This systematic review and meta-analysis evaluates whether mind-body therapies are associated with pain reduction and opioid-related outcome improvement in adults using opioids for pain.
Key Points
Question
Are mind-body therapies (ie, meditation, hypnosis, relaxation, guided imagery, therapeutic suggestion, and cognitive behavioral therapy) associated with pain reduction and opioid-related outcome improvement among adults using opioids for pain?
Findings
In this systematic review and meta-analysis of 60 randomized clinical trials with 6404 participants, mind-body therapies were associated with improved pain (Cohen d = −0.51; 95% CI, −0.76 to −0.27) and reduced opioid dose (Cohen d = −0.26; 95% CI, −0.44 to −0.08).
Meaning
Practitioners should be aware that mind-body therapies may be associated with moderate improvements in pain and small reductions in opioid dose.
Abstract
Importance
Mind-body therapies (MBTs) are emerging as potential tools for addressing the opioid crisis. Knowing whether mind-body therapies may benefit patients treated with opioids for acute, procedural, and chronic pain conditions may be useful for prescribers, payers, policy makers, and patients.
Objective
To evaluate the association of MBTs with pain and opioid dose reduction in a diverse adult population with clinical pain.
Data Sources
For this systematic review and meta-analysis, the MEDLINE, Embase, Emcare, CINAHL, PsycINFO, and Cochrane Library databases were searched for English-language randomized clinical trials and systematic reviews from date of inception to March 2018. Search logic included (pain OR analgesia OR opioids) AND mind-body therapies. The gray literature, ClinicalTrials.gov, and relevant bibliographies were also searched.
Study Selection
Randomized clinical trials that evaluated the use of MBTs for symptom management in adults also prescribed opioids for clinical pain.
Data Extraction and Synthesis
Independent reviewers screened citations, extracted data, and assessed risk of bias. Meta-analyses were conducted using standardized mean differences in pain and opioid dose to obtain aggregate estimates of effect size with 95% CIs.
Main Outcomes and Measures
The primary outcome was pain intensity. The secondary outcomes were opioid dose, opioid misuse, opioid craving, disability, or function.
Results
Of 4212 citations reviewed, 60 reports with 6404 participants were included in the meta-analysis. Overall, MBTs were associated with pain reduction (Cohen d = −0.51; 95% CI, −0.76 to −0.26) and reduced opioid dose (Cohen d = −0.26; 95% CI, −0.44 to −0.08). Studies tested meditation (n = 5), hypnosis (n = 25), relaxation (n = 14), guided imagery (n = 7), therapeutic suggestion (n = 6), and cognitive behavioral therapy (n = 7) interventions. Moderate to large effect size improvements in pain outcomes were found for meditation (Cohen d = −0.70), hypnosis (Cohen d = −0.54), suggestion (Cohen d = −0.68), and cognitive behavioral therapy (Cohen d = −0.43) but not for other MBTs. Although most meditation (n = 4 [80%]), cognitive-behavioral therapy (n = 4 [57%]), and hypnosis (n = 12 [63%]) studies found improved opioid-related outcomes, fewer studies of suggestion, guided imagery, and relaxation reported such improvements. Most MBT studies used active or placebo controls and were judged to be at low risk of bias.
Conclusions and Relevance
The findings suggest that MBTs are associated with moderate improvements in pain and small reductions in opioid dose and may be associated with therapeutic benefits for opioid-related problems, such as opioid craving and misuse. Future studies should carefully quantify opioid dosing variables to determine the association of mind-body therapies with opioid-related outcomes.
Introduction
The opioid crisis is being addressed with heightened urgency at both clinical and policy levels. For much of the 20th century, opioids were prescribed primarily for postoperative and cancer-related pain.1 In the 1990s, prescription of opioids to treat all forms of pain became standard care.1 Consequently, opioid prescriptions increased to 208 million by 2011.1 Currently, more than 35% of the US adult population is prescribed opioids in a given year.2 This marked increase in opioid prescriptions was paralleled by an increasing incidence of opioid use disorder (OUD), which now affects approximately 2 million individuals in the United States,3 and opioid misuse, which affects 12 million individuals in the United States overall.2 Since 2006, US deaths due to opioid overdose have tripled, increasing to 42 200 in 2016,4 and are projected to reach 82 000 by 2025, resulting in 700 000 additional deaths in the United States.5
The opioid crisis arose in part because of well-intentioned efforts to alleviate untreated pain. Although opioids are considered to be useful in managing a wide continuum of pain, including acute, procedural, and chronic pain, evidence of their long-term efficacy and safety is limited.6 To help combat the opioid crisis, guidelines encourage practitioners to consider nonopioid pain management options, including mind-body therapies (MBTs).7 Mind-body therapies target “interactions among the brain, mind, body, and behavior, with the intent to use the mind to affect physical functioning and promote health.”8 Mind-body therapies might ameliorate pain and prevent downstream transitions from long-term opioid use to OUD. Thus, the National Institutes of Health initiative Helping to End Addiction in the Long Term (HEAL) has called for studies of MBTs as interventions for pain and OUD.
The efficacy of MBTs should be examined across the pain continuum. Reviews9,10,11,12,13,14 demonstrate that MBTs may be associated with significantly alleviated clinical pain. Few of the studies reviewed measured opioid use, and reviews included patients who were not prescribed opioids. However, no review, to date, has examined the efficacy of MBTs specifically for the subset of patients prescribed opioid analgesics. Given the importance of this population, we provide, to our knowledge, the first systematic review of MBTs for opioid-treated pain. Because of the urgency of the opioid crisis, we reviewed all studies of MBTs for patients with opioid-treated pain regardless of the study quality or clinical population to provide comprehensive evidence to prescribers, patients, payers, and policy makers.7
Methods
Literature Search
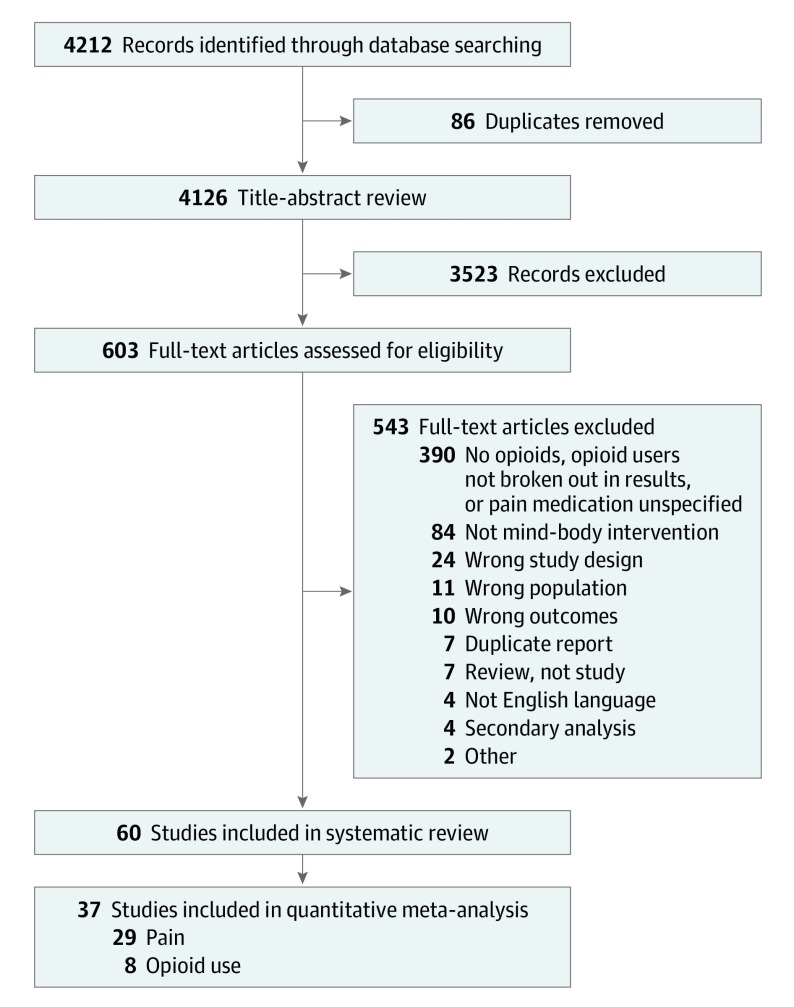
For this systematic review and meta-analysis, the following bibliographic databases were searched for English-language randomized clinical trials and systematic reviews from the date of inception to March 2018: MEDLINE, Embase, CINAHL, Emcare, PsychINFO, and Cochrane Library. Search logic included (pain OR analgesia OR opioids) AND mind body therapies (eMethods in the Supplement). We searched gray literature and ClinicalTrials.gov and performed hand searches of relevant bibliographies. The methods and reporting of this systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Figure 1).15
Figure 1. Preferred Reporting Items for Systematic Review and Meta-analysis Flow Diagram of Literature Search and Study Inclusion.
Inclusion and Exclusion Criteria
Randomized clinical trials of MBTs were included if they involved adults (aged ≥18 years) prescribed opioids for chronic, acute, procedural, or cancer pain. Because we were focused on both pain and opioid use outcomes, studies that did not include pain-related outcomes were excluded (eg, studies of individuals with OUD who did not report pain). Studies were excluded if they collected data on pain medicine or analgesics without specifying that these medications were opioids.
To constrain the considerable heterogeneity of MBTs, we limited our review to studies of psychologically oriented MBTs that prioritize using mental techniques to ameliorate pain, including meditation, hypnosis, guided imagery, relaxation, therapeutic suggestion, and cognitive behavioral therapy (CBT). Meditation involves practices, such as mindfulness, to cultivate present-moment focused attention and meta-awareness, as well as acceptance of thoughts, emotions, and body sensations.16 Hypnosis involves induction of an altered state of consciousness in which focused attention and reduced peripheral awareness enhance the capacity for responding to suggestions for changing thoughts, emotions, and sensations.17 Guided imagery involves active imagination of visual, auditory, and somatic sensations and perceptions.18 Relaxation involves the use of the mind to systematically release muscle tension throughout the body.19 Therapeutic suggestion involves provision of suggestions to change thoughts, emotions, and sensations without directly inducing an hypnotic altered state.20 Cognitive behavioral therapy involves the use of logic to challenge and change negative thinking patterns, thereby decreasing negative emotions and promoting adaptive behaviors.21
Although acupuncture and spinal manipulation are sometimes labeled MBTs, given that these approaches rely on physical (eg, needling and musculoskeletal adjustment) rather than psychological techniques, we did not include studies of these therapies in our review. Similarly, studies of yoga or Tai Chi without formal meditation practice were excluded. We included studies of physical mind-body modalities or other complementary therapies only if 50% or more of the intervention involved delivery of psychologically oriented MBT techniques. We elected to focus our review on MBTs that primarily use mental techniques because they may be more accessible to people whose mobility is compromised by pain or used for pain relief during inpatient procedures when patients are immobilized.
Types of Outcome Measures
The primary outcome was pain severity or intensity. Secondary outcomes were opioid use measured by prescription record, self-report, or urine toxicologic screening; opioid misuse and craving; and disability or functional impairment.
Data Extraction and Analysis
Abstracts and full texts were screened and data extracted independently by 2 reviewers (E.L.G., C.E.B., A.W.H., E.J.R., R.M.A., S.A.G., K.R.F., J.Y., and/or M.F.) via Covidence (https://www.covidence.org/home). Risk of bias was assessed in Covidence using the Cochrane risk of bias tool by 2 independent reviewers (E.L.G., C.E.B., A.W.H., E.J.R., R.M.A., S.A.G., K.R.F., J.Y., and/or M.F.). Disagreements were resolved by a third reviewer (E.L.G., C.E.B., A.W.H., E.J.R., R.M.A., S.A.G., K.R.F., J.Y., or M.F.) or by discussion. To prevent conflict of interest, studies written by review authors were assessed by other members of the author team.
Mixed-effects meta-analyses were performed using the R Metafor package22 for pain and opioid dose outcomes. After sending email requests for missing data to authors of studies included in the review who did not provide sufficient data in the original publication, 29 studies19,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 were included in the pain meta-analysis and 8 studies29,30,35,37,38,39,42,43 in the opioid dose meta-analysis. In studies with more than 1 MBT arm, data from both MBTs were included. Studies that reported P values but did not report numerical means and SDs for baseline or postintervention pain or opioid use could not be included in the meta-analysis. Pain values were standardized using a 0- to 10-point numeric rating scale, and opioid dose was standardized into morphine equivalents using standard equianalgesic conversion tables.7 Change scores were created by subtracting the baseline value from the most proximal postintervention end point; this end point was selected because it was consistently collected despite great variability in time points across studies. The SDs of the change scores were imputed via Cochrane best practices.51 Effect size estimates were calculated as standardized mean differences.22 Study heterogeneity was investigated using Baujat plots in conjunction with the Q and I2 statistics.52,53 Publication bias was examined with funnel plots and the Egger test.53,54 Although we performed quantitative meta-analyses on all studies for which we could extract data, the entire body of studies was systematically reviewed in a qualitative manner (summary study data19,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86 are presented in Table 1 and detailed study data in the eMethods in the Supplement).
Table 1. Mind-Body Therapy Study Descriptions.
| Source | Clinical Target | No. of Patients | Mind-Body Therapy | Comparator(s) | Session Extensiveness, Format | Pain-Related and Opioid Outcomes (Length of Follow-up) |
|---|---|---|---|---|---|---|
| Meditation Studies | ||||||
| Dindo et al,55 2018 | Orthopedic surgery | 76 | Acceptance and Commitment Therapy | Usual care | Single, in person | |
| Esmer et al,27 2010 | Failed back surgery | 44 | Mindfulness-Based Stress Reduction | Waiting list | Multiple, in person | |
| Garland et al,25,56 2014 and Garland et al,26 2017 | Chronic pain and taking opioids most days for 90 d | 115 | Mindfulness-Oriented Recovery Enhancement | Support group | Multiple, in person | |
| Garland et al,26 2017 | Hospital inpatients reporting intolerable pain or inadequate pain control | 244 | (1) Mindfulness training, (2) hypnotic suggestion | Pain education | Single, in person | |
| Zgierska et al,57 2016 | Back pain | 35 | Meditation-based CBT | Usual care | Multiple, in person | |
| Hypnosis Studies | ||||||
| Ashton et al,58 1997 | Cardiac surgery | 32 | Self-hypnosis | Usual care | Single, in person | |
| Akgul et al,46 2016 | Cardiac surgery | 44 | Hypnosis | Usual care | Single, in person | |
| Askay et al,31 2007 | Burn pain | 46 | Hypnosis | Attention control | Single, in person | |
| Enqvist et al,59 1997 | Dental surgery | 69 | Hypnosis | Usual care | Single, recording | |
| Everett et al,32 1993 | Burn pain | 32 | (1) Hypnosis plus placebo, (2) hypnosis plus lorazepam | (1) Psychological intervention plus placebo, (2) psychological intervention plus lorazepam | Single, in person | |
| Faymonville et al,47 1997 | Elective plastic surgery | 60 | Hypnosis | Stress-reducing strategies | Single, in person | |
| Frenay et al,28 2001 | Burn pain | 30 | Hypnosis | Stress reduction | Multiple, in person |
|
| Garland et al,26 2017 | Hospital inpatients reporting intolerable pain or inadequate pain control | 244 | (1) Hypnotic suggestion, (2) nindfulness training | Pain education | Single, in person | |
| Ghoneim et al,60 2000 | Dental surgery | 60 | Hypnosis | Usual care | Single, recording | |
| Joudi et al,61 2016 | Postoperative pain and analgesic use | 120 | Hypnosis | Usual care | Single, recording | |
| Lang et al,62 1996 | Interventional radiology procedures | 30 | Hypnosis | Usual care | Single, in person | |
| Lang et al,63 2000 | Arterial, venous, and renal surgery | 241 | Hypnosis | (1) Structured attention, (2) usual care | Single, in person | |
| Lang et al,64 2008 | Percutaneous tumor treatment | 201 | Hypnosis | (1) Empathic attention, (2) usual care | Single, in person | |
| Mackey et al,65 2010 | Outpatient third molar extraction | 91 | Hypnosis plus music plus IV sedation | Music plus IV sedation | Single, recording | |
| Mackey et al66 2018 | Outpatient third molar extraction | 119 | Hypnosis plus music plus IV sedation | Music plus IV sedation | Single, recording | |
| Marc et al,48 2008 | Surgical abortion | 350 | Hypnosis | Usual care | Single, in person | |
| Montgomery et al,67 2007 | Breast surgery | 200 | Hypnosis | Attention control | Single, in person | |
| Patterson et al,33 1992 | Burn, wound debridement | 30 | Hypnosis | (1) Attention control, (2) usual care | Single, in person | |
| Patterson et al,34 2010 | Hospitalized for traumatic injury | 21 | VR hypnosis | (1) Usual care, (2) VR distraction | Single, in person | |
| Surman et al,68 1974 | Cardiovascular surgery | 40 | Hypnosis | Usual care | Single, in person | |
| Syrjala et al,29 1992 | Cancer pain (undergoing bone marrow transplant) | 45 | (1) Hypnosis, (2) CBT coping skills | (1) Therapist contact, (2) usual care | Multiple, in person | |
| Wang et al,69 2015 | Lung cancer surgery | 60 | Hypnosis plus relaxation plus music | Usual care | Multiple, recording | |
| Wright et al,30 2000 | Burn pain | 30 | Hypnosis | Usual care | Multiple, in person | |
| Relaxation Studies | ||||||
| Anderson et al,70 2006 | Cancer pain | 57 | (1) PMR, (2) positive imagery | (1) Distraction, (2) waitlist | Multiple, recording |
|
| Gavin et al,37 2006 | Spinal surgery | 49 | Relaxation | Usual care | Single, in person | |
| Good,38 1995 | Abdominal surgery | 84 | (1) Relaxation, (2) relaxation plus music | (1) Music, (2) usual care | Multiple, recording | |
| Good et al,19 1999 | Abdominal surgery | 500 | (1) Relaxation, (2) relaxation plus music | (1) Music, (2) attention control | Multiple, recording | |
| Good et al,50 2010 | Abdominal surgery | 517 | Relaxation plus music | Patient teaching | Single, recording | |
| Haase et al,71 2005 | Colorectal cancer surgery | 60 | (1) Relaxation, (2) guided imagery | Usual care | Multiple, recording | |
| Konstantatos et al,35 2009 | Burn wound dressing changes | 86 | VR relaxation | Usual care | Single, recording | |
| Kwekkeboom et al,72 2008 | Cancer pain during hospitalization | 40 | (1) PMR, (2) guided imagery | Information | Multiple, recording | |
| Mandle et al,73 1990 | Femoral angiography | 45 | Relaxation | (1) Music tape, (2) blank tape | Single, recording | |
| Manyande et al,74 1998 | Major abdominal or abdominal-perineal surgery | 118 | Relaxation | Informational tape | Single, recording | |
| Rejeh et al,36 2013 | Elective abdominal surgery | 124 | Systematic relaxation | Usual care | Single, recording | |
| Roykulcharoen and Good,24 2004 | Abdominal surgery | 102 | Systematic relaxation | Lying still in bed | Single, in person | |
| Sloman et al,75 1994 | Cancer pain | 67 | (1) Relaxation in person, (2) relaxation by tape | Usual care | Multiple, in person plus recording | |
| Syrjala et al,39 1995 | Cancer pain | 94 | (1) Relaxation plus imagery, (2) relaxation plus imagery plus CBT | (1) Therapist contact, (2) usual care | Multiple, in person | |
| Wang et al,40 2008 | Postembolization pain | 262 | Relaxation plus psychotherapy | Usual care | NR, in person |
|
| Wilson et al,76 1981 | Surgery, cholecystectomy, and hysterectomy | 70 | (1) Relaxation, (2) relaxation plus information | (1) Information, (2) usual care | Single, recording | |
| Guided Imagery Studies | ||||||
| Anderson et al,70 2006 | Cancer pain | 57 | (1) Positive imagery, (2) PMR | (1) Distraction, (2) waitlist | Single, in person |
|
| Antall et al,77 2004 | Joint replacement surgery | 13 | Guided imagery | Usual care | Single, recording | |
| Forward et al,78 2015 | Joint replacement surgery | 225 | Guided imagery | (1) Massage, (2) usual care | Multiple, in person | |
| Gonzales et al,42 2010 | Head and neck surgical procedures | 44 | Guided imagery | Usual care | Multiple, recording | |
| Haase et al,71 2005 | Colorectal cancer surgery | 60 | (1) Guided imagery, (2) relaxation | Usual care | Multiple, recording | |
| Kwekkeboom et al,79 1998 | Surgery for breast or gynecologic malignancy | 75 | Guided imagery | Usual care | Single, recording | |
| Kwekkeboom et al,72 2008 | Cancer pain during hospitalization | 40 | (1) PMR, (2) guided imagery | Information | Multiple, recording | |
| Pijl et al,41 2016 | Laproscopic cholecystectomy for gall stones | 140 | Guided imagery | Usual care | Multiple, recording | |
| Tusek et al,80 1997 | Colorectal surgery | 130 | Guided imagery plus music | Usual care | Multiple, recording | |
| Suggestion Studies | ||||||
| Block et al,23 1991 | Heterogeneous sample of anesthetized surgical patients | 209 | Therapeutic suggestion | Blank tape | Single, recording | |
| van der Laan et al,81 1996 | Gynecologic surgery | 60 | Therapeutic suggestion | Story control | Single, recording | |
| Melzack et al,49 1996 | Surgery, cholecystectomy and hysterectomy | 20 | Positive suggestion plus music | Scientific information plus music | Single, recording | |
| McLintock et al,82 1990 | Hysterectomy surgery | 63 | Positive suggestion | Blank tapes | Single, recording | |
| Nilsson et al,432001 | Abdominal surgery | 90 | Therapeutic suggestion plus music | (1) Music, (2) operating sounds | Single, recording | |
| Nilsson et al,83 2003 | Varicose vein or open inguinal hernia repair surgery | 182 | Therapeutic suggestion plus music | (1) Music alone, (2) blank tape | Single, recording | |
| CBT Studies | ||||||
| Jamison et al,44 2010 | Chronic back and/or neck pain and history of or high risk for prescription opioid misuse | 62 | Cognitive behavioral substance misuse counseling | Usual care | Multiple, in person | |
| Kroenke et al,84 2009 | Comorbid chronic musculoskeletal pain and depression (opioid users analyzed separately) | 134 | Pain self-management | Usual care | Multiple, in person |
|
| Naylor et al,85 2010 | Chronic musculoskeletal pain (opioid users analyzed separately) | 32 | Group CBT followed by therapeutic interactive voice response | Group CBT followed by usual care | Multiple, in person plus recording | |
| Rolving et al,86 2016 | Undergoing lumbar spinal fusion for degenerative spinal disorders | 90 | Preoperative CBT | Usual care | Multiple, in person | |
| Syrjala et al,29 1992 | Cancer pain (undergoing bone marrow transplant) | 45 | (1) CBT coping skills, (2) hypnosis | (1) Therapist contact, (2) usual care | Multiple, in person | |
| Syrjala et al,39 1995 | Cancer pain | 94 | (1) Relaxation plus imagery, (2) relaxation plus imagery plus CBT coping skills | (1) Therapist contact, (2) usual care | Multiple, in person | |
| Wilson et al,45 2016 | Chronic noncancer pain plus prescribed opioids | 92 | Internet-based pain self-management | Usual care | Multiple, recording (online self led) | |
Abbreviations: CBT, cognitive-behavioral therapy; EMA, ecologic momentary assessment; IV, intravenous; NR, not reported; PMR, progressive muscle relaxation; VR, virtual reality.
Statistically significant between-groups difference favoring the mind-body therapy over the control condition for that particular measurement point.
Nonsignificant between-groups difference.
Results
Overview of Studies
We screened 4212 citations and 603 full-text articles. Sixty studies with a total of 6404 participants were ultimately included in the review (Figure 1). The 60 studies focused on various clinical pain targets: procedural pain (n = 39), burn pain (n = 7), cancer pain (n = 5), chronic pain (n = 8), or heterogeneous acute pain conditions (n = 1). Sample sizes ranged from 13 to 500. Studies tested meditation (n = 5), hypnosis (n = 25), relaxation (n = 14), guided imagery (n = 7), therapeutic suggestion (n = 6), and CBT (n = 7) interventions. Studies used a range of control conditions, including another MBT (n = 4), psychotherapy comparators (n = 11), attention control (n = 10), information control (n = 7), music controls (n = 6), waiting list control (n = 2), usual care (n = 20), or other control conditions (n = 3) (eTables 1-6 in the Supplement).
Mindfulness or Meditation Studies
Association of Meditation With Pain Outcomes
All 5 mindfulness or meditation studies25,26,27,55,57 (100%) reported significant improvements in pain severity, pain unpleasantness, interference, thermal pain sensitivity, and/or cessation of postsurgical pain. Meta-analytic results indicated that meditation had a significant strong association with pain reduction (Cohen d = –0.70; 95% CI, −1.09 to −0.31; P < .001) (eFigure 1 in the Supplement), with homogeneity of effect sizes (Q [χ2 = 4.59, P = .10]; I2 = 56.20%).
Association of Meditation With Opioid-Related Outcomes
Four of the 5 studies (80%) reported significant improvements in opioid misuse,25 opioid craving,25,26 time to opioid cessation,55 and/or opioid use27; 1 of these studies reported reduced opioid analgesic use,27 but the analgesic outcome was an imprecise categorical variable. One study57 failed to find effects on opioid dose, and 2 other studies25,26 were unable to consistently and reliably collect opioid dosing data.
Intervention Characteristics and Clinical Pain Targets
Three of the 5 studies (60%) examined multiple-session mindfulness-based interventions: Mindfulness-Oriented Recovery Enhancement,25 meditation-based CBT,57 and Mindfulness-Based Stress Reduction.27 Two studies examined single-session interventions: mindful breathing26 and Acceptance and Commitment Therapy with meditation.55 Four of the 5 studies25,27,55,57 (80%) focused on chronic pain conditions.
Hypnosis Studies
Association of Hypnosis With Pain Outcomes
Fifteen of the 23 hypnosis studies26,29,30,31,33,34,47,61,62,63,64,65,66,67,69 (65%) reported statistically significant improvements in pain intensity, pain unpleasantness, and/or pain affect. Meta-analytic results indicated that hypnosis had a significant moderate association with pain reduction (Cohen d = −0.54; 95% CI, −0.87 to −0.20; P < .001) (eFigure 2 in the Supplement), with some heterogeneity of effect sizes (Q [χ2 = 38.16, P < .001]; I2 = 73.90%).
Association of Hypnosis With Opioid-Related Outcomes
Twelve hypnosis studies26,30,46,59,61,62,63,64,65,66,69 (63%) reported statistically significant improvements in opioid dose, desire for opioids, and/or time to first postoperative opioid dose.
Intervention Characteristics and Clinical Pain Targets
Four studies28,29,30,69 (17%) examined multiple-session hypnotic interventions, with the remainder26,31,32,33,34,46,47,48,58,59,60,61,62,63,64,65,66,67,68 examining single-session hypnotic inductions. Seventeen studies29,34,46,47,48,58,59,60,61,62,63,64,65,66,67,68,69 focused on presurgical, postsurgical, or procedural pain; 5 studies focused28,30,31,32,33 on burn pain; and 1 study26 focused on acute pain.
Relaxation Studies
Association of Relaxation With Pain Outcomes
Twelve of the 16 relaxation studies19,24,36,37,39,40,50,72,73,74,75,76 (75%) reported statistically significant improvements in pain intensity or severity, pain sensation, pain distress, and/or nurse-assessed pain. In 1 study,35 pain intensity was reported as significantly worse in a virtual reality relaxation group compared with a morphine-only comparison group during burn dressing change. Meta-analytic results indicated that relaxation did not have a significant association with pain reduction (Cohen d = −0.45; 95% CI, −1.13 to 0.22; P = .19) (eFigure 3 in the Supplement), with some heterogeneity of effect sizes (Q [χ2 = 218.62, P < .001]; I2 = 96.96%).
Association of Relaxation With Opioid-Related Outcomes
Three studies36,73,74 (19%) reported significant therapeutic effects of relaxation on procedural opioid dose, postoperative opioid dose, and number of patients receiving opioids. Two studies (14%) reported significantly worse opioid-related outcomes, including postoperative opioid dose37 and recovery dose.74
Intervention Characteristics and Clinical Pain Targets
Seven studies19,38,39,71,72,75 examined multiple-session relaxation interventions, with the remainder24,35,36,37,40,50,73,74,76 examining single-session relaxation interventions and 1 study40 not reporting that information. Relaxation interventions included progressive muscle relaxation, systematic relaxation, and jaw relaxation. Eleven studies19,24,36,37,38,40,50,71,73,74,76 focused on surgical or procedural pain, 4 studies39,70,72,75 focused on cancer pain, and 1 study35 focused on burn dressing change pain.
Guided Imagery Studies
Association of Guided Imagery With Pain Outcomes
Three of the 9 guided imagery studies72,78,80 (33%) reported statistically significant improvements in pain intensity. There were insufficient numbers of guided imagery studies with pain values to perform a meta-analysis.
Association of Guided Imagery With Opioid-Related Outcomes
Two studies41,80 (29%) reported statistically significant effects of guided imagery on opioid dose.
Intervention Characteristics and Clinical Pain Targets
Six studies41,42,71,72,78,80 examined multiple-session guided imagery interventions, with the remainder70,77,79 examining single-session interventions. Seven studies41,42,71,77,78,79,80 focused on surgical pain, and 2 studies70,72 focused on cancer pain.
Therapeutic Suggestion Studies
Association of Suggestion With Pain Outcomes
Two of the 6 therapeutic suggestion studies23,83 (33%) reported statistically significant improvements in pain intensity. No other studies reported comparative improvements in pain outcomes, including pain intensity or pain unpleasantness. Meta-analytic results indicated that suggestion had a significant moderate association with pain reduction (Cohen d = −0.68; 95% CI, −1.18 to −0.18; P = .008) (eFigure 4 in the Supplement), with some heterogeneity of effect sizes (Q [χ2 = 5.75, P = .056]; I2 = 63.66%).
Association of Suggestion With Opioid-Related Outcomes
Three studies23,43,82 (50%) reported significant therapeutic effects of suggestion on opioid dose.
Intervention Characteristics and Clinical Pain Targets
All 6 studies23,43,49,81,82,83 examined single-session, audio-recorded suggestions and focused on surgical pain.
CBT Studies
Association of CBT With Pain Outcomes
Three studies29,39,44 (43%) reported statistically significant improvements in pain intensity. One study86 (14%) reported statistically significantly improvements in postoperative mobility. No other studies reported comparative improvements in pain outcomes including pain intensity or pain disability. Meta-analytic results indicated that CBT had a significant moderate association with pain reduction (Cohen d = −0.43; 95% CI, −0.71 to −0.15; P = .002) (eFigure 5 in the Supplement), with homogeneity of effect sizes (Q [χ2 = 2.07, P = .55]; I2 = 0.0%).
Association of CBT With Opioid-Related Outcomes
Four of the 7 CBT studies44,45,85,86 (57%) reported significant therapeutic effects of CBT on opioid dose, use, or misuse.
Intervention Characteristics and Clinical Pain Targets
All 7 studies29,39,44,45,84,85,86 of CBT interventions examined multiple-session CBT interventions. Interventions used in-person therapists,29,39,44,86 pain self-management,45,84 and interactive voice response.85 Four studies44,45,84,85 focused on chronic pain, 2 studies29,39 focused on cancer pain, and 1 study86 focused on surgical pain.
Overall Meta-analysis
Characteristics of the Overall Meta-analysis
Two meta-analyses were performed on all studies for which data could be extracted to determine the association of MBTs with reduced pain and opioid use. Inspection of Baujat plots (eFigure 6 in the Supplement) revealed that 2 studies,23,24 both of which demonstrated significant clinical efficacy in favor of MBTs, were appropriate for removal as outliers: 1 in the pain meta-analysis and 1 in the opioid use meta-analysis. We chose to remove those studies to obtain stable and reliable meta-analytic effect size estimates per best practice guidelines.87
Pain-Related Outcome Results
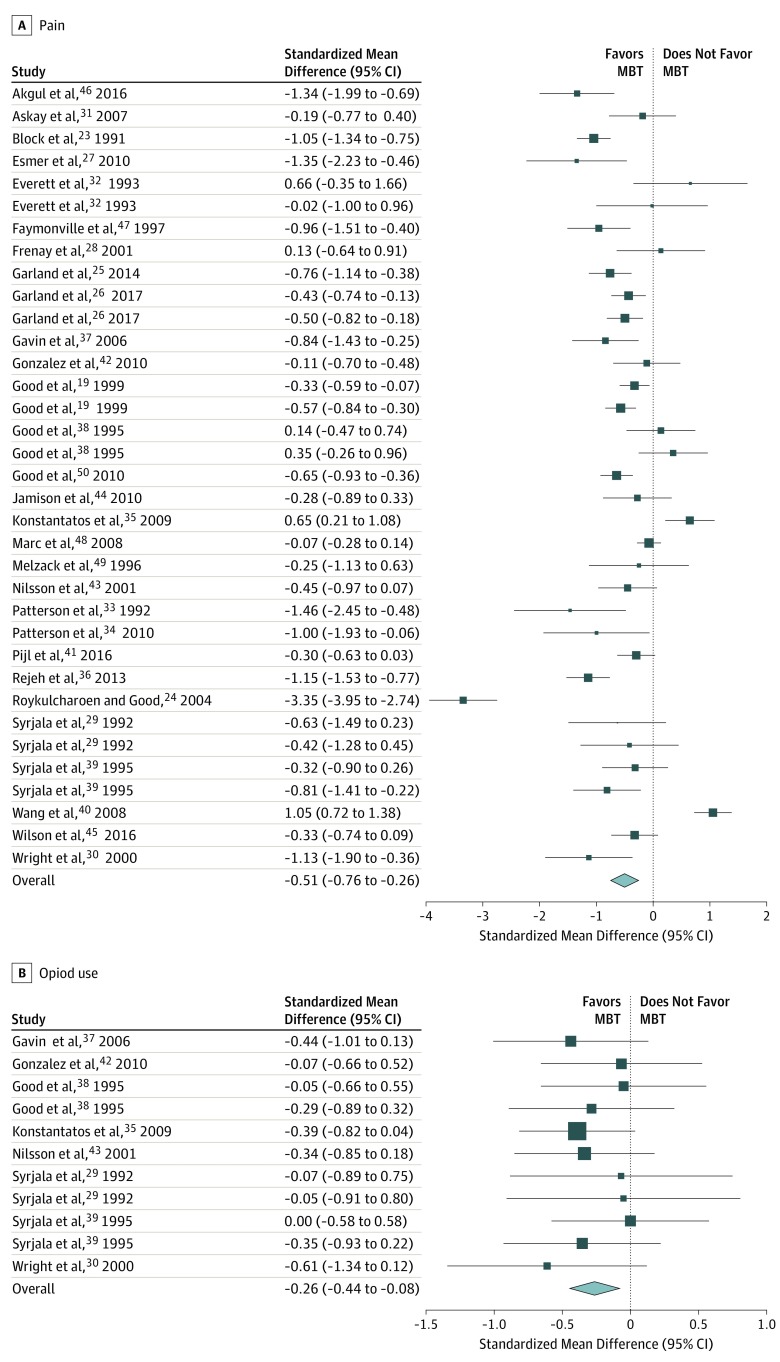
Overall, MBTs had a significant, moderate association with reduced pain (Cohen d = −0.51; 95% CI, −0.76 to −0.27; P < .001) (Figure 2A). Computation of the Q (χ2 = 287.21, P < .001) and I2 (90.53%) statistics showed some heterogeneity of effect sizes. These data were derived from 29 studies (n = 2916), with 1679 patients receiving an MBT. A funnel plot (eFigure 7 in the Supplement) and the Egger statistic (z = −0.65, P = .52) did not indicate publication bias.
Figure 2. Summary of Studies Examining the Association of Mind-Body Therapies With Pain and Opioid Use .
Squares indicate point estimates, with the size of the squares indicating weight. Horizontal lines indicate 95% CIs. The diamond indicates the pooled effect estimate with the tips of the diamond indicating the 95% CI. MBT indicates mind-body therapy.
Opioid-Related Outcome Results
Overall, MBTs had a significant, small association with opioid use (Cohen d = −0.26; 95% CI, −0.44 to −0.08; P = .01) (Figure 2B). Computation of the Q (χ2 = 6.70, P = .82) and I2 (0.0%) statistics showed homogeneity of effect sizes. These data were derived from 8 distinct studies (n = 435), with 250 patients receiving an MBT. A funnel plot (eFigure 8 in the Supplement) and the Egger statistic (z = −0.30, P = .76) did not indicate publication bias.
Discussion
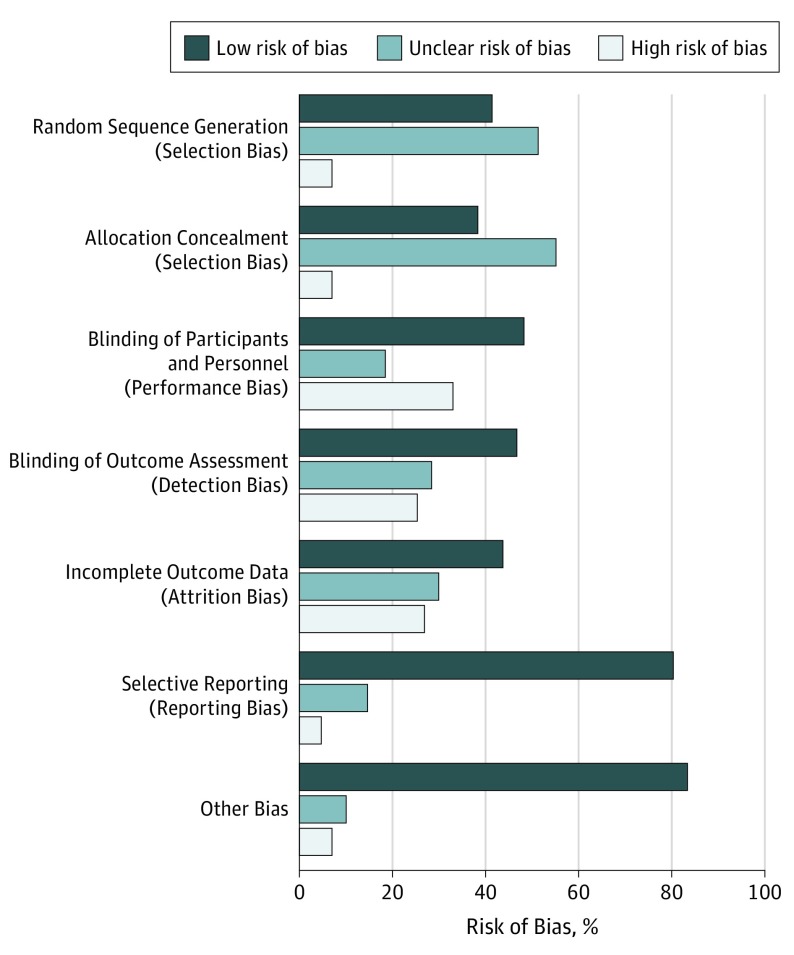
To our knowledge, this study represents the first systematic evaluation of the therapeutic benefits of MBTs for opioid-treated clinical pain in studies including more than 6000 patients. Overall meta-analytic results revealed that MBTs had a statistically significant, moderate association with reduced pain intensity and a statistically significant, small association with reduced opioid dosing compared with a range of control arms. The strength of the evidence for the therapeutic effects of MBTs on pain and opioid dose reduction was moderate, although this evidence varied by specific MBT. Taken together with descriptive results from this systematic review, MBTs overall may be associated with improved pain and opioid-related outcomes for a variety of painful health conditions. Most studies used active or placebo controls and had low risk of bias (Figure 3 and eFigures 9-14 in the Supplement), increasing confidence that the reported benefits are not solely the result of nonspecific therapeutic factors.
Figure 3. Risk of Bias.
Present review authors' judgments about each risk of bias item presented as percentages across all included studies.
From a more granular perspective, differences emerged regarding the efficacy of the specific types of MBTs studied. Most studies of meditation, hypnosis, and CBT reported significant therapeutic associations with opioid-related outcomes, including opioid dosing, craving, and opioid misuse, whereas comparatively fewer studies of suggestion, imagery, and relaxation reported significant associations with opioid-related outcomes. Of note, 2 studies37,74 reported significantly worsened opioid dosing outcomes after relaxation, suggesting the possibility of adverse effects. However, few studies reported adverse effects or harms of MBTs. Because of insufficient statistical power from the paucity of studies reporting opioid dose data, we could not conduct separate meta-analyses for each type of MBT on opioid dosing.
A different pattern emerged with regard to pain outcomes. Separate meta-analyses by specific MBT type demonstrated significant associations of meditation, hypnosis, CBT, and suggestion with pain outcomes, with the largest effect sizes observed for meditation studies. Differences in therapeutic efficacy between MBTs could be ascertained through rigorous comparative effectiveness trials. Although several of the studies26,29,39,70,71,72 in this review compared 2 MBTs, they were not sufficiently powered to detect what are likely to be small effect size differences between bona fide treatments. Furthermore, many of the MBTs reviewed involved combinations of approaches, including some with CBT. Dismantling trials could unpack multimodal MBTs and determine the differential effects of their various treatment components.
Differences also emerged with regard to foci of MBT clinical pain targets. Most of the meditation-based intervention studies focused on treating chronic noncancer pain (eg, low back pain). In contrast, most hypnosis, relaxation, imagery, and suggestion studies focused on treating acute, procedural, or cancer-related pain. It is plausible that MBTs have differential associations with acute vs chronic pain as well as opioid use depending on their mechanisms of action. In that regard, mindfulness training aims to increase acceptance, decrease catastrophizing, and facilitate a shift from affective to sensory processing of pain sensations by reappraising pain as innocuous sensory information rather than an emotionally laden threat to bodily integrity.88 These mechanisms might be especially efficacious for chronic pain conditions in which pain exacerbation occurs through the development of cognitive schemas, attentional hypervigilance, and distress intolerance. In contrast, techniques such as hypnosis and guided imagery aim to reduce pain through dissociation or imaginal superimposition of pleasurable sensations onto the painful body part.89 These mechanisms might instead be efficacious for acute pain conditions or procedural pain where nociceptive peripheral or visceral afference during noxious stimulation causes suffering. However, mindfulness and hypnosis appear to help alleviate pain via corticothalamic modulation of ascending nociceptive input.90,91,92,93 Additional studies are needed to disentangle the unique and overlapping mechanisms of MBTs.
Recommendations for future research are detailed in Table 2. Future studies should collect data needed to obtain quantitative estimates of opioid dosing, including opioid type, dose per unit, dosage form, dosage frequency, and duration of use. Because participant self-report is unreliable, if possible, data should be extracted from electronic health records and prescription drug monitoring programs. Trials that examine the effect of MBT on opioid misuse should triangulate data from self-reports, practitioner evaluation, and toxicologic screening. Psychophysiologic measures could also be used to assess the association of MBT with opioid cue reactivity, and such measures have been reported to be sensitive to the use of MBTs in patients with opioid-treated pain.56,94
Table 2. Limitations of Existing Studies of MBTs and Suggestions for Future Research in this Area.
| Limitations of Existing Studies | Suggestions for Future Research |
|---|---|
| Insufficient reporting of opioid dosing outcomes | Record the type of opioid agent prescribed, the dose per unit, the dose form, dose frequency, and duration of opioid use |
| Outcomes for opioid-using subgroups were not analyzed separately in the results | Conduct a priori subgroup analyses for opioid users in future clinical trials |
| High levels of intervention heterogeneity preclude examination of effect modifiers, including intervention dosage and delivery format | Increase the number of studies of each type of MBT of various dosages (brief vs multiweek MBT) and delivery formats (delivered in person by practitioner vs audio recording or internet); randomly assign participants to different MBT dosages and delivery formats |
| High levels of heterogeneity in study design preclude determinations of the durability of treatment effects | Use standardized and consistent assessment points and outcome measures to facilitate meta-analytic comparisons across studies |
| Some studies have small sample sizes | Increase sample size to ensure full power to detect treatment effects |
| Some studies had risk of bias because of a lack of blinding of participants, personnel, and assessors | Blind participants, personnel, and assessors, as well as use double-blind or active placebo-controlled designs whenever possible |
| Some studies had risk of bias because of a lack of intent-to-treat analysis | Use intent-to-treat analyses to assess primary and secondary outcomes |
| Some studies relied on self-report of opioid dosing or opioid misuse–related outcomes | Triangulate data from self-reports, practitioner evaluation, PDMPs, and urine toxicologic screenings via modeling strategies capable of analyzing latent dependent variables composed of multiple observed indicators (eg, structural equation modeling); use psychophysiologic testing to detect addictive tendencies toward opioids |
Abbreviations: MBT, mind-body therapy; PDMPs, prescription drug monitoring plans.
Extant evidence from controlled trials suggests that MBTs can improve clinical pain and opioid-related outcomes. Practitioners should consider presenting MBTs as nonpharmacologic adjuncts to opioid analgesic therapy. The observed findings on procedural pain are especially notable; if MBTs can reduce procedural pain, they may serve as an important form of primary prevention of long-term opioid use and OUD. Among MBTs, meditation-based interventions and CBT may be particularly useful given their association with reduced pain severity and functional interference, their potential to improve opioid-related outcomes, their broad public appeal, and the comparatively larger numbers of practitioners already trained to deliver these modalities. These interventions may also increase patient self-efficacy in that they involve developing self-management skills that patients can use independently after an initial brief training period. Moreover, because MBTs can be delivered via audio-recorded formats and in person by social workers and nurses for relatively low cost, they may prove to have a significant economic advantage in future cost-effectiveness research. Behavioral health care professionals working alongside physicians could feasibly integrate MBTs into standard medical practice through coordinated care management, colocated care on site with some system integration, or a fully integrated, onsite care model (eg, behavioral health integration into primary care). Insofar as MBTs are associated with pain relief and opioid use reduction among patients prescribed opioids for a range of pain conditions, MBTs may help alleviate the opioid crisis.
Limitations
This study has limitations. We could not draw quantitative conclusions about outcome modifiers, such as dose or delivery format, or about durability of treatment effects because of high levels of study heterogeneity. Outcomes ranged from immediate postintervention acute pain outcomes to outcomes that lasted 3 months or longer. Approximately one-third of studies had small samples and therefore may have been underpowered. Although most studies had low risk of bias, a number of trials had biases, such as lack of blinding of participants, personnel, and/or outcomes assessors, and lack of intention-to-treat analysis. Given that nearly approximately half of the trials reviewed were conducted before publication of the revised CONSORT statement in 2001,95 some studies were missing clinical trial reporting information. Funnel plots and the Egger statistic indicated some publication bias for meditation and suggestion studies.
Another limitation was the insufficient reporting of opioid dosing in the MBT literature. A number of studies, including high-impact trials,96 could not be included because the type of analgesic was unspecified and/or outcomes for opioid users were not analyzed separately. Of the trials reviewed, less than one-fifth yielded opioid dosing data of sufficient detail to be meta-analyzed.
Conclusions
The findings suggest that MBTs are associated with moderate improvements in pain and small reductions in opioid dose and may be associated with therapeutic benefits for opioid-related problems, such as opioid craving and misuse. Future studies should carefully quantify opioid dosing variables to determine the association of mind-body therapies with opioid-related outcomes.
eMethods. Supplementary Methods
eTable 1. Characteristics of Meditation Studies
eTable 2. Findings of Meditation Studies
eTable 3. Characteristics of Hypnosis Studies
eTable 4. Findings of Hypnosis Studies
eTable 5. Characteristics of Relaxation Studies
eTable 6. Characteristics of Guided Imagery Studies
eTable 7. Findings of Guided Imagery Studies
eTable 8. Characteristics of Therapeutic Suggestion Studies
eTable 9. Findings of Therapeutic Suggestion Studies
eFigure 1. Meta-analysis of Meditation Studies on Pain Outcomes
eFigure 2. Meta-analysis of Hypnosis Studies on Pain Outcomes
eFigure 3. Meta-analysis of Relaxation Studies on Pain Outcomes
eFigure 4. Meta-analysis of Suggestion Studies on Pain Outcomes
eFigure 5. Meta-analysis of Cognitive-Behavioral Therapy Studies on Pain Outcomes
eFigure 6. Baujut Plots
eFigure 7. Funnel Plots
eFigure 8. RoB Across All Studies
eFigure 9. RoB for Meditation Studies
eFigure 10. RoB for Hypnosis Studies
eFigure 11. RoB for Relaxation Studies
eFigure 12. RoB for Guided Imagery Studies
eFigure 13. RoB for Therapeutic Suggestion Studies
eFigure 14. RoB for CBT Studies
References
- 1.National Academies of Sciences, Engineering, and Medicine. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 2.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293-301. doi: 10.7326/M17-0865 [DOI] [PubMed] [Google Scholar]
- 3.Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425-426. doi: 10.1001/jama.2017.8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. 2018;108(4):500-502. doi: 10.2105/AJPH.2017.304265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. 2019;2(2):e187621-e187621. doi: 10.1001/jamanetworkopen.2018.7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276-286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 7.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Complementary and Integrative Health The Science of Mind-Body Therapies. https://nccih.nih.gov/video/series/mindbody. Accessed May 8, 2019.
- 9.Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166(7):493-505. doi: 10.7326/M16-2459 [DOI] [PubMed] [Google Scholar]
- 10.Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199-213. doi: 10.1007/s12160-016-9844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendrick C, Sliwinski J, Yu Y, et al. Hypnosis for acute procedural pain: a critical review. Int J Clin Exp Hypn. 2016;64(1):75-115. doi: 10.1080/00207144.2015.1099405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anheyer D, Haller H, Barth J, Lauche R, Dobos G, Cramer H. Mindfulness-based stress reduction for treating low back pain: a systematic review and meta-analysis. Ann Intern Med. 2017;166(11):799-807. doi: 10.7326/M16-1997 [DOI] [PubMed] [Google Scholar]
- 13.Ball EF, Nur Shafina Muhammad Sharizan E, Franklin G, Rogozińska E. Does mindfulness meditation improve chronic pain? a systematic review. Curr Opin Obstet Gynecol. 2017;29(6):359-366. doi: 10.1097/GCO.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 14.Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99:298-310. doi: 10.1016/j.neubiorev.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163-169. doi: 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: the revised APA Division 30 definition of hypnosis. Int J Clin Exp Hypn. 2015;63(1):1-9. doi: 10.1080/00207144.2014.961870 [DOI] [PubMed] [Google Scholar]
- 18.Posadzki P, Ernst E. Guided imagery for musculoskeletal pain: a systematic review. Clin J Pain. 2011;27(7):648-653. doi: 10.1097/AJP.0b013e31821124a5 [DOI] [PubMed] [Google Scholar]
- 19.Good M, Stanton-Hicks M, Grass JA, et al. Relief of postoperative pain with jaw relaxation, music and their combination. Pain. 1999;81(1-2):163-172. doi: 10.1016/S0304-3959(99)00002-0 [DOI] [PubMed] [Google Scholar]
- 20.Kekecs Z, Varga K. Positive suggestion techniques in somatic medicine: a review of the empirical studies. Interv Med Appl Sci. 2013;5(3):101-111. doi: 10.1556/IMAS.5.2013.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT. Cognitive therapy: a 30-year retrospective. Am Psychol. 1991;46(4):368-375. doi: 10.1037/0003-066X.46.4.368 [DOI] [PubMed] [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 23.Block RI, Ghoneim MM, Sum Ping ST, Ali MA. Efficacy of therapeutic suggestions for improved postoperative recovery presented during general anesthesia. Anesthesiology. 1991;75(5):746-755. doi: 10.1097/00000542-199111000-00005 [DOI] [PubMed] [Google Scholar]
- 24.Roykulcharoen V, Good M. Systematic relaxation to relieve postoperative pain. J Adv Nurs. 2004;48(2):140-148. doi: 10.1111/j.1365-2648.2004.03181.x [DOI] [PubMed] [Google Scholar]
- 25.Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82(3):448-459. doi: 10.1037/a0035798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland EL, Baker AK, Larsen P, et al. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. J Gen Intern Med. 2017;32(10):1106-1113. doi: 10.1007/s11606-017-4116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmer G, Blum J, Rulf J, Pier J. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc. 2010;110(11):646-652. [PubMed] [Google Scholar]
- 28.Frenay MC, Faymonville ME, Devlieger S, Albert A, Vanderkelen A. Psychological approaches during dressing changes of burned patients: a prospective randomised study comparing hypnosis against stress reducing strategy. Burns. 2001;27(8):793-799. doi: 10.1016/S0305-4179(01)00035-3 [DOI] [PubMed] [Google Scholar]
- 29.Syrjala KL, Cummings C, Donaldson GW. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: a controlled clinical trial. Pain. 1992;48(2):137-146. doi: 10.1016/0304-3959(92)90049-H [DOI] [PubMed] [Google Scholar]
- 30.Wright BR, Drummond PD. Rapid induction analgesia for the alleviation of procedural pain during burn care. Burns. 2000;26(3):275-282. doi: 10.1016/S0305-4179(99)00134-5 [DOI] [PubMed] [Google Scholar]
- 31.Askay SW, Patterson DR, Jensen MP, Sharar SR. A randomized controlled trial of hypnosis for burn wound care. Rehabil Psychol. 2007;52(3):247-253. doi: 10.1037/0090-5550.52.3.247 [DOI] [Google Scholar]
- 32.Everett JJ, Patterson DR, Burns GL, Montgomery B, Heimbach D. Adjunctive interventions for burn pain control: comparison of hypnosis and ativan: the 1993 Clinical Research Award. J Burn Care Rehabil. 1993;14(6):676-683. doi: 10.1097/00004630-199311000-00014 [DOI] [PubMed] [Google Scholar]
- 33.Patterson DR, Everett JJ, Burns GL, Marvin JA. Hypnosis for the treatment of burn pain. J Consult Clin Psychol. 1992;60(5):713-717. doi: 10.1037/0022-006X.60.5.713 [DOI] [PubMed] [Google Scholar]
- 34.Patterson DR, Jensen MP, Wiechman SA, Sharar SR. Virtual reality hypnosis for pain associated with recovery from physical trauma. Int J Clin Exp Hypn. 2010;58(3):288-300. doi: 10.1080/00207141003760595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantatos AH, Angliss M, Costello V, Cleland H, Stafrace S. Predicting the effectiveness of virtual reality relaxation on pain and anxiety when added to PCA morphine in patients having burns dressings changes. Burns. 2009;35(4):491-499. doi: 10.1016/j.burns.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 36.Rejeh N, Heravi-Karimooi M, Vaismoradi M, Jasper M. Effect of systematic relaxation techniques on anxiety and pain in older patients undergoing abdominal surgery. Int J Nurs Pract. 2013;19(5):462-470. doi: 10.1111/ijn.12088 [DOI] [PubMed] [Google Scholar]
- 37.Gavin M, Litt M, Khan A, Onyiuke H, Kozol R. A prospective, randomized trial of cognitive intervention for postoperative pain. Am Surg. 2006;72(5):414-418. [PubMed] [Google Scholar]
- 38.Good M. A comparison of the effects of jaw relaxation and music on postoperative pain. Nurs Res. 1995;44(1):52-57. doi: 10.1097/00006199-199501000-00010 [DOI] [PubMed] [Google Scholar]
- 39.Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain. 1995;63(2):189-198. doi: 10.1016/0304-3959(95)00039-U [DOI] [PubMed] [Google Scholar]
- 40.Wang Z-X, Liu S-L, Sun C-H, Wang Q. Psychological intervention reduces postembolization pain during hepatic arterial chemoembolization therapy: a complementary approach to drug analgesia. World J Gastroenterol. 2008;14(6):931-935. doi: 10.3748/wjg.14.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pijl AJ, de Gast HM, Mats J, Hoen MB. Guided imagery intervention does not affect surgical outcome of patients undergoing laparoscopic cholecystectomy: a multi-centre randomised controlled study. J Patient Care. 2016;2(03):3-5. doi: 10.4172/2573-4598.1000119 [DOI] [Google Scholar]
- 42.Gonzales EA, Ledesma RJA, McAllister DJ, Perry SM, Dyer CA, Maye JP. Effects of guided imagery on postoperative outcomes in patients undergoing same-day surgical procedures: a randomized, single-blind study. AANA J. 2010;78(3):181-188. [PubMed] [Google Scholar]
- 43.Nilsson U, Rawal N, Uneståhl LE, Zetterberg C, Unosson M. Improved recovery after music and therapeutic suggestions during general anaesthesia: a double-blind randomised controlled trial. Acta Anaesthesiol Scand. 2001;45(7):812-817. doi: 10.1034/j.1399-6576.2001.045007812.x [DOI] [PubMed] [Google Scholar]
- 44.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150(3):390-400. doi: 10.1016/j.pain.2010.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson M, Roll JM, Corbett C, Barbosa-Leiker C. Empowering patients with persistent pain using an internet-based self-management program. Pain Manag Nurs. 2015;16(4):503-514. doi: 10.1016/j.pmn.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 46.Akgul A, Guner B, Çırak M, Çelik D, Hergünsel O, Bedirhan S. The Beneficial Effect of Hypnosis in Elective Cardiac Surgery: A Preliminary Study. Thorac Cardiovasc Surg. 2016;64(7):581-588. doi: 10.1055/s-0036-1580623 [DOI] [PubMed] [Google Scholar]
- 47.Faymonville ME, Mambourg PH, Joris J, et al. Psychological approaches during conscious sedation. Hypnosis versus stress reducing strategies: a prospective randomized study. Pain. 1997;73(3):361-367. doi: 10.1016/S0304-3959(97)00122-X [DOI] [PubMed] [Google Scholar]
- 48.Marc I, Rainville P, Masse B, et al. Hypnotic analgesia intervention during first-trimester pregnancy termination: an open randomized trial. Am J Obstet Gynecol. 2008;199(5):469.e1-469.e9. doi: 10.1016/j.ajog.2008.01.058 [DOI] [PubMed] [Google Scholar]
- 49.Melzack R, Germain M, Belanger E, Fuchs PN, Swick R. Positive intrasurgical suggestion fails to affect postsurgical pain. J Pain Symptom Manage. 1996;11(2):103-107. doi: 10.1016/0885-3924(95)00157-3 [DOI] [PubMed] [Google Scholar]
- 50.Good M, Albert JM, Anderson GC, et al. Supplementing relaxation and music for pain after surgery. Nurs Res. 2010;59(4):259-269. doi: 10.1097/NNR.0b013e3181dbb2b3 [DOI] [PubMed] [Google Scholar]
- 51.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England; Hoboken, NJ: Wiley-Blackwell; 2008. doi: 10.1002/9780470712184 [DOI] [Google Scholar]
- 52.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 53.Quintana DS. From pre-registration to publication: a non-technical primer for conducting a meta-analysis to synthesize correlational data. Front Psychol. 2015;6:1549. doi: 10.3389/fpsyg.2015.01549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dindo L, Zimmerman MB, Hadlandsmyth K, et al. Acceptance and commitment therapy for prevention of chronic postsurgical pain and opioid use in at-risk veterans: a pilot randomized controlled study. J Pain. 2018;19(10):1211-1221. doi: 10.1016/j.jpain.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garland EL, Froeliger B, Howard MO. Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl). 2014;231(16):3229-3238. doi: 10.1007/s00213-014-3504-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zgierska AE, Burzinski CA, Cox J, et al. Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: pilot findings from a randomized controlled trial. Pain Med. 2016;17(10):1865-1881. doi: 10.1093/pm/pnw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashton C Jr, Whitworth GC, Seldomridge JA, et al. Self-hypnosis reduces anxiety following coronary artery bypass surgery. A prospective, randomized trial. J Cardiovasc Surg (Torino). 1997;38(1):69-75. [PubMed] [Google Scholar]
- 59.Enqvist B, Fischer K. Preoperative hypnotic techniques reduce consumption of analgesics after surgical removal of third mandibular molars: a brief communication. Int J Clin Exp Hypn. 1997;45(2):102-108. doi: 10.1080/00207149708416112 [DOI] [PubMed] [Google Scholar]
- 60.Ghoneim MM, Block RI, Sarasin DS, Davis CS, Marchman JN. Tape-recorded hypnosis instructions as adjuvant in the care of patients scheduled for third molar surgery. Anesth Analg. 2000;90(1):64-68. doi: 10.1097/00000539-200001000-00016 [DOI] [PubMed] [Google Scholar]
- 61.Joudi M, Fathi M, Izanloo A, Montazeri O, Jangjoo A. An Evaluation of the Effect of Hypnosis on Postoperative Analgesia following Laparoscopic Cholecystectomy. Int J Clin Exp Hypn. 2016;64(3):365-372. doi: 10.1080/00207144.2016.1171113 [DOI] [PubMed] [Google Scholar]
- 62.Lang EV, Joyce JS, Spiegel D, Hamilton D, Lee KK. Self-hypnotic relaxation during interventional radiological procedures: effects on pain perception and intravenous drug use. Int J Clin Exp Hypn. 1996;44(2):106-119. doi: 10.1080/00207149608416074 [DOI] [PubMed] [Google Scholar]
- 63.Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive non-pharmacological analgesia for invasive medical procedures: a randomised trial. Lancet. 2000;355(9214):1486-1490. doi: 10.1016/S0140-6736(00)02162-0 [DOI] [PubMed] [Google Scholar]
- 64.Lang EV, Berbaum KS, Pauker SG, et al. Beneficial effects of hypnosis and adverse effects of empathic attention during percutaneous tumor treatment: when being nice does not suffice. J Vasc Interv Radiol. 2008;19(6):897-905. doi: 10.1016/j.jvir.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackey EF. An Extension Study Using Hypnotic Suggestion as an Adjunct to Intravenous Sedation. Am J Clin Hypn. 2018;60(4):378-385. doi: 10.1080/00029157.2017.1416279 [DOI] [PubMed] [Google Scholar]
- 66.Mackey EF. Effects of hypnosis as an adjunct to intravenous sedation for third molar extraction: a randomized, blind, controlled study. Int J Clin Exp Hypn. 2010;58(1):21-38. doi: 10.1080/00207140903310782 [DOI] [PubMed] [Google Scholar]
- 67.Montgomery GH, Bovbjerg DH, Schnur JB, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J Natl Cancer Inst. 2007;99(17):1304-1312. doi: 10.1093/jnci/djm106 [DOI] [PubMed] [Google Scholar]
- 68.Surman OS, Hackett TP, Silverberg EL, Behrendt DM. Usefulness of psychiatric intervention in patients undergoing cardiac surgery. Arch Gen Psychiatry. 1974;30(6):830-835. doi: 10.1001/archpsyc.1974.01760120082012 [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Tang H, Guo Q, et al. Effects of intravenous patient-controlled sufentanil analgesia and music therapy on pain and hemodynamics after surgery for lung cancer: a randomized parallel study. J Altern Complement Med. 2015;21(11):667-672. doi: 10.1089/acm.2014.0310 [DOI] [PubMed] [Google Scholar]
- 70.Anderson KO, Cohen MZ, Mendoza TR, Guo H, Harle MT, Cleeland CS. Brief cognitive-behavioral audiotape interventions for cancer-related pain: immediate but not long-term effectiveness. Cancer. 2006;107(1):207-214. doi: 10.1002/cncr.21964 [DOI] [PubMed] [Google Scholar]
- 71.Haase O, Schwenk W, Hermann C, Müller JM. Guided imagery and relaxation in conventional colorectal resections: a randomized, controlled, partially blinded trial. Dis Colon Rectum. 2005;48(10):1955-1963. doi: 10.1007/s10350-005-0114-9 [DOI] [PubMed] [Google Scholar]
- 72.Kwekkeboom KL, Wanta B, Bumpus M. Individual difference variables and the effects of progressive muscle relaxation and analgesic imagery interventions on cancer pain. J Pain Symptom Manage. 2008;36(6):604-615. doi: 10.1016/j.jpainsymman.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandle CL, Domar AD, Harrington DP, et al. Relaxation response in femoral angiography. Radiology. 1990;174(3 pt 1):737-739. doi: 10.1148/radiology.174.3.2406782 [DOI] [PubMed] [Google Scholar]
- 74.Manyande A, Salmon P. Effects of pre-operative relaxation on post-operative analgesia: immediate increase and delayed reduction. Br J Health Psychol. 1998;3(3):215-224. doi: 10.1111/j.2044-8287.1998.tb00568.x [DOI] [Google Scholar]
- 75.Sloman R, Brown P, Aldana E, Chee E. The use of relaxation for the promotion of comfort and pain relief in persons with advanced cancer. Contemp Nurse. 1994;3(1):6-12. doi: 10.5172/conu.3.1.6 [DOI] [PubMed] [Google Scholar]
- 76.Wilson JF. Behavioral preparation for surgery: benefit or harm? J Behav Med. 1981;4(1):79-102. doi: 10.1007/BF00844849 [DOI] [PubMed] [Google Scholar]
- 77.Antall GF, Kresevic D. The use of guided imagery to manage pain in an elderly orthopaedic population. Orthop Nurs. 2004;23(5):335-340. doi: 10.1097/00006416-200409000-00012 [DOI] [PubMed] [Google Scholar]
- 78.Forward JB, Greuter NE, Crisall SJ, Lester HF. Effect of structured touch and guided imagery for pain and anxiety in elective joint replacement patients: a randomized controlled trial: M-TIJRP. Perm J. 2015;19(4):18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwekkeboom K. The role of imaging ability in successful use of guided imagery for cancer related pain. Res Nurs Health. 1998;21(3):189-198. doi: [DOI] [PubMed] [Google Scholar]
- 80.Tusek DL, Church JM, Strong SA, Grass JA, Fazio VW. Guided imagery: a significant advance in the care of patients undergoing elective colorectal surgery. Dis Colon Rectum. 1997;40(2):172-178. doi: 10.1007/BF02054983 [DOI] [PubMed] [Google Scholar]
- 81.van der Laan WH, van Leeuwen BL, Sebel PS, Winograd E, Baumann P, Bonke B. Therapeutic suggestion has not effect on postoperative morphine requirements. Anesth Analg. 1996;82(1):148-152. [DOI] [PubMed] [Google Scholar]
- 82.McLintock TT, Aitken H, Downie CF, Kenny GN. Postoperative analgesic requirements in patients exposed to positive intraoperative suggestions. BMJ. 1990;301(6755):788-790. doi: 10.1136/bmj.301.6755.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nilsson U, Rawal N, Enqvist B, Unosson M. Analgesia following music and therapeutic suggestions in the PACU in ambulatory surgery; a randomized controlled trial. Acta Anaesthesiol Scand. 2003;47(3):278-283. doi: 10.1034/j.1399-6576.2003.00064.x [DOI] [PubMed] [Google Scholar]
- 84.Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: prevalence, disability, and health care use. Arch Intern Med. 2010;170(18):1686-1694. doi: 10.1001/archinternmed.2010.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naylor MR, Naud S, Keefe FJ, Helzer JE. Therapeutic Interactive Voice Response (TIVR) to reduce analgesic medication use for chronic pain management. J Pain. 2010;11(12):1410-1419. doi: 10.1016/j.jpain.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Preoperative cognitive-behavioural intervention improves in-hospital mobilisation and analgesic use for lumbar spinal fusion patients. BMC Musculoskelet Disord. 2016;17(1):217. doi: 10.1186/s12891-016-1078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 88.Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. 2012;35(6):591-602. doi: 10.1007/s10865-011-9391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129(4):495-521. doi: 10.1037/0033-2909.129.4.495 [DOI] [PubMed] [Google Scholar]
- 90.Del Casale A, Ferracuti S, Rapinesi C, et al. Hypnosis and pain perception: an activation likelihood estimation (ALE) meta-analysis of functional neuroimaging studies. J Physiol Paris. 2015;109(4-6):165-172. doi: 10.1016/j.jphysparis.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 91.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968-971. doi: 10.1126/science.277.5328.968 [DOI] [PubMed] [Google Scholar]
- 92.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540-5548. doi: 10.1523/JNEUROSCI.5791-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci. 2016;1373(1):114-127. doi: 10.1111/nyas.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garland EL, Howard MO. Mindfulness-oriented recovery enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. J Psychother Psychosom. 2013;82(5):311-318. doi: 10.1159/000348868 [DOI] [PubMed] [Google Scholar]
- 95.Moher D, Schulz KF, Altman DG; CONSORT . The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1(1):2. doi: 10.1186/1471-2288-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240-1249. doi: 10.1001/jama.2016.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods
eTable 1. Characteristics of Meditation Studies
eTable 2. Findings of Meditation Studies
eTable 3. Characteristics of Hypnosis Studies
eTable 4. Findings of Hypnosis Studies
eTable 5. Characteristics of Relaxation Studies
eTable 6. Characteristics of Guided Imagery Studies
eTable 7. Findings of Guided Imagery Studies
eTable 8. Characteristics of Therapeutic Suggestion Studies
eTable 9. Findings of Therapeutic Suggestion Studies
eFigure 1. Meta-analysis of Meditation Studies on Pain Outcomes
eFigure 2. Meta-analysis of Hypnosis Studies on Pain Outcomes
eFigure 3. Meta-analysis of Relaxation Studies on Pain Outcomes
eFigure 4. Meta-analysis of Suggestion Studies on Pain Outcomes
eFigure 5. Meta-analysis of Cognitive-Behavioral Therapy Studies on Pain Outcomes
eFigure 6. Baujut Plots
eFigure 7. Funnel Plots
eFigure 8. RoB Across All Studies
eFigure 9. RoB for Meditation Studies
eFigure 10. RoB for Hypnosis Studies
eFigure 11. RoB for Relaxation Studies
eFigure 12. RoB for Guided Imagery Studies
eFigure 13. RoB for Therapeutic Suggestion Studies
eFigure 14. RoB for CBT Studies