This randomized clinical trial compares the effect of intensive weight management combined with group medical visits vs group medical visits alone on glycemia in patients with type 2 diabetes.
Key Points
Question
Does adding intensive weight management to group medical visits improve glycemia compared with group medical visits alone while also enhancing weight loss and decreasing medication intensity in patients with type 2 diabetes?
Findings
In this randomized clinical trial of 263 individuals with diabetes and mean hemoglobin A1c level of 9.1% at baseline, during the 48 weeks, hemoglobin A1c level improved in both study arms (8.2% and 8.3%). Weight management added to group medical visits also led to reduced diabetes medication use, greater weight loss, and fewer hypoglycemic events.
Meaning
For persons with diabetes who attended group medical visits, adding intensive weight management using low-carbohydrate nutrition counseling showed comparable glycemic improvement plus advantages in several clinically important outcomes.
Abstract
Importance
Traditionally, group medical visits (GMVs) for persons with diabetes improved glycemia by intensifying medications, which infrequently led to weight loss. Incorporating GMVs with intensive dietary change could enable weight loss and improve glycemia while decreasing medication intensity.
Objective
To examine whether a program of GMVs combined with intensive weight management (WM) is noninferior to GMVs alone for change in glycated hemoglobin (HbA1c) level at 48 weeks (prespecified margin of 0.5%) and superior to GMVs alone for hypoglycemic events, diabetes medication intensity, and weight loss.
Design, Setting, and Participants
This randomized clinical trial identified via the electronic medical record 2814 outpatients with type 2 diabetes, uncontrolled HbA1c, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 27 or higher from Veterans Affairs Medical Center clinics in Durham and Greenville, North Carolina. Between January 12, 2015, and May 30, 2017, 263 outpatients started the intervention.
Interventions
Participants randomized to the GMV group (n = 136) received counseling about diabetes-related topics with medication optimization every 4 weeks for 16 weeks, then every 8 weeks (9 visits). Participants randomized to the WM/GMV group (n = 127) received low-carbohydrate diet counseling with baseline medication reduction and subsequent medication optimization every 2 weeks for 16 weeks followed by an abbreviated GMV intervention every 8 weeks (13 visits).
Main Outcomes and Measures
Outcomes included HbA1c level, hypoglycemic events, diabetes medication effect score, and weight at 48 weeks analyzed using hierarchical generalized mixed models to account for clustering within group sessions.
Results
Among 263 participants (mean [SD] age, 60.7 [8.2] years; 235 [89.4%] men; 143 [54.4%] black), baseline HbA1c level was 9.1% (1.3%) and BMI was 35.3 (5.1). At 48 weeks, HbA1c level was improved in both study arms (8.2% in the WM/GMV arm and 8.3% in the GMV arm; mean difference, −0.1%; 95% CI, −0.5% to 0.2%; upper 95% CI, <0.5% threshold; P = .44). The WM/GMV arm had lower diabetes medication use (mean difference in medication effect score, −0.5; 95% CI, −0.6 to −0.3; P < .001) and greater weight loss (mean difference, −3.7 kg; 95% CI, −5.5 to −1.9 kg; P < .001) than did the GMV arm at 48 weeks and approximately 50% fewer hypoglycemic events (incidence rate ratio, 0.49; 95% CI, 0.27 to 0.71; P < .001) during the 48-week period.
Conclusions and Relevance
In GMVs for diabetes, addition of WM using a low-carbohydrate diet was noninferior for lowering HbA1c levels compared with conventional medication management and showed advantages in other clinically important outcomes.
Trial Registration
ClinicalTrials.gov identifier: NCT01973972
Introduction
Group medical visits (GMVs) are a health care system redesign strategy in which groups of patients who share a common chronic condition meet to receive education, self-management skills training, and medication management to improve clinical outcomes. Meta-analyses of randomized clinical trials have shown that GMVs for patients with type 2 diabetes improve glycated hemoglobin (HbA1c) levels by approximately 0.5% (to convert to proportion of total hemoglobin, multiply by 0.01).1,2 The GMVs improve HbA1c levels primarily via medication intensification (which can have undesirable adverse effects, such as weight gain and hypoglycemia) that may attenuate the macrovascular benefits of glycemic control.1,2,3
Weight management (WM) is one of the factors most associated with glycemic control4,5,6 and is associated with lower risk for diabetes complications independent of glycemic control.7,8 Because weight loss in patients with type 2 diabetes can improve glycemic control while reducing diabetes medication needs, subsequent risk for hypoglycemia may also be lower.6,9,10 Emerging research has shown potential advantages to weight loss with a low-carbohydrate diet in these domains.11,12 However, 1 meta-analysis found no effect of GMVs for diabetes on weight, and no previous studies of GMVs have used a low-carbohydrate diet to our knowledge.2
Therefore, we tested an evidence-based GMV program alone vs an evidence-based WM program using a low-carbohydrate diet combined with the GMV program (WM/GMV) in an outpatient clinical setting. Because diabetes medication use may be reduced with WM, offsetting some of the glycemic improvement, this study was designed as a noninferiority trial for the primary outcome of HbA1c level. However, we hypothesized the WM/GMV intervention would be superior to GMV alone with regard to hypoglycemic events, medication burden, and weight.
Methods
Study Design
This 2-arm randomized clinical trial took place at 2 sites (Durham and Greenville, North Carolina) and included outpatient participants with uncontrolled type 2 diabetes followed up for 48 weeks (Trial Protocol in Supplement 1).13 Participants in both arms received a GMV intervention, with 1 arm focusing on weight management (WM/GMV) and the other on medication intensification (GMV) for glycemic control. The study was approved by the Durham Veterans Affairs Health Care System institutional review board. A data monitoring committee monitored recruitment and adverse events. All participants provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Setting and Participants
Participants included veterans enrolled from Veterans Affairs Medical Center clinics between January 12, 2015, and May 30, 2017. Eligibility was assessed by electronic health record or telephone, followed by in-person screening, at which point written informed consent was obtained.13 Key eligibility criteria were type 2 diabetes, HbA1c level of at least 8.0% (≥7.5% if age <55 years), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 27 or higher, and interest in losing weight. Key exclusion criteria were (1) age of at least 75 years; (2) hemoglobinopathy; (3) certain chronic or unstable diseases; (4) pregnancy, breastfeeding, or lack of birth control if premenopausal; (5) dementia, unstable psychiatric illness, or substance abuse; and (6) enrollment in another study that might affect the main outcomes. To increase slower than expected enrollment, the following changes were made: (1) removal of HbA1c upper limit of 12.0% and decreased lower limit to at least 7.5% if younger than 55 years, (2) removal of recent weight loss exclusion, (3) shortened exclusion for recent unstable heart disease to 1 month, and (4) addition of the Greenville site.
Randomization
Eligible participants were randomized using a computerized random number generator in blocks of 2 (study personnel other than statisticians blinded to block size) within strata defined by baseline HbA1c level (7.5%-8.9% vs ≥9%) and insulin use (multiple types vs 1 type or none). After a participant was assigned to a study arm, a staff person blinded to assignment informed the participant by telephone of the initial small group visit. Study arm assignment was not revealed until the initial visit to avoid participants starting behavior changes before initiation of the intervention and dropout associated with not receiving the preferred treatment. Participants were considered to be randomized when they learned of their assignment at the initial visit, which was when baseline study outcomes were collected and the interventions began. Participants who dropped out before the first visit were not included in data analyses.
Interventions
Participants were assigned to intervention groups (goal of 8-15 participants per group) based on their arm assignment. Sessions for both arms lasted approximately 1.5 to 2 hours and started with outcome data collection by research staff, followed by group counseling led by a registered nurse or registered dietitian. Sessions ended with a one-on-one meeting with a study physician assigned to the group.13 The study physicians included 2 general internists and 4 endocrinologists. Participants in both arms received a nutrient handbook and similar information emphasizing adequate hydration and physical activity.14,15
Participants in the GMV groups met every 4 weeks for 16 weeks and every 8 weeks thereafter for diabetes counseling and medication management. The goal of the GMV intervention was to enhance overall diabetes management. A nurse led the class in discussing topics associated with diabetes self-management, such as foot care, managing hypoglycemia, and preventing diabetes complications.13 After the class, each participant met with a study physician to review blood glucose and hypoglycemic episode data, address any health concerns, and optimize medications for glycemic control and cardiovascular disease prevention using a study-specific algorithm and clinical guidelines.13,16
Participants in the WM/GMV groups met every 2 weeks for 16 weeks for WM counseling and medication management and every 8 weeks thereafter for diabetes counseling, medication management, and continued WM support. The WM/GMV intervention included low-carbohydrate nutrition, physical activity, and weight management counseling. A dietitian provided the nutritional counseling using a book and handouts.17 Initially, carbohydrate intake was restricted to approximately 20 to 30 g per day with no specified caloric restriction.18,19 Participants were taught how to add to daily carbohydrate intake gradually as they approached their weight goal or if adherence was threatened.18,19,20 Classes covered topics, such as grocery shopping, restaurant eating, and recipe makeovers, and incorporated behavioral techniques to improve adherence. Physical activity was emphasized at later visits to aid weight maintenance, with discussions on overcoming barriers and demonstrations of home exercises.15 With use of an algorithm, doses of diabetes medications related to hypoglycemia (insulin and sulfonylureas) and/or weight gain (thiazolidinediones) were reduced by a study physician during the first 16 weeks and optimized for glycemic control thereafter. Because of the diuresis that occurs at diet initiation, low-dose diuretics were discontinued and higher doses were reduced; doses were reinstated at future visits if blood pressure or peripheral edema warranted.
Fidelity to the interventions was assessed by research personnel using a checklist of the key topics covered at each group meeting. Medication adjustments were reviewed periodically by the collective study physicians with the data monitoring committee members to enhance individual physician adherence to the algorithm.
Measurements
Study measurements were collected by trained research personnel at parallel time points in both arms. Weight and hypoglycemic events were additionally assessed at weeks 2, 6, 10, and 14 in the WM/GMV arm for safety and medication adjustment purposes but were not used as outcomes. Because of the nature of the interventions and the coincident collection of outcomes (eg, hypoglycemia) at group sessions, it was not possible to blind patients, interventionists (dietitian, nurse, and clinicians), or research personnel to intervention assignment. However, measurement of the primary outcome, HbA1c level, and other laboratory tests were performed by central laboratory technicians who were unaware of the intervention assignment.
Body weight was measured at every visit using a standardized digital scale. Participants recorded any medication changes since the previous visit on a printed sheet of their medications that was updated after every visit. Medications for diabetes, hypertension, and hyperlipidemia (with doses and schedules) were confirmed by a study physician. The medication effect score was used to summarize each participant’s diabetes medication regimen, allowing comparison across different patient regimens.21 The medication effect score is based on the doses and potencies of medications in a patient’s regimen; it is calculated as the percentage taken of the maximum dose multiplied by the expected HbA1c-lowering effect for each of a participant’s medications, and these products were then summed across the patient’s medication regimen. Participants logged hypoglycemic events using a standardized form to improve recall of events.22 Hypoglycemic events were assessed with a questionnaire at every visit or by telephone if the participant missed a visit. The questionnaire distinguished between asymptomatic and symptomatic hypoglycemic events and unassisted and assisted events.23 Diabetes-related emotional distress was assessed using the Problem Areas in Diabetes (PAID) scale, a sensitive and responsive 20-item measure with demonstrated reliability, validity (compared with general emotional distress, glycemic control, fear of hypoglycemia, and self-care behaviors), and responsiveness. Problem Areas in Diabetes scores range from 0 to 100, with higher scores indicating higher levels of diabetes-related emotional distress.24,25 Dietary intake was assessed at baseline and every 16 weeks by 3-day food records, with data verified and entered by a registered dietitian and analyzed using Food Processor software, version 11.0.124 (ESHA Research); physical activity was assessed on the same schedule using the International Physical Activity Questionnaire, long version.26 Blood pressure was measured twice (and averaged) at each visit using an automatic sphygmomanometer with an appropriately sized cuff after the participant was seated quietly for 5 minutes.
The cost of delivering each intervention was assessed by having study staff record time spent on intervention-related activities, calculating the cost of nonlabor inputs (eg, laboratory tests and participant materials), and applying a 30% indirect rate for utilities, office space, and other administrative services. Market laboratory costs (instead of Veterans Administration laboratory costs, which are less expensive) were applied.
Statistical Analysis
For the primary noninferiority analysis, we conducted both an intention-to-treat analysis and a per-protocol analysis for the subset of patients who attended at least 75% of visits.27,28 Our secondary analyses were superiority tests and were conducted on an intention-to-treat basis regardless of intervention attendance using all data available. For intention-to-treat analyses with implicit- and explicit-imputed outcomes for missing data, we assumed continuation of assigned treatment.29
For continuous longitudinal outcomes (HbA1c level, medication effect score, weight, and Problem Areas in Diabetes scale), hierarchical linear mixed-effects models were used to test hypotheses of treatment differences over time with restricted likelihood estimation.30 The final models included fixed effects for linear, quadratic, and/or cubic time (depending on best fit) (eMethods in Supplement 2); associated time-by-arm interaction terms; and the randomization stratification variables. A random effect was fit to account for the clustering of counseling group over time, and covariance terms were fit for the repeated measures over time. The best covariance structure for each outcome was determined using Akaike Information Criteria model selection criteria.31 We tested the noninferiority hypothesis for the primary outcome by estimating the difference in HbA1c levels between the WM/GMV and GMV groups at 48 weeks from model measures and examining the upper limit of the 95% CI of the estimate for inclusion of the 0.5% HbA1c noninferiority threshold; this threshold was considered to be the minimum clinically important difference.32,33 Longitudinal models used all available data, including data from participants who had missing observations and/or were lost to attrition, with the estimation procedure implicitly accommodating missing values when related to previous outcomes or to other baseline covariates in the model (ie, missing at random). For the per-protocol analysis, we fit the primary model to the subset of patients in each arm who attended at least 75% of group sessions. To assess the primary model’s robustness to missing observations, we multiply imputed missing longitudinal HbA1c measurements using a Markov chain Monte Carlo algorithm incorporating additional variables (eMethods in Supplement 2) beyond those in the linear mixed-effects models to strengthen the missing-at-random assumption. For the per-person count of hypoglycemic events for 48 weeks, a hierarchical generalized linear mixed model with a negative binomial distribution and log link was used with a random effect for counseling group, including fixed effects for randomization arm, baseline number of hypoglycemic events, and randomization stratification variables.34 Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute) and R Project (R Foundation for Statistical Computing). Additional details of statistical methods are given in the eMethods in Supplement 2.
Sample size calculations accounted for clustering due to counseling groups using an intraclass correlation coefficient of 0.01 and assumed a within-patient correlation of HbA1c level of 0.50 to adjust the variance of a 1-sided, 2-sample difference-in-means test (ie, the difference in HbA1c level at 48 weeks between the WM/GMV and GMV arms) with a significance level of 0.025, an SD of 1.5%, and a 20% final dropout rate.35,36,37 Based on these assumptions, we estimated that 308 participants (154 per arm) were needed to identify less than 0.5% difference in HbA1c level (noninferiority threshold) between the 2 treatment arms. Because of lower than expected recruitment rates (primarily owing to patients’ inability to attend sessions or improved glycemic control), higher than assumed retention for the primary outcome, and lower than assumed SD of HbA1c level, we conducted an updated sample size calculation for a cost extension proposal assuming a 15% loss to follow-up and an SD of 1.4% for HbA1c level. With these updated assumptions, sample sizes ranging from 254 to 290 participants yielded 80% power for the noninferiority hypothesis. Sample size calculations were conducted using PASS, version 11 (NCSS Statistical Software).
Results
Participants, Retention, and Attendance
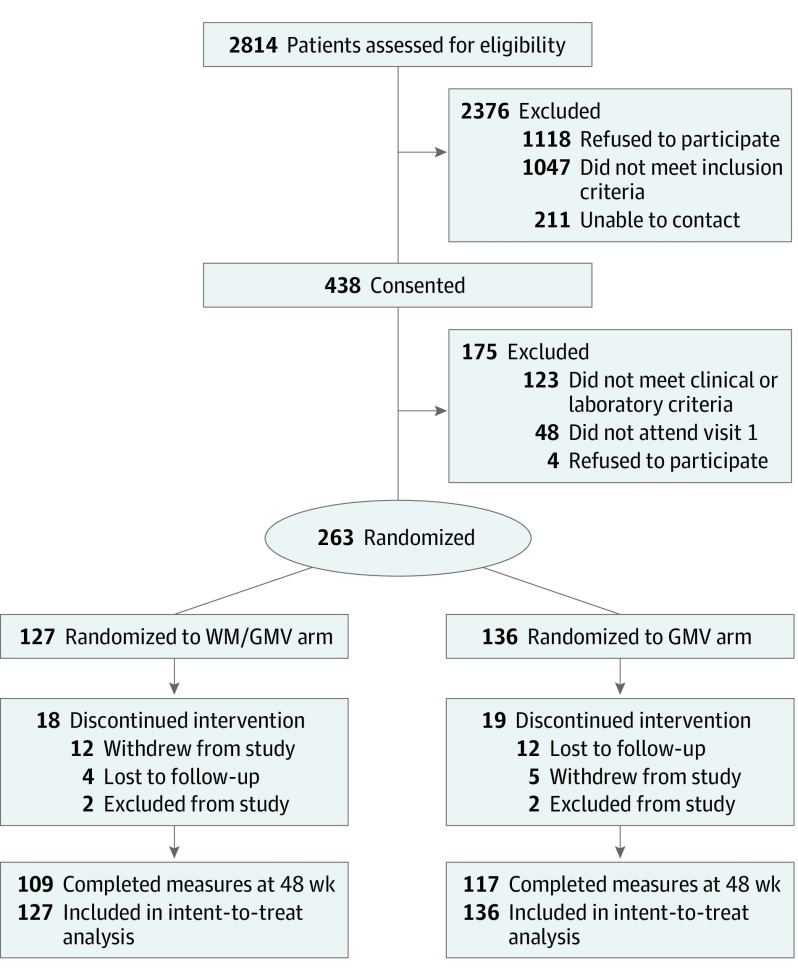
We assessed 2814 veterans for eligibility and consented 438 participants (Figure 1). Of these, 263 participants were eligible and attended the first group session. At baseline, participants had a mean (SD) age of 60.7 (8.2) years, HbA1c level of 9.1% (1.3%), and BMI of 35.3 (5.1); 143 (54.4%) had black race/ethnicity, and 235 (89.4%) were men (Table 1). At 48 weeks, 109 of 127 WM/GMV participants (85.8%) and 117 of 136 GMV participants (86.0%) completed HbA1c measurements.
Figure 1. Participant Flow.
GMV indicates group medical visit; WM, weight management.
Table 1. Baseline Participant Characteristics.
| Characteristic | All (n = 263) | WM/GMV Group (n = 127) | GMV Group (n = 136) |
|---|---|---|---|
| Age, mean (SD), y | 60.7 (8.2) | 61.0 (8.1) | 60.4 (8.3) |
| Age at diabetes diagnosis, mean (SD), ya | 47.4 (10.3) | 47.3 (10.1) | 47.5 (10.6) |
| Men, No. (%) | 235 (89.4) | 110 (86.6) | 125 (91.9) |
| Race/ethnicity, No. (%) | |||
| White | 112 (42.6) | 60 (47.2) | 52 (38.2) |
| African American | 143 (54.4) | 64 (50.4) | 79 (58.1) |
| Other | 8 (3.0) | 3 (2.4) | 5 (3.7) |
| College degree, No. (%) | 113 (43.0) | 61 (48.0) | 52 (38.2) |
| Clinical measures, mean (SD) | |||
| HbA1c level, % | 9.1 (1.3) | 9.0 (1.3) | 9.2 (1.3) |
| Body weight, kg | 108.5 (19.6) | 109.1 (20.7) | 107.9 (18.5) |
| Body mass indexb | 35.3 (5.1) | 35.6 (5.4) | 35.0 (4.8) |
| Waist circumference, cm | 46.2 (5.1) | 46.5 (5.3) | 45.9 (5.0) |
| Hypoglycemic events, No.c | 1.3 (3.2) | 1.4 (3.7) | 1.1 (2.7) |
| Diabetes regimen, No. (%) | |||
| Metformin | 217 (82.5) | 105 (82.7) | 112 (82.3) |
| Sulfonylureas | 119 (45.2) | 53 (41.7) | 66 (48.5) |
| Thiazolidinedione | 8 (3.0) | 5 (3.9) | 3 (2.2) |
| DPP-4 inhibitors | 10 (3.8) | 8 (6.3) | 2 (1.5) |
| GLP-1 receptor agonists | 7 (2.7) | 3 (2.4) | 4 (2.9) |
| SGLT-2 inhibitors | 4 (1.5) | 1 (0.8) | 3 (2.2) |
| No insulin | 101 (38.4) | 51 (40.2) | 50 (36.8) |
| Basal insulin alone | 65 (24.7) | 28 (22.0) | 37 (27.2) |
| Prandial with or without basal insulin | 97 (36.9) | 48 (37.8) | 49 (36.0) |
| Receiving hypertension medication, No. (%) | 235 (89.4) | 116 (91.3) | 119 (87.5) |
| Receiving cholesterol medication, No. (%) | 209 (79.5) | 106 (83.5) | 103 (75.7) |
| Medication effect score, mean (SD) | 2.3 (1.1) | 2.2 (1.1) | 2.3 (1.1) |
| PAID score, mean (SD)d | 30.5 (21.6) | 29.9 (19.9) | 31.0 (23.2) |
Abbreviations: DPP-4 indicates dipeptidyl peptidase 4; GLP-1, glucagon-like peptide-1; GMV, group medical visit; HbA1c, glycated hemoglobin; PAID, Problem Areas in Diabetes; SGLT-2, sodium-glucose co-transporter-2; WM, weight management.
Patients (GMV [n = 2] and WM/GMV [n = 2]) who were missing age at diabetes diagnosis.
Calculated as weight in kilograms divided by height in meters squared.
Patients (GMV [n = 2] and WM/GMV [n = 4]) who were missing data on hypoglycemic events.
Patients (GMV [n = 4]) who were missing a PAID score. Scores range from 0 to 100, with higher scores indicating higher levels of diabetes-related emotional distress.
The mean (SD) number of group sessions attended was 9.1 (3.9) of 13 sessions for WM/GMV participants and 6.0 (2.6) of 9 sessions for GMV participants. Seventy-seven of WM/GMV participants (60.6%) and 75 of GMV participants (55.2%) attended at least 75% of sessions. Of these participants, only 1 person was missing an HbA1c measurement at 48 weeks.
HbA1c Outcomes
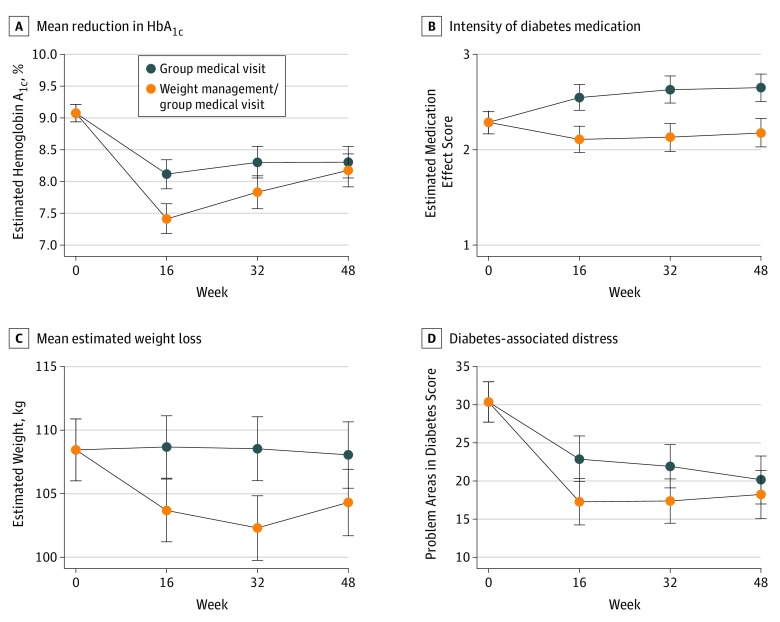
In the intention-to-treat analysis, WM/GMV was found to be noninferior compared with GMV at 48 weeks for HbA1c levels (estimated mean difference, −0.1%; 95% CI, −0.5% to 0.2%; upper 95% CI, <0.5%) but not superior to GMV (P = .44). Between baseline and 48 weeks, HbA1c level decreased 0.9% (95% CI, −1.2% to −0.6%) in the WM/GMV group and decreased 0.8% (95% CI, −1.0% to −0.5%) in the GMV group. Similar results were found in the per-protocol analysis and using multiple imputed data sets (eMethods in Supplement 2). The mean reduction in HbA1c level was greater in the WM/GMV group compared with the GMV group at 16 weeks (estimated mean difference, −0.7%; 95% CI, −1.0 to −0.4) and at 32 weeks (estimated mean difference, −0.5%; 95% CI, −0.8 to −0.1), but the arms converged at 48 weeks (estimated mean difference, –0.1%; 95% CI, –0.5% to 0.2%) (Figure 2A). At 48 weeks, 48 of 109 WM/GMV participants (44.0%) and 34 of 117 GMV participants (29.1%) achieved an HbA1c level below 7.5%, the minimum level allowed to enroll in the study. Regarding a definition used for diabetes remission, 12 of 109 WM/GMV participants (11.0%) and no GMV participants achieved an HbA1c level <6.5% after receiving metformin alone or no diabetes medication.11 The estimated mean intraclass correlation coefficient for HbA1c level accounting for group clustering across time was 0.008.
Figure 2. Estimated Trajectories by Arm for Hemoglobin A1c Level, Medication Effect Score, Weight, and Diabetes Distress Score at Measurement Times From Hierarchical Linear Mixed Models.
Secondary Outcomes
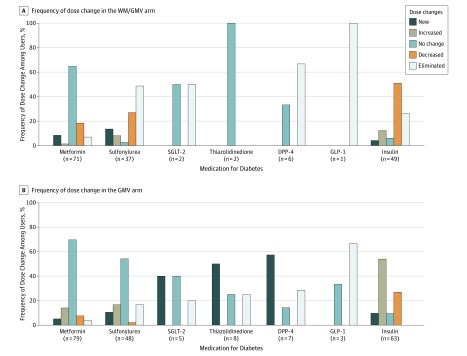
The WM/GMV participants reported significantly fewer hypoglycemic events compared with GMV participants during 48 weeks (incidence rate ratio, 0.49; 95% CI, 0.27-0.71; P < .001). The estimated mean number of hypoglycemic events among WM/GMV participants during 48 weeks was 3.2 (95% CI, 2.1-4.4; approximately 1 event/16 weeks) and among GMV participants was 6.6 (95% CI, 4.5-8.6; approximately 1 event/8 weeks), assuming no baseline events. There were few individuals with hypoglycemic events who needed assistance from family (7 persons in the WM/GMV arm and 9 persons in the GMV arm) or medical personnel (0 in the WM/GMV arm and 6 in the GMV arm). During 48 weeks, the estimated baseline medication effect score of 2.3 points (95% CI, 2.2-2.4 points) decreased to 2.2 (95% CI, 2.0-2.3) in the WM/GMV arm, whereas it increased to 2.7 (95% CI, 2.5-2.8) in the GMV arm (estimated mean difference, −0.5; 95% CI, −0.6 to −0.3; P < .001) (Table 2 and Figure 2B). Figure 3 details medication dose changes for each medication class by arm for the 168 participants who attended week 48. Mean estimated weight loss was 3.7 kg (95% CI, −5.5 to −1.9 kg; P < .001) greater in the WM/GMV arm (−4.1 kg) compared with the GMV arm (−0.4 kg) at 48 weeks (Figure 2C). Diabetes-associated distress as measured by Problem Areas in Diabetes score decreased more in the WM/GMV arm than in the GMV arm at 16 and 32 weeks but was similar between the 2 arms at week 48 (estimated difference, –2.0; 95% CI, –6.1 to 2.2; P = .35) (Figure 2D). The estimated intervention cost per patient was $1513.42 (95% CI, $1451.34-$1575.49) for patients in the WM/GMV arm and $1264.49 (95% CI, $1204.39-$1324.59) for patients in the GMV arm.
Table 2. Estimated Means and Mean Differences (95% CI) of Outcomes for WM/GMV and GMV Arms by Time Point.
| Measurement | WM/GMV Group | GMV Group | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| HbA1c level, %a | ||||
| Baseline | 9.1 | 9.1 | NA | NA |
| 16 wk | 7.4 | 8.1 | −0.7 (−1.0 to −0.4) | NA |
| 32 wk | 7.8 | 8.3 | −0.5 (−0.8 to −0.1) | NA |
| 48 wk | 8.2 | 8.3 | −0.1 (−0.5 to 0.2) | .44 |
| Medication effect scoreb | ||||
| Baseline | 2.3 | 2.3 | NA | NA |
| 16 wk | 2.1 | 2.5 | −0.4 (−0.6 to −0.3) | NA |
| 32 wk | 2.1 | 2.6 | −0.5 (−0.7 to −0.3) | NA |
| 48 wk | 2.2 | 2.7 | −0.5 (−0.6 to −0.3) | <.001 |
| Body weight, kgc | ||||
| Baseline | 108.4 | 108.4 | NA | NA |
| 16 wk | 103.7 | 108.7 | −5.0 (−5.9 to −4.1) | NA |
| 32 wk | 102.3 | 108.5 | −6.2 (−7.6 to −4.9) | NA |
| 48 wk | 104.3 | 108.0 | −3.7 (−5.5 to −1.9) | <.001 |
| Problem Areas in Diabetes scored,e | ||||
| Baseline | 30.5 | 30.5 | NA | NA |
| 16 wk | 17.5 | 23.1 | −5.6 (−9.3 to −1.9) | NA |
| 32 wk | 17.4 | 22.5 | −5.1 (−8.7 to −1.5) | NA |
| 48 wk | 18.3 | 20.3 | −2.0 (−6.1 to 2.2) | .35 |
Abbreviations: GMV, group medical visit; HbA1c, glycated hemoglobin; NA, not applicable; WM, weight management.
Follow-up data at week 16 are missing for 36 participants (WM/GMV [n = 17] and GMV [n = 19]), at week 32 for 49 participants (WM/GMV [n = 24] and GMV [n = 25]), and at week 48 for 37 participants (WM/GMV [n = 18] and GMV [n = 19]).
Follow-up data at week 16 are missing for 68 participants (WM/GMV [n = 32] and GMV [n = 36]), at week 32 for 106 participants (WM/GMV [n = 53] and GMV [n = 53]), and at week 48 for 95 participants (WM/GMV [n = 48] and GMV [n = 47]).
Follow-up data at week 16 are missing for 41 participants (WM/GMV [n = 19] and GMV [n = 22]), at week 32 for 65 participants (WM/GMV [n = 31] and GMV [n = 34]), and at week 48 for 54 participants (WM/GMV [n = 26] and GMV [n = 28]).
Baseline data are missing for 4 GMV participants. Follow-up data at week 16 are missing for 48 participants (WM/GMV [n = 23] and GMV [n = 25]), at week 32 for 69 participants (WM/GMV [n = 33] and GMV [n = 36]), and at week 48 for 59 participants (WM/GMV [n = 26] and GMV [n = 33]).
Scores range from 0 to 100. with higher scores indicating higher levels of diabetes-related emotional distress.
Figure 3. Medication Dose Changes From Baseline for Each Diabetes Medication Class by Arm for Participants Who Attended Week 48.
DPP-4 indicates dipeptidyl peptidase 4 inhibitor; GLP-1, glucagon-like peptide-1 receptor agonist; GMV, group medical visit; SGLT-2, sodium-glucose co-transporter-2 inhibitors; and WM, weight management.
Results for dietary intake, physical activity, blood pressure, and additional laboratory measurements (fasting serum cholesterol profile and serum creatinine level) are included in eTables 1-3 in Supplement 2. High-density lipoprotein cholesterol levels improved more during 48 weeks in the WM/GMV arm than in the GMV arm. There were 27 serious adverse events in each arm; of these, 2 adverse events in the WM/GMV arm and 11 adverse events in the GMV arm were related to cardiac events. There were 2 deaths in the WM/GMV arm (1 each related to esophageal tear and cirrhosis) and 2 deaths in the GMV arm (1 each related to myocardial infarction and stroke).
Discussion
This study compared 2 GMV-based counseling approaches to diabetes management: 1 approach focusing on diabetes medication management for glycemic control (GMV) and the other focusing on intensive WM using a low-carbohydrate diet in addition to diabetes medication management (WM/GMV). Although the patients receiving the WM/GMV approach had greater initial improvement in glycemic control, by 48 weeks, glycemic control converged to the improvement seen with medication management alone. However, the WM/GMV approach led to greater weight loss, reduced diabetes medication use, and fewer hypoglycemic events during 48 weeks. When GMVs are used for diabetes, WM/GMV should be considered as an alternative, noninferior approach for glycemic management that has additional clinical advantages.
Other recent studies11,12,38 have tested lifestyle WM strategies for diabetes. In the Diabetes Remission Clinical Trial (DiRECT),38 a low-calorie meal replacement program led to weight loss of 8.8 kg compared with usual care during 12 months, as well as reduction in receiving both diabetes and hypertension medications. In a nonrandomized study11 using a similar low-carbohydrate diet approach as the present study, weight loss was 13.8 kg at 1 year, and rate of diabetes medication use other than metformin declined from 57% to 30%. Greater improvement in HbA1c level, weight loss, and diabetes medication reduction were also seen using a low-carbohydrate approach in a small randomized trial12 during 12 months. Our study contrasted with these studies by using an active comparison, with more similar attention to participants between arms, and both interventions aimed at improving HbA1c levels.
The greatest differences between the 2 approaches of the present study occurred at 16 weeks after the intensive initial phase of the WM/GMV program. At that point, the WM/GMV intervention decreased HbA1c level by 1.7% from baseline, which was 0.7% lower than the GMV arm. The WM/GMV intervention also led to a 5.6-point difference in Problem Areas in Diabetes score compared with GMV at this interim point. The improvement in diabetes distress symptoms likely was associated with the WM/GMV participants’ weight loss and medication reduction at that point since both have been associated previously with improved quality of life.39,40 A remaining question is whether these differences may have been more durable had the intervention persisted at a higher frequency (than every 2 months), which increases long-term adherence to lifestyle changes.41
Compared with the weight and medication use outcomes, the lower rate of hypoglycemic events in the WM/GMV arm was more unexpected and deserves acknowledgment. Hypoglycemia is commonly identified by people with diabetes as a cause for apprehension and is correlated with lower quality of life.42,43 In addition, fear of hypoglycemia can interfere with efforts at more intensive glycemic control.44 Because hypoglycemia is often associated with a mismatch between certain diabetes medications and carbohydrate intake, dietary change, particularly carbohydrate reduction, can reduce glycemia and diabetes medication dose concurrently, thereby reducing the chance for such a mismatch.11,12,21,45,46
Limitations
The different frequency of meetings in the 2 arms during the first 16 weeks was a limitation of this study. The more frequent meetings in the WM/GMV group was consistent with guideline recommendations for WM interventions; the lower initial GMV intervention frequency was consistent with published approaches and chosen to provide a valid comparison of 2 different diabetes management approaches, recognizing that more frequent meetings could lead to a participant perceiving either increased attention or increased burden.1,41,47 Another limitation was that study physicians could not feasibly be blinded to the study arm because recent dietary intake and weight trajectory can factor into decision-making for medication adjustment. We tried to minimize any associated bias by having multiple study physicians, using medication management algorithms, and monitoring fidelity to the algorithms. In addition, mean weight change was modest in the WM/GMV arm, and target HbA1c levels per guidelines were not achieved in either group48; however, the changes observed were adequate to lead to health improvements. The study’s low enrollment rate raises concerns about generalizability, but people with uncontrolled diabetes have known barriers to health care that also may limit enrollment in research.49 In this study at a major referral center, distance from the intervention site was an additional barrier to recruitment.
Conclusions
The results from this study indicate that intensive WM using a low-carbohydrate diet can be as effective for glycemic improvement as medication intensification in GMVs. Furthermore, the WM program had additional benefits by reducing weight, medication burden, and hypoglycemic events. Given these benefits and the knowledge that lifestyle changes can be difficult to maintain,50 we should continue to develop strategies that help patients sustain these improvements.
Trial Protocol
eMethods. Statistical Methods Technical Appendix
eTable 1. Mean (SD) Calorie and Macronutrient Intake for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
eTable 2. Minutes of Activity Per Week for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
eTable 3. Estimated Means and Mean Differences (95% CI) of Clinical and Laboratory Outcomes for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
Data Sharing Statement
References
- 1.Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW Jr. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Dept of Veterans Affairs; 2012. [PubMed] [Google Scholar]
- 2.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185(13):E635-E644. doi: 10.1503/cmaj.130053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi: 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63. doi: 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross SA, Dzida G, Vora J, Khunti K, Kaiser M, Ligthelm RJ. Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin. 2011;27(7):1431-1438. doi: 10.1185/03007995.2011.585396 [DOI] [PubMed] [Google Scholar]
- 6.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117(10):762-774. doi: 10.1016/j.amjmed.2004.05.024 [DOI] [PubMed] [Google Scholar]
- 7.Kramer H, Reboussin D, Bertoni AG, et al. ; Look Ahead Research Group . Obesity and albuminuria among adults with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2009;32(5):851-853. doi: 10.2337/dc08-2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn MW, Budiman-Mak E, Lee TA, Oh E, Stuck RM. Significant J-shaped association between body mass index (BMI) and diabetic foot ulcers. Diabetes Metab Res Rev. 2011;27(4):402-409. doi: 10.1002/dmrr.1193 [DOI] [PubMed] [Google Scholar]
- 9.Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91-100. doi: 10.1016/j.jada.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Franz MJ, Powers MA, Leontos C, et al. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110(12):1852-1889. doi: 10.1016/j.jada.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 11.Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi: 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saslow LR, Daubenmier JJ, Moskowitz JT, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. 2017;7(12):304. doi: 10.1038/s41387-017-0006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley MJ, Edelman D, Voils CI, et al. Jump starting shared medical appointments for diabetes with weight management: rationale and design of a randomized controlled trial. Contemp Clin Trials. 2017;58:1-12. doi: 10.1016/j.cct.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borushek A. The Calorie King Calorie, Fat & Carbohydrate Counter 2013. Costa Mesa, CA: Family Health Publications; 2013. [Google Scholar]
- 15.Haskell WL, Lee IM, Pate RR, et al. ; American College of Sports Medicine; American Heart Association . Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081-1093.doi: 10.1161/CIRCULATIONAHA.107.185649 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(suppl 1):S14-S80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 17.Westman EC, Phinney SD, Volek JS. The New Atkins for a New You. New York, NY: Fireside; 2010. [Google Scholar]
- 18.Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. 2002;113(1):30-36. doi: 10.1016/S0002-9343(02)01129-4 [DOI] [PubMed] [Google Scholar]
- 19.Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140(10):769-777. doi: 10.7326/0003-4819-140-10-200405180-00006 [DOI] [PubMed] [Google Scholar]
- 20.Yancy WS Jr, Westman EC, McDuffie JR, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170(2):136-145. doi: 10.1001/archinternmed.2009.492 [DOI] [PubMed] [Google Scholar]
- 21.Mayer SB, Jeffreys AS, Olsen MK, McDuffie JR, Feinglos MN, Yancy WS Jr. Two diets with different haemoglobin A1c and antiglycaemic medication effects despite similar weight loss in type 2 diabetes. Diabetes Obes Metab. 2014;16(1):90-93. doi: 10.1111/dom.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zammitt NN, Streftaris G, Gibson GJ, Deary IJ, Frier BM. Modeling the consistency of hypoglycemic symptoms: high variability in diabetes. Diabetes Technol Ther. 2011;13(5):571-578. doi: 10.1089/dia.2010.0207 [DOI] [PubMed] [Google Scholar]
- 23.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245-1249. doi: 10.2337/diacare.28.5.1245 [DOI] [PubMed] [Google Scholar]
- 24.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760. doi: 10.2337/diacare.18.6.754 [DOI] [PubMed] [Google Scholar]
- 25.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69-72. doi: 10.1046/j.1464-5491.2003.00832.x [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 27.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152-1160. doi: 10.1001/jama.295.10.1152 [DOI] [PubMed] [Google Scholar]
- 28.Pocock SJ. The pros and cons of noninferiority trials. Fundam Clin Pharmacol. 2003;17(4):483-490. doi: 10.1046/j.1472-8206.2003.00162.x [DOI] [PubMed] [Google Scholar]
- 29.Little R, Kang S. Intention-to-treat analysis with treatment discontinuation and missing data in clinical trials. Stat Med. 2015;34(16):2381-2390. doi: 10.1002/sim.6352 [DOI] [PubMed] [Google Scholar]
- 30.Goldstein H. Multilevel Statistical Models 3rd ed. London: United Kingdom: Hodder Arnold; 2003. [Google Scholar]
- 31.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716-723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 32.Lenters-Westra E, Schindhelm RK, Bilo HJ, Groenier KH, Slingerland RJ. Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Neth J Med. 2014;72(9):462-466. [PubMed] [Google Scholar]
- 33.Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) Steering Committee . Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi: 10.1373/clinchem.2010.148841 [DOI] [PubMed] [Google Scholar]
- 34.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons Inc; 2006. [Google Scholar]
- 35.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234-1238. doi: 10.1016/j.jclinepi.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37.Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 38.Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541-551. doi: 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 39.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478-2483. doi: 10.2337/dc07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin RR, Wadden TA, Bahnson JL, et al. ; Look AHEAD Research Group . Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37(6):1544-1553. doi: 10.2337/dc13-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(11):1163-1171. doi: 10.1001/jama.2018.13022 [DOI] [PubMed] [Google Scholar]
- 42.Lavernia F, Kushner P, Trence D, Rice D, Dailey G, Kuritzky L. Recognizing and minimizing hypoglycemia: the need for individualized care. Postgrad Med. 2015;127(8):801-807. doi: 10.1080/00325481.2015.1086628 [DOI] [PubMed] [Google Scholar]
- 43.Lopez JM, Annunziata K, Bailey RA, Rupnow MF, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer Adherence. 2014;8:683-692. doi: 10.2147/PPA.S58813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leiter LA, Yale J-F, Chiasson J-L, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29:186-192. [Google Scholar]
- 45.Westman EC, Yancy WS Jr, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5:36. doi: 10.1186/1743-7075-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yancy WS Jr, Foy M, Chalecki AM, Vernon MC, Westman EC. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34. doi: 10.1186/1743-7075-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25)(suppl 2):S102-S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Diabetes Association 6. Glycemic targets: Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42(suppl 1):S61-S70. doi: 10.2337/dc19-S006 [DOI] [PubMed] [Google Scholar]
- 49.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1-9. doi: 10.1016/j.diabres.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 50.Ludwig DS, Ebbeling CB. Weight-loss maintenance—mind over matter? N Engl J Med. 2010;363(22):2159-2161. doi: 10.1056/NEJMe1011361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Statistical Methods Technical Appendix
eTable 1. Mean (SD) Calorie and Macronutrient Intake for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
eTable 2. Minutes of Activity Per Week for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
eTable 3. Estimated Means and Mean Differences (95% CI) of Clinical and Laboratory Outcomes for Weight Management/Group Medical Visit (WM/GMV) and Group Medical Visit (GMV) Arms by Time Point
Data Sharing Statement