Abstract
Purpose
This research aimed to compare the efficacy of combination treatment of transcatheter arterial chemoembolization (TACE) with apatinib versus TACE-alone for intermediate and advanced-stage hepatocellular carcinoma (HCC) cases refractory to TACE.
Patients and methods
A total of 125 patients with TACE refractory intermediate or advanced-stage HCC were enrolled and classified as TACE-apatinib group and TACE-alone group. One-to-one matched pairs between two groups were generated using propensity score matching (PSM). Associations of treatment modality with overall survival (OS) and progression-free survival (PFS) were determined by Cox regression. Adverse effects (AEs) were compared between two treatment groups to assess the safety of apatinib.
Results
Before PSM analysis, the median OS and PFS were 17.0 and 7.0 months in the TACE-apatinib group, while 8.5 and 2.5 months in the TACE-alone group (P<0.05). After PSM analysis, 29 pairs of patients were generated with no significant difference in baseline characteristics. The median OS and PFS were 17.0 and 7.0 months in the TACE-apatinib group, while 10.7 and 2.0 months in the TACE-alone group (P<0.001). Multivariate analyses showed that TACE-apatinib treatment was a positive prognostic factor of both OS (hazard ratio [HR]=0.280, 95% confidence interval [95% CI] =0.158–0.499; P<0.001) and PFS (HR=0.348, 95% CI=0.223–0.544; P<0.001). Tumor size≥5 cm (HR=1.732, 95% CI=1.086–2.760; P=0.021), presence of portal vein tumor thrombus (HR=2.297, 95% CI=1.379–3.827; P=0.001) and distant metastasis (HR=1.962, 95% CI=1.223–3.148; P=0.005) were independent hazard factors of OS. Three patients in TACE-apatinib group appeared grade 3/4 AEs while their symptoms could be alleviated by dosage reduction and symptomatic treatments.
Conclusion
TACE combined with apatinib demonstrated a superior therapeutic efficacy than TACE alone for improved OS and PFS toward the TACE refractory HCC. Apatinib could be recommended for HCC patients when TACE refractoriness occurs after further validation.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, apatinib, prognosis
Introduction
According to the updated report, the incidence of primary hepatocellular carcinoma (HCC) is still alarming and ranks the sixth place for new cancer cases as well as fourth for the mortality of cancer cases worldwide, essentially suggesting that it is still the main cause of cancer-related deaths and more effective treatments are urgently needed.1 The Barcelona Clinic Liver Cancer (BCLC) clinical staging system recommends that the current standard treatment for HCCs in BCLC stage B is transcatheter arterial chemoembolization (TACE),2 a common nonsurgical therapy which is popular in Asian countries such as China. For HCC patients in BCLC stage C with Child-Pugh class A or B liver function, Chinese guideline for diagnosis and treatment of primary liver cancer (2017 Edition) also recommends the use of TACE.3 Overall, TACE is a critical palliative therapy for unresectable HCCs and the majority of patients will receive TACE until the decompensation of their liver function occurs.
Among those with a history of undergoing repeated TACE, some patients showed poor efficacy of this treatment by appearing sustained enlargement of the lesion, vascular invasion or extrahepatic spread. This state was first termed as TACE failure or refractory and proposed by the Japan Society of Hepatology (JSH).4 In 2014, JSH and the Liver Cancer Study Group of Japan (LCSGJ) proposed a clear definition of “refractoriness or failure to TACE”.5 In this definition, two criteria apply to intrahepatic lesions: (1) two or more consecutive insufficient responses of the treated tumor (viable lesion>50%) even after changing the chemotherapeutic agents and/or reanalysis of the feeding artery seen on response evaluation CT/MRI at 1–3 months after having adequately performed selective TACE; (2) two or more consecutive progressions in the liver (tumor number increases as compared to tumor number before the previous TACE procedure) even after having changed the chemotherapeutic agents and/or reanalysis of the feeding artery seen on response evaluation CT/MRI at 1–3 months after having adequately performed selective TACE. Other criteria include (1) continuous elevation of tumor markers immediately after TACE even though slight transient decrease is observed; (2) appearance of vascular invasion; (3) appearance of extrahepatic spread. The recurrence and progression of the tumor might be attributed to the elevation of vascular endothelial growth factor (VEGF) and neo-angiogenesis mediated by TACE-induced hypoxia.6 Therefore, adjuvant treatments to suppress post-TACE angiogenesis is considered as a critical supplement.
Several studies have explored the efficacy of different treatments prescribed to patients with refractoriness of TACE. Sorafenib is a multi-kinase inhibitor which inhibits neo-angiogenesis in tumors by blocking several tumor-related signal pathways including vascular endothelial growth factor receptors (VEGFR).7 Its therapeutic effect had been proved in the advanced HCC patients,8 and researchers subsequently found that the combination of TACE and sorafenib was a safe treatment with superior efficacy for unresectable HCC as compared to sorafenib-alone.9,10 Having been inspired by previous researches and the possible mechanism of post-TACE recurrence, retrospective studies from different institutions was carried out and they separately reported that the switch to sorafenib or combining TACE with sorafenib would prolong the overall survival (OS) of patients or raise the 5-year survival rate after the failure of TACE.11–14 Finally, a prospective study on a large scale of TACE refractory cases (n=507) confirmed that the median OS in sorafenib group was longer than that of TACE-alone group in all four test applying different criteria of TACE refractoriness (American Association for the Study of Liver Diseases: 27.6 vs 12.4 months; protocol-specified: 16.2 vs 12.1 months; protocol-specified excluding Eastern Cooperative Oncology Group performance status: 19.3 vs 13.0 months; JSH: 15.2 vs 11.8 months).15 However, the response rate of sorafenib was relatively low,8,16 and the drug resistance was common.17 Moreover, this targeted drug is expensive for a large proportion of Chinese patients. Therefore, a lot of TACE refractory HCC patients in China kept on receiving TACE rather than undergoing the treatment of sorafenib.
Apatinib, a new kind of targeted drug, has higher selectivity to VEGFR-2 than sorafenib based on the laboratory results that the IC50 of apatinib is significantly smaller than that of sorafenib (0.001μmol/L vs 0.090μmol/L).7,18 In 2014, a phase II clinical study on apatinib in HCC was completed and showed that apatinib monotherapy could prolong the OS of patients with intermediate and advanced HCC.19 Besides, studies have revealed that the number of VEGF-positive cells in residual liver cancer tissues increased after the TACE surgery,20,21 suggesting the potential of apatinib in cooperation with TACE. Considering that apatinib may effectively inhibit post-TACE neo-angiogenesis, combining these two treatments may also improve the anti-tumor efficacy and the prognosis of patients with refractoriness of TACE.
Therefore, the goal of this retrospective study was to compare the efficacy of TACE–apatinib combination treatment with TACE-alone treatment in HCC patients refractory to TACE. For conducting comparisons with similar compositions of patients, a 1:1 ratio propensity score matching (PSM) was adopted to homogenize the baseline clinical characteristic of different groups.
Materials And Methods
Patients Population
From January 1st, 2013 to March 1st, 2017, 586 intermediate-stage HCC patients were admitted and received TACE at the Sun Yat-sen University Cancer Center (SYSUCC). All the HCC patients were diagnosed by radiological findings (contrast-enhanced computed tomography [CT] or magnetic resonance imaging [MRI]) or biopsy. This study was approved by the SYSUCC Hospital Ethics Committee, which waived the need for written informed consent because of the retrospective nature of this study. The information of all participants is maintained with confidentiality.
The inclusion criteria were: (1) HCC patients in stage B or C according to the BCLC staging system;22 (2) patients with Child-Pugh Class A or B liver function; (3) patients with performance status of 0 or 1; (4) patients who encountered TACE refractoriness based on the JSH-LCSGJ criteria (2014 update).5 The patients’ AFP level was examined before the first TACE as the baseline level and reexamined no later than 2 months after the first TACE. The continuous elevation of AFP was defined as two or more appearance of an increase of >20% from the previous result. However, only patients with a baseline level of >20 ng/mL would be considered to adopt this criterion.
The exclusion criteria were: (1) patients had inadequate bone marrow function (leukocyte count<3,000/μL, platelet<40,000/μL, absolute neutrophil count<1,500/μL); (2) patients had other primary malignancies or the immune deficiency; (3) patients had received other antineoplastic treatments except for TACE or apatinib before the disease progression which occurred after TACE refractoriness; (4) the interval between the confirmation of TACE refractoriness and administration of apatinib was longer than 15 days.
Therapeutic Method
All TACE refractory patients enrolled in this study had been informed of the choice of taking apatinib and they made the decision based on the full communication between patients and doctors as well as their personal willingness. According to their decision of treatments, patients were classified into two groups, the TACE-apatinib group or the TACE-alone group.
TACE Method
The TACE was conducted by multidisciplinary teams of our hospital including specialists with more than 10 years’ experience in the following procedure. Femoral artery puncture and intubation were performed via the Seldinger method. Guided by the angiography of proper hepatic and related vessels, a super-selective microcatheter was inserted into the feeding artery of tumors. Once the catheterization was done, 5–15 mL of lipiodol, 30–50 mg pirarubicin and 30–50 mg lobaplatin (the exact dose of each patient depended on their respective embolization condition) were mixed together and infused via the catheter. Finally, feeding arteries were embolized with absorbable gelatin sponge particles. The efficacy of TACE was examined by contrast-enhanced CT or MRI 1 month later and this operation would be recommended to repeat once the radiology examination revealed that the lipiodol deposition shrank and residual lesions occurred. Decisions of undergoing subsequent TACE were also made after discussions between patients and surgical teams.
Apatinib Administration
To accurately evaluate the effect of apatinib, the administration pattern of apatinib was strictly defined in advance. It was taken 500 mg/day orally and no later than 15 days after the confirmation of TACE refractoriness. If patients could not tolerate side effects, dose reduction to 250 mg/day was recommended but was reverted to the original dosage once side effects were alleviated or they could tolerate them. If patients could not tolerate side effects or disease progression was confirmed, the administration of apatinib was not continued.
Follow Up, Endpoints And Evaluation Of The Therapeutic Effect
Patients were regularly followed-up and reexamined by abdominal contrast-enhanced CT or MRI as well as blood tests every 1 to 3 months during the treatment. The primary endpoint of this study was OS, which started from the time of being TACE refractory to the time of death by any cause. The secondary endpoint was progression-free survival (PFS), defined as the interval between the time of being TACE refractory and the time of disease progression or death. Patients’ imaging examination (CT or MR) results were evaluated by at least two diagnostic radiologists having more than 5-year experience according to the modified response evaluation criteria in solid tumor (MRECIST) with 4 levels: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).23 In this study, disease control rate (DCR) = (CR+PR+SD)/total number of cases *100%. All AEs were recorded and assessed according to the Common Terminology Criteria for Adverse Events (version 4.0).
Statistics Analysis
The analysis of this study was conducted through the statistical software SPSS 24.0. The Intergroup comparison of baseline clinical characteristics (sex, age, alpha-fetoprotein [AFP] level, Hepatitis B Virus [HBV] infection, Child-Pugh Class, tumor size, number of lesions, portal vein tumor thrombus [PVTT], distant metastasis, BCLC stage and number of previous TACE) was conducted by chi-square test, Fisher’s exact test or Wilcoxon rank-sum test before and after matching. To avoid selection bias due to discrepant compositions of patients, a 1:1 ratio PSM was conducted to match the baseline characteristics of two treatment groups. OS and PFS were estimated by the Kaplan–Meier method and compared between two groups using the log rank test and the survival rate curve. The effect of TACE-apatinib and other baseline characteristics were assessed by Cox proportional hazard regression model to calculate the hazard ratio of each variable. Only variables with P-value<0.10 in univariate analysis were considered for multivariate analysis of independent prognostics factors affecting OS or PFS. A 95% confidence interval (95% CI) was also evaluated and added to the statistical results. Inspection level was set as α=0.05 and P-value<0.05 was considered statistically significant.
Results
Baseline Characteristics Of Patients In This Study
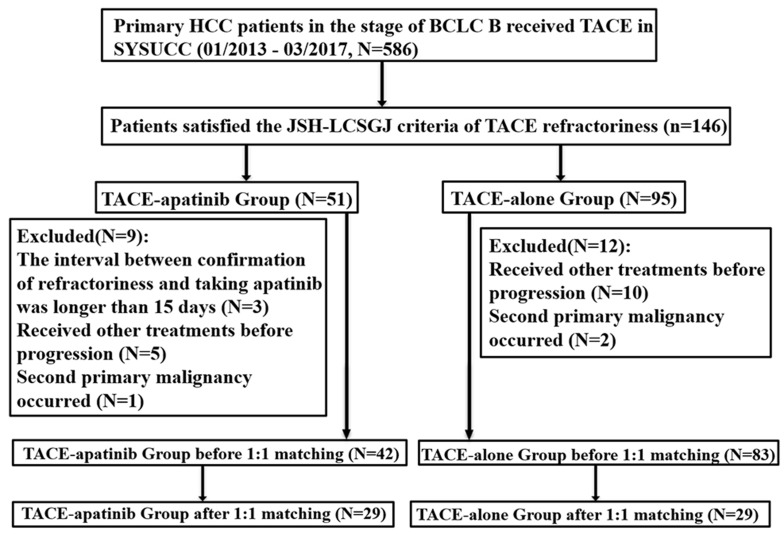
According to the inclusion and exclusion criteria, 125 patients were enrolled in this study (Figure 1). Among them, 42 and 83 patients received TACE-apatinib and TACE-only before disease progression. The baseline characteristics of two treatment groups (sex, age, AFP level, HBV infection, Child-Pugh class, tumor size, number of lesions, PVTT, distant metastasis, BCLC stage and number of previous TACE) are listed and compared in Table 1. After the 1:1 ratio PSM, 29 pairs of patients were generated. No statistical significance was found between any of the characteristics in two treatment groups and their compositions became more similar.
Figure 1.
The flow diagram of this study.
Abbreviations: HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; JSH, Japan Society of hepatology; LCSGJ, Liver Cancer Study Group of Japan; TACE, transcatheter arterial chemoembolization.
Table 1.
Comparison Of Two Treatment Groups Of Patients With Baseline Characteristics (Before And After PSM)
| Characteristics | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| n, (%) | TACE-Apatinib Group(n=42) | TACE-Alone Group(n=83) | P value | TACE-Apatinib Group(n=29) | TACE-Alone Group(n=29) | P value |
| Sex | 0.142 | 1.000 | ||||
| Male | 41(97.6) | 73(88.0) | 28(96.6) | 27(93.1) | ||
| Female | 1(2.4) | 10(12.0) | 1(3.4) | 2(6.9) | ||
| Age (years) | 0.343 | 1.000 | ||||
| <56 | 23(54.8) | 38(45.8) | 14(48.3) | 14(48.3) | ||
| 56 | 19(45.2) | 45(54.2) | 15(51.7) | 15(51.7) | ||
| AFP (ng/mL) | 0.952# | 0.602# | ||||
| <400 | 20(47.6) | 40(48.2) | 14(48.3) | 16(55.2) | ||
| ≥400 | 22(52.4) | 43(51.8) | 15(51.7) | 13(44.8) | ||
| HBV | 0.590 | 1.000 | ||||
| Infected | 39(92.9) | 73(88.0) | 27(93.1) | 28(96.6) | ||
| Uninfected | 3(7.1) | 10(12.0) | 2(6.9) | 1(3.4) | ||
| Child-Pugh | 0.633 | 1.000 | ||||
| Class A | 36(85.7) | 75(90.4) | 25(86.2) | 26(89.7) | ||
| Class B | 6(14.3) | 8(9.6) | 4(13.8) | 3(10.3) | ||
| Tumor Size (cm) | 0.938 | 0.597 | ||||
| <5 | 17(40.5) | 33(39.8) | 12(41.4) | 14(48.3) | ||
| ≥5 | 25(59.5) | 50(60.2) | 17(58.6) | 15(51.7) | ||
| Number of lesions | 0.206 | 0.703 | ||||
| Single | 8(19.0) | 9(10.8) | 5(17.2) | 3(10.3) | ||
| Multiple | 34(81.0) | 74(89.2) | 24(82.8) | 26(89.7) | ||
| PVTT | <0.001 | 0.788 | ||||
| Present | 23(54.8) | 17(20.5) | 11(37.9) | 12(41.4) | ||
| Absent | 19(45.2) | 66(79.5) | 18(62.1) | 17(58.6) | ||
| Metastasis | 0.229 | 0.189 | ||||
| Present | 27(64.3) | 44(53.0) | 17(58.6) | 12(41.4) | ||
| Absent | 15(35.7) | 39(47.0) | 12(41.4) | 17(58.6) | ||
| BCLC | 0.121 | 0.256 | ||||
| Stage B | 9(21.4) | 29(34.9) | 7(24.1) | 11(37.9) | ||
| Stage C | 33(78.6) | 54(65.1) | 22(75.9) | 18(62.1) | ||
| Number of Previous TACE | 0.144 | 0.780 | ||||
| ≤2 | 29(69.0) | 67(80.7) | 20(69.0) | 19(65.5) | ||
| >2 | 13(31.0) | 16(19.3) | 9(31.0) | 10(34.5) | ||
Notes: #Rank sum test. Before PSM, there was a significant difference in the presence of PVTT between the two groups. After PSM, no statistical significance was found in any of the characteristics and compositions of the two groups became more similar.
Abbreviations: PSM, propensity score matching; TACE, transcatheter arterial chemoembolization; AFP, alpha-fetoprotein; HBV, Hepatitis B Virus; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer.
Adverse Events And Safety Assessment
During the whole course of this study, different kinds of adverse events (AEs) appeared in two cohorts and symptomatic treatments were adopted to alleviate patients’ symptoms. All AEs are listed and their incidences were compared between two groups in Table 2. Hepatic failure and postembolization syndrome, such as fever, abdominal pain, nausea and vomiting were recorded while no significant difference was found between two treatment groups. No grade 4 AEs appeared in the TACE-alone group. Oppositely, three patients in the TACE-apatinib group discontinued taking apatinib because of intolerable AEs. Two of them experienced grade 3/4 hand-foot syndrome and one experienced grade 4 diarrhea but were gradually relieved after stopping taking apatinib. The other 39 patients also experienced a series of apatinib-related AEs but were of lower and tolerable grades. The number of apatinib-related AEs can be summed up as follows: hand-foot syndrome (HFS) (20 patients, 47.6%), hypertension (13 patients, 31.0%), diarrhea (11 patients, 26.2%), epistaxis (3 patients, 7.1%), hoarseness (4 patients, 9.5%), oral or anus ulcer (8 patients, 19.0%) and proteinuria (1 patient, 2.4%). Totally, 13 patients (31.0%) had reduced the prescription dosage from 500 mg to 250 mg or even transiently quitted the use of apatinib and 6 (14.3%) of them resumed the initial dosage after the AEs had been alleviated or they could tolerate them. Compared with the TACE-alone group, the incidences of apatinib-related AEs (HFS, hypertension, diarrhea, hoarseness, oral or anal ulcer) were significantly higher in the TACE-apatinib group. No hepatic failure or grade 5 AEs (death caused by the medicine) were observed.
Table 2.
The Comparison Of AEs Between Two Treatment Groups
| AEs | TACE-Apatinib (n=42) | TACE-Alone(n=83) | P-value |
|---|---|---|---|
| Fever | 19(45.2%) | 51(61.4%) | 0.085 |
| Hand-foot syndrome | 20(47.6%) | 0(0%) | <0.001* |
| Fatigue | 12(28.6%) | 21(25.3%) | 0.695 |
| Hypertension | 13(31.0%) | 2(2.4%) | <0.001 |
| Nausea and vomiting | 9(21.4%) | 13(15.7%) | 0.424 |
| Diarrhea | 11(26.2%) | 6(7.2%) | 0.003 |
| Abdominal pain | 8(19.0%) | 19(22.9%) | 0.622 |
| Hoarseness | 4(9.5%) | 0(0%) | 0.012* |
| Epistaxis | 3(7.1%) | 0(0%) | 0.036* |
| Oral or anal ulcer | 8(19.0%) | 0(0%) | <0.001* |
| Proteinuria | 1(2.4%) | 0(0%) | 0.336* |
| Hepatic failure | 0(0%) | 0(0%) | - |
Notes: *Fisher’s exact test. Compared with the TACE-alone group, the incidences of apatinib-related AEs (hand-foot syndrome, hypertension, diarrhea, hoarseness, oral or anal ulcer) were significantly higher in the TACE-apatinib group.
Abbreviations: AEs, adverse events; TACE, transcatheter arterial chemoembolization.
Comparison Of OS Between Two Treatment Groups
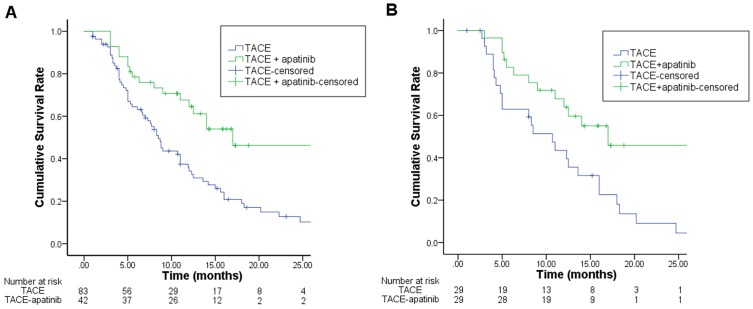
Before the PSM, the median OS was 17.0 months (95% CI: 9.9–24.1 months) for the TACE-apatinib group and 8.5 months (95% CI: 7.2–9.8 months) for the TACE-alone group (P=0.003). After PSM analysis, the modified median OS were 17.0 months (95% CI:12.0–22.0 months) for the TACE-apatinib group and 10.7 months (95% CI: 6.4–15.0 months) for the TACE-alone group (P=0.027) (Figure 2).
Figure 2.
Kaplan–Meier curves of OS for TACE+apatinib and TACE groups before (A) and after PSM (B).
Notes: The significant increase of OS can be both observed in the TACE-apatinib group compared with the TACE-alone group before (A) and after PSM (B).Abbreviations: OS, overall survival; TACE, transcatheter arterial chemoembolization; PSM, propensity score matching.
Comparison Of PFS Between Two Treatment Groups
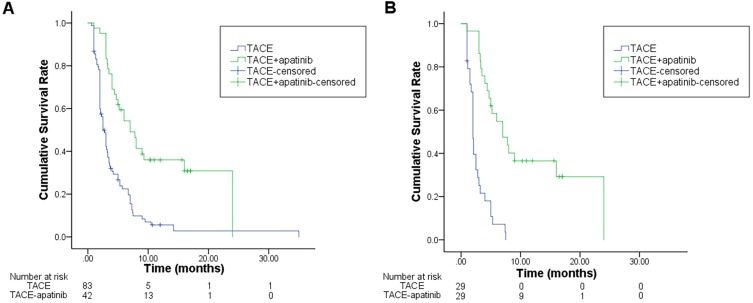
Before the PSM, the median PFS was 7.0 months (95% CI:4.6–9.4 months) for TACE-apatinib group and 2.5 months (95% CI: 2.0–3.0 months) for the TACE-alone group (P<0.001). After the PSM, the modified median PFS was found to be 7.0 months (95% CI:3.7–10.3 months) in TACE-apatinib group and 2.0 months (95% CI:1.7–2.3 months) in TACE-alone group (P<0.001) (Figure 3).
Figure 3.
Kaplan–Meier curves of PFS for TACE+apatinib and TACE groups before (A) and after PSM (B).
Notes: The significant increase of PFS can be observed in the TACE-apatinib group compared with the TACE-alone group, especially after PSM.
Abbreviations: PFS, progression-free survival; TACE, transcatheter arterial chemoembolization; PSM, propensity score matching.
Prognostic Factors Affecting OS And PFS
Considering the interference of confounding factors, univariate and multivariate analyses of prognostic factors for OS and PFS including treatments as well as other baseline characteristics were separately conducted using the Cox proportional hazard regression model.
Univariate analysis revealed that tumor size, AFP level, PVTT, distant metastasis, and treatments in this study were factors associated with OS (all P<0.10). However, multivariate analysis confirmed that only tumor size, PVTT, distant metastasis and treatments in this study were independent prognostic factors of OS. More importantly, TACE-apatinib was identified as a positive factor for improved OS while tumor size≥5 cm, presence of PVTT and distant metastasis were hazard factors of OS (Table 3). As for PFS, both univariate and multivariate analyses showed that the treatment was the only independent prognostic factor for PFS in this study (Table 4). Consequently, TACE-apatinib was the only positive factor for improved OS and PFS.
Table 3.
Univariate And Multivariate Analysis Of Prognostics Factors Affecting OS For Two Treatment Cohorts
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Numbers | HR | P value | HR | P value | ||
| Sex (Male vs Female) | 114/11 | 0.645(0.322–1.293) | 0.217 | – | – | |
| Age (≥56 vs <56) | 64/61 | 0.759(0.491–1.173) | 0.214 | – | – | |
| AFP level (≥400 ng/mL vs <400 ng/mL) | 65/60 | 1.644(1.050–2.572) | 0.030 | – | – | |
| HBV (Present vs Absent) | 112/13 | 1.231(0.592–2.561) | 0.578 | – | – | |
| Child Pugh Class (B vs A) | 14/111 | 0.805(0.398–1.626) | 0.545 | – | – | |
| Tumor size (≥5 cm vs <5 cm) | 75/50 | 1.794(1.135–2.837) | 0.012 | 1.732(1.086–2.760) | 0.021 | |
| Number of lesions (Multiple vs Single) | 108/17 | 1.676(0.807–3.481) | 0.166 | – | – | |
| PVTT (Present vs Absent) | 40/85 | 1.591(1.001–2.528) | 0.050 | 2.297(1.379–3.827) | 0.001 | |
| Distant metastasis (Present vs Absent) | 71/54 | 1.580(1.005–2.485) | 0.048 | 1.962(1.223–3.148) | 0.005 | |
| Number of Previous TACE (>2 VS ≤2) | 29/96 | 0.709(0.413–1.214) | 0.210 | – | – | |
| Treatments (TACE-apatinib vs TACE) | 42/83 | 0.459(0.274–0.770) | 0.003 | 0.280(0.158–0.499) | <0.001 | |
Notes: Tumor size, PVTT, distant metastasis and treatments in this study were independent prognostic factors of OS.
Abbreviations: OS, overall survival; AFP, alpha-fetoprotein; HBV, Hepatitis B Virus; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer; TACE, transcatheter arterial chemoembolization.
Table 4.
Univariate And Multivariate Analysis Of Prognostics Factors Affecting PFS For Two Treatment Cohorts
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Numbers | HR | P value | HR | P value | ||
| Sex (Male vs Female) | 114/11 | 0.864(0.449–1.662) | 0.661 | – | – | |
| Age (≥56 vs <56) | 64/61 | 0.780(0.529–1.148) | 0.208 | – | – | |
| AFP level (≥400 ng/mL vs <400 ng/mL) | 65/60 | 0.986(0.669–1.455) | 0.945 | – | – | |
| HBV (Present vs Absent) | 112/13 | 1.013(0.542–1.895) | 0.968 | – | – | |
| Child Pugh Class (B vs A) | 14/111 | 1.112(0.607–2.038) | 0.731 | – | – | |
| Tumor size (≥5 cm vs <5 cm) | 75/50 | 1.408(0.941–2.106) | 0.096 | – | – | |
| Number of lesions (Multiple vs Single) | 108/17 | 2.038(1.083–3.834) | 0.027 | – | – | |
| PVTT (Present vs Absent) | 40/85 | 0.971(0.637–1.482) | 0.892 | – | – | |
| Distant metastasis (Present vs Absent) | 71/54 | 0.945(0.639–1.398) | 0.776 | – | – | |
| Number of Previous TACE (>2 VS ≤2) | 29/96 | 0.714(0.444–1.146) | 0.163 | |||
| Treatments (TACE-apatinib vs TACE) | 42/83 | 0.348(0.223–0.544) | <0.001 | 0.348(0.223–0.544) | <0.001 | |
Notes: The treatment was the only independent prognostic factor of PFS in this study.
Abbreviations: PFS, progression-free survival; AFP, alpha-fetoprotein; HBV, Hepatitis B Virus; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer; TACE, transcatheter arterial chemoembolization.
Comparison Of Tumor Response Between Two Treatment Groups
According to mRECIST, in the TACE-apatinib group, 2 patients (4.8%) showed CR, 5 patients (11.9%) showed PR, 27 patients (64.3%) showed SD and 8 patients (19.0%) showed PD. In the TACE-alone group, 3 patients (3.6%) showed CR, 4 patients (4.8%) showed PR, 37 patients (44.6%) showed SD and 39 patients (47.0%) showed PD. The DCR was significantly different between the two groups (81.0% vs 53.0%, P=0.002).
Follow-Up Treatments
Follow-up treatments of two treatment groups (after PSM) after disease progression in cohorts are listed and compared in Table 5. No significant differences were found in the number of following treatments.
Table 5.
The Comparison Of Post-Treatments Between Two Groups After PSM
| Post Treatments | TACE-Apatinib (n=29) | TACE-Alone(n=29) | P-value |
|---|---|---|---|
| New targeted drugs | 1(3.4%) | 0(0%) | 1.000* |
| Surgical resection | 1(3.4%) | 1(3.4%) | 1.000 |
| Ablative therapies | 10(34.5%) | 9(31.0%) | 0.780 |
| Intra-arterial infusion | 5(17.2%) | 2(6.9%) | 0.420 |
| Radiotherapy | 1(3.4%) | 0(0%) | 1.000* |
| Immunotherapy | 2(6.9%) | 3(10.3%) | 1.000 |
| Radioactive seed implanting | 1(3.4%) | 0(0%) | 1.000* |
Notes: *Fisher’s exact test. No significant difference in post-treatments between the TACE-apatinib group and the TACE-alone group (after PSM) was found.
Abbreviations: TACE, transcatheter arterial chemoembolization. PSM, propensity score matching.
Discussion
The management of HCC patients with TACE refractoriness is an increasingly prominent issue. Several published reports have indicated that sorafenib could be an effective adjuvant therapy to TACE.11–15 However, the efficacy of this targeted drug for HCC is reported to be limited due to the observed low response rate8,16 and the development of resistance to this drug.17 Once the resistance to sorafenib takes place in patients being refractory to TACE, few therapeutic choices are available for treatment continuation and this has been the stimulus for researchers to seek for surrogates.
Apatinib, a new anti-angiogenesis molecular drug, selectively binds with tumor-related VEGFR2 with a significantly higher affinity than that of sorafenib.7,18 Its efficacy in HCC has been proved by a phase II study,19 and a phase III study (NCT02329860) is also being performed. With the previous finding that VEGF expression increases in residual tumor tissues after TACE,20,21 apatinib is assumed as a new targeted drug suitable for combining with TACE. Therefore, Lu W et al conducted the first randomized control test evaluating the effects of TACE combined with apatinib in unresectable HCC and concluded that apatinib could prolong the PFS of HCC patients in BCLC stage B & C (median PFS:12.5 months vs 6 months).24 Chen S et al also reported that patients in BCLC stage C would have a longer OS when treated with TACE-apatinib compared to TACE-alone (median OS:13.0 months vs 9.9 months).25 Accordingly, apatinib is considered as a promising candidate for treating HCC with TACE.
To explore the exact effectiveness and further application of this promising new drug, we conducted this study, being the first to evaluate the efficacy of TACE-apatinib in HCC patients being refractory to TACE. Our results revealed that patients who received TACE-apatinib would have survival prolongations both in OS and PFS when compared with those who only received TACE (median OS: 17.0 months vs 8.5 months, median PFS: 7.0 months vs 2.5 months). This result was confirmed by the PSM (modified median OS: 17.0 months vs 10.7 months, modified median PFS: 7.0 months vs 2.0 months) and the Cox proportional hazard regression model, which revealed that TACE-apatinib was a positive prognostic factor of both OS and PFS. Referring to safety assessment, only three patients in the TACE-apatinib group developed grade 3/4 apatinib-related AEs and quitted the administration. The rest finally could tolerate them through dosage reduction and symptomatic treatments. Although apatinib-related AEs (HFS, hypertension, diarrhea, oral or anal ulcer) had significantly higher incidences in TACE-apatinib group than TACE-alone group, they were controllable and no death due to AEs appeared. A recent study comparing the efficacy and safety of sorafenib versus apatinib revealed that the targeted drug-related-AEs were significantly different in the sorafenib and apatinib group. The common AEs in apatinib group included proteinuria, hypertension, elevated transaminase and HFS. In sorafenib group, patients were more likely to suffer from HFS, diarrhea, anorexia and alopecia. Overall, 5 grade 3–4 AEs (elevated transaminase[n=2], proteinuria[n=2] and HFS[n=1]) were observed in the apatinib group while 6 in the sorafenib group (HFS[n=2], hypertension[n=1], diarrhea[n=1], elevated transaminase[n=1] and anorexia[n=1]); however, all the events could be well managed.26
The result of our study shed light on the treatment of TACE refractory HCC and provided another choice for patients in this state of disease. The length of the median OS in our study was consistent with previous studies on the efficacy of sorafenib towards TACE refractory HCC. The discrepancy on the length of median PFS may be attributed to the differences in sample size, population, BCLC stage, TACE procedure, and the criteria of TACE refractory. For instance, a study by Wu et al comparing the efficacy of TACE-sorafenib and TACE defined TACE refractoriness as tumor progression or a tumor shrinkage rate<25% of the corresponding hypervascular lesions.14 Under this criterion, patients were included in the cohort earlier and may have a longer PFS or TTP compared with patients in our study. Up to now, there have been at least three criteria of TACE refractoriness with different emphases, leading to the heterogeneity of findings in similar studies.2,5,27 Besides, the definition of continuous elevation of tumor markers can be different between studies even they all adopt the JSH-LCSGJ criterion. Therefore, a distinct and universal accepted consensus on the definition of TACE refractoriness is needed to guide future studies.
Although our study showed statistically significances in OS and PFS between two treatment groups, several limitations should be taken into consideration. First, the present study was retrospectively performed and comprised of data from only one hospital, which may contain certain selective bias despite performing PSM. Second, our results may be affected by the recall bias from patients. To confirm the efficacy of TACE-apatinib treatment in TACE refractory HCC patients compared with TACE-alone, it is necessary to conduct a prospective randomized control test in a large sample size and multiple centers. Presently, the most recommended treatment for HCC refractory to TACE is sorafenib or levantinib. The next stage of our research will be to compare the effectiveness between apatinib and other targeted drugs towards TACE refractory HCC. Furthermore, combining TACE with immunotherapy could be a reasonable idea, as TACE will cause necrosis in tumors and increase the exposure of tumor-related antigens, which might improve the efficacy of PD1/PD-L1 antibodies.
In conclusion, our study revealed that the curative effect of TACE-apatinib was superior to TACE-alone on OS and PFS for TACE refractory HCC patients. Grade 4 AEs rarely occurred and we confirmed the clinical safety of using apatinib in these patients. Therefore, apatinib can be recommended for HCC patients when TACE refractoriness occurs after validating the findings of this study in larger and prospective study cohorts.
Acknowledgments
Here, we thank Dr. Suyi Zhang for statistical advice, Dr. Qi Zhong and Ms Yuwen Du for writing advice. We also thank Dr. Xiaojun He and Dr. Rusi Zhang for their irreplaceable support for Zhiyu Qiu on the road to an oncology doctor. Zhiyu Qiu, Lujun Shen, and Shuanggang Chen are co-first authors for this study.
Statement Of Ethics
In this study, the protocol was approved by the research institute’s committee and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi: 10.1159/000488035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi: 10.1159/000327577 [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142 [DOI] [PubMed] [Google Scholar]
- 6.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–921. doi: 10.1111/j.1572-0241.2007.01712.x [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 9.Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136(6):1458–1467. doi: 10.1002/ijc.29126 [DOI] [PubMed] [Google Scholar]
- 10.Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269(2):603–611. doi: 10.1148/radiol.13130150 [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330–341. doi: 10.1159/000365993 [DOI] [PubMed] [Google Scholar]
- 12.Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with Transcatheter Arterial Chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262. doi: 10.1159/000367743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohki T, Kondo M, Karasawa Y, et al. Evaluation of the efficacy of sorafenib on overall survival in patients with hepatocellular carcinoma using FT rate: a devised index. Adv Ther. 2017;34(5):1097–1108. doi: 10.1007/s12325-017-0524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Li A, Yang J, Lu Y, Li J. Efficacy and safety of TACE in combination with sorafenib for the treatment of TACE-refractory advanced hepatocellular carcinoma in Chinese patients: a retrospective study. Onco Targets Ther. 2017;10:2761–2768. doi: 10.2147/OTT.S131022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck-Radosavljevic M, Kudo M, Raoul JL, Lee HC, Decaens T, Heo J. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): global OPTIMIS final analysis. J Clin Oncol. 2018;36(15):abstract4018. doi: 10.1200/JCO.2018.36.15_suppl.4018 [DOI] [Google Scholar]
- 16.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 17.Mendez-Blanco C, Fondevila F, Garcia-Palomo A, et al. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):134–142. doi: 10.1038/s12276-018-0159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin S, Ouyang X, Bai Y, et al. Apatinib in Chinese patients with advanced hepatocellular carcinoma: a phase II randomized, open-label trial. J Clin Oncol. 2014;32(suppl 5):abstract4019. doi: 10.1200/JCO.2013.54.6911 [DOI] [Google Scholar]
- 20.Hsieh MY, Lin ZY, Chuang WL. Serial serum VEGF-A, angiopoietin-2, and endostatin measurements in cirrhotic patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Kaohsiung J Med Sci. 2011;27(8):314–322. doi: 10.1016/j.kjms.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi: 10.1080/02841850801958890 [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 23.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18(6):433–438. doi: 10.1080/15384047.2017.1323589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Yu W, Zhang K, Liu W. Comparison of the efficacy and safety of transarterial chemoembolization with and without apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer. 2018;18(1):1131–1138. doi: 10.1186/s12885-018-5081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Gou Q, Xu R, Chen X, Zhou Z. Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study. Onco Targets Ther. 2018;11:3407–3413. doi: 10.2147/OTT.S161023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474. doi: 10.1007/s12072-010-9165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]