Abstract
Introduction
Acute epididymo-orchitis (AEO) is a male urological emergency without an approved clinical classification. We aimed to determine the clinical value of proposed in 2012 local AEO classification system and summarize results of partner's follow-up.
Material and methods
A total of 293 patients with AEO were enrolled into our study. Based on the investigated AEO classification, they were divided into four groups: 118 patients (40.3%) with Stage I AEO; 97 patients (33.1%) with Stage II AEO; 42 patients (14.3%) with Stage IIIA AEO; 36 patients (12.3%) with Stage IIIB AEO.
If after 72 hours of conservative treatment there was no clinical improvement, AEO patients underwent surgery. We analyzed the clinical value of the investigated classification system and results of partner's follow-up.
Results
Only 3 (2.5%) patients with Stage I AEO required surgery. In patients with Stage II AEO, conservative treatment was effective in 79 (81.4%) cases. A total of 27 (64.3%) patients with Stage IIIA and 36 (100%) patients with stage IIIB AEO underwent surgery. Sexually transmitted infections (STIs) were detected in 176 (60.1%) cases among 293 patients with AEO and bacterial infection in 117 (39.9%) cases. We registered a statistically lower incidence rate of trichomoniasis in AEO patients compared to their sole female partners (13.8% vs. 23.3%, p <0.05). Distribution of other STIs in AEO patients and their sole partners was similar.
Conclusions
The investigated classification system enables the determination of a treatment strategy in patients with AEO. Partner's follow-up allows for the clarification of the etiology of disease, complete evaluation of STIs and prevents reinfection in couples.
Keywords: acute epididymo-orchitis, classification, follow-up, sexually transmitted diseases, trichomoniasis
INTRODUCTION
Acute epididymo-orchitis (AEO) is a male emergent urological pathology that is characterized by inflammation of the epididymis and ipsilateral testis. Usually this condition involves the epididymis at first and afterwards results in orchitis. Sexually transmitted infections (STIs) as well as indwelling urethral catheters have been established as the main causes of AEO, even as far back as in 1805 by Bolby A.A. [1].
Treatment should be started immediately after diagnosis and includes empirical antibiotics administration, before microbiological identification of the pathogen, which can then be revised and corrected afterwards, as well as surgery in cases with conservative treatment failure [2, 3, 4].
In contrast to other urologic diseases, there is no strict approved acute epididymo-orchitis clinical classification system that enables the determination of the most appropriate treatment (conservative or surgery) immediately after diagnosis.
Previously, a local AEO staging system with treatment recommendations was presented and published in CEJU [5].
From 2016, the approach that was proposed by authors has been actively reviewed and mentioned as expedient in the contemporary EAU Guidelines [2, 6, 7].
The follow-up in patients with AEO caused by STIs as well as in their partners is currently recommended [8].
We aimed to determine the clinical value of local AEO classification system and summarize results of partner's follow-up.
MATERIAL AND METHODS
A total of 293 patients with AEO were enrolled into our prospective study from 2013 until 2019. Patients with AEO due to tuberculosis or mumps were not included in the investigation. Basing on clinical signs, scrotum ultrasound investigation (SUI) results and according to local AEO classification and pointed stages (Table 1) the patient were divided into four groups:
Table 1.
Staging of acute epididymo-orchitis [5]
| Stage | Palpation | SUI | ||
|---|---|---|---|---|
| E/T | Malacia | Hydrocele | Abscess | |
| I | + | – | – | No |
| II | + | – | + | No, one or a few abscesses, each up to 0.5 cm in greatest dimension |
| IIIA | – | – | + | |
| IIIB | – | + – * |
+ | One or more abscesses, each above 0.5 cm in greatest dimension |
SUI – scrotum ultrasound investigation
E/T – palpatory differentiation between epididymis and testis
+ present, palpation evaluates both enlarged painful epididymis and normal or insignificantly enlarged testis
– absent, enlarged epididymis is not differentiated from enlarged, painful testis
– * malacia absence only in cases of large hydrocele and inability of epididymis/testis palpation
Group 1, 118 patients (40.3%) with Stage I AEO.
Group 2, 97 patients (33.1%) with Stage II AEO.
Group 3, 42 patients (14.3%) with Stage IIIA AEO.
Group 4, 36 patients (12.3%) with Stage IIIB AEO.
In case of AEO diagnosis, we immediately administered empirical antibacterial treatment and analgesics. Sexually active men and all their available recent sexual partners were examined for STIs. If after 72 hours of conservative treatment there was no clinical improvement, patients underwent surgery.
After confirmation of STIs in our AEO patients, we performed the follow-up of their female partners. The duration of look-back for sexual contact tracing of patients with infectious AEO (IAEO) was 6 months for confirmed Chlamydia trachomatis IAEO or Ureaplasma urealyticum IAEO and 3 months for confirmed Mycoplasma genitalium IAEO. In other cases, thought to be STIs other than those abovementioned, as well as for diagnosed Neisseria gonorrhoeae IAEO, the time span of look-back was 2 months as recommended [8–12]. For STIs diagnosis we performed microscopy of a Gram-stained /methylene blue-stained urethral smear showing >5 polymorphonuclear leucocytes (PMNLs) per high power field (HPF, 1000x) or a spun down sample from first pass urine Gram stained showing >10 PMNLs per HFP (1000x) as well as polymerase chain reaction (PCR). In female sexual partners of AEO patients we also performed PCR combined with microscopy of vaginal secretions. Trichomoniasis was diagnosed by the wet-mount examination of vaginal secretions or culture in females and by PCR in males that combined with microscopy of penile meatal swabs and the first-voided urine sediment with careful specimen preservation and immediate investigation [13, 14, 15].
IAEO patients were instructed to abstain from sexual activity until they and all their sexual partners were treated completely with final laboratory confirmation.
If sexually transmitted infections were confirmed in an IAEO patient and/or his sexual partner or partners, they were all given appropriate antibacterial treatment for eradication of pathogens (Table 2). In cases of STI confirmation in a patient but not in their partner or vice versa, both partners were treated.
Table 2.
Antibacterial treatment in IAEO patients and their partners
| IAEO etiology | Treatment |
| Chlamydia trachomatis | Doxycycline 100 mg twice daily for 14 days |
| Neisseria gonorrhoeae | Ceftriaxone 500 mg intramuscular injection following by Doxycycline 100 mg twice daily for 14 days |
| Trichomonas vaginalis | Metronidazole 2.0 g in single dose orally or 500 mg twice a day for 7 days or Tinidazole (or Secnidazole or Ornidazole) 2.0 g in single dose orally (for all partners) plus Doxycycline 100 mg twice daily or Ofloxacin 200 mg twice daily for 14 days (for AEO patient) |
| Other STIs | Doxycycline 100 mg twice daily or Ofloxacin 200 mg twice daily or Levofloxacin 500 mg once daily for 14 days |
IAEO – infectious acute epididymo-orchitis; STI – sexually transmitted infection
When epididymo-orchitis was caused by enteric organisms, we prescribed Ofloxacin 200 mg twice daily or Levofloxacin 500 mg once daily for 14 days.
We analyzed the clinical value of the investigated system by the efficacy of conservative treatment for each stage and thereby the classification's influence on treatment tactics.
We also noted obtained laboratory findings in IAEO patients and compared them with results of their sexual partners registered during follow-up.
Two-sample t-test, chi-square or Fisher's exact tests, and univariate and multivariate logistic regression were performed. A p-value <0.05 represented a significant statistical difference. The study was approved by the local Ethics Committee. All included patients declared their informed consent in writing.
RESULTS
A total of 293 patients with a mean age of 33 ±12.6 years (ranged 18–82 yrs) were included in the study. Sexually transmitted diseases were detected in 176 (60.1%) of them and bacterial infection (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and others) in 117 (39.9%) cases. Among the 176 cases of infectious AEO, mixed sexually transmitted infection was diagnosed in 32 (18.2%) patients. Monoinfection was identified in 144 (81.8%) IAEO patients. Among them, Chlamydia trachomatis was detected in 52 (36.1%) cases, Mycoplasma genitalium in 28 (19.4%) cases, Neisseria gonorrhoeae in 25 (17.4%) cases, Ureaplasma urealyticum in 21 (14.6%) cases and Trichomonas vaginalis in 18 (12.5%) cases.
Only 3 (2.5%) patients with Stage I AEO required surgery. The remaining 115 (97.5%) patients were successfully treated by antibiotics with body temperature decreasing at the 3rd day of treatment.
In patients with Stage II AEO, conservative treatment was effective in 79 (81.4%) patients. Another 18 (18.6%) patients without clinical improvement underwent surgery.
A total of 27 (64.3%) patients with Stage IIIA and 36 (100%) patients with stage IIIB AEO did not demonstrate improvement after 72 hours of antibacterial treatment, so they underwent surgery.
Multivariate analysis demonstrated that presence of malacia during testis palpation (OR = 4.378, 95% CI: 1.027–8.692, P = 0.001), absence of palpatory differentiation between epididymis and testis (OR = 3.546, 95% CI: 1.016–7.831, P = 0.006) and presence of one or more abscesses above 0.5 cm in its greatest dimension (OR = 4.242, 95% CI: 1.013–9.613, P=0.014), were significant independent risk factors for surgery in AEO patients.
Stage I of AEO was independently associated with success of conservative AEO treatment (OR = 5.467, 95% CI: 1.048–13.235, P = 0.003), while Stage III – with necessity of surgery (OR = 4.632, 95% CI: 1.035–12.462, P = 0.012).
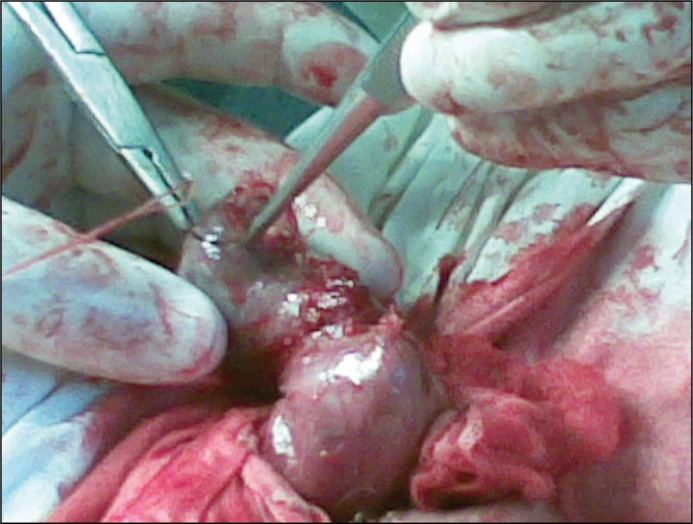
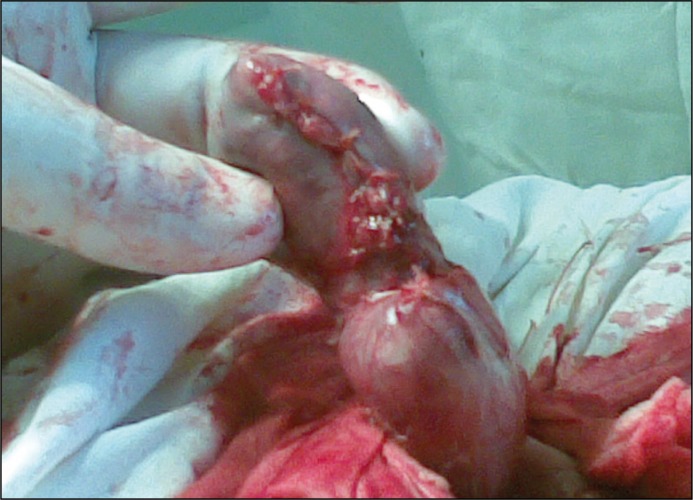
Among the 84 patients who were operated, orchiectomy was performed in 29 (34.5%) cases, epididymotomy in 24 (28.6%) cases, epididymectomy (removal of a part or all of the epididymis) in 15 (17.9%) cases and epididymectomy with resection of testis in 16 (19.0%) patients. Goals of surgical treatment via Bergman's operation were: excision of the hydrocele sac, abscess drainage with removal of purulent destructed parts and maximally possible preservation of healthy testicular parenchyma. During resection of testis after removal of destroyed testicular tissues the parenchyma was inspected for haemostasis, edges of the testicular tunica albuginea were carefully matched (Figure 1) and closed with numerous sutures (Figure 2) followed by testicle replacement into the scrotum.
Figure 1.
Careful matching of the testicular tunica albuginea edges during epididymectomy with resection of testis.
Figure 2.
Tunica albuginea is closed tightly with numerous sutures.
During follow-up, 29 (16.5%) patients with IAEO did not inform their doctor about their sexual partners because of reluctance, loss or total absence of contact with them. Among the remaining 147 patients, 116 (78.9%) patients with IAEO informed urologists about one sexual partner during look-back and 31 (21.1%) patients about more than one partner. For correct comparing of laboratory findings in IAEO patients and their partners, we excluded cases with multiple partners and investigated only those with sole partner during performed look-back, n = 116. The pathogens that were isolated in IAEO patients and their sole partners are presented in Table 3.
Table 3.
Pathogens that have been isolated in IAEO patients and their sole partners
| Pathogen | IAEO patients | Partners | P | ||
|---|---|---|---|---|---|
| n | % | N | % | ||
| Chlamydia trachomatis | 34 | 29.3 | 32 | 27.6 | >0.05 |
| Mycoplasma genitalium | 19 | 16.4 | 14 | 12.1 | >0.05 |
| Neisseria gonorrhoeae | 15 | 12.9 | 13 | 11.2 | >0.05 |
| Ureaplasma urealyticum | 13 | 11.2 | 11 | 9.5 | >0.05 |
| Trichomonas vaginalis | 12 | 10.3 | 20 | 17.2 | <0.05 |
| Mixed STIs | 23 | 19.8 | 21 | 18.1 | >0.05 |
| No STIs | 0 | 0 | 5 | 4.3 | <0.05 |
| Total | 116 | 100 | 116 | 100 | >0.05 |
IAEO – infectious acute epididymo-orchitis; STI – sexually transmitted infection
As presented in Table 3, only in 5 (4.3%) IAEO cases their sole partners did not demonstrate presence of STIs at all after laboratory examinations, but they were given preventive treatment accordingly their male partner's results. Correspondingly, 111 (95.7%) sole sexual partners of sexually active patients with IAEO had confirmed STIs that were treated completely. A structure of STIs in a cohort of men with IAEO and monoinfection is similar to their sole female partners results except cases with trichomoniasis that were diagnosed in 10.3% and 17.2% cases correspondingly (p <0.05).
The distribution of STIs in cases of mixed infection among the IAEO patients and their sole partners is presented in Table 4.
Table 4.
Distribution of STIs in cases of mixed infection among the IAEO patients and their sole partners
| Pathogen | IAEO patients | Partners | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Chlamydia trachomatis | 9 | 39.1 | 8 | 38.1 | >0.05 |
| Mycoplasma genitalium | 7 | 30.4 | 6 | 28.6 | >0.05 |
| Neisseria gonorrhoeae | 6 | 26.1 | 6 | 28.6 | >0.05 |
| Trichomonas vaginalis | 4 | 17.4 | 7 | 33.3 | <0.05 |
| Ureaplasma urealyticum | 6 | 26.1 | 5 | 23.8 | >0.05 |
| Total (patients) | 23 | 100 | 21 | 100 | >0.05 |
IAEO – infectious acute epididymo-orchitis; STI – sexually transmitted infection
Hence, during diagnostics and follow-up, a total number of confirmed trichomoniasis among IAEO patients (n = 116) was 16 (13.8%) and presented by 12 cases of monoinfection plus 4 cases in the mixed group. Among their sole partners, trichomoniasis was confirmed totally in 27 (23.3%) cases and presented by 20 cases of monoinfection plus 7 cases in the mixed group. We registered a statistically higher incidence rate of trichomoniasis in IAEO sole female partners compare to IAEO patients (23.3% vs. 13.8%, p <0.05). Distribution of other STIs in IAEO patients and their sole partners was similar.
DISCUSSION
AEO usually develops by local spreading of infection into the epididymis from the prostate, urethra or bladder. This mechanism was described as ‘metastatic’ or ‘sympathetic’, which means the transfer of the inflammatory process from one urogenital gland to another by way from the prostatic urethra to the ejaculatory ducts and thence along the vasa deferentia [1]. In sexually active men aged under 35 years, AEO is usually caused by sexually transmitted pathogens (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Ureaplasma urealyticum, Trichomonas vaginalis). Gram-negative enteric organisms in men engaging in unprotected anal intercourse also can be a cause of AEO. In sexually active older patients, the abovementioned aetiologic factors are relevant too, but because of healthy constant sexual partners or abstinence in aging males, AEO more often occurs as a complication of bacterial urinary tract infection (E. coli, St. aureus, Pseudomonas etc.) [8, 16–20]. So, sexually active patients with AEO should be screened for STIs regardless of their age [21].
According to Eshiobo et al., in their local the risk factors of AEO development were: prostatitis (3.4%), bladder outlet obstruction (62.1%), trauma/instrumentation (5.2%), and multiple sexual partners (17.2%). No risk factors have been documented by authors in 12.1% cases among 58 adult men from Nigeria [22].
Patients with indwelling urethral catheters are at a high risk of multiple drug resistance and should be treated empirically with both a fluoroquinolone and a third-generation cephalosporin until antimicrobial susceptibility testing has been completed [4].
Mumps and other viral infections, tuberculosis, brucellosis and even candidosis are less common but possible AEO causes [23–27].
AEO is a potentially dangerous condition for male health because of its possible future negative influence on fertility or even on development of testicular cancer or total testicular infarction. A population-based case-control study demonstrates that patients with testicular cancer had higher odds of prior epididymo-orchitis than those without cancer even after adjustment for potential confounders [28, 29, 30].
According our data, mixed STIs were diagnosed in 18.2% patients with IAEO. This fact advocates changes in antibacterial treatment strategy according to obtained findings during follow-up.
Despite the gonorrhea prevalence among IAEO patients that have been declared in previous early studies, in our representative cohort the percentage of this STI did not significantly differ from other infections except chlamydiosis (Tables 3 and 4). Obtained data can be explained by changing trends with local differences in STI epidemiology throughout the world and by the decrease in gonorrhoea morbidity in our region due to active treatment and prophylaxis during the last decades [31, 32].
We registered a statistically higher incidence rate of trichomoniasis in female partners cohort collated to IAEO patients (23.3% vs. 13.8%, p <0.05). Acquired results could be ascribed by relative difficulties of trichomoniasis evaluation in males compared to females thanks to a relatively higher sensitivity of wet-mount examination of vaginal secretions in comparison with male urethral discharges or first-voided urine microscopy. So, the role of trichomoniasis in IAEO development should not be underestimated. Moreover, it seems that trichomoniasis eradication could be more widely included into empirical AEO treatment in cases of STI anamnesis, objective signs or its suspicion.
Obviously, follow-up in partners of IAEO patients is obligatory because of necessity of complete STIs elimination in both persons. Absence of adequate partner's treatment leads to future reinfection and possible IAEO recurrence or other manifestation of STIs in couples.
Because AEO is an emergent pathology, sometimes there is no technical possibility to examine correctly the AEO patient prior to start of immediate empirical treatment (absence of urethral discharge at the moment of diagnosis, reluctance to urinate or presence of urethral catheter). As presented in Tables 3 and 4, the pathogens in males with IAEO and their sole female partners mostly coincide except for trichomoniasis. In this regard, the results that were obtained from untreated female partners could be transferred for determination of the AEO causes and for the choice of the most effective drug.
Our study has confirmed the clinical value of the investigated AEO staging system because of strict differences in clinical outcomes between stages. According to our data, in AEO patients with Stage I the efficacy of conservative treatment is high (97.5%). In Stage II AEO the need of surgical treatment after the 72nd hour of conservative treatment arose in 18.6% cases. It seems that in Stage III B, surgical treatment should be performed immediately after diagnosis while patients with AEO Stage III A should be informed about high probability of surgery (approximately 64.3%) after the 3rd day of treatment. Hence, the analyzed classification could be proposed for wide use by urologists in choosing the best approach to treatment of acute epididymo-orchitis.
CONCLUSIONS
The investigated classification system enables the determination of a treatment strategy in patients with AEO and the prediction of efficacy of conservative treatment in Stage I with an accuracy of 97.5%. Surgery should be predominantly recommended in Stage IIIA AEO and absolutely in Stage IIIB without waiting for a time period of 72 hours.
Almost all sole sexual partners (95.7%) of patients with infectious epididymo-orchitis have confirmed STIs with similar pathogen distribution, except trichomoniasis, that required specific diagnostics and appropriate treatment. Partner's follow-up allows for the clarification of the etiology of disease, complete evaluation of sexually transmitted diseases and prevention of reinfection in couples.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Bolby A.A. Surgical Pathology and Morbid Anatomy. 3rd Edition. London: J and A Churchill; 1805. pp. 459–460. [Google Scholar]
- 2.Pickard R, Bartoletti RR, Bjerklund-Johansen TE, et al. EAU Guidelines on Urological Infections. 2016. p. 7. [Google Scholar]
- 3.Walker NA, Challacombe B. Managing epididymo-orchitis in general practice. Practitioner. 2013;257:21–25. 2-3. [PubMed] [Google Scholar]
- 4.Pilatz A, Boecker M, Schuppe H-C, Wagen- lehner F. AktuelleAspekte der Epididymo-Orchitis. Aktuel Urol. 2016;47:237–242. doi: 10.1055/s-0042-104803. [DOI] [PubMed] [Google Scholar]
- 5.Banyra O, Shulyak A. Acute epididymo-orchitis: staging and treatment. Cent European J Urol. 2012;65:139–143. doi: 10.5173/ceju.2012.03.art8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonkat G, Pickard R, Bartoletti R, et al. EAU Guidelines on Urological Infections. 2018. p. 33. [Google Scholar]
- 7.Bonkat G, Bartoletti R, Bruyère F, et al. EAU Guidelines on Urological Infections. 2019. https://uroweb.org/guideline/urological-infections/#3.
- 8.Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. International Journal of STD & AIDS. 2017;28:744–749. doi: 10.1177/0956462417699356. [DOI] [PubMed] [Google Scholar]
- 9.Radcliffe KW, Flew S, Poder A, Cusini M. 2012 European guideline for the organisation of a consultation for sexually transmitted infections. Int J STD AIDS. 2012;23:609–612. doi: 10.1258/ijsa.2012.012115. [DOI] [PubMed] [Google Scholar]
- 10.Bignell C, IUSTI/WHO 2009 European (IUSTI/ WHO) guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2009;20:453–457. doi: 10.1258/ijsa.2009.009160. [DOI] [PubMed] [Google Scholar]
- 11.Lanjouw E, Ossewaarde JM, Stary A, Boag F, van der Meijden WI. 2010 European guideline for the management of Chlamydia trachomatis infections. Int J STD AIDS. 2010;21:729–737. doi: 10.1258/ijsa.2010.010302. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JS CM, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30:1650–1656. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 13.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommen Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 14.Frobenius W, Bogdan C. Diagnostic Value of Vaginal Discharge, Wet Mount and Vaginal PH – an Update on the Basics of Gynecologic Infectiology. GeburtshilfeFrauenheilkd. 2015;75:355–366. doi: 10.1055/s-0035-1545909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs MM, Lapple DM, Lawing LF, et al. Methods for detection of Trichomonas vaginalis in the male partners of infected women: implications for control of trichomoniasis. J Clin Microbiol. 2006;44:3994–3999. doi: 10.1128/JCM.00952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Sexually transmitted infection guidelines. 2010. http://www.cdcgov/std/treatment/2010/epididymitis.html (accessed 3 May 2019).
- 17.Weidner W, Schiefer HG, Garbe C. Acute nongonococcal epididymitis. Aetiological and therapeutic aspects. Drugs. 1987;34:111–117. doi: 10.2165/00003495-198700341-00024. [DOI] [PubMed] [Google Scholar]
- 18.Bradford R, Farry S. Antibiotic therapy for Epididymitis. US Pharm. 2015;40:39–43. [Google Scholar]
- 19.Trojian Th, Lishnak TS, Heiman D. Epididymitis and Orchitis: An Overview. American Family Physician. 2009;79:583–587. [PubMed] [Google Scholar]
- 20.Ito S, Tsuchiya T, Yasuda M, Yokoi S, Nakano M, Deguchi T. Prevalence of genital mycoplasmas and ureaplasmas in men younger than 40 years-of-age with acute epididymitis. Int J Urol. 2012;19:234–238. doi: 10.1111/j.1442-2042.2011.02917.x. [DOI] [PubMed] [Google Scholar]
- 21.Çek M, Sturdza L, Pilatz A. Acute and Chronic Epididymitis. European Urology Supplements. 2017;16:124–131. [Google Scholar]
- 22.Eshiobo I, Ogbetere F, Esezobor E. Acute epididymorchitis: a study of the predisposing factors and immediate management outcome in adult men in Irrua, Nigeria. Clinical Audit. 2016;8:1–6. [Google Scholar]
- 23.Emerson C, Dinsmore WW, Quah SP. Are we missing mumps epididymo-orchitis? Int J STD AIDS. 2007;18:341–342. doi: 10.1258/095646207780749754. [DOI] [PubMed] [Google Scholar]
- 24.Badmos KB. Tuberculous epididymo-orchitis mimicking a testicular tumour: a case report. Afr Health Sci. 2012;12:395–397. doi: 10.4314/ahs.v12i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Tawfiq JA. Brucella epididymo-orchitis: a consideration in endemic area. Int Braz J Urol. 2006;32:313–315. doi: 10.1590/s1677-55382006000300011. [DOI] [PubMed] [Google Scholar]
- 26.Gordon DL, Maddern J. Treatment of candida epididymo-orchitis with oral fluconazole. Med J Aust. 1992;156:744. doi: 10.5694/j.1326-5377.1992.tb121548.x. [DOI] [PubMed] [Google Scholar]
- 27.Michel V, Pilatz A, Hedger MP, Meinhardt А. Epididymitis: revelations at the convergence of clinical and basic sciences. Asian J Androl. 2015;17:756–763. doi: 10.4103/1008-682X.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fijak M, Pilatz A, Hedger MP, et al. Infectious, inflammatory and 'autoimmune' male factor infertility: how do rodent models inform clinical practice? Human Reproduct Update. 2018;24:416–441. doi: 10.1093/humupd/dmy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao L-T, Lin H-Ch, Chung Sn-D, Huang Ch-Yu. Association between Testicular Cancer and Epididymo-orchitis: A Population-Based Case-Control Study. 2016; Sci. Rep. 2016;6:23079. doi: 10.1038/srep23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C-D, Lin J-W, Lee C-C, et al. Acute epididymo-orchitis-related global testicular infarction: clinical and ultrasound findings with an emphasis on the juxta-epididymal string-of-bead sign. Ultrasound Q. 2016;3:283–289. doi: 10.1097/RUQ.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 31.Uusküla A, Puur A, Toompere K, DeHovitz J. Trends in the epidemiology of bacterial sexually transmitted infections in eastern Europe, 1995-2005. Sex Transm Infect. 2010;86:6–14. doi: 10.1136/sti.2009.037044. [DOI] [PubMed] [Google Scholar]
- 32.Carmona-Gutierrez D, Kainz K, Madeo F. Sexually transmitted infections: old foes on the rise. Microbial Cell. 2016;3:361–362. doi: 10.15698/mic2016.09.522. [DOI] [PMC free article] [PubMed] [Google Scholar]