Abstract
Natural killer (NK) cells are critical effector lymphocytes mediating tumor immune surveillance and clearance. They do so by direct tumor killing using cytolytic granules and death receptors, and by interfacing with and potentiating adaptive immune responses through the production of cytokines. From a therapeutic perspective, NK cells have been shown to exert graft-versus-leukemia activity in the context of hematopoietic stem cell transplantation and are important in the clinical efficacy of antibodies. Advances in basic and translational NK cell biology have led to multiple potential strategies to augment their in vivo activity to improve antitumor responses. Despite their potent effects, NK cells have been shown to be safe for adoptive cell therapy in both the autologous and allogeneic settings, with promising, but so far limited, clinical efficacy. This review will provide an overview of strategies being pursued to improve NK cell activity and efficacy, focusing on cell source, NK cell activation, and in vivo persistence.
Keywords: NK cells, immunotherapy, cytokines, adaptive NK cells, microenvironment
Introduction
Cytotoxic lymphocytes are critical components of the antitumor defense system that exist within adaptive and innate arms of the immune system. Specifically, CD8+ T cells require antigen presentation in the context of MHC class I and require priming through antigen-presenting cells (APCs). In contrast, NK cells are innate effectors that are not antigen-specific and can be active without immunologic priming. Tumors have been known to downregulate beta-2-microglobulin, leading to impaired MHC presentation, as a mechanism of T-cell escape (1) but these are better targets for NK cell killing because of their “missing self”. NK cell activation is regulated by a complex balance of activating and inhibitory receptors (2). Briefly, the major inhibitory receptors include killer cell immunoglobulin–like receptors (KIRs), which recognize classical HLA (human leukocyte antigens A/B/C) and the heterodimer CD94/NKG2A, which recognizes non-classical HLA-E. Both NKG2A and NKG2C recognize HLA-E with different affinities leading to inhibition and activation, respectively (3). Other activating receptors include stress induced ligands (e.g. NKG2D recognizing MICA/B, ULBP), antibodies (e.g. CD16 against the Fc portion of IgG1), and natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46.
Due to their diverse targeting capabilities, NK cells are an attractive product for immunotherapy. In contrast to T cells, they have been shown to be safe without evidence of graft-versus-host disease (GVHD) in the allogeneic setting. This feature offers the potential for off-the-shelf application. NK cells were first tested against cancer in the 1980’s in the form of lymphokine-activated killer cells, a mixture of T and NK cells expanded ex vivo with high-dose IL2 (4). Since that time, advances in the understanding of NK biology have improved the safety profile and efficacy of adoptive cellular therapies. Since the late 1990’s, the feasibility and safety of NK cell adoptive transfer has been established by our group and others. The translational aspects arising from these important biological insights serve as the focus of this review. Specifically, attempts to improve NK cell efficacy can be broadly categorized into (1) developing an optimized NK cell source for adoptive cell immunotherapy, (2) improving NK cell activity through priming, activation, targeting, and overcoming immunosuppressive mechanisms, and (3) prolonging persistence (Fig. 1).
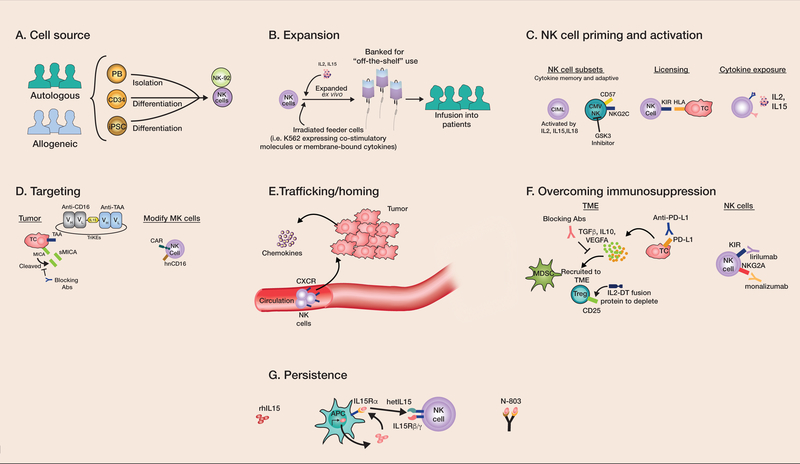
Figure 1: Strategies to improve NK cell immunotherapy.
(A) NK cells can be derived from autologous or allogeneic sources. Although most autologous NK cells are blood derived, allogeneic sources include PB NKs, CD34-, and iPSC-differentiated NK cells. PB NK: peripheral blood NKs; CD34: CD34+ hematopoietic stem cells; iPSC: induced pluripotent stem cells. (B) Ex vivo expansion is typically accomplished with cytokines such as IL2 or IL15, with many also incorporating irradiated feeder cells (typically using genetically modified K562 cells). The expanded NK cells can be used fresh or banked and frozen to be available on demand. To improve NK cell antitumor activity further, (C) cytokine-primed viral or small molecular–primed NK cells can be used, which include those with a memory phenotype, licensed subsets, and those generally exposed to gamma-chain cytokine activating cytokines. CIML: cytokine-induced memory-like; CMV-exposed NK: NK cells from cytomegalovirus seropositive individuals; GSK3: glycogen synthase kinase 3, KIR: killer cell immunoglobulin-like receptor, HLA: human leukocyte antigen. (D) Tumor targeting can be accomplished through increasing tumor expression of activating ligands (e.g. MICA) via upregulation or preventing cleavage. Tumor-associated antigens (TAAs) can also be targeted using therapeutic antibodies, engager molecules (e.g. tri-specific killer engagers (TriKEs)), and chimeric antigen receptors (CARs). sMICA: soluble MICA; hnCD16: high affinity, ADAM17 non-cleavable CD16. (E) Expression of chemokine receptors (like CXCL4) on NK cells can improve homing to tumor sites. (F) Strategies to overcome the immunosuppressive TME include blockade of inhibitory receptor interactions, interruption of negative immunoregulatory cytokines, and addressing suppressive immune cells such as Tregs and MDSCs through targeted depletion. IL-2-DT: IL2-diphtheria toxin fusion protein. (G) Improving NK cell persistence utilizing pro-survival and proliferative cytokines that do not stimulate Tregs, such as IL15 or modified versions (e.g. hetIL15, N-803), may mimic physiologic IL15 trans-presentation by antigen presenting cells (APCs). rhIL15: recombinant human IL15.
NK cell source
Identifying and developing an optimal source of NK cells is complex but much has been learned in the context of hematopoietic transplantation, where NK cells are the first lymphocyte to reconstitute (5). The importance of promoting “missing self” through KIR/KIR-ligand mismatch serves as proof-of-concept for the efficacy of NK cell therapy (6–8). NK cell adoptive immunotherapy can be broadly divided into autologous and allogeneic approaches. Initial studies demonstrated safety of adoptively transferred autologous NK cells, but efficacy was disappointing, likely due to the presence of inhibitory receptor ligands, insufficient MHC downregulation in tumors, and the redundancy in the MHC system (9,10). To overcome this limitation, we hypothesized that the use of allogeneic NK cells would allow at least some NK cells to persist from the donor product that would not be inhibited by host tumor residual MHC. Our initial study also compared various conditioning regimens and found that lymphodepletion was important for NK cell expansion and persistence, likely due to production of homeostatic cytokines including IL15. This initial study led to ~25% complete remissions in patients with refractory acute myeloid leukemia (AML) and served as proof-of-concept for this approach (11).
In the allogeneic setting, multiple sources are being investigated (Fig. 1A). A frequent source of mature peripheral blood (PB) NK cells are haploidentical donors, which are half-matched for HLA from a sibling or child (11). NK cells can also be derived from CD34+ hematopoietic cells, typically from umbilical cord blood (12), and also induced pluripotent stem cells (iPSCs)(13). NK cell lines, such as NK-92, derived from a patient with non-Hodgkin lymphoma are also being evaluated. One limitation of using NK-92 cells is that it is a transformed line that is aneuploidy. Therefore, for safety reasons, it requires irradiation prior to infusion to render them unable to proliferate (14). It has not yet been determined which cell source is best from a clinical standpoint. In terms of function, historically, PB NK cells have been the most potent in terms of antitumor activity (13). However, advances in in vitro NK cell differentiation, expansion, and activation have improved progenitor-derived NK cell function to rival that of PB NK cells (15). One additional advantage with progenitors to manufacture an NK cell product is the relative ease of gene modification (16) compared to PB NK cells (17) or cord blood–derived NK cells (18).
From a technical aspect, billions of NK cells are needed for adoptive transfer, requiring weeks of ex vivo expansion, depending on the starting cell source and number (Fig. 1B). An important advance in ex vivo expansion has been the use of irradiated feeder cells. One source of feeder cells that has been used both in bench research and for clinical trials is the erythroleukemic cell line K562, which can be transduced to express a variety of costimulatory molecules including 4–1BBL and membrane-bound cytokines including IL15 (19) and IL21 (20). Utilizing such irradiated feeder cell lines, clinically scalable expansions to over 1000-fold have been achieved without evidence of NK cell exhaustion or senescence. To address potential safety concerns, feeder cell–free expansion systems are being developed, including the use of plasma membrane particles derived from K562 cells, which can retain expression of stimulatory ligands and cytokines (21).
Enhancing NK cell activity
Priming/activation
Multiple factors have been identified that impact NK cell activation. Although NK cells do not require immunologic priming, certain conditions have been shown to lower their threshold of activation and enhance antitumor immunity. These include cytokine stimulation, viral infection, and the paradox of functional acquisition through inhibitory receptors (i.e. licensing)(Fig. 1C). Not all activating receptors are equally potent. Using agonist antibodies to trigger single-receptor signaling, only anti-CD16 was able to activate resting NK cells. However, culturing NK cells with cytokines lowers the threshold for activation, whereby single-receptor triggering beyond CD16 is now sufficient (22). Cytokines such as IL2 and IL15 provide not only a proliferative signal to NK cells, but also enhance function. IL15 is more selective for NK cell proliferation (compared to CD8+ T cells) and is presented in trans by APCs. In an attempt to improve IL15 stability and trans-presentation, natural IL15/IL15Rαheterodimers (hetIL15) and IL15/IL15Rα-Fc complexes (N-803) have been tested (23). Monomeric rhIL15 has been tested alone (24) and in combination with haploidentical NK cell infusions, resulting in 35% complete responses in refractory AML. Unique toxicities were seen with this combination, including cytokine-release syndrome and neurotoxicity, explained by subcutaneous administration leading to IL15 accumulation from decreased elimination as a result of lymphodepletion (25). These various formulations are undergoing further fine-tuning to minimize toxicity and avoid NK cell exhaustion (26). Also, when comparing various feeder cell phenotypes, K562 cells expressing membrane-bound IL15 leads to robust expansion of NK cells followed by senescence, which is not observed with membrane-bound IL21 (20).
Cytokines have also been shown to induce memory-like NK cells. A brief 16-hour exposure to a combination of IL12, IL15, and IL18 is able to induce long-lived, functional changes in NK cells (27). These cytokine-induced memory-like (CIML) NK cells have been tested clinically in relapsed/refractory AML, leading to responses in 5 of 9 treated patients (28). NK cell memory is also induced by CMV, where a distinct subset of NK cells has been identified. These cells express the maturation marker CD57 and the activating receptor NKG2C (with a reciprocal downregulation of NKG2A) and an epigenetic signature that is reminiscent of memory CD8+ T cells. Decreased expression of signaling molecules FcεR1γ, SYK, EAT-2, and the transcription factor PLZF are part of this signature (29). These cells have been termed “adaptive” NK cells,” given that they are long-lived, expand further with CMV reactivation, and are also more broadly primed against cancer targets. In a first clinical link to these cells and clinical outcomes, reconstitution of CD57+NKG2C+ NK cells following transplantation in those with CMV reactivation was associated with improved disease-free survival due to relapse protection (30,31). Efforts to selectively expand or enrich these adaptive cells led to the identification of the small molecule glycogen synthase kinase 3 (GSK3) inhibitor, which when combined with IL15 and NK cells from CMV seropositive donors, results in highly activated mature NK cells with enhanced cytotoxicity in vitro across a panel of solid tumor cell lines (lung, ovarian, and pancreas)(32). These findings have been translated into an ongoing Phase I clinical trial ().
NK cell licensing is another factor that contributes to the activation state. In animal models, germline knockout of MHC abrogates NK cell function, indicating that NK cell education via exposure to self-MHC ligands is important in their development (33). Such NK cells that cannot undergo education are termed unlicensed, comprising up to 50% of NK cells in mice and over 20% in humans. Metabolically, unlicensed NK cells are dependent on oxidative phosphorylation, whereas licensed NK cells are able to utilize glycolysis and glutaminolysis (reminiscent of central memory CD8+ T cells)(34). Therefore, licensed NK cells are better able to sustain activation. However, in the context of self-MHC–expressing infected and transformed cells, unlicensed NK cells may have some advantages compared to licensed NK cells (33). Ultimately, whether licensing will affect clinical outcomes in adoptive NK cell immunotherapy has not been determined.
Targeting
For NK cells to kill a tumor target, engagement of an activating receptor(s) is required. This can be achieved through endogenous receptor-ligand binding or redirected killing through engager molecules and chimeric antigen receptors (Fig. 1D). NKG2D is an activating receptor that typically recognizes stress-induced ligands such as MICA/B. To improve tumor clearance, stress-induced ligand expression can be upregulated by treatment with hypomethylating agents such as decitabine (35). However, tumor cells have developed strategies to cleave MICA from their surface to avoid NK cell recognition. Antibodies targeting the proteolytic cleavage site can prevent MICA clipping, restoring sensitivity to NK cell killing (Fig. 1D). Treatment with this antibody can skew the NK cell phenotype within the tumor microenvironment (TME) towards activation with increased expression of eomesodermin, granzyme B, and perforin (36). MICA shedding can also be antagonized by small molecule inhibitors of the metalloprotease ADAM17 (37). CD16 is an important activating receptor on NK cells, mediating specificity through antibody targeting. Polymorphisms in the gene for CD16 (38,39), accounting for inhibitory Fc receptors and Fc modifications, will enhance signaling through CD16A, the dominant isoform on NK cells (40). CD16 is also subject to proteolytic cleavage by ADAM17 upon NK cell activation (41), and inhibition of ADAM17 with antibodies, small molecule inhibitors, or genetic modification of the cleavage site can maintain CD16 expression and should augment antibody-dependent cellular cytotoxicity (ADCC)(42).
Another approach to (re)direct NK cell killing utilizes “engager” molecules. These small molecules combine two different antibody-targeting domains (e.g. variable fragments). One of these domains targets an activating receptor of choice, such as CD16/CD16A, while the other targets a tumor antigen or stress ligand (43–46). Therefore, these engagers can improve the immunologic synapse and cell activation, at least in part by engendering higher NK receptor binding affinity and specificity (Fig. 1D). These engagers can be further modified with the addition of IL15 between the two targeting domains, providing additional co-stimulation. A variety of tri-specific killer engagers (TriKEs) have been tested in vitro including against CLL (CD19)(46) and myeloid neoplasms (CD33)(45). In each of these settings, TriKEs selectively induced NK cell proliferation and augmented antitumor toxicity better than constructs without IL15. Genetic modifications to NK cells have the potential to improve their specificity and reactivity. For example, expression of the high-affinity and/or cleavage-resistant variants of CD16A have been transduced on the NK-92 cell line, as well as progenitor-derived NK cells, both of which have been shown to express low or absent CD16 natively (41). Chimeric antigen receptors have been successfully expressed on NK cells, and an optimal signaling domain of NKG2D and 2B4 (18,47)(Fig. 1D).
NK cells are infrequently found within the TME, but increased numbers have been associated with improved outcomes in solid tumors including lung, esophageal, and colon (48). Various strategies to enhance NK cell homing have focused on chemokines and chemokine receptors (Fig. 1E). For example, forced expression of CXCR4 improves NK cell trafficking into and control of glioblastoma tumors, which secrete CXCL12/SDF-1α (49). For tumors that do not express chemokines, strategies to artificially create a chemotaxis gradient have been developed, including targeting mesothelin expressed by pancreatic cancer to a cleavable linker to the chemokine CXCL16 (50). Other approaches to enhance homing would lead to enhanced NK cell antitumor efficacy.
Dampening immune suppression
A significant hurdle in antitumor immunity is the immunosuppressive TME. Tumors themselves can directly secrete suppressive cytokines such as TGFβ, IL10, and VEGFA, as well as express inhibitory ligands including PD-L1. Tumor-derived factors can skew APCs towards a more immunosuppressive subtype, and recruit immunoregulatory cells including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). With respect to NK cell–specific mechanisms, various blocking antibodies have been developed to counter inhibitory signals (Fig. 1F). Lirilumab is an antibody that targets KIRs (specifically KIR2DL1/2/3), and monalizumab targets NKG2A. These antibodies, as well as those targeting TIGIT, TIM-3, and LAG3, are all checkpoint inhibitors in various phases of clinical development (2). Combinations are also being tested. For example, targeting multiple mechanisms with dual checkpoint blockade against NKG2A/HLA-E and PD-1/PDL-1 axes (e.g. monalizumab plus durvalumab in ), or checkpoint blockade plus therapeutic antibody (e.g. monalizumab plus an anti-EGFR antibody in ) are being tested (51,52).
Other innovative strategies to address immunosuppression have used fusion proteins such as diphtheria toxin conjugated to IL2 (IL2-DT; Fig. 1F). This molecule preferentially binds to Tregs, which express the high-affinity IL2Rα (CD25). IL2-DT has been shown to partially deplete Tregs leading to improved NK cell persistence and anti-leukemia responses (53). Another approach is the expression of a dominant-negative TGFβ receptor on NK cells, which makes them resistant to TGFβ–mediated suppression, evidenced by maintained expression of activating receptors NKG2D and DNAM1, and preservation of antitumor activity in vitro (54). It is also important to highlight that adaptive NK cells have been shown to be relatively resistant to the suppressive effects of Tregs and MDSCs, which may have important clinical implications (55,56).
Persistence
We and others have shown that NK cell persistence correlates with clinical outcomes. In the setting of adoptive cell therapy with haploidentical NK cells, persistence greater than 7 days is associated with improved disease control (11,53,57). The importance of persistence has also been observed in CAR T-cell trials (58). The loss of the NK cell persistence is mediated by two primary mechanisms: (i) insufficient survival/proliferation signals and (ii) allo-rejection. Lymphodepletion helps by increasing endogenous levels of homeostatic cytokines such as IL15. Exogenous cytokine supplementation with IL2, IL15, or rhIL15 may also promote NK cell maintenance, but this is not without limitations as IL2 can stimulate Tregs cells. One could hypothesize that endogenous production of cytokines through genetic modification of NK cells or APCs to mimic IL15 trans-presentation could be optimal (18)(Fig. 1G), including, as discussed earlier, hetIL15 and IL15/IL15Rα-Fc complexes (N-803)(23).
Allo-rejection is another concern and degrees of HLA-mismatch or specific HLA alleles may increase the clearance of allogeneic NK cell infusion. Attempts at immunologic stealth are also ongoing in the sphere of progenitor stem cells, which requires evasion from multiple arms of the immune system. For example, knocking out HLA A/B/C and class II, overexpressing PD-L1 to avoid T-cell clearance, and overexpressing HLA-G and CD47 to avoid NK cell and macrophage clearance, respectively, results in hypo-immune pluripotent stem cells resistant to allo-rejection (59). It would be interesting to see whether a similar strategy would improve persistence of the various allogeneic NK cell platforms. These strategies could be important for proposed “universal” off-the-shelf options for multi-dosing, but this remains to be tested.
Conclusions
NK cells are potent mediators of antitumor immunity. Their use in cellular therapy has been shown to be safe, with minimal toxicities both in preclinical murine models and in clinical trials. Although results from NK cell trials have been promising, responses will need to be more durable. It is likely a multi-pronged approach that addresses current limitations to NK cell therapy will be optimal, including addressing issues of potency, specificity, and persistence balanced with safety. We are in important and exciting times for cell-based immunotherapy and NK cell–targeted approaches may rival those already seen in the T-cell field. This remains to be tested and will be the focus of ongoing and future clinical trials.
Acknowledgements
K.V. Woan was supported by a National Institute of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Hematology in Research training grant (T32HL007062–42). J.S. Miller was supported by NIH National Cancer Institute (NCI) grants R35CA197292, P01CA111412, and P01CA065493.
References
- 1.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 2017;8(1):1136 doi 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JS, Lanier LL. Natural Killer Cells in Cancer Immunotherapy. Annual Review of Cancer Biology 2019;3(1):77–103 doi 10.1146/annurev-cancerbio-030518-055653. [DOI] [Google Scholar]
- 3.Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci U S A 2008;105(18):6696–701 doi 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West EJ, Scott KJ, Jennings VA, Melcher AA. Immune activation by combination human lymphokine-activated killer and dendritic cell therapy. Br J Cancer 2011;105(6):787–95 doi 10.1038/bjc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minculescu L, Marquart HV, Friis LS, Petersen SL, Schiødt I, Ryder LP, et al. Early Natural Killer Cell Reconstitution Predicts Overall Survival in T Cell-Replete Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2016;22(12):2187–93 doi 10.1016/j.bbmt.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295(5562):2097–100 doi 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 7.Velardi A Natural killer cell alloreactivity 10 years later. Curr Opin Hematol 2012;19(6):421–6 doi 10.1097/MOH.0b013e3283590395. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer BC, Hsu KC. How important is NK alloreactivity and KIR in allogeneic transplantation? Best Pract Res Clin Haematol 2016;29(4):351–8 doi 10.1016/j.beha.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant 1997;3(1):34–44. [PubMed] [Google Scholar]
- 10.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant 2003;32(2):177–86 doi 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 11.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051–7 doi 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 12.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One 2010;5(2):e9221 doi 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med 2013;2(4):274–83 doi 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klingemann H, Boissel L, Toneguzzo F. Natural Killer Cells for Immunotherapy - Advantages of the NK-92 Cell Line over Blood NK Cells. Front Immunol 2016;7:91 doi 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng J, Tang SY, Toh LL, Wang S. Generation of “Off-the-Shelf” Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells. Stem Cell Reports 2017;9(6):1796–812 doi 10.1016/j.stemcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Bjordahl R, Gaidarova S, Rogers P, Blum R, Kaufman D, et al. Genetically Engineered Pluripotent Cell-Derived Natural Killer Cell Therapy Provides Enhanced Antibody Dependent Cellular Cytotoxicity Against Hematologic Malignancies and Solid Tumors in Combination with Monoclonal Antibody Therapy. Blood 2017;130(Suppl 1):4452–. [Google Scholar]
- 17.Carlsten M, Childs RW. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front Immunol 2015;6:266 doi 10.3389/fimmu.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32(2):520–31 doi 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009;69(9):4010–7 doi 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012;7(1):e30264 doi 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyer JL, Pandey V, Igarashi RY, Somanchi SS, Zakari A, Solh M, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: Clinical implications for cancer treatment. Cytotherapy 2016;18(5):653–63 doi 10.1016/j.jcyt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006;107(1):159–66 doi 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131(23):2515–27 doi 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33(1):74–82 doi 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Advances 2019;3(13):1970–80 doi 10.1182/bloodadvances.2018028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018;3(3) doi 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009;106(6):1915–9 doi 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8(357):357ra123 doi 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015;42(3):443–56 doi 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016;30(2):456–63 doi 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cichocki F, Taras E, Chiuppesi F, Wagner JE, Blazar BR, Brunstein C, et al. Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight 2019;4(2) doi 10.1172/jci.insight.125553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cichocki F, Valamehr B, Bjordahl R, Zhang B, Rezner B, Rogers P, et al. GSK3 Inhibition Drives Maturation of NK Cells and Enhances Their Antitumor Activity. Cancer Res 2017;77(20):5664–75 doi 10.1158/0008-5472.CAN-17-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu MM, Mahmoud AB, Makrigiannis AP. Licensed and Unlicensed NK Cells: Differential Roles in Cancer and Viral Control. Front Immunol 2016;7:166 doi 10.3389/fimmu.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer JR, Salzillo TC, Chakravarti N, Kararoudi MN, Trikha P, Foltz JA, et al. Education-dependent activation of glycolysis promotes the cytolytic potency of licensed human natural killer cells. J Allergy Clin Immunol 2019;143(1):346–58.e6 doi 10.1016/j.jaci.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 35.Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood 2016;127(23):2879–89 doi 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari de Andrade L, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018;359(6383):1537–42 doi 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arai J, Goto K, Tanoue Y, Ito S, Muroyama R, Matsubara Y, et al. Enzymatic inhibition of MICA sheddase ADAM17 by lomofungin in hepatocellular carcinoma cells. Int J Cancer 2018;143(10):2575–83 doi 10.1002/ijc.31615. [DOI] [PubMed] [Google Scholar]
- 38.Gavin PG, Song N, Kim SR, Lipchik C, Johnson NL, Bandos H, et al. Association of Polymorphisms in FCGR2A and FCGR3A With Degree of Trastuzumab Benefit in the Adjuvant Treatment of ERBB2/HER2-Positive Breast Cancer: Analysis of the NSABP B-31 Trial. JAMA Oncol 2017;3(3):335–41 doi 10.1001/jamaoncol.2016.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99(3):754–8. [DOI] [PubMed] [Google Scholar]
- 40.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res 2007;67(18):8882–90 doi 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 41.Jing Y, Ni Z, Wu J, Higgins L, Markowski TW, Kaufman DS, et al. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One 2015;10(3):e0121788 doi 10.1371/journal.pone.0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Mishra HK, Walcheck B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J Leukoc Biol 2019. doi 10.1002/JLB.2MR1218-501R. [DOI] [PMC free article] [PubMed]
- 43.Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019;177(7):1701–13.e16 doi 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Märklin M, Hagelstein I, Koerner SP, Rothfelder K, Pfluegler MS, Schumacher A, et al. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J Immunother Cancer 2019;7(1):143 doi 10.1186/s40425-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarhan D, Brandt L, Felices M, Guldevall K, Lenvik T, Hinderlie P, et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv 2018;2(12):1459–69 doi 10.1182/bloodadvances.2017012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felices M, Kodal B, Hinderlie P, Kaminski MF, Cooley S, Weisdorf DJ, et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv 2019;3(6):897–907 doi 10.1182/bloodadvances.2018029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23(2):181–92.e5 doi 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen SK, Gao Y, Basse PH. NK cells in the tumor microenvironment. Crit Rev Oncog 2014;19(1–2):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller N, Michen S, Tietze S, Töpfer K, Schulte A, Lamszus K, et al. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-secreting Glioblastoma. J Immunother 2015;38(5):197–210 doi 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Kang TH, Yoo W, Choi H, Jo S, Kong K, et al. An Antibody Designed to Improve Adoptive NK-Cell Therapy Inhibits Pancreatic Cancer Progression in a Murine Model. Cancer Immunol Res 2019;7(2):219–29 doi 10.1158/2326-6066.CIR-18-0317. [DOI] [PubMed] [Google Scholar]
- 51.André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018;175(7):1731–43.e13 doi 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Montfoort N, Borst L, Korrer MJ, Sluijter M, Marijt KA, Santegoets SJ, et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 2018;175(7):1744–55.e15 doi 10.1016/j.cell.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014;123(25):3855–63 doi 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy 2017;19(3):408–18 doi 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, et al. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res 2016;76(19):5696–706 doi 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res 2018;6(7):766–75 doi 10.1158/2326-6066.CIR-17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grzywacz B, Moench L, McKenna D, Tessier KM, Bachanova V, Cooley S, et al. Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates With Better Leukemia Control. J Immunother 2019;42(2):65–72 doi 10.1097/CJI.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood 2016;127(26):3312–20 doi 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A 2019;116(21):10441–6 doi 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]