Abstract
Objective
To evaluate the association between gifts from pharmaceutical companies to French general practitioners (GPs) and their drug prescribing patterns.
Design
Retrospective study using data from two French databases (National Health Data System, managed by the French National Health Insurance system, and Transparency in Healthcare).
Setting
Primary care, France.
Participants
41 257 GPs who in 2016 worked exclusively in the private sector and had at least five registered patients. The GPs were divided into six groups according to the monetary value of the received gifts reported by pharmaceutical, medical device, and other health related companies in the Transparency in Healthcare database.
Main outcome measures
The main outcome measures were the amount reimbursed by the French National Health Insurance for drug prescriptions per visit (to the practice or at home) and 11 drug prescription efficiency indicators used by the National Health Insurance to calculate the performance related financial incentives of the doctors. Doctor and patient characteristics were used as adjustment variables. The significance threshold was 0.001 for statistical analyses.
Results
The amount reimbursed by the National Health Insurance for drug prescriptions per visit was lower in the GP group with no gifts reported in the Transparency in Healthcare database in 2016 and since its launch in 2013 (no gift group) compared with the GP groups with at least one gift in 2016 (−€5.33 (99.9% confidence interval −€6.99 to −€3.66) compared with the GP group with gifts valued at €1000 or more reported in 2016) (P<0.001). The no gift group also more frequently prescribed generic antibiotics (2.17%, 1.47% to 2.88% compared with the ≥€1000 group), antihypertensives (4.24%, 3.72% to 4.77% compared with the ≥€1000 group), and statins (12.14%, 11.03% to 13.26% compared with the ≥€1000 group) than GPs with at least one gift between 2013 and 2016 (P<0.001). The no gift group also prescribed fewer benzodiazepines for more than 12 weeks (−0.68%, −1.13% to −0.23% compared with the €240-€999 group) and vasodilators (−0.15%, −0.28% to −0.03% compared with the ≥€1000 group) than GPs with gifts valued at €240 or more reported in 2016, and more angiotensin converting enzyme (ACE) inhibitors compared with all ACE and sartan prescriptions (1.67%, 0.62% to 2.71%) compared with GPs with gifts valued at €1000 or more reported in 2016 (P<0.001). Differences were not significant for the prescription of aspirin and generic antidepressants and generic proton pump inhibitors.
Conclusion
The findings suggest that French GPs who do not receive gifts from pharmaceutical companies have better drug prescription efficiency indicators and less costly drug prescriptions than GPs who receive gifts. This observational study is susceptible to residual confounding and therefore no causal relation can be concluded.
Trial registration
OSF register OSF.IO/8M3QR.
Introduction
Healthcare professionals are susceptible to the marketing and promotional activities of pharmaceutical companies. Evidence suggests that doctors’ exposure to such activities has a negative impact on the quality and quantity of drugs they prescribe, resulting in lower quality of care, unjustified risks to patients, and more costly prescriptions.1 2 To tackle this problem, some countries have implemented legislations, such as the US Physician Payments Sunshine Act, to regulate the interactions between the pharmaceutical industry and doctors.3 The relevance of these policies on drug prescription patterns is controversial.4 5 In France, following the health scandal of the benfluorex (Mediator), marketed off-label by Servier as an appetite suppressant despite thousands estimated deaths due to the medicine, the French version of the Sunshine Act legislation was implemented in 2011, including the introduction of the Transparency in Healthcare database.1 6
The National Health Data System (Système National des Données de Santé) can be used to analyse drug prescribing by French doctors.7 This database is used for multiple purposes, including the calculation of drug prescription efficiency indicators for doctors’ performance related financial incentives (Rémunération sur Objectif de Santé Publique).8
The Transparency in Healthcare and National Health Data System databases (box 1) offer an opportunity to investigate the influence of gift donations on the prescribing patterns of French general practitioners (GPs). We evaluated the association between gifts reported in the Transparency in Healthcare database and prescribing by French GPs in 2016. As promotional activities by pharmaceutical companies are expected to influence the quality, quantity, and cost of drug prescribing, we hypothesised that an association exists between the donations of gifts and poorer quality and more costly prescribing patterns.
Box 1. Databases and definitions.
Transparency in Healthcare
This database was created after the introduction of the French “Sunshine Act” in 2011 and is managed by the French ministry of health
Since 2013, all data declared by pharmaceutical, medical device, and other health related companies on their financial links with healthcare professionals and organisations are collected and made accessible
Companies must complete a declaration
Data are searchable through a French government public website (transparence.sante.gouv.fr) and can be downloaded (data.gouv.fr)
Gifts
One of the information types reported in Transparency in Healthcare
Any type of present or payment given by a company to a healthcare professional without any counterpart, such as performing a work or a service
Gifts include donations of equipment, invitations, catering expenses, travel expenses, and cash payments such as commissions, rebates, or reimbursement of expenses
Presents and payments must be declared by companies, starting from €10.00 (£8.60; $11.00) including taxes (date, amount, type of donation, identity of the receiver, identity of the company)
National Health Data System
This database was created and is managed by the French National Health Insurance system
Information is collected on all reimbursement claims from French healthcare insurances
The database includes, for example, anonymised data on patients, data on prescribers, visits to the practice or at home, dispensed and reimbursed drugs, chronic medical conditions
99% of the French population is covered by the database
The data are used to calculate the drug prescription efficiency indicators for the French performance related financial incentives programme
Anonymous data are accessible for research purposes on a secure portal, after authorisation
French performance related financial incentives programme
This programme is based on data from the National Health Data System and was launched in 2011
The aim of the programme for doctors (Rémunération sur Objectifs de Santé Publique) is to encourage more efficient drug prescription patterns and to control health expenditure
The programme uses drug prescription indicators to assess GPs’ prescription patterns
Over-the-counter drugs are not included in the drug prescription indicators
Methods
Data sources
This retrospective study was conducted using data on gifts reported in the Transparency in Healthcare database and data from the National Health Data System. From the Transparency in Healthcare database (www.data.gouv.fr), we calculated the total monetary value of the gifts listed for each French GP in 2016. We also included GPs without any gift listed for 2016. For such GPs, we calculated the total monetary value of the gifts received from 2013 to 2015, to differentiate between those who did not and those who did receive gifts between 2013 and 2016.
Under an agreement with the National Health Insurance (the French system that offers healthcare coverage to all citizens), one of its data officers extracted from the National Health Data System the drug prescription efficiency indicators used for the performance related financial incentives, the amount reimbursed for drug prescriptions per visit (to the practice or at home) in 2016, and data on the sociodemographic characteristics of the GPs and patients. Data in the National Health Data System are anonymised.
We focused on 2016 data because of the long administrative procedure to obtain authorisation to access the National Health Data System. Our first application was submitted on 31 August 2017, and a protocol detailing the analysis plan was registered on 17 January 2018. Ethical approval for the study was provided on 24 May 2018. Data were available on a secured remote portal on 21 November 2018.
Study population
We included GPs who had worked in metropolitan France or overseas territories from 1 January 2016 to 31 December 2016. To obtain a homogeneous study population, we excluded those who were not family doctors (eg, allergists, angiologists, geriatricians, emergency doctors, and others listed by the National Health Insurance), did not work exclusively in the private sector (unlike standard practice in France; that is, GPs employed by a public healthcare institution or who worked in both the private sector and a public healthcare institution), worked for part of 2016, and had fewer than five registered patients. These exclusion criteria were based on information from the National Health Data System.
Study groups
To preserve anonymity the study population was divided into six groups according to the amount (or the lack) of gifts reported in the Transparency in Healthcare database. The first group included GPs without reported gifts in 2016 or since the launch of the Transparency in Healthcare database in 2013 (no gift group). The second group included GPs without reported gifts in 2016, but with at least one gift between 2013 and 2015 (pre-2016 gift group). The third group included doctors with considerable gifts donated in 2016, arbitrarily defined as a cumulative monetary value of €1000 (£864; €1105) or more, including taxes. The remaining GPs were divided into three groups of equivalent size to study the influence of small donations (€10-€69, €70-€239, €240-€999). We considered all gifts reported for 2016, except for those smaller than €10, including taxes, because these are not reported in the Transparency in Healthcare database.
Database linkage
We included GPs from the National Council of the College of Physicians’ (Conseil National de l’Ordre des Médecins) list received on 17 August 2017. Firstly, we matched the GPs with the data from the Transparency in Healthcare database downloaded on 5 April 2018, using their unique identification number in the National Healthcare Professional Registry (Répertoire Partagé des Professionnel de Santé, RPPS). The National Health Insurance’s data officer then matched the GPs in our list with those in the National Health Data System using family name, first name, and postal code of practice as the National Health Data System does not contain the RPPS identification numbers. Finally, 3338 (6.2%) of the listed GPs could not be included because further matching was not possible on their name and the postal code of their practice.
Explanatory variables and outcomes of interest
Every year the National Health Insurance calculates various indicators for the GPs’ performance related financial incentives (see box 1). In 2016, 11 efficiency indicators for drug prescriptions were used with the aim of promoting or limiting the prescription of some drug classes according to their benefit-risk balance, or of limiting the cost of prescriptions, particularly by promoting generic drugs.8 We considered these 11 efficiency indicators (11 variables) and the amount reimbursed by the National Health Insurance for the drugs prescribed and dispensed per visit in 2016 (one variable) as outcomes of interest. Table 1 and supplementary appendix 1 provide details on the indicators.
Table 1.
Outcomes of interest. Data are for patients registered with each general practitioner (GP) in 2016
| Outcomes of interest | Calculations | What high values indicate |
|---|---|---|
| Amount reimbursed for drug prescriptions/visit (€) | Cost of all drugs prescribed by GP, dispensed and reimbursed to patient by the National Health Insurance, divided by number of visits by the GP (€) | More health expenditures |
| 11 indicators used for the performance related financial incentives programme | ||
| Antibiotics to 16-65 year olds (%) | Number of antibiotic prescriptions to 16-65 year old patients without chronic disease compared with total number of 16-65 year old patients without chronic disease (%) | More side effects or more misuse |
| Benzodiazepines >12 weeks (%) | Number of patients with a new prescription of benzodiazepines for more than 12 weeks compared with total number of patients (%) | |
| Benzodiazepines to >65 year olds (%) | Number of patients older than 65 years with one or more prescriptions of long half-life benzodiazepines compared with total number of patients older than 65 years (%) | |
| Vasodilators to >65 year olds (%) | Number of patients older than 65 years with one or more prescriptions of vasodilators compared with total number of patients older than 65 years (%) | |
| Angiotensin converting enzyme (ACE) inhibitors/ACE inhibitors+sartans (%) | Number of ACE inhibitors items prescribed compared with total number of ACE inhibitors and sartans items prescribed (%) | Fewer health expenditures |
| Antiplatelets (%) | Number of patients with prescriptions for low dose aspirin compared with total number of patients treated with antiplatelet drugs (%) | |
| Generic antibiotics (%) | Number of antibiotic items prescribed as generic drugs compared with total number of antibiotic items prescribed (%) | |
| Generic antidepressants (%) | Number of antidepressant items prescribed as generic drugs compared with total number of antidepressant items prescribed (%) | |
| Generic antihypertensives (%) | Number of antihypertensive items prescribed as generic drugs compared with total number of antihypertensive items prescribed (%) | |
| Generic proton pump inhibitors (PPIs) (%) | Number of PPI items prescribed as generic drugs compared with total number of PPI items prescribed (%) | |
| Generic statins (%) | Number of statin items prescribed as generic drugs compared with total number of statin items prescribed (%) | |
Covariates
We adjusted for several covariates in the National Health Data System that could influence drug prescriptions. The covariates related to the GPs’ characteristics were sex, age, size of the city where the practice is located (<2000 or ≥2000 inhabitants), number of visits to the practice or at home, and number of registered patients. Moreover, the patient related covariates were age distribution and proportion of patients with medical fee exemption status because of low income or chronic diseases.9
Statistical analyses
To describe the study population, we assessed differences among GP groups using the χ2 test for qualitative variables and analysis of variance for quantitative variables. We then used a two step strategy to answer our research question. First (primary analyses) we identified significant differences among at least two GP groups for each of the 12 variables to be explained. Then (secondary analyses), for variables with significant differences between groups in the primary analyses, we compared each group to the no gift group.
Primary analyses
A linear regression model was used for the primary analyses. Specifically, after univariate analysis, we performed a multivariate analysis of the different GP groups and the potential confounding factors (covariates: GPs and patients’ characteristics) identified as being associated with the outcome (threshold: P=0.25). We used a step-by-step strategy to retain the most parsimonious model and verified the application conditions of the final model. A significance threshold of 0.001 was chosen for statistical analyses, with an omnibus test from the linear model. This is slightly more conservative than Bonferroni’s correction, with a threshold of 0.05 that accounts for the 12 different outcomes analysed (0.05/12=0.00416).
Secondary analyses
From literature data, we hypothesised that the no gift group should display the most efficient prescribing patterns. This group was therefore used as the reference group to explore the differences detected with the omnibus test in the primary analyses (significance threshold 0.001).
Additional analyses
We performed two additional exploratory analyses that were not planned in the initial protocol. Firstly, to determine whether the associations observed across GP groups could be explained by the amount of reported gifts, we performed multivariate analyses by replacing the different groups by the median monetary value of gifts for each group (the no gift group and the pre-2016 gift group (ie, no reported gifts in 2016, but at least one gift between 2013 and 2015) were grouped together as having received no gift in 2016). To test the effect of extreme values on the results we performed the post hoc sensitivity analyses after excluding the first and last centiles of each explanatory variable and covariate.
Statistical analyses were performed using SAS software 9.4 (SAS Institute, Cary NC). As we were not allowed to export the National Health Data System data, we analysed the information in the National Health Data System remote portal.
Changes to the protocol
We updated the Transparency in Healthcare data on 5 April 2018. This slightly changed the size of the different GP groups. As GPs with fewer than five patients were not eligible for performance related incentives, we excluded them from the analysis. In multivariate analyses we had to split patients in to two age groups (<60 and ≥60 years) because the population aged 60 years or more seemed to be the most important group concerning drug prescriptions.9
Patient and public involvement
We did not include patients as study participants. Patients were not involved in the research question or the study design. We do not plan to involve patients in the dissemination of results, and we will not disseminate results directly to patients.
Results
Selection of study population
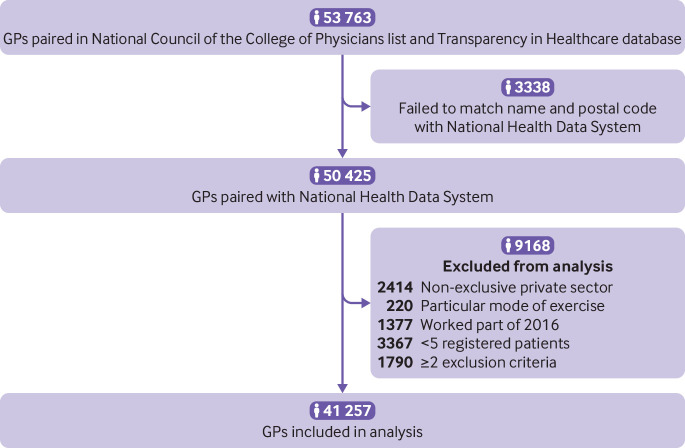
Among the 53 763 French GPs identified in the National Council of the College of Physicians list, 41 257 were included for analysis. The name and practice postal code for 3338 listed GPs (6.2% of the sample) could not be matched to data in the National Health Data System. These doctors belonged more often to the no gift group (16.4%) than to the other groups (range 2.5-5.3%). A further 9168 GPs were excluded according to the exclusion criteria (fig 1). Supplementary appendix 2 summarises the steps leading to the selection of the GP groups.
Fig 1.
Study flowchart. GPs=general practitioners
Description of study population
The mean age of included GPs was 53.5 (SD 10.2) years, 26 614 (64.5%) were men and 12 857 (31.2%) practised in rural areas. In 2016, the GPs carried out a mean 5359 (SD 2510) visits and had 1177 (SD 577) registered patients. Among the 41 257 GPs included in the study, 27 512 (66.7%) were listed in the Transparency in Healthcare database as having received gifts in 2016, and 36 232 (87.8%) as having received gifts since the launch of the database in 2013. Comparison of the GPs and patients’ characteristics (variables used for adjustment) using the analysis of variance and χ2 tests highlighted significant differences among the GP groups (P<0.001) for all covariates (see supplementary appendix 3). GPs in the group that did not receive gifts had the lowest mean number of visits (4623 (SD 2525)) and the smallest mean number of registered patients (1006 (SD 611)), whereas the group that received gifts to the value of €1000 or more had the highest number of visits (6140 (SD 2577)) and the largest number of registered patients (1293 (SD 586)). GPs in the group gifted €1000 or more were mostly men (76.5%), and they had the highest percentage of patients with chronic conditions (30.4% (SD 10.1%)). Multivariate analyses were adjusted for GPs’ workload (number of visits and number of registered patients in 2016), as well as for all covariates. Between 0% and 0.90% of data were missing for these variables.
Primary analyses
Univariate and multivariate analyses (table 2) showed significant differences among GP groups for amount reimbursed for drug prescriptions per visit, proportion of antibiotic treatments for 16 to 65 year olds, percentage of patients treated with benzodiazepines for more than 12 weeks, percentage of patients aged more than 65 years treated with a long half-life benzodiazepine or with vasodilators, percentage of prescriptions for angiotensin converting enzyme (ACE) inhibitors compared with all ACE inhibitors and sartan prescriptions, and percentage of prescriptions for generic antibiotics, antihypertensives, and statins. Missing data for the 12 outcomes of interest (11 efficiency indicators and the amount reimbursed by the National Health Insurance for the drugs prescribed and dispensed per visit in 2016) ranged from 0.60% to 3.06%.
Table 2.
Primary analyses. Values are means (standard deviations)
| Outcomes | Study groups | All | P values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No gift | Pre-2016 gift | €10-€69 | €70-€239 | €240-€999 | ≥€1000 | Univariate | Multivariate | ||
| Amount reimbursed for drug prescriptions/visit (€)* | 45.8 (40.1) | 47.8 (24.4) | 48.2 (21.8) | 49.4 (21.5) | 51.5 (22.0) | 53.2 (21.0) | 49.1 (25.3) | <0.001 | <0.001 |
| Antibiotics to 16-65 year olds (%)* | 35.6 (30.0) | 35.8 (23.0) | 36.8 (24.9) | 37.3 (21.0) | 38.1 (19.1) | 41.0 (21.9) | 37.2 (23.2) | <0.001 | <0.001 |
| Benzodiazepines >12 weeks (%)† | 14.0 (9.3) | 14.2 (7.8) | 14.4 (8.1) | 14.6 (7.7) | 14.8 (6.9) | 14.8 (6.5) | 14.5 (7.7) | <0.001 | <0.001 |
| Benzodiazepines to >65 year olds (%)† | 9.7 (7.9) | 9.5 (5.9) | 9.5 (5.9) | 9.6 (5.7) | 9.7 (5.0) | 10.0 (5.0) | 9.6 (5.9) | <0.001 | <0.001 |
| Vasodilators to >65 year olds (%)† | 0.8 (1.8) | 0.8 (1.6) | 0.8 (1.6) | 0.8 (1.6) | 0.9 (1.9) | 1.0 (1.8) | 0.8 (1.7) | <0.001 | <0.001 |
| ACE inhibitors/ACE inhibitors+sartan (%)† | 44.3 (17.1) | 44.0 (14.9) | 44.2 (14.2) | 44.7 (13.4) | 43.9 (12.4) | 42.9 (12.7) | 44.1 (14.1) | <0.001 | <0.001 |
| Antiplatelets (%)‡ | 87.3 (10.8) | 86.9 (9.2) | 87.2 (9.1) | 87.1 (8.0) | 86.8 (7.3) | 86.7 (6.9) | 87.0 (8.6) | 0.006 | 0.004 |
| Generic antibiotics (%)† | 87.3 (10.8) | 86.6 (9.7) | 86.5 (9.4) | 86.1 (9.2) | 85.5 (8.9) | 85.1 (8.9) | 86.2 (9.5) | <0.001 | <0.001 |
| eric antidepressants (%)‡ | 91.3 (10.5) | 91.4 (8.9) | 91.4 (8.7) | 91.3 (7.8) | 91.3 (7.2) | 90.9 (7.2) | 91.3 (8.4) | 0.16 | 0.15 |
| Generic antihypertensives (%)† | 84.7 (8.2) | 84.1 (7.3) | 83.7 (7.1) | 83.2 (6.6) | 82.1 (6.3) | 80.3 (7.0) | 83.2 (7.1) | <0.001 | <0.001 |
| Generic PPIs (%)† | 99.9 (0.9) | 99.9 (0.8) | 99.9 (0.8) | 99.9 (0.5) | 99.9 (0.8) | 99.9 (0.4) | 99.9 (0.7) | 0.02 | 0.03 |
| Generic statins (%)‡ | 77.6 (16.3) | 74.8 (15.4) | 73.2 (15.5) | 71.9 (14.2) | 69.2 (13.7) | 65.3 (14.0) | 72.3 (15.2) | <0.001 | <0.001 |
1.00 (£0.86; $1.10).
ACE=angiotensin converting enzyme; PPIs=proton pump inhibitors.
<1% of missing data.
1% to 2.5% of missing data.
>2.5% to 4% of missing data.
Threshold P=0.001 (Bonferroni correction for P value: 0.05/12=0.0042)
Secondary analyses
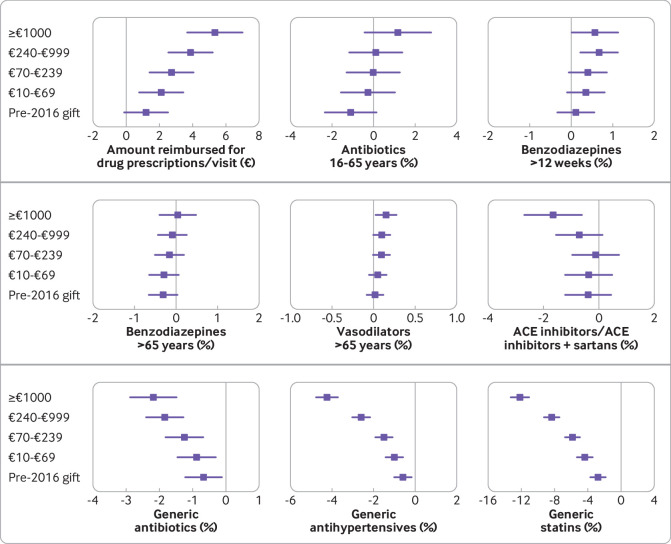
Figure 2 and supplementary appendix 4 present the analysis of the differences between the no gift group and the other GP groups for the nine variables identified in the primary analyses. Compared with the no gift group, the amount reimbursed for prescribed drugs per visit significantly increased, and the percentage of prescriptions for generic antibiotics, antihypertensive, and statins significantly decreased for most groups.
Fig 2.
Comparison of explanatory variables with the no gift group in multivariate analysis. Values are adjusted mean differences and corresponding 99.9% confidence intervals (data are reported in supplementary appendix 4). Threshold P=0.001 (Bonferroni correction for P=0.05/(9×5)=0.0011). ACE=angiotensin converting enzyme
Additional analyses
Except for the proportion of patients aged more than 65 treated with benzodiazepines with a long half-life (P=0.01), the associations observed in the primary analyses were also found using the median monetary value of gifts for each group (instead of the group as a class), suggesting a dose dependent association between gifts and eight outcomes. This concerned the amount reimbursed for drug prescriptions per visit, proportion of antibiotic treatments for 16 to 65 year old patients, percentage of patients treated with benzodiazepines for more than 12 weeks compared with patients treated with benzodiazepines, percentage of patients aged more than 65 years treated with vasodilators, percentage of prescriptions for ACE inhibitors compared with all ACE inhibitors and sartan prescriptions, and percentage of prescriptions for generic antibiotics, antihypertensives, and statins (see supplementary appendix 5).
Similar results were obtained in sensitivity analyses performed after excluding the first and last centiles of each explanatory variable and covariate (data not shown).
Discussion
In this retrospective study using data from two French databases, the Transparency in Healthcare and the National Health Data System, we found an association between gifts paid by pharmaceutical companies to French GPs and nine of 12 outcomes studied for 2016 (11 efficiency indicators and the amount reimbursed by the National Health Insurance for the drugs prescribed and dispensed per visit (to the practice and at home)).
The amount reimbursed for prescribed drugs per visit was significantly lower for the group that did not receive gifts compared with the groups that received €10-€69, €70-€239, €240-€999, and €1000 or more. GPs in the no gift group prescribed significantly more generic antibiotics, antihypertensives, and statins than the other groups, including those without reported gifts in 2016 but with at least one gift between 2013 and 2015 (pre-2016 gift group). They also prescribed significantly fewer benzodiazepines for more than 12 weeks and vasodilators compared with the €240-€999 and €1000 or more groups and significantly fewer ACE inhibitors compared with the €1000 or more group. Conversely, we did not find any association for the percentage of patients treated with low dose aspirin among those treated with antiplatelets, and for prescribed generic antidepressants and proton pump inhibitors. The high proportion of prescriptions for generic proton pump inhibitors (around 99.9% in all groups) and antidepressants (around 91% in all groups) and the absence of differences between groups could be explained because in 2016 proton pump inhibitors did not have a patented originator molecule, and the few patented antidepressants on the market were established drugs and so not actively promoted by pharmaceutical companies.10 Finally, compared with the no gift group no difference was found in the percentage of antibiotic prescribed for patients aged 16 to 65 years and of long half-life benzodiazepines for patients older than 65 years.
Strengths and limitations of this study
This study has several strengths. We tried to reduce confounding bias by taking into account the main available confounders in the multivariate analysis.9 The use of databases is an effective way to explain and highlight differences in prescribing patterns that are minimised in declarative studies where doctors are directly interviewed on the influence of promotional activities by pharmaceutical companies.2 The external validity of our results is shown by concordance between the mean values for the indicators in our population and those of the national averages.11
Our study has some limitations. We do not know whether all gifts were mentioned or the extent of misinformation in the Transparency in Healthcare database because data are only based on the statements made by the pharmaceutical companies without effective control.5 The 6.2% of failed matches for GPs might have introduced some bias, especially because the matching failure rate was different among the GP groups.
Confounding is a major problem in observational research. Methods, such as computation of E-values (minimum strength of association that an unmeasured confounder would need to have with both treatment and outcome to fully explain away a specific treatment-outcome association, conditional on the measured covariates), have been proposed but have caveats.12 Despite the adjustment of the analysis to various available factors that might influence prescribing patterns, we could not take all of them into account. For example, we did not include type of GP activity (in a group or alone), status of university internship supervisor, or other methods of pharmaceutical promotion. The study is therefore prone to residual confounding.
In addition, in the absence of clinical data we could not fully understand the reasoning leading to each drug prescription.13 Other studies have shown a link between payments from industry and specific prescriptions (eg, opioids, gabapentinoids).14 15 As the Transparency in Healthcare database does not specify the marketed drug or drugs associated with each listed gift, we could not link a gift to a specific prescription.5 Our study explores prescription patterns in a more global way. Indeed, providing gifts is not the only way industry uses to promote drugs. For instance, sponsored medical press, advertising directed towards doctors, sponsored continuing medical education courses (possibly with key opinion leaders), one-to-one visits from sales representatives, and sample delivery are many other methods to influence doctors’ prescribing patterns. Receiving a gift from a company might reflect a higher acceptance, an interest in novelty, a belief in the promotion, a favourable image of the firms, and a belief in using drugs as a first solution to health problems.2 In other words, receiving gifts from a company might not only be the direct cause of a prescription pattern, but also be a symptom of a general behaviour.
Our literature search did not find a unique or validated indicator to evaluate the GPs’ drug prescription quality in a general way.16 Therefore, we used multiple indicators validated by the French National Health Insurance to assess prescribing patterns.8 Some of them are also used in drug utilisation studies.17 Their validity is, however, questionable.18
Performance related financial incentive indicators were not available for 0.64% to 3.04% of GPs, which roughly corresponds to the 2.8% of refusals from doctors to collaborate in this process.19 Such missing data may be associated with the gifts perceived and also with the GP’s pattern of drug prescribing.
French GPs may benefit from industry (eg, gifts) and from the National Health Insurance through the performance related financial incentive programme. Gifts from industry and the performance related financial incentives programme cannot be directly linked to each other because no indicator assessed GPs’ financial links with industry. A GP might receive the maximum remuneration from the performance related financial incentive programme and concomitantly many gifts from industry. Nevertheless, if we assume that the desire of profit is a confounder, our results are not suggesting optimisation trade-offs by GPs. Indeed, in our study GPs with the best drug prescription indicator results and therefore the highest National Health Insurance financial incentive for these indicators seemed to receive fewer gifts from industry.
Finally, it is not possible to conclude that the different drug prescription patterns are the result of the promotional activities by pharmaceutical companies. An alternative explanation could be that GPs who receive gifts may be more targeted or receptive to pharmaceutical marketing because they have specific prescribing patterns.2 All these possible caveats suggest that these findings should be interpreted with caution, and they preclude any definitive conclusion in terms of causality.
Results consistent with the literature
These results are consistent with recent meta-analyses and systematic reviews showing an association between gifts from pharmaceutical companies and more frequent, lower quality and more costly drug prescriptions.14 20 21 22 23 24 Two recent studies also found a lower prescription rate of generic drugs by doctors who benefit from pharmaceutical companies.25 26 According to Health Action International, no study has shown health benefits from promotional activities of pharmaceutical companies.1 2
Our post hoc analyses suggest a possible dose-effect association between gifts paid by pharmaceutical companies and the cost of drug prescriptions per visit and drug prescription efficiency indicators. A similar observation was reported by two large retrospective studies in the United States in 2016.24 27
Our study shows a significant difference between the group that did not receive gifts and the pre-2016 gift group for generic drug prescription of antibiotics, antihypertensive agents, and statins, suggesting that the observed association could also be observed over time.
Health and economic impacts
The more frequent use of some drugs, such as benzodiazepines and vasodilators, increases the risk of well known adverse effects of these drug classes, with occasional serious or fatal consequences. Our data suggest that prescription of these drug classes increases slowly but progressively from the no gift group to the €1000 or more group. Prescriptions of brand name drugs instead of generic drugs represent an additional cost for the National Health Insurance with no proved benefit for the patient. In France, the price of a generic drug is at least 60% lower than the price of the original drug.28 With an additional €1.2 to €5.3 reimbursed per drug prescription, GPs with gifts reported in the Transparency in Healthcare database are associated with an important additional charge for the National Health Insurance compared with GPs who did not have any gift reported. Notably, among the 12 outcomes we used, the most significant were directly linked with economic factors: cost of drug prescription per visit, and generic drug prescription. This is in line with studies showing that pharmaceutical promotion targets especially market issues.1 2
In our study, associations were also significant for the “€10-€69” group. Differences in prescribing behaviours after donation of small gifts have been reported by several studies and are based on donation and counter-donation mechanisms that have been well described by humanities and social science studies.29 30 31 Gifts lead to a sense of accountability and ultimately negatively influence prescribing habits.32 More generally, for most doctors the amount of gifts reported represents a small part of their annual income, and for pharmaceutical industries a small financial engagement compared with their benefits in terms of selling drugs.2
Results in favour of the influence of gifts on drug prescriptions
Before the creation of the Transparency in Healthcare database, the scope and frequency of gifts paid by pharmaceutical companies to French GPs were not easily accessible. Our study shows that gifts to GPs are common and associated with less rational drug prescriptions for patients and more expenses for the National Health Insurance. Although causality must not be assumed, the results of our study are in line with the existing literature and reinforce the hypothesis that pharmaceutical companies influence GPs’ prescribing patterns. Future research should assess the association between prescribing patterns and conventions, another link of interests reported in the Transparency in Healthcare database involving obligations on both sides (such as speaker at a conference), and evaluate these features also in specialist doctors and particularly among the so-called key opinion leaders.2
Perhaps the time has come for interventional studies to test the impact of restrictive policies on physicians’ drug prescription patterns prospectively.
What is already known on this topic
Drug promotion is linked to less rational and more costly drug prescriptions
French general practitioners (GPs) may receive gifts from pharmaceutical companies (eg, donated equipment, meals, transport, accommodation)
What this study adds
The findings of this study suggest that French GPs who do not receive gifts from pharmaceutical companies have better drug prescription efficiency indicators (as defined by France’s National Healthcare Insurance) and less costly drug prescriptions than those who receive gifts
Acknowledgments
We thank Medhi Gabbas for extracting data from the National Health Insurance database, Elisabetta Andermarcher for English language editing, and Emmanuel Allory, Irène Frachon, Barbara Mintzes, Emmanuel Oger, Dominique Somme, and Bruno Toussaint for their help.
Web extra.
Extra material supplied by authors
Supplementary material: additional information
Contributors: BG and PF initiated and designed the study, searched the literature, interpreted the results, and wrote the manuscript. FB performed the analysis and interpreted results. FN and ME contributed to the study design and interpreted the results. BB contributed to the study design. AC contributed to the study design and offered institutional support. PF is guarantor. All authors have critically revised the manuscript for important intellectual content and approved the manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: None.
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; AC reports personal fees and non-financial support from Roche, personal fees and non-financial support from Novartis Pharma, and personal fees from Congrès colloques conventions, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the French Commission Nationale Informatique et Libertés (authorisation CNIL DR 2018-089). A letter has been delivered to all French URPS (Unions Régionales des Professionnels de Santé) to inform general practitioners of the objective of the research and give them the right to oppose.
Data sharing: Data from Transparency in Healthcare database are available on www.data.gouv.fr. We cannot share National Health Data System data as they are only available on a secure portal. Authorisation to access this portal needs registration and clearance.
The guarantor (PF) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspect of the study was omitted; and that any discrepancies from the study as originally planned have been explained.
References
- 1.Health Action International. Fact or Fiction: What Healthcare Professionals Need to Know about Pharmaceutical Marketing in the European Union – Health Action International. 2016. https://haiweb.org/publication/fact-or-fiction-pharmaceutical-marketing-in-the-european-union/
- 2.World Health Organization, Health Action International. Understanding and responding to pharmaceutical promotion: a practical guide. 2009. http://haiweb.org/wp-content/uploads/2015/05/Pharma-Promotion-Guide-English.pdf
- 3. Fabbri A. Santos Ancel la, Mezinska S, Mulinari S, Mintzes B. Sunshine Policies and Murky Shadows in Europe: Disclosure of Pharmaceutical Industry Payments to Health Professionals in Nine European Countries. Int J Health Policy Manag 2018;7:504-9 10.15171/ijhpm.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson M. Is transparency really a panacea? J R Soc Med 2014;107:216-7 10.1177/0141076814532744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauray B. Dispositifs de transparence et régulation des conflits d’intérêts dans le secteur du médicament. Rev Fr Adm Publique 2018;(1):49-61. [Google Scholar]
- 6.French Law n° 2011-2012 du 29 décembre 2011 relative au renforcement de la sécurité sanitaire du médicament et des produits de santé. 2011-2012 Dec 29, 2011.
- 7. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017;26:954-62 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 8.European Observatory on Health Systems and Policies Series. Paying for Performance in Health Care: Implications for Health System Performance and Accountability. Open University Press; 2014. 338 p. www.oecd-ilibrary.org/employment/paying-for-performance-in-health-care_9789264224568-en
- 9. Darmon D, Belhassen M, Quien S, Langlois C, Staccini P, Letrilliart L. Factors associated with drug prescription in general practice: a multicenter cross-sectional study. Sante Publique 2015;27:353-62 10.3917/spub.153.0353. [DOI] [PubMed] [Google Scholar]
- 10.Assurance Maladie. Medic’AM. Données mensuelles et annuelles sur les médicaments remboursés par l’Assurance Maladie. www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/medicament/medic-am/medic-am-mensuel-2016.php
- 11.Assurance Maladie. La rémunération sur objectif de santé publique -Bilan à 5 ans et présentation du nouveau dispositif. 2017 Apr p.29. www.ameli.fr/fileadmin/user_upload/documents/DP_bilan_ROSP_2016_du_21_avril_def.pdf
- 12. Ioannidis JPA, Tan YJ, Blum MR. Limitations and Misinterpretations of E-Values for Sensitivity Analyses of Observational Studies. Ann Intern Med 2019;170:108 10.7326/M18-2159. [DOI] [PubMed] [Google Scholar]
- 13.Scailteux L-M, Droitcourt C, Balusson F, Nowak E, Kerbrat S, Dupuy A, et al. French administrative health care database (SNDS): The value of its enrichment. Therap. 2018 Oct 25; 10.1016/j.therap.2018.09.072 [DOI] [PubMed]
- 14. Hadland SE, Rivera-Aguirre A, Marshall BDL, Cerdá M. Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. JAMA Netw Open 2019;2:e186007 10.1001/jamanetworkopen.2018.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee TG, Ross JS. Association Between Industry Payments to Physicians and Gabapentinoid Prescribing. JAMA Intern Med. 2019 Jul 8. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2737748 [DOI] [PMC free article] [PubMed]
- 16. Chapman S, Durieux P, Walley T. Good prescribing practice. In: Regulating pharmaceuticals in europe: Striving for efficiency, equity and quality. Open University Press, 2004: 144-57. [Google Scholar]
- 17. Walker AJ, Curtis HJ, Croker R, Bacon S, Goldacre B. Measuring the Impact of an Open Web-Based Prescribing Data Analysis Service on Clinical Practice: Cohort Study on NHS England Data. J Med Internet Res 2019;21:e10929 10.2196/10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prescrire Pay for performance: financial rewards without improving quality of care. Prescrire Int 2015;24:279. [PubMed] [Google Scholar]
- 19.Quotidien du Médecin. P4P, téléservices, dépassements, maîtrise, le patron de la CNAM à l’offensive sur tous les fronts. Quotidien du médecin. 2012 Jan 30. www.lequotidiendumedecin.fr/actualites/article/2012/01/30/le-patron-de-la-cnam-loffensive-sur-tous-les-fronts_591466
- 20. Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010;7:e1000352 10.1371/journal.pmed.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brax H, Fadlallah R, Al-Khaled L, et al. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: A systematic review and meta-analysis. PLoS One 2017;12:e0175493 10.1371/journal.pone.0175493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open 2017;7:e016408 10.1136/bmjopen-2017-016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mejia J, Mejia A, Pestilli F. Open data on industry payments to healthcare providers reveal potential hidden costs to the public. Nat Commun 2019;10:1-8 10.1038/s41467-019-12317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma M, Vadhariya A, Johnson ML, Marcum ZA, Holmes HM. Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data. BMC Health Serv Res 2018;18:236 10.1186/s12913-018-3043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of Industry Payments to Physicians With the Prescribing of Brand-name Statins in Massachusetts. JAMA Intern Med 2016;176:763-8 10.1001/jamainternmed.2016.1709. [DOI] [PubMed] [Google Scholar]
- 26. Qian J, Hansen RA, Surry D, Howard J, Kiptanui Z, Harris I. Disclosure of industry payments to prescribers: industry payments might be a factor impacting generic drug prescribing. Pharmacoepidemiol Drug Saf 2017;26:819-26 10.1002/pds.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perlis RH, Perlis CS. Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PLoS One 2016;11:1 10.1371/journal.pone.0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministère de l’économie. Trésor-éco: Quelle politique pour poursuivre la diffusion des médicaments génériques? 2017 Jun. Report No: 199. www.tresor.economie.gouv.fr/Articles/2017/06/20/tresor-eco-quelle-politique-pour-poursuivre-la-diffusion-des-medicaments-generiques
- 29. Grande D, Frosch DL, Perkins AW, Kahn BE. Effect of Exposure to Small Pharmaceutical Promotional Items on Treatment Preferences. Arch Intern Med 2009;169:887-93 10.1001/archinternmed.2009.64. [DOI] [PubMed] [Google Scholar]
- 30. Lo B, Grady D. Payments to Physicians: Does the Amount of Money Make a Difference? JAMA 2017;317:1719-20 10.1001/jama.2017.1872. [DOI] [PubMed] [Google Scholar]
- 31. DeJong C, Aguilar T, Tseng C-W, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical Industry–Sponsored Meals and Physician Prescribing Patterns for Medicare Beneficiaries. JAMA Intern Med 2016;176:1114 10.1001/jamainternmed.2016.2765. [DOI] [PubMed] [Google Scholar]
- 32. Sah S, Fugh-Berman A. Physicians under the influence: social psychology and industry marketing strategies. J Law Med Ethics 2013;41:665-72 10.1111/jlme.12076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: additional information