Abstract
Introduction
The aim of this study was to describe age- related prostate cancer (PCa) characteristics in men after radical prostatectomy (RP).
Material and methods
There were 2,373 men who underwent RP for clinically localized PCa between 2002 and 2017 and had complete data that were included into the study. Among them, 315 (13.3%) men aged ≤55 years (GR-1), 1,098 (46.3%) men aged between 56 to 65 years (GR-2) and 960 (40.4%) men aged older than 65 years (GR-3) were identified. All preoperative and pathological parameters were compared between all three groups and between each group separately. High-risk prostate cancer (HRPCa) cases were analyzed separately. Regression analysis was used to evaluate the impact of age on cancer aggressiveness.
Result
Clinical stage (cT), biopsy Gleason score and D'Amico risk groups were different comparing age-related study groups (all p <0.01), respectively. Preoperatively cT1 and Gleason 6 were in the highest rate for GR-1 in comparison with GR-3: 35.9 vs. 27.1%, p = 0.003 and 65.1% vs. 56.7%, p = 0.008, respectively. Analyzing pathological parameters, only Gleason 9–10 was different between GR-1 and GR-3–3.8 vs. 7.6%, p = 0.02. There were 921 (38.8%) HRPCa cases identified. Age was a significant predictor for HRPCa (p = 0.019) in the regression analysis. The oldest men (GR-3) had up to 1.5 fold increased risk for HRPCa detection in comparison with the youngest one (p = 0.008, HR1.44. 95% CI 1.098–1.87).
Conclusions
Younger, ≤55-year-old men, are more likely to present with less aggressive clinical and pathological PCa features in comparison with the older ones. Increasing age has a significant influence on HRPCa detection after RP.
Keywords: cancer of prostate, radical prostatectomy, age
INTRODUCTION
Prostate cancer (PCa) is a disease of the elderly with 80% of men diagnosed at the age ≥65 [1]. However, PCa diagnosis is not uncommon in younger men, and the proportion of patients with PCa aged <50 years has increased from 1% in the 1970s to 5% in the prostate-specific antigen (PSA) era [2]. Different autopsy studies show high rates of latent PCa in the fourth and fifth decades of age. The prevalence of latent PCa in younger men varies markedly among different autopsy series – from 2.6% in the Greek series [3] to a much higher 27% prevalence in Hungary [4] and up to 34% in USA [5]. Altogether, the study shows that about 20–30% of 40–50-year-old men would harbor a PCa [6]. The increase of PCa diagnosis at young age raises a number of important questions about its aggressiveness, biology and treatment modalities. A 60-year-old patient with a low risk and Gleason score 6 PCa is a suitable candidate for active surveillance. However, a similar scenario in men aged 45–55 might prompt immediate intervention in the majority of cases because of the opinion that PCa detected at young age might behave more aggressively [7] and because young men are more likely to undergo radical prostatectomy (RP) [8]. Data about young age men with PCa treatment outcomes are controversial. According to the pre-PSA era studies, younger men are likely to have a more aggressive disease and carry a worse prognosis [9, 10]. However, more recent studies suggest higher rates of indolent PCa with more favorable outcomes in young men after RP compared to their older counterparts [11, 12, 13]. If this is the case, we can propose that for the select group of young men, RP is an overtreatment. The aim of the present study was to identify aggressiveness of PCa in men according to their age of RP.
MATERIAL AND METHODS
Between 2002 and 2017, 2,437 men were treated with RP for clinically localized PCa at a single university hospital center. Sixty-four men with incomplete clinical or pathological data or who underwent neoadjuvant treatment were excluded from the study. Among the 2,373 men included into the final analysis, 315 men aged ≤55 years (Group 1 – GR-1), 1,098 men aged from 56 to 65 years (Group 2 – GR-2) and 960 men aged more than 65 years (Group 3 – GR-3) at the time of RP were identified. Clinical characteristics such as prostate-specific antigen (PSA) level (<10 vs. 10.1–20.0 vs. >20.0 ng/ml), clinical stage (cT1 vs. cT2 vs. cT3), biopsy Gleason scores (6, 3+4, 4+3, 8 and 9-10) and D'Amico risk groups were reported before RP. Pathological parameters (pathological stage (pT2 vs. pT3a vs. pT3b), Gleason score, surgical margin status, lymph nodes status, number of positive and removed lymph nodes) were collected after surgery. Pathological stage was assessed using the 2002 TNM system, and tumor grading was classified using the Gleason grading system (2001–2005) and the revised 2005 Gleason grading system afterwards [14]. The high-risk PCa (HRPCa) cases according to high risk factors (HRF) – PSA >20 ng/ml, Gleason score 8-10 and pT3a-b – were identified [15]. HRPCa cohort was divided according to the number of HRF (one of three, two of three and all three) into 3 groups.
Clinical and pathological data were compared among the three study groups. The Chi-square test for nominal variables and the Mann-Whitney test for continuous variables were used to compare baseline clinical and pathological characteristics. The 2-sided Fisher's exact test was used to compare values between two groups: GR1 vs. GR2, GR1 vs. GR3 and GR2 vs. GR3. Logistic regression analysis was done to identify the risk of age groups on high-risk PCa detection.
The universities ethical committee approved prospective collection of the data, and all patients signed a consent form provided before RP.
RESULTS
The number of men aged ≤55 years treated with RP at our center increased from 5.9% in 2002 to 21.6% in 2016. On the other hand, reduction of the number of surgical treatment in the oldest counterparts (>65 years) was detected – from 62.4% in 2002 to 26.4% in 2016. Within the present study cohort, clinical stage, biopsy and pathological Gleason score and preoperative D'Amico risk groups were different comparing pre- and postoperative parameters among groups (Table 1 and Table 2).
Table 1.
Clinical characteristics of patients
| Parameter | Age ≤55 (n = 315) | Age 56–65 (n = 1,098) | Age >65 (n = 960) | p | Total (n = 2,373) |
|---|---|---|---|---|---|
| Age (yr) | 53 (51–54) | 61 (59–64) | 69 (67–71) | 64 (59–69) | |
| PSA (ng/mL) ≤10 ng/mL 10–20 ng/mL >20 ng/mL |
5.8 (4.5–9.2) 245 (77.8) 54 (15.8) 16 (5.1) |
6.2 (4.9–9.1) 869 (79.1)* 173 (15.8)* 56 (5.1) |
6.6 (5.0–10.0) 722 (75.2)* 198 (20.6)* 40 (4.2) |
0.06 | 6.7 (4.9–10.3) 1,836 (77.4) 425 (17.9) 112 (4.7) |
| Clinical stage cT1 cT2 cT3 |
113 (35.9) 174 (55.2) 28 (8.9*#) |
332 (30.2) 612 (55.8) 154 (14.0*) |
260 (27.1) 556 (57.9) 144 (15.0#) |
0.009 |
705 (29.7) 1,342 (56.6) 326 (13.7) |
| Biopsy GS 3+3 3+4 4+3 8 9-10 |
205 (65.1*#) 87 (27.6) 11 (3.5) 6 (1.9*) 6 (1.9) |
632 (57.6*) 361 (32.9) 49 (4.5) 34 (3.1) 22 (2.0) |
544 (56.7#) 289 (30.1) 42 (4.4) 61 (6.4*) 24 (2.5) |
0.002 |
1,381 (58.2) 737 (31.1) 102 (4.3) 101 (4.3) 5.2 (2.2) |
| D’Amico groups Low risk Intermediate risk High risk |
83 (26.3)*# 192 (61.0) 40 (12.7)*# |
238 (21.7)* 653 (59.5) 207 (18.9)*) |
171 (17.8)# 570 (59.4) 219 (22.8)# |
0.0001 |
492 (20.7) 1,415 (59.6) 466 (19.6) |
Data presented as median (quartiles) or number (%), PSA – prostate specific antigen, GS – Gleason Score
p <0.05
Table 2.
Pathological characteristics of patients
| Parameter | Age ≤55 (n = 315) | Age 56–65 (n = 1,098) | Age >65 (n = 960) | p | Total (n = 2,373) |
|---|---|---|---|---|---|
| Pathological stage pT2 pT3a pT3b |
216 (68.6) 83 (26.3) 16 (5.1) |
717 (65.3) 293 (26.7) 88 (8.0) |
604 (62.9) 277 (28.9) 79 (8.2) |
0.23 |
1,537 (64.8) 653 (27.5) 183 (7.7) |
| Pathological GS 3+3 3+4 4+3 8 9–10 |
83 (26.3) 172 (54.6) 35 (11.1) 13 (4.1) 12 (3.8*) |
286 (26.0) 602 (54.8) 124 (11.3) 36 (3.3) 50 (4.6) |
257 (26.8) 485 (50.5) 87 (9.1) 58 (6.0) 73 (7.6*) |
0.002 |
626 (26.4) 1,259 (53.1) 246 (10.4) 107 (4.5) 135 (5.7) |
| HRPCa | 105 (33.3*) | 415 (37.8) | 401 (41.8*) | 0.018 | 921 (38.8) |
| PLND | 82 (26.0*) | 321 (29.3#) | 375 (39.1*#) | 0.001 | 779 (32.8) |
| Positive LN | 13/82 (15.9) | 32/321 (10.0) | 37/375 (9.9) | 0.225 | 82/779 (10.6) |
| LN removed | 7 (4–12) | 7 (4–11) | 6 (4–9) | 6 (4–10) | |
| Positive LN removed | 2 (2–3) | 2 (1–2) | 1 (1–2) | 2 (1–2.5) |
Data presented as median (quartiles) or number (%), GS – Gleason Score
p<0.05
HRPCa – high-risk prostate cancer, PLND – pelvic lymph node dissection, LN – lymph nodes
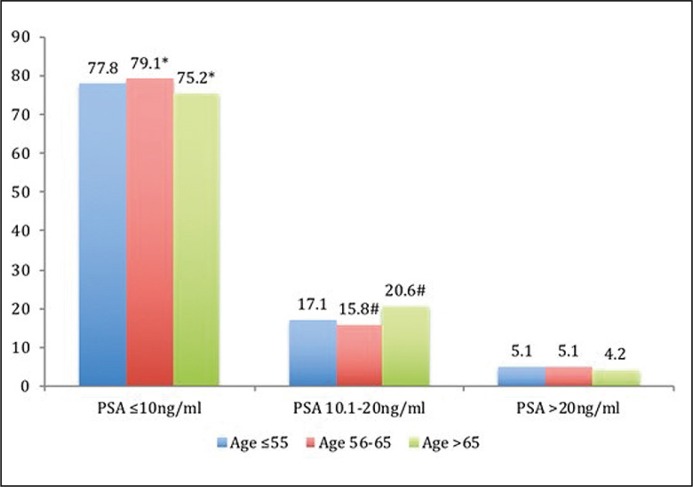
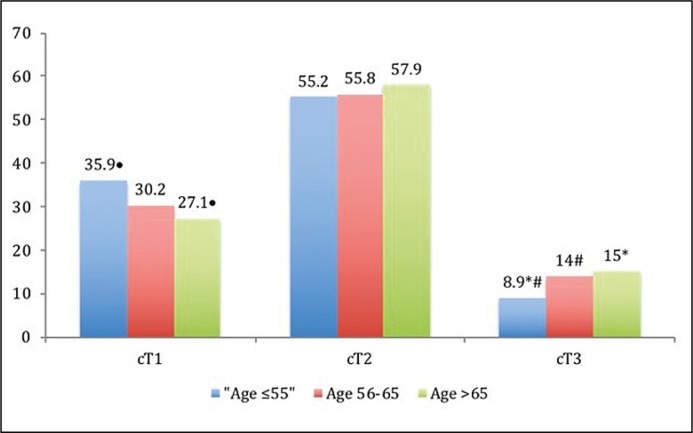
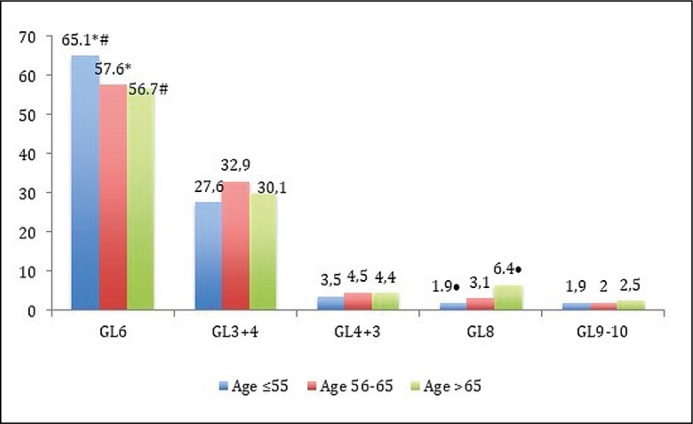
The comparison of parameters group by group has revealed that GR-3 has a different PSA ≤10 ng/ml and 10.1–20 ng/ml rates in comparison with GR-2 – p = 0.035 and p = 0.005, respectively (Figure 1A). The youngest men (GR-1) were more likely to present with a low clinical stage: the rate of cT3 was different in comparison with GR-2 (8.9 vs. 14.0%, p = 0.01) and with GR-3 (8.9 vs. 15.0%, p = 0.006, Figure 1B). The rate of Gleason score 6 at biopsy was higher in GR-1 in comparison with GR-2 (65.1 vs. 57.6%, p = 0.02) and with GR-3 (65.1 vs. 56.7%, p = 0.008, Figure 1C). The youngest men have the lowest rate of biopsy Gleason scores 8 and 9–10, which associates with cancer aggressiveness, in comparison with GR-2 and with GR-3: 3.8 vs. 5.1%, p = 0.45 and 3.8 vs. 8.9%, p = 0.003, respectively.
Figure 1A.
Comparison of prostate-specific antigen (PSA) rates (%) between study groups (* – p = 0.035, # – p = 0.005).
Figure 1B.
Comparison of clinical stage (cT) rates (%) between study groups (• – p = 0.003,* – p = 0.006, # – p = 0.01).
Figure 1C.
Comparison of Gleason Score (GL) rates (%) between study groups (• – p =0.001, * – p = 0.02, # – p = 0.008).
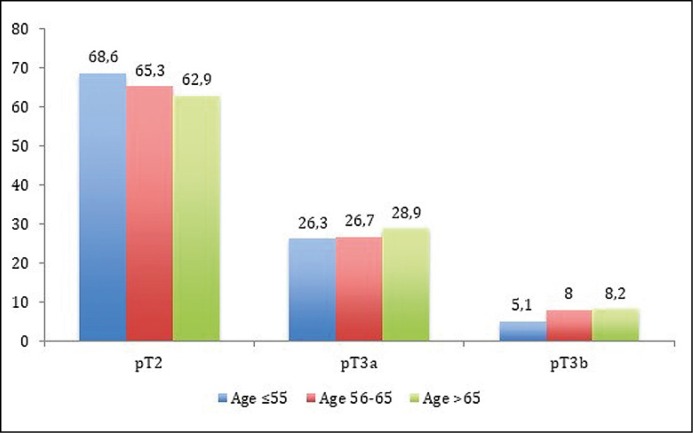
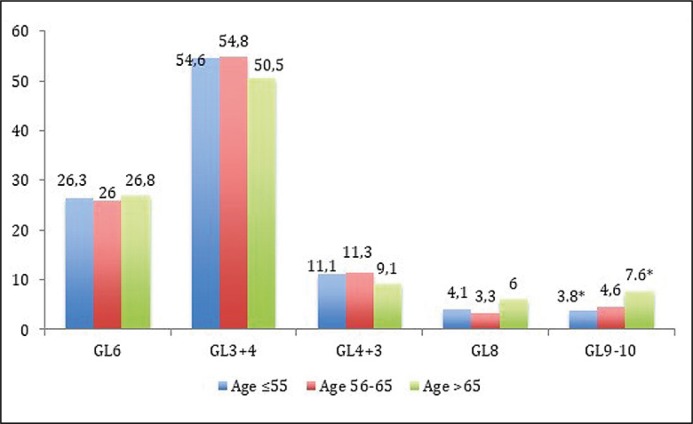
Pathological stages (pT2 vs. pT3a vs. pT3b) were not different among each study group (Figure 2A). Despite that, a tendency that locally advanced cancer (pT3) was more common in GR-3 in comparison with GR-1 has been detected: 37.1 vs. 31.4%, p = 0.078. No differences among age groups and pathological Gleason scores were estimated (Figure 2B), except that Gleason 9–10 comparing GR-1 with GR-3 (p = 0.02). The difference between these groups becomes more evident when Gleason 8 and 9–10 are analyzed together – 7.9 vs. 13.6%, p = 0.007, respectively.
Figure 2A.
Comparison of pathological stage (pT) rates (%) between study groups.
Figure 2B.
Comparison of pathological Gleason Score (GL) rates (%) between study groups (* – p = 0.019).
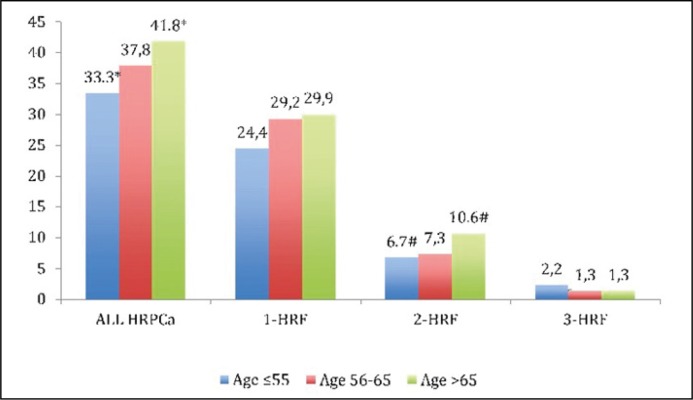
Among all study men, the HRPCa was detected in 921 (38.8%) cases (Figure 2C) with difference between GR-1 and GR-3 (33.3 vs. 41.8%, p = 0.008). One HRF had 685 (28.9%) men with no difference between groups. Two HRF were detected in 203 (8.6%) men with difference comparing GR-1 with GR-3 (6.7 vs. 10.6%, p = 0.047). Three HRF were detected in 33 (1.4%) men with no difference between each age group. Logistic regression analysis shows that age is a significant predictor for HRPCa detection – p = 0.005, HR 1.2, 95% CI 1.05–1.35. The influence of age on HRPCa according to detected 1, 2 or 3 HRF is presented in Table 3. The risk to have HRPCa increased in each study group comparing the youngest men in GR-1 with GR-2 – HR 1.21, 95% CI 0.93–1.58, p = 0.15 and with GR-3 – HR 1.44, 95% CI 1.10–1.87, p = 0.008.
Figure 2C.
Distribution of high-risk prostate cancer (HRPCa) between study groups (* – p = 0.008, # – p = 0.047). HRF – high-risk factor.
Table 3.
Logistic regression analysis: impact of age for high-risk prostate cancer detection
| No. of cases | Hazard ratio | 95%CI | p | |
|---|---|---|---|---|
| One High-Risk PCa Factor | 685 | 1.11 | 0.975–1.268 | 0.11 |
| Two High-Risk PCa Factors | 203 | 1.37 | 1.096–1.707 | 0.006 |
| Three High-Risk PCa Factors | 33 | 0.77 | 0.474–1.266 | 0.31 |
| All High-Risk PCa Cases | 921 | 1.2 | 1.055–1.348 | 0.005 |
PCa – prostate cancer
DISCUSSION
Age at the detection of cancer is a well-recognized prognostic factor in patients with the majority of localizations of malignancy. Although a few studies have demonstrated an association of young age and high stage of PCa with worse prognosis [9, 10], data from recent studies have shown that earlier diagnosis of PCa in young men is associated with a low grade and stage disease or even with superior outcome [11, 12, 13]. This suggests that the data regarding pathological findings after RP in young men are still controversial and the link between the patient's age and the aggressiveness of PCa has not been well investigated so far.
We should agree that the aggressiveness of cancer plays a crucial role in choosing treatment modality. Until now, for both for most clinicians and patients, cancer detected at young age was associated with a higher stage, worse prognosis and a need of more aggressive treatment. It was clearly demonstrated in the Kinnear et al. study analyzing the South Australian Prostate Cancer Clinical Outcomes Collaborative database [8]. These authors detected that in their cohort, men ≤50 years with PCa had less aggressive clinical characteristics, but were more likely to undergo RP. Selection bias for radical treatment of younger men can be seen from the present study data. Younger men before RP had the highest rate of cT1 and the lowest rate cT3 disease in comparison with the oldest counterparts – 35.9 vs. 27.1%, p = 0.003 and 8.9 vs. 15.0%, p = 0.006, respectively. Additionally, biopsy Gleason score 6 and 3+4, which are directly associated with low PCa aggressiveness, were present in GR-1 with statistically significant differences in comparison with GR-3 (92.7 vs. 86.8%, p = 0.007). Finally, in the youngest male group HRPCa (according to the preoperative D'Amico criteria) was detected at a significantly lower rate in comparison with GR-2 (12.7 vs. 18.9%, p = 0.02) and GR-3 (12.7 vs. 22.8%, p = 0.003), respectively. All these findings allow us to assume that the preconception about the aggressiveness of cancer at a young age plays a significant role in making a surgical treatment decision. Do pathological data and outcomes after radical treatment support this paradigm?
Becker et al. presented data of more than 13 thousand men who underwent RP at a single center [13]. The authors compared men aged <50 and ≥50 years and detected a significant difference in the pathological grade and stage between the groups, showing favorable results in the younger patients. Similar data have also been published in other studies [11, 12]. Twiss et al. demonstrated opposite results in their analysis of 790 men after RP. These authors did not detect difference in preoperative and pathologic predictors of organ-confined disease and biochemical recurrence (BCR) between men aged <50 years and older [16]. On the other hand, these authors did not also confirm that PCa at a younger age is more aggressive. Our previously published data showed that younger men had similar pathological findings and the same risk for PSA relapse in comparison with the oldest male cohorts [17]. More detailed and extended present study data have shown that the proportion between locally advanced (37.1 vs.31.4%, p= 0.08) and high-grade (Gleason score ≥8–13.6% vs. 7.9%, p = 0.007) disease was different when comparing the oldest and the youngest male counterparts, respectively. Moreover, the HRPCa occurred more often in the oldest male group in comparison with the younger one (41.8 vs. 33.3, p = 0.008), and the logistic regression analysis has demonstrated that age was a significant predictor for HRPCa. The intermediate age group had no difference in the risk for aggressive PCa in comparison with the younger group; however, the men over 65 years had 1.5 fold (p = 0.008) increased risk for HRPCa. Our results are in concordance with the Becker et al. study when younger patients had less aggressive PCa pathological stage and grade [13]. This confirms data presented in recent studies that pathological PCa characteristics in younger men are not more or even less aggressive than the characteristics in older men.
Other important issues concerning PCa aggressiveness is follow-up data. Long-term biochemical progression-free survival rate (BFSR) for the younger men cohort presented in various studies is high. Becker et al. reported 80.7% and 63.0% estimated 5- and 10-year BFSR for men aged <50 years, and it was significantly higher (p = 0.006) compared to the older counterparts [13]. Freedland et al. presented 6-year BFSR data according to the decade of life in 1,753 men after RP and showed that men younger than 50 years of age had significantly higher BFSR compared to other groups [11]. Parker et al. also detected a significantly lower BCR rate and higher BPFS among men aged <50 years versus all other age groups in their analysis of 5,195 men after RP [12]. Our recent study showed that 5- and 8-year BFSR was 77.9% and 72.4%, respectively, for young men, but the difference compared to men aged >55 years was not significant (p = 0.57) [17]. Having looked at the data of all publications mentioned above, we would like to emphasize that younger men are not at the increased risk for early biochemical recurrence.
An important finding of the present study that should be mentioned when discussing the aggressiveness of PCa is the increased positive lymph nodes rate in the youngest male group. Despite the fact that significant differences comparing every study group to each other was not reached (15.9 vs. 10%, p = 0.16 and 15.9, 6% vs. 9.9%, p = 0.08), it raises some additional questions about real aggressiveness of PCa at a young age. We cannot explain it by the possible selection bias for pelvic lymph node dissection because lymphonodectomy rate for younger men was the lowest (26 vs. 29.3 vs. 39.1%) in comparison with other groups. Also, the median number of lymph nodes removed was similar among GR-1, GR-2 and GR3 (7 vs. 7 vs. 6), respectively. The rates of postoperative Gleason 9-10 and invasion to seminal vesicles (stage pT3b), which are direct positive lymph nodes risk predictors, were the lowest in the youngest male group (Figures 2A and 2B). All aforementioned clinical data do not show higher risk for cancer metastasis in lymph nodes. Moreover, the age at logistic regression analysis was not founded as a significant predictor for positive lymph nodes (data not shown). One possible explanation could be higher HRPCa with all three HRF (PSA >ng/mL, pT3 and Gleason score 8–10) rate (Figure 2C), but it hardly provides an entirely convincing answer. The key for better understanding of such cancer behavior lies in biological studies.
In their review of young-age prostate cancer, Hussein et al. noticed that young-age PCa has several biological and genetic features that are distinct from the elderly-onset cancer, but in the majority of cases young men tend to have a low grade and stage disease. On the other hand, the authors note that early-onset PCa could represent a subset of young-age and familial PCa with a more aggressive disease and a higher prostate-cancer-specific death rate [6]. Biological difference of PCa between younger and older men was very recently presented by Jedroszka et al. [18]. Epithelial-to-mesenchymal transition (EMT) is a basic mechanism that plays a central role in the development, tissue regeneration, architecture and remodeling in physiology, as well as, pathological migratory properties of cancer cells. The authors examined the significance of EMT and signaling via androgen receptors and estrogen receptors status in the prostate according to the patient's age and Gleason score. The major observation according to these authors was that PCa from patients under 50 years old compared to the older ones has entirely different EMT gene expression profiles showing potentially more aggressive invasive phenotypes despite Gleason score classification. This indicates that similar clinical parameters at a different age can harbor real aggressiveness of PCa and this can serve as a more complete explanation of PCa spread to lymph nodes in younger men at less aggressive clinical and pathological parameters.
Summarizing, our group-by-group analysis shows that PCa at a younger age presents with less aggressive clinical and pathological features in comparison with the oldest counterparts. Men over 65 years of age are associated with an increasing risk for HRPCa. However, younger men are at increasing risk for positive lymph node detection. More clinical studies are needed to confirm our findings.
CONCLUSIONS
The present analysis of a large, single center's cohort of men after RP indicates that younger, up to 55-year-old men, are more likely to present with less aggressive clinical and PCa features in comparison with the older ones. Increasing age has a significant influence on HRPCa detection after RP because of clinically localized PCa.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. Ca Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Li J, German R, King J, et al. Recent trends in prostate cancer testing and incidence among men under age 50. Cancer Epidemic. 2012;36:122–127. doi: 10.1016/j.canep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Stamatiou K, Alevizos A, Agapitos E, Sofras F. Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcenomatous lesions in Greek male population: An autopsy study. Prostate. 2006;66:319–1328. doi: 10.1002/pros.20339. [DOI] [PubMed] [Google Scholar]
- 4.Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The prevalence of prostate carcino-ma and its precursor in Hungary: an auto- psy study. Eur Urol. 2005;48:739–744. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Sakr W, Haas G, Cassin B, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150:379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 6.Hussein S, Satturwar S, Van der Kwast T. Young-age prostate cancer. J Cain Pathol. 2015;68:511–515. doi: 10.1136/jclinpath-2015-202993. [DOI] [PubMed] [Google Scholar]
- 7.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnear NJ, Kichenadasse G, Plagakis S, et al. Prostate cancer in men aged less 50 years at diagnosis. World J Urol. 2016;34:1533–1539. doi: 10.1007/s00345-016-1824-4. [DOI] [PubMed] [Google Scholar]
- 9.Tjaden HB, Culp DA, Floks RH. Clinical adenocarcinoma of the prostate in patients under 50 years of age. J Urol. 1965;93:618–621. doi: 10.1016/S0022-5347(17)63840-0. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DE, Lanieri JP, Ayala AG. Prostatic adenocarcinoma occurring in men under 50 years of age. J Surg Oncol. 1972;4:207–216. doi: 10.1002/jso.2930040305. [DOI] [PubMed] [Google Scholar]
- 11.Freedland JS, Presti JC, Kane CJ, et al. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63:518–522. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Parker PM, Rice KR, Sterbis JR, et al. Prostate cancer in men less than the age of 50: a comparison of race and outcomes. Urology. 2011;781:110–115. doi: 10.1016/j.urology.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Becker A, Tennstedt P, Hansen J, et al. Functional and oncological outcomes of patients aged <50 years treated with radical prostatectomy for localised prostate cancer in a European population. BJU Int. 2014;114:38–45. doi: 10.1111/bju.12407. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer: Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Twiss C, Slova D, Lepor H. Outcomes for men younger than 50 years undergoing radical prostatectomy. Urology. 2005;66:141–146. doi: 10.1016/j.urology.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Milonas D, Venclovas Z, Gudinaviciene I, Zviniene K, Matjosaitis AJ. Long-Term Oncological Outcomes for Young Men Undergoing Radical Prostatectomy for Localized Prostate Cancer. Biomed Res Int. 2017;2017:9858923. doi: 10.1155/2017/9858923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jedroszka D, Orzechowska M, Hamouz R, Gorrniak K, Bednarek AK. Markers of epithelial-to-mesenchymal transition reflect tumor biology according to patient age and Gleason score in prostate cancer. PLoS One. 2017;12:e0188842. doi: 10.1371/journal.pone.0188842. [DOI] [PMC free article] [PubMed] [Google Scholar]