Abstract
Background:
Pulmonary disease remains the primary cause of morbidity and mortality for individuals with cystic fibrosis (CF). Variants at a locus on the X-chromosome containing the type 2 angiotensin II receptor gene (AGTR2) were identified by a large GWAS as significantly associating with lung function in CF patients. We hypothesized that manipulating the angiotensin-signaling pathway may yield clinical benefit in CF.
Methods:
Genetic subset analysis was conducted on a local CF cohort to extend the GWAS findings. Next, we evaluated pulmonary function in CF mice with a deleted AGTR2 gene, and in those who were given subcutaneous injections of PD123,319, a selective AGTR2 antagonist for 12 weeks beginning at weaning.
Results:
The genetic subset analysis replicated the initial GWAS identified association, and confirmed the association of this locus with additional lung function parameters. Studies in genetically modified mice established that absence of the AGTR2 gene normalized pulmonary function indices in two independent CF mouse models. Further, we determined that pharmacologic antagonism of AGTR2 improved overall pulmonary function in CF mice to near wild-type levels.
Conclusions:
These results identify that reduced AGTR2 signaling is beneficial to CF lung function, and suggest the potential of manipulating the angiotensin-signaling pathway for treatment and/or prevention of CF pulmonary disease. Importantly, the beneficial effects were not CF gene mutation dependent, and were able to be reproduced with pharmacologic antagonism. As there are clinically approved drugs available to target the renin-angiotensin signaling system, these findings may be quickly translated to human clinical trials.
Introduction
While cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR)1, there is large natural variability in CF pulmonary disease. Heritability studies indicate that 50-80% of this variation is due to genetic differences not related to CFTR.2 A genome-wide association study (GWAS) conducted by the International Cystic Fibrosis Gene Modifier Consortium identified variants within an interval on the X chromosome containing the type 2 angiotensin II receptor gene (AGTR2) as significantly associating with a longitudinal measure of lung function based on forced expiratory volume in 1 second (FEV1) in individuals with CF.3 This analysis identified multiple single nucleotide polymorphisms (SNPs) in the interval on the X chromosome containing AGTR2 that were in linkage disequilibrium, all associating with CF pulmonary disease severity. The most significantly associating SNP in this region was rs5952223, a C/T polymorphism with a minor allele frequency of 0.26.
AGTR2 is part of the renin-angiotensin signaling (RAS) pathway that has been widely studied for its role in blood-pressure regulation and in renal and cardiovascular health. Much less is known about its involvement in pulmonary function and disease. In the airway, the precursor, angiotensinogen, is secreted by airway smooth muscle cells and apoptotic epithelial cells, and cleaved to angiotensin I by renin. Angiotensin converting enzyme (ACE) further cleaves angiotensin I to the biologically active angiotensin II, which can then signal through two receptors, the type-1 angiotensin II receptor (AGTR1) and AGTR2.
In addition to the GWAS identification of AGTR2-linked loci as modifiers of CF pulmonary disease, there is evidence that the RAS pathway is altered in CF. For example, CF patients demonstrate increased activation of RAS in response to salt deprivation that is thought to compensate for salt depletion and prevent dehydration.4 However, increased serum ACE levels are associated with a more rapid decline in pulmonary function in CF patients5, suggesting that decreasing angiotensin signaling may be beneficial. To establish the viability of manipulating the angiotensin signaling pathway for clinical benefit in CF, we extended the GWAS findings by confirming the association of this X-chromosome locus with additional lung function parameters. Using genetically modified mice, we examined the effect of absence of the AGTR2 gene on pulmonary function indices in CF mice to identify whether loss of AGTR2 would be beneficial or deleterious. Subsequently, we examined the impact of AGTR2 pharmacologic intervention for CF lung disease treatment, establishing the translational relevance of manipulating the angiotensin-signaling pathway for treatment and/or prevention of CF pulmonary disease.
Methods
Human CF cohort.
All research subjects were cared for at the Rainbow Babies and Children’s Hospital Cystic Fibrosis Center in Cleveland, Ohio between 2005 and 2013. Individuals with CF who chose to participate were consented for study enrollment during routine outpatient clinic visits and provided buccal swabs for research purposes. Specimens were matched with pulmonary function data available from a curated research database which contains phenotypic data (including spirometry values). All available values of FEV1 percent predicted, FVC percent predicted, and FEF25-75 percent predicted were included for each participant; the number of individual measurements for each subject varied.
Human genotyping.
DNA extraction was preformed immediately upon collection of the buccal swab using a Qiagen DNeasy kit (Qiagen) following the manufacturer’s protocol. Resultant DNA was stored at −20°C for batch analysis. Genotyping was performed using custom Taqman SNP genotyping assays to the AGTR2 SNP rs5952223 (AB Life Technologies) on a Taqman StepOne plus thermocycler per manufacturer instructions. Genotype assignments were made using Applied Biosystems Taqman Genotyper software.
Statistical analysis of human pulmonary function.
All spirometric variables and changes over time were examined using box plots and LOWESS line plots over time. Frequency analysis was used to check and validate data quality. Distributions of spirometry measurements (FEV1 percent predicted, FVC percent predicted, and FEF25-75 percent predicted) were found to be skewed and unable to be transformed into symmetrical distributions. Thus, we used simultaneous quantile (median) regression analysis for studying effects of AGTR2 genotype on these longitudinal outcome variables. For this analysis, AGTR2 genotype was coded as risk genotype (C [males] or CC [females]) or low-risk genotypes (CT [females], T [males] or TT [females]). To capture the correlations among the repeated measurements, we used clustering in time of the measurements and computed the standard errors of the estimates using a bootstrap method. We performed 500 bootstraps for estimating the standard error of the estimates. Point estimates are presented as well as normal-based 95% confidence intervals (CI) of the AGTR2 genotype coefficient and other regression coefficients in the multivariable or adjusted quantile regression models. All the analyses were performed using statistical software Stata 15.0.
Mouse models.
Several genetically altered mouse models were utilized in these studies. The mice harbored either the F508del Cftr mutation (Cftrtm1kth)6, the R117H Cftr mutation (Cftrtm2Uth)7 or an Agtr2 mutation (Agtr2tm1Tin)8. In addition, the Agtr2 and Cftr mutations were bred on the same background to give an F508del/Agtr2 strain and the R117H/Agtr2 strain. Wild type (WT) littermates from each strain were used as controls. Mice were allowed unrestricted access to chow (Harlan Teklad 7960; Harlan Teklad Global Diets, Madison, WI) and sterile water and were maintained on a 12 hour light/dark cycle at a mean ambient temperature of 22°C. Mice with Cftr mutations were given access to sterile water with an osmotic laxative, Colyte (Schwarz Pharma, Milwaukee, WI), to decrease the incidence of intestinal obstruction due to loss of CFTR function.
Respiratory mechanics.
Lung mechanics measurements were made using automated maneuvers and analysis algorithms as described previously using a computer-controlled mechanical ventilator (flexiVent system, SCIREQ).9 Briefly, following induction of anesthesia via intraperitoneal injection of ketamine (150 mg/kg) and xylazine (15 mg/kg), mice were intubated and mechanically ventilated at a rate of 150 breaths/minute with tidal volumes of 10mL/kg and positive end-expiratory pressure of 3 cmH2O. Single-frequency forced oscillation maneuvers were used to calculate overall respiratory system compliance (Crs). Automated measurements made during a broadband low-frequency forced oscillation maneuver with a mixed frequency forcing function comprised of multiple prime frequencies ranging from 0.25 to 20Hz were fit to a constant phase model to determine tissue damping (G) and tissue elastance (H). Parameters from individual data sets were included in the analysis if the coefficient of determination assessing the fit of the model to the experimental data was ≥0.95. Pressure-volume curves were produced by stepped increases and subsequent decreases in airway pressure. Static lung compliance (Cst) was calculated from the slope of each curve and a shape parameter (K) describing the deflation limb of the pressure-volume loop was quantified.
Histology.
Following CO2 euthanasia in separate groups of animals, lungs were pressure inflated to 30cmHg with 10% buffered formalin for 30 minutes. Resected lungs were fixed in 10% buffered formalin for ten days and subsequently embedded in paraffin prior to sectioning in 150μm increments at a thickness of 5μm. Lung sections were mounted on glass slides and stained with hematoxylin/eosin (H&E) for assessments of distal airspaces.
Distal airspace enlargement.
A Mean Linear Intercept (MLI, Lm) calculation was used to quantitatively assess distal airspace enlargement using an indirect method as described previously.9 Briefly, software generated a test-line across multiple 20x fields of view representing ~5% of the total lung area. The Lm was determined by calculating the ratio of line endpoints within parenchyma, including airspaces and ductal spaces [P(A) + P(duct)], to points intersecting the septum [I(A)] across the known-length test line (d) (excluding nonparenchyma structures) 10, as indicated by the following formula 11: Lm = d · [P(A) + P(duct)]/I(A)
AGTR2 pharmacologic inhibition.
Weanling wild-type and F508del mice were weighed at 3 weeks of age for dosing of AGTR2 inhibitor PD123,319 (Sigma). PD123,319 has been well established as an AGTR2 antagonist.12-14 Lyophilized PD123,319 was resuspended in filtered 0.9% saline. Injections were performed with 27G TB syringe (BD) subcutaneously in the interscapular region every 24 hours with 2mg/kg/day (5-day-a-week protocol) for 12 weeks. Weights were recorded bi-weekly during weanling growth phases, and once weekly at 6 weeks of age to maintain dosing accuracy. The administered volume was diluted as necessary to deliver an accurate and consistent weight-based bolus between 200 and 400uL throughout the treatment course.
Statistical analysis of murine data.
Descriptive statistics were used to summarize pulmonary function measurements and MLI distances in mice. Differences in group means were quantified by analysis of variance (ANOVA) using the statistical software package SigmaStat. When significant differences were detected, a pairwise comparison of the means of each combination of groups was conducted assuming equal variances. The Student-Newman-Keuls procedure was used to account for all pairwise comparisons. Corrected p-values ≤ 0.05 were considered significant.
Study approval.
Relevant human studies were approved by the University Hospitals Cleveland Medical Center Institutional Review Board (protocol 11-67-200). Informed consent was obtained from all participants. Mouse studies were approved by the Case Western Reserve University Institutional Care and Use Committee under protocol 2015-0181.
Results
The rs5952223 association was evaluated in a local CF cohort of 126 participants in whom additional measures of lung function were available. The mean (±SD) age at enrollment was 9.3±2.3 years, and 46.8% of the participants were male. No subjects were excluded because of race or ethnic background; 96.7% of the patients were self-identified as white. Basic demographic and clinical characteristics of the participants are detailed in Table 1. The risk genotype of rs5952223 that associated with worse pulmonary function in the previous GWAS (C [males] or CC [females]) was also significantly associated with lower FEV1 percent predicted values in this population (7.1% lower, 95% Confidence Interval (CI), −5.1 to −9.0). In addition, the same risk genotype was significantly associated with a reduction in the percent predicted values of forced vital capacity (FVC) (4.4% lower, 95% CI, −2.5 to −6.2) and forced expiratory flow over the middle one half of the FVC (FEF25-75) (5.3% lower, 95% CI, −2.2 to −8.5). These findings, summarized in Table 2, are consistent with the prior GWAS analyses.3, 15 Moreover, they extend the association between genetic variation in this region and two additional, functionally related measures of pulmonary function and replicate the genetic findings in a younger cohort.
Table 1.
Cystic Fibrosis Patient Cohort Characteristics
| Characteristics | Total Study Population | rs5952223 (C/CC) risk genotype |
rs5952223 (CT/T/TT) non-risk genotypes |
|---|---|---|---|
| N | 126 | 73 | 53 |
| Male Sex - n (%) | 59 (46.8) | 36 (49.3) | 23(43.4) |
| Age - years | 9.3 ± 2.3 | 8.9±1.7 | 9.9±2.8 |
| FEV 1 (% predicted) | 83.7 ± 23.4 | 80.8 ± 22.4 | 87.6 ± 24.4 |
| FVC (% predicted) | 96.9 ± 18.4 | 95.8 ± 16.2 | 98.6 ± 21.2 |
| FEF 25-75 (% predicted) | 69.6 ± 31.3 | 65.8 ± 28.3 | 74.7 ± 34.6 |
Table 2.
AGTR2 Risk Genotype Associates with Lower Spirometry Measurements in Individuals with CF
| Pulmonary Function | Regression Coefficient |
Bootstrap Standard Error |
z-score | p-value | 95% Confidence Interval |
|---|---|---|---|---|---|
| FEV 1 (% predicted) | −7.1* | 1.01 | 7.01 | <0.001 | −5.09 – −9.04 |
| FVC (% predicted) | −4.4* | 0.93 | 4.67 | <0.001 | −2.52 – −6.18 |
| FEF 25-75 (% predicted) | −5.3* | 1.6 | 3.32 | 0.001 | −2.18 – −8.47 |
= risk genotype (C/CC) worse than low-risk genotype (CT/T/TT)
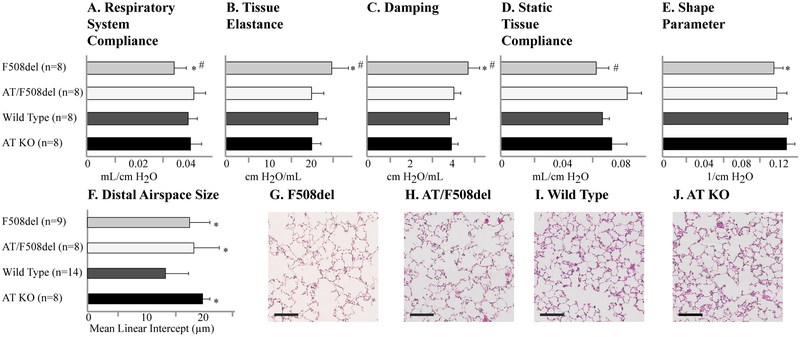
To further establish whether AGTR2 is mechanistically linked to lung function in CF, we crossed a CF mouse model (F508del; Cftrtm1Kth)6 with an Agtr2 knock out mouse model (Agtr2tm1Tin).8 Overall survival was not impacted in the resultant CF/Agtr2 double mutants (F508del/Agtr2 KO). Our objective was to determine if absence of Agtr2 would either positively or negatively impact the pulmonary phenotype in a mouse model of early CF lung disease.9, 16, 17 Using a computer-controlled mechanical ventilator, automated maneuvers showed that the absence of Agtr2 restores respiratory system compliance, tissue elastance, tissue damping and static tissue compliance to near wild type (WT) levels in F508del mice (Figure 1). In contrast, absence of Agtr2 did not improve distal airspace enlargement or the delayed stepwise lung deflation observed in CF mice.9 Thus, these results indicate that some but not all CF-specific changes in the distal lung compartment are improved by Agtr2 inactivation (Figure 1).
Figure 1.
An inactivating deletion in AGTR2 prevents aspects of pulmonary dysfunction in F508del CF mice. (A-E) Lung function and (F-J) distal airspace size were quantified in F508del CF mice, F508del CF mice with the genetic deletion of the AGTR2 gene (double knockout: AT/F508del), wild type C57bl/6 mice and AGTR2 knockout mice with wild type CFTR (AT KO). (G-J) Representative histologic images are displayed (size bars indicate 100 microns). Data are presented as means, with error bars denoting standard deviations. *P<0.05 compared to wild type; #P<0.05 compared to AT/F508del.
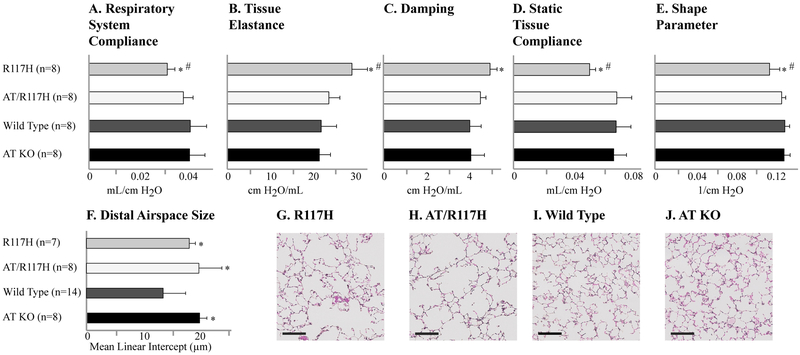
To ensure that the restoration of pulmonary function resulting from the absence of Agtr2 was not specific to the F508del mouse model, we crossed the Agtr2 KO mice with a second, independent mouse model with a human CFTR mutation (R117H; Cftrtm2Uth).7 We observed a similarly beneficial effect on pulmonary function in the R117H/Agtr2 KO mice (Figure 2), suggesting that reduction in Agtr2 may impact lung pathophysiology via a CFTR-genotype independent mechanism. Taken together, these findings support the idea that genetic inactivation of AGTR2 is beneficial to lung function in CF.
Figure 2.
An inactivating deletion in AGTR2 aspects of pulmonary dysfunction in R117H CF mice. (A-E) Lung function and (F-J) distal airspace size were also quantified in CF mice homozygous for the R117H CFTR mutation (R117H), and compared to CF mice with the genetic deletion of the AGTR2 gene (double knockout: AT/R117H), wild type C57bl/6 mice and AGTR2 knockout mice with wild type CFTR (AT KO). (G-J) Representative histologic images are displayed (size bars indicate 100 microns). Data are presented as means, with error bars denoting standard deviations. *P<0.05 compared to wild type; #P<0.05 compared to AT/F508del.
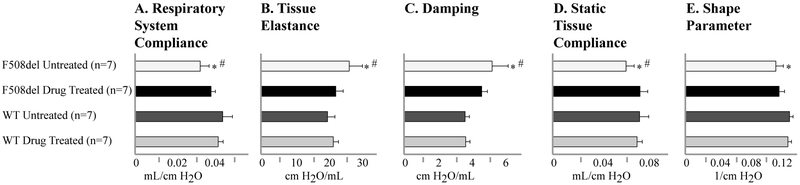
To extend these pre-clinical studies and test whether the improvements in lung function could be achieved with pharmacologic inhibition of Agtr2 function, we treated WT and F508del mice by subcutaneous injections of a selective Agtr2 antagonist (PD123,319).12-14 A 5-day-a-week regimen (2mg/kg/day) was started after weaning (age 3-4 weeks) and was continued for 12 weeks. Following this pharmacologic intervention, CF mice had lung function that was not significantly different from WT levels, and was similar to what was achieved by complete genetic inactivation of the Agtr2 gene (Figure 3). In contrast, Agtr2 antagonism had no detectable effect on lung function in WT mice.
Figure 3.
Pharmacologic antagonism of AGTR2 prevents the development of CF-related pulmonary dysfunction. (A-E) Lung function was quantified in drug treated F508del CF mice and compared to untreated CF mice and wild type C57bl/6 mice (both treated and untreated). *P<0.05 compared to wild type; #P<0.05 compared to AT/F508del.
Discussion
The objective of this study was to establish the potential for manipulation of angiotensin signaling as a novel therapeutic strategy to be pursued for the treatment of CF pulmonary disease. Key findings include: 1) replicating the prior GWAS analysis on a younger CF cohort; 2) extending the genetic association of the X-chromosome locus containing AGTR2 with additional pulmonary function parameters in CF patients (FVC and FEF25-75 percent predicted); 3) determining that an inactivating deletion of Agtr2 prevents some but not all pathologic distal lung compartment phenotypes in a mouse model of early CF lung disease; 4) establishing that the beneficial impact of Agtr2 absence on pulmonary function is independent of the CFTR mutation; and 5) identifying that pharmacologic antagonism of Agtr2 function yielded similarly beneficial effects in CF mice.
The GWAS analyses that motivated this work included genotyping over 8 million genetic variants in over 6,000 individuals with CF from 3 independent cohorts with an average age of 19.5±9.4 years.3 Our findings, although based on a smaller cohort of CF individuals, extended the association of the X-chromosome locus to additional lung function parameters. This increases the likelihood that this locus may be acting as a modifier of pulmonary function in CF. Further, the replication of the association in younger individuals suggests the modifying effects may be occurring early in the disease process. Although the AGTR2 gene is located on the X-chromosome, males and females were combined in this analysis because there is no evidence of differential expression of the AGTR2 gene in males and females.18 There are important limitations to any genetic association study including difficulties controlling for socioeconomic status, environmental factors and other non-genetic modifiers of CF lung disease. This X-chromosome region has been examined in CF related diabetes (CFRD) and was not found to be associated, but its potential association with other aspects of CF pathophysiology are unknown.19 Future studies will include an examination of the additional GWAS-identified modifying regions in the cohort, as well as other aspects of CF-related phenotypes such as pseudomonas colonization.
While GWAS analyses are hypothesis-generating, genetic association does not prove a gene’s involvement as a genetic modifier of the examined phenotype. The region on the X-chromosome associating with CF lung disease was located in a non-coding region 3’ of the AGTR2 gene. This suggests that the modifying effect is likely due to variation in gene regulation rather than a difference in protein coding sequence. Further, the genetic analyses do not indicate whether increasing or decreasing expression of a candidate gene like AGTR2 would be beneficial. We determined that an inactivating deletion of Agtr2 in two independent CF mouse models improved a CF-specific distal lung compartment phenotype of decreased respiratory system compliance, increased tissue elastance and damping, and reduced static tissue compliance. Importantly, there is high homology (95.3%) between mouse and human AGTR2, underscoring a potential relevance to human disease. Taken together, these findings established that AGTR2 is a modifier of lung function in CF mice independent of CFTR mutation type, and more importantly, determined that reduced AGTR2 was beneficial.
The CF mouse exhibits a complex pulmonary phenotype characterized by changes in lung mechanics, increased respiratory rate, histological differences including distal airspace enlargement, exaggerated inflammatory cell recruitment and altered responses to infection and other pathologic stimuli.7, 9, 16, 17, 20-22 However, the mouse model has relevant limitations. For example, although CF mice do develop spontaneous pulmonary infections,23 the involved microorganisms are not those typically observed in CF patients. Further, the changes in lung mechanics observed in CF mice are mild compared to human adult CF lung disease,9 and the CF mouse does not naturally progress to more severe disease pathology, including bronchiectasis and mucus plugging of the airways. However, it has been suggested that the pulmonary phenotype observed in the CF mouse may serve as an animal model of early CF lung disease, which is now recognized as an important time period for clinical intervention.24-31 The association of the genetic region studied with small airways disease (decreased FEF25-75) is consistent with this perspective. Decreased respiratory system compliance in the CF mouse indicates that additional work will be required to exchange a given volume of air during breathing. Increased tissue elastance and tissue damping localizes the phenotype to the peripheral lung compartment.9 A similar phenotype has been reported in CF patients with spirometry within normal limits.32-34
Complete genetic ablation of the Agtr2 gene was beneficial in a CF mouse, but this approach is not translationally viable. The data presented here indicate that the AGTR2 antagonist administered beginning at a young age results in similar improvements in pulmonary function compared to the genetic models. Furthermore, this pharmacologic approach to treatment may potentially benefit all CF patients, regardless of CFTR genotype, as our genetic results indicated improvements in CF mouse models with two independent CFTR mutations.
Pharmacologic agents targeting the renin-angiotensin signaling pathway exist, and therefore translation of our findings to clinical benefit may be a possibility. Though there is not currently an FDA-approved antagonist for AGTR2, ACE inhibitors would likely result in reduced AGTR2 activity, as they reduce the amount of ligand available. In contrast, angiotensin receptor blockers (ARBs) selectively antagonize AGTR1 and thus may result in increased angiotensin II binding to AGTR2, raising the possibility that they could even worsen lung function in CF patients. Although there is still a great deal to be understood about this pathway in relationship to CF lung disease, clinicians might exercise caution when selecting agents to treat hypertension in CF patients. Careful clinical trials will be required to evaluate the efficacy of AGTR2 antagonism in humans, and to determine optimal dose, timing, threshold for initiation, and synergy with other medications for either ACE inhibitors, or clinical application of AGTR2 blockers as therapies for CF lung disease.
While the results reported here provide an exciting opportunity for potential therapeutic development, the mechanism of these benefits remains unknown. The renin-angiotensin signaling pathway is involved in tissue growth, inflammation, apoptosis, tissue repair, and vascular responsiveness. AGTR2 expression has been localized in the lung, where is it found in bronchi35 and airway epithelial cells, mucous glands, vascular endothelial cells, fibroblasts, chondrocytes, and macrophages.36 Increased AGTR2 signaling has been shown to be detrimental in other pulmonary diseases. AGTR2 antagonism attenuates lung fibrosis37 and decreases migration and proliferation of fibrotic fibroblasts38 in bleomycin-induced murine lung fibrosis. The expression of both angiotensin II receptors (AGTR1 and AGTR2) is increased in the lungs of human adults with chronic obstructive pulmonary disease (COPD) compared to both healthy controls and non-COPD smokers.36 The lung tissue from individuals with idiopathic pulmonary fibrosis also had elevated amounts of both receptor types, with a relatively greater increase in AGTR2.38 Further, hypoxia-induced collagen synthesis of human lung fibroblasts is attenuated by the antagonism of AGTR2.39 A meta-analysis of asthma susceptibility genes in the Chinese population identified an insertion/deletion polymorphism in ACE as one of seven SNPs significantly associated with the risk of asthma.40 Studies in rats have identified activation of the p42/44 signaling pathway by angiotensin as a part of the hyper-responsiveness of the bronchial smooth muscle.41 While other data also support that AGTR2 signaling contributes to the pathogenesis of lung disease,36, 38, 42, 43 the mechanism behind these contributions remains elusive. Here we show reduction of AGTR2 signaling may be effective for the treatment of CF lung disease, though the exact mechanism of this association will be the focus of future studies.
In conclusion, our data indicate that impeding AGTR2 signaling is beneficial to CF lung disease in a mouse model. These studies are an exciting example of validation of a novel therapeutic target identified by GWAS. Successfully determining the direction of effect and establishing the feasibility of pharmacologic manipulation of the signaling pathway in a pre-clinical model set the stage for first-in-human trials of AGTR2 blockade for treatment and prevention of CF lung disease.
Supplementary Material
Acknowledgements
We are grateful to the individuals with cystic fibrosis and their families for contributing to this study. We thank John Dunn for assistance with preparation of the figures. This work was supported by Cystic Fibrosis Foundation grants DARRAH17PO and DARRAH1610 (to RJD), as well as DRUMM15RO and DRUMM15R1 (to MLD); Gilead Sciences Research Scholars Program in Cystic Fibrosis award (to RJD); as well as VA Research Service BLR&D Merit Review Award I01BX000873 (to FJJ).
Footnotes
Conflicts of Interest
Authors Darrah, Jacono, Mitchell and Drumm are named on a patent application for the use of AGTR2 inhibition as a therapy for CF lung disease. The authors declare no other relevant conflicts of interest.
References
- 1.Ong T and Ramsey BW, Update in Cystic Fibrosis 2014. American journal of respiratory and critical care medicine, 2015. 192(6): p. 669–75. [DOI] [PubMed] [Google Scholar]
- 2.Vanscoy LL, et al. , Heritability of lung disease severity in cystic fibrosis. American journal of respiratory and critical care medicine, 2007. 175(10): p. 1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corvol H, et al. , Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nature communications, 2015. 6: p. 8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legris GJ, et al. , Sodium space and intravascular volume: dietary sodium effects in cystic fibrosis and healthy adolescent subjects. Pediatrics, 1998. 101(1 Pt 1): p. 48–56. [DOI] [PubMed] [Google Scholar]
- 5.Arkwright PD, et al. , End-organ dysfunction in cystic fibrosis: association with angiotensin I converting enzyme and cytokine gene polymorphisms. American journal of respiratory and critical care medicine, 2003. 167(3): p. 384–9. [DOI] [PubMed] [Google Scholar]
- 6.Zeiher BG, et al. , A mouse model for the delta F508 allele of cystic fibrosis. The Journal of clinical investigation, 1995. 96(4): p. 2051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heeckeren AM, et al. , Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. American journal of physiology. Lung cellular and molecular physiology, 2004. 287(5): p. L944–52. [DOI] [PubMed] [Google Scholar]
- 8.Ichiki T, et al. , Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature, 1995. 377(6551): p. 748–50. [DOI] [PubMed] [Google Scholar]
- 9.Darrah RJ, et al. , Early pulmonary disease manifestations in cystic fibrosis mice. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society, 2016. 15(6): p. 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Cai BQ, and Zhu YJ, Pathogenesis of cigarette smoke-induced chronic obstructive pulmonary disease and therapeutic effects of glucocorticoids and N-acetylcysteine in rats. Chinese medical journal, 2004. 117(11): p. 1611–9. [PubMed] [Google Scholar]
- 11.Knudsen L, et al. , Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. Journal of applied physiology, 2010. 108(2): p. 412–21. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Anand P, and Rice AS, Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: preclinical and clinical studies. Pain, 2016. 157 Suppl 1: p. S33–41. [DOI] [PubMed] [Google Scholar]
- 13.Porter AJ, et al. , The angiotensin converting enzyme inhibitor, captopril, prevents the hyperactivity and impulsivity of neurokinin-1 receptor gene ‘knockout’ mice: Sex differences and implications for the treatment of attention deficit hyperactivity disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MT, Wyse BD, and Edwards SR, Small molecule angiotensin II type 2 receptor (AT(2)R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. Pain medicine, 2013. 14(5): p. 692–705. [DOI] [PubMed] [Google Scholar]
- 15.Wright FA, et al. , Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nature genetics, 2011. 43(6): p. 539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent G, et al. , Lung disease in mice with cystic fibrosis. The Journal of clinical investigation, 1997. 100(12): p. 3060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JC, et al. , The “Goldilocks effect” in cystic fibrosis: identification of a lung phenotype in the cftr knockout and heterozygous mouse. BMC genetics, 2004. 5: p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tukiainen T, et al. , Landscape of X chromosome inactivation across human tissues. Nature, 2017. 550(7675): p. 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackman SM, et al. , Genetic modifiers of cystic fibrosis-related diabetes. Diabetes, 2013. 62(10): p. 3627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruscia EM, et al. , Increased susceptibility of Cftr−/− mice to LPS-induced lung remodeling. American journal of physiology. Lung cellular and molecular physiology, 2016. 310(8): p. L711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonfield TL, et al. , Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. Journal of leukocyte biology, 2012. 92(5): p. 1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darrah RJ, et al. , Ventilatory pattern and energy expenditure are altered in cystic fibrosis mice. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society, 2013. 12(4): p. 345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darrah R, Bonfield T, LiPuma JJ, Litman P, Hodges CA, Jacono F, and Drumm M, Cystic Fibrosis Mice Develop Spontaneous Chronic Bordetella Airway Infections. Journal of Infectious Pulmonary Diseases, 2017. 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan S, et al. , Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax, 2005. 60(2): p. 159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren CL, et al. , Analysis of the associations between lung function and clinical features in preschool children with cystic fibrosis. Pediatric pulmonology, 2012. 47(6): p. 574–81. [DOI] [PubMed] [Google Scholar]
- 26.Marostica PJ, et al. , Spirometry in 3- to 6-year-old children with cystic fibrosis. American journal of respiratory and critical care medicine, 2002. 166(1): p. 67–71. [DOI] [PubMed] [Google Scholar]
- 27.Vilozni D, et al. , Spirometry in early childhood in cystic fibrosis patients. Chest, 2007. 131(2): p. 356–61. [DOI] [PubMed] [Google Scholar]
- 28.Castile RG, Iram D, and McCoy KS, Gas trapping in normal infants and in infants with cystic fibrosis. Pediatric pulmonology, 2004. 37(5): p. 461–9. [DOI] [PubMed] [Google Scholar]
- 29.Beydon N, et al. , Pulmonary function tests in preschool children with cystic fibrosis. American journal of respiratory and critical care medicine, 2002. 166(8): p. 1099–104. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TT, et al. , Evolution of lung function during the first year of life in newborn screened cystic fibrosis infants. Thorax, 2014. 69(10): p. 910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerby GS, et al. , Lung function distinguishes preschool children with CF from healthy controls in a multi-center setting. Pediatric pulmonology, 2012. 47(6): p. 597–605. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowska WJ, et al. , Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. American journal of respiratory and critical care medicine, 2008. 178(1): p. 42–9. [DOI] [PubMed] [Google Scholar]
- 33.Hoo AF, et al. , Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax, 2012. 67(10): p. 874–81. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey BW, et al. , Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report. American journal of respiratory and critical care medicine, 2012. 185(8): p. 887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavel J, et al. , Increased angiotensin II AT1 receptor mRNA and binding in spleen and lung of AT2 receptor gene disrupted mice. Regulatory peptides, 2009. 158(1-3): p. 156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bullock GR, et al. , Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochemistry and cell biology, 2001. 115(2): p. 117–24. [DOI] [PubMed] [Google Scholar]
- 37.Waseda Y, et al. , Angiotensin II type 2 receptor antagonist reduces bleomycin-induced pulmonary fibrosis in mice. Respiratory research, 2008. 9: p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konigshoff M, et al. , The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. American journal of respiratory cell and molecular biology, 2007. 37(6): p. 640–50. [DOI] [PubMed] [Google Scholar]
- 39.Liu SS, et al. , Hypoxia-induced collagen synthesis of human lung fibroblasts by activating the angiotensin system. International journal of molecular sciences, 2013. 14(12): p. 24029–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, et al. , Asthma susceptible genes in Chinese population: a meta-analysis. Respiratory research, 2010. 11: p. 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai H, et al. , Angiotensin II induces hyperresponsiveness of bronchial smooth muscle via an activation of p42/44 ERK in rats. Pflugers Archiv : European journal of physiology, 2010. 460(3): p. 645–55. [DOI] [PubMed] [Google Scholar]
- 42.Wagenaar GT, et al. , Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. American journal of physiology. Lung cellular and molecular physiology, 2014. 307(3): p. L261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, et al. , Chronic Activation of the Renin-Angiotensin System Induces Lung Fibrosis. Scientific reports, 2015. 5: p. 15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.