A. The Requirement for Nucleoporin NUP153 during Human Immunodeficiency Virus Type 1 Infection Is Determined by the Viral Capsid. J. Virol. 2011;85:7818–7827. doi: 10.1128/JVI.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu P., Ng S.B.H., Mahalingam S., Balasundaram D., Dorairajoo D.S.K., Gandotra S., Varadarajan P. The Functionally Conserved Nucleoporins Nup124p from Fission Yeast and the Human Nup153 Mediate Nuclear Import and Activity of the Tf1 Retrotransposon and HIV-1 Vpr. Mol. Biol. Cell. 2005;16:1823–1838. doi: 10.1091/mbc.E04-07-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dewangan P.S., Sonawane P.J., Chouksey A.R., Chauhan R. The Nup62 Coiled-Coil Motif Provides Plasticity for Triple-Helix Bundle Formation. Biochemistry. 2017;56:2803–2811. doi: 10.1021/acs.biochem.6b01050. [DOI] [PubMed] [Google Scholar]
- 131.Chopra K., Bawaria S., Chauhan R. Evolutionary Divergence of the Nuclear Pore Complex from Fungi to Metazoans. Prot. Sci. 2019;8:571–586. doi: 10.1002/pro.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalverda B., Pickersgill H., Shloma V.V., Fornerod M. Nucleoporins Directly Stimulate Expression of Developmental and Cell-Cycle Genes Inside the Nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 133.Koh Y., Wu X., Ferris A.L., Matreyek K.A., Smith S.J., Lee K., KewalRamani V.N., Hughes S.H., Engelman A. Differential Effects of Human Immunodeficiency Virus Type 1 Capsid and Cellular Factors Nucleoporin 153 and LEDGF/P75 on the Efficiency and Specificity of Viral DNA Integration. J. Virol. 2013;87:648–658. doi: 10.1128/JVI.01148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Emerman M. HIV-1 Regulatory/Accessory Genes: Keys to Unraveling Viral and Host Cell Biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 135.Ptak R.G. HIV-1 Regulatory Proteins: Targets for Novel Drug Development. Expert Opin. Investigl. Drugs. 2005;11:1099–1115. doi: 10.1517/13543784.11.8.1099. [DOI] [PubMed] [Google Scholar]
- 136.Truant R., Cullen B.R. The Arginine-Rich Domains Present in Human Immunodeficiency Virus Type 1 Tat and Rev Function as Direct Importin β-Dependent Nuclear Localization Signals. Mol. Biol. 1999;19:1210–1217. doi: 10.1128/MCB.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barboric M., Fujinaga K. The Two Sides of Tat. ELife. 2016;19:e12686. doi: 10.7554/eLife.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dayton A.I. Within You, without You: HIV-1 Rev and RNA Export. Retrovirology. 2004;1:35. doi: 10.1186/1742-4690-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Szebeni A., Baumann A., Olson M.O.J., Mehrotra B., Adam S.A., Wingfield P.T. Nucleolar Protein B23 Stimulates Nuclear Import of the HIV-1 Rev Protein and NLS-Conjugated Albumin. Biochemistry. 1997;36:3941–3949. doi: 10.1021/bi9627931. [DOI] [PubMed] [Google Scholar]
- 140.Zolotukhin A.S., Felber B.K. Nucleoporins Nup98 and Nup214 Participate in Nuclear Export of Human Immunodeficiency Virus Type 1 Rev. J. Virol. 1999;73:120–127. doi: 10.1128/jvi.73.1.120-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hutten S., Kehlenbach R.H. Nup214 Is Required for CRM1-Dependent Nuclear Protein Export In Vivo. Mol. Cell Biol. 2006;26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neville M., Stutz F., Lee L., Davis L., Rosbash M. The Importin-Beta Family Member Crm1p Bridges the Interaction between Rev and the Nuclear Pore complex during nuclear export. Curr. Biol. 1997;7:767–775. doi: 10.1016/S0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 143.Hofmann W., Reichart B., Ewald A., Müller E., Schmitt I., Stauber R.H., Lottspeich F., Jockusch B.M., Scheer U., Hauber J., et al. Cofactor Requirements for Nuclear Export of Rev Response Element (RRE)- and Constitutive Transport Element (CTE)-Containing Retroviral RNAs: An Unexpected Role for Actin. J. Cell Biol. 2001;152:895–910. doi: 10.1083/jcb.152.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Strebel K. Virus-Host Interactions: Role of HIV Proteins Vif, Tat, and Rev. AIDS. 2003;17:S25–S34. doi: 10.1097/00002030-200317004-00003. [DOI] [PubMed] [Google Scholar]
- 145.Hezwani M., Fahrenkrog B. The Functional Versatility of the Nuclear Pore Complex Proteins. Semin. Cell Dev. Biol. 2017;68:2–9. doi: 10.1016/j.semcdb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 146.Jühlen R., Fahrenkrog B. Moonlighting Nuclear Pore Proteins: Tissue-Specific Nucleoporin Function in Health and Disease. Histochem. Cell Biol. 2018;150:593–605. doi: 10.1007/s00418-018-1748-8. [DOI] [PubMed] [Google Scholar]
- 147.Borlido J., D’Angelo M.A. Nup62-mediated nuclear import of p63 in squamous cell carcinoma. EMBO Rep. 2018;19:3–4. doi: 10.15252/embr.201745497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodriguez-Bravo V., Pippa R., Song W., Carceles-Cordon M., Dominguez-Andres A., Fujiwara N., Woo J., Koh A.P., Ertel A., Lokareddy R.K., et al. Nuclear Pores Promote Lethal Prostate Cancer by Increasing POM121-Driven E2F1, MYC, and AR Nuclear Import. Cell. 2018;174:1200–1215. doi: 10.1016/j.cell.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chan E.Y., Diamond D.L., Katze M.G., Qian W.-J., Liu T., Gritsenko M.A., Monroe M.E., Camp II D.G., Smith R.D. Quantitative Analysis of Human Immunodeficiency Virus Type 1-Infected CD4+ Cell Proteome: Dysregulated Cell Cycle Progression and Nuclear Transport Coincide with Robust Virus Production. J. Virol. 2007;88:7571–7583. doi: 10.1128/JVI.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chan E.Y., Sutton J.N., Jacobs J.M., Bondarenko A., Smith R.D., Katze M.G. Dynamic Host Energetics and Cytoskeletal Proteomes in Human Immunodeficiency Virus Type 1-Infected Human Primary CD4 Cells: Analysis by Multiplexed Label-Free Mass Spectrometry. J. Virol. 2009;83:9283–9295. doi: 10.1128/JVI.00814-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ori A., Banterle N., Iskar M., Andrés-Pons A., Escher C., Khanh H., Sparks L., Solis-Mezarino V., Rinner O., Bork P. Cell Type-Specific Nuclear Pores: A Case in Point For Context-Dependent Stoichiometry Of Molecular Machines. Mol. Sys. Biol. 2013;9:648. doi: 10.1038/msb.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kane M., Rebensburg S.V., Takata M.A., Zang T.M., Yamashita M., Kvaratskhelia M., Bieniasz P.D. Nuclear Pore Heterogeneity Influences HIV-1 Infection and the Antiviral Activity of MX2. ELife. 2018;7:e35738. doi: 10.7554/eLife.35738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.D’Angelo M.A., Gomez-Cavazos J.S., Mei A., Lackner D.H., Hetzer M.W. A Change in Nuclear Pore Complex Composition Regulates Cell Differentiation. Dev. Cell. 2012;22:446–458. doi: 10.1016/j.devcel.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raices M., D’Angelo M.A. Nuclear Pore Complex Composition: A New Regulator of Tissue-Specific and Developmental Functions. Nat. Rev. Mol. Cell Biol. 2012;13:687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 155.Gorjánácz M. Nuclear Assembly as a Target for Anti-Cancer Therapies. Nucleus. 2014;5:47–55. doi: 10.4161/nucl.27928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kosyna F., Depping R. Controlling the Gatekeeper: Therapeutic Targeting of Nuclear Transport. Cells. 2018;7:221. doi: 10.3390/cells7110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E.P., Wolff B., Yoshida M., Horinouchi S. Leptomycin B Inactivates CRM1/Exportin 1 by Covalent Modification at a Cysteine Residue in the Central Conserved Region. Proc. Natl. Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dickmanns A., Monecke T., Ficner R. Structural Basis of Targeting the Exportin CRM1 in Cancer. Cells. 2015;4:538–568. doi: 10.3390/cells4030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Newlands E.S., Rustin G.J., Brampton M.H. Phase I trial of elactocin. Br. J. Cancer. 1996;74:648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Levin A., Loyter A., Bukrinsky M. Strategies to Inhibit Viral Protein Nuclear Import: HIV-1 as a Target. Biochim. Biophys. Acta-Mol. Cell Res. 2011;1813:1646–1653. doi: 10.1016/j.bbamcr.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levin A., Armon-Omer A., Rosenbluh J., Melamed-Book N., Graessmann A., Waigmann E., Loyter A. Inhibition of HIV-1 Integrase Nuclear Import and Replication by a Peptide Bearing Integrase Putative Nuclear Localization Signal. Retrovirology. 2009;6:112. doi: 10.1186/1742-4690-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hayouka Z., Levin A., Maes M., Hadas E., Shalev D.E., Volsky D.J., Loyter A., Friedler A. Mechanism of Action of the HIV-1 Integrase Inhibitory Peptide LEDGF. Biochem. Biophys. Res. Comm. 2010;394:260–265. doi: 10.1016/j.bbrc.2010.02.100. [DOI] [PubMed] [Google Scholar]