Abstract
PAR2 has been proposed to contribute to lesion formation and intense itch in atopic dermatitis. Here, we tested the ability of a cell-penetrating pepducin, PZ-235, to mitigate the potentially deleterious effects of PAR2 in models of atopic dermatitis. PZ-235 significantly inhibited PAR2-mediated expression of inflammatory factors NF-κB, TSLP, TNF-α, and differentiation marker K10 by 94%–98% (P < 0.001) in human keratinocytes and suppressed IL-4 and IL-13 by 68%–83% (P < 0.05) in mast cells. In delayed pepducin treatment models of oxazolone- and DNFB-induced dermatitis, PZ-235 significantly attenuated skin thickening by 43%–100% (P < 0.01) and leukocyte crusting by 57% (P < 0.05), and it inhibited ex vivo chemotaxis of leukocytes toward PAR2 agonists. Daily PZ-235 treatment of filaggrin-deficient mice exposed to dust mite allergens for 8 weeks significantly suppressed total leukocyte and T-cell infiltration by 50%–68%; epidermal thickness by 60%–77%; and skin thickening, scaling, excoriation, and total lesion severity score by 46%–56%. PZ-235 significantly reduced itching caused by wasp venom peptide degranulation of mast cells in mice by 51% (P < 0.05), which was comparable to the protective effects conferred by PAR2 deficiency. Taken together, these results suggest that a PAR2 pepducin may confer broad therapeutic benefits as a disease-modifying treatment for atopic dermatitis and itch.
INTRODUCTION
PAR2 is a cell surface sensor of extracellular and environmental proteases that are particularly abundant in atopic and inflammatory conditions in the skin (Seeliger et al., 2003). PAR2 is widely expressed in different cutaneous cell types and has been implicated as playing multifaceted roles in epidermal homeostasis, barrier formation, immune responses, and the sensations of pain and itch (Lee et al., 2010). PAR2 is a member of the protease-activated receptor family, which includes PAR1–4, which are activated by N-terminal proteolytic cleavage and unmasking of a tethered ligand that activates the cleaved receptor via an unusual intramolecular liganding mechanism (Seeley et al., 2003). PAR2 is activated by specific serine and cysteine proteases such as tryptase, kallikreins, cathepsins, and dust mite allergen proteases (Lee et al., 2010). Unique among the four PAR family members, PAR2 is not directly cleaved by thrombin but can be trans-activated as a heterodimer with thrombin-cleaved PAR1 (Kaneider et al., 2007; Sevigny et al., 2011a, 2011b).
Accumulating research implicates PAR2 as a major contributor to atopic dermatitis, a chronic inflammatory skin disease affecting over 32 million people with a prevalence of 10% in the United States (Silverberg and Hanifin, 2013). As an atopic response to environmental allergens, atopic dermatitis skin lesions in both humans and mice involve enhanced inflammatory networks that are largely driven through T-cell–dependent recruitment and degranulation of mast cells, basophils, and eosinophils (Graham and Nadeau, 2014). Atopic dermatitis is typically treated with steroids or calcineurin inhibitors to suppress mast cell and T-cell activation; however, these therapies are often not fully effective and carry the risk of adverse effects, especially when used long-term or in children (Eichenfield et al., 2017; Frankel and Qureshi, 2012). Moreover, steroids and calcineurin inhibitors do not directly suppress itch or address skin barrier dysfunction; thus, there remains a great clinical need for nonsteroidal treatments for atopic dermatitis that can simultaneously treat the debilitating itch and skin lesions that are characteristic of this chronic disease.
Aberrantly high protease activity, PAR2 up-regulation, and PAR2 activation have been shown to be involved in several key aspects of atopic dermatitis pathophysiology (Steinhoff et al., 1999, 2003). First, the breakdown of the epidermal barrier in atopic dermatitis patients allows penetration of external allergens and proteases, including PAR2 agonist proteases from dust mites that are thought to be a major trigger of atopic dermatitis lesion onset (Chapman et al., 2007; Kato et al., 2009). Barrier deficits further enhance the activity of endogenous epidermal PAR2 protease agonists like kallikrein-5 and −14, cathepsins, and mast cell tryptase (Hachem et al., 2006; Lee et al., 2010). Second, PAR2 is highly expressed in keratinocytes and cutaneous immune cells in atopic dermatitis patients, where it induces IL-6, ICAM-1, and TSLP inflammatory mediators that amplify leukocyte infiltration and activation (Briot et al., 2009; Seeliger et al., 2003; Steinhoff et al., 1999). Third, exogenous and endogenous proteases activate PAR2 to increase release of pruritogenic mediators from keratinocytes and mast cells and stimulate firing of TRPV1 and TRPA1-positive pruriceptive sensory endings (Kempkes et al., 2014; Wilson et al., 2013). Thus, a PAR2 antagonist would be of great interest as a potential disease-modifying agent for the treatment of atopic dermatitis because of its broad effects on epidermal homeostasis, barrier function, inflammation, and itch.
Here, we tested the ability of a PAR2-targeted pepducin, PZ-235, to suppress skin lesion thickening, inflammation, and itch in acute and chronic models of atopic dermatitis. PZ-235 is a member of a class of cell-penetrating lipidated peptides based on receptor intracellular loop domains, which flip across the plasma membrane of cells where they bind to their cognate receptor to modulate signaling to intracellular G proteins (O’Callaghan et al., 2012a; Zhang et al., 2015). PAR2 exhibits high constitutive activity, and PZ-235 acts as an allosteric antagonist that requires the presence of its cognate receptor to exert its inhibitory effects (Michael et al., 2013; Sevigny et al., 2011b; Shearer et al., 2016). In this study, we show that targeting PAR2 with PZ-235 significantly attenuates cutaneous inflammation and itch in both acute and chronic models of atopic dermatitis and reduces expression of proinflammatory mediators in keratinocytes and mast cells to give marked overall improvement in skin lesions.
RESULTS
PZ-235 inhibits PAR2-calcium responses and K10 differentiation marker in human keratinocytes
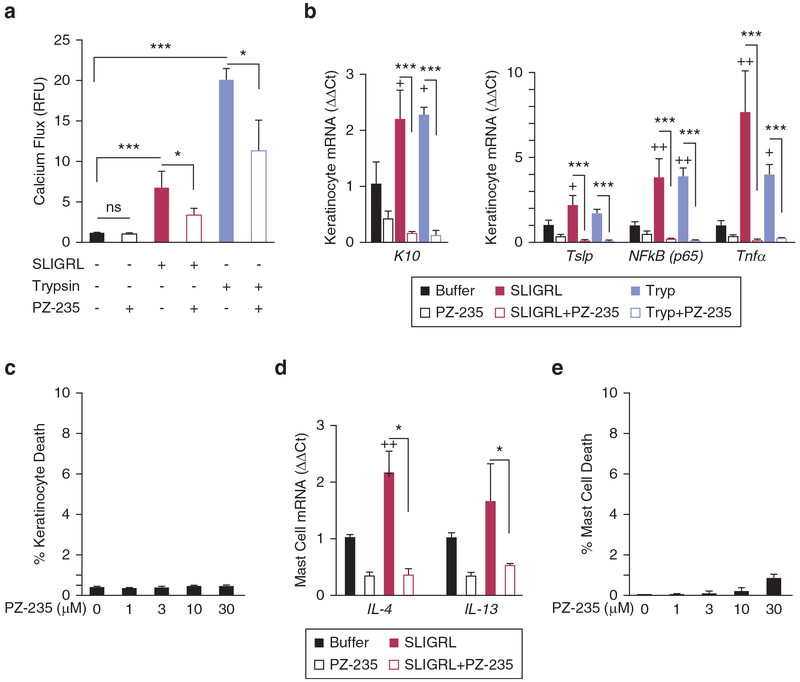
PAR2 activation has been shown to increase differentiation of keratinocytes, along with enhancing the disruption of epidermal barrier formation (O’Regan and Irvine, 2010; Stefansson et al., 2008). Intracellular calcium flux is essential for regulating the proliferation and differentiation of keratinocytes and for forming the epidermal barrier. Because PAR2 expression is normally segregated to suprabasal differentiated keratinocytes, cells were differentiated for 3 days before treatment with PAR2 agonists. The PAR2 agonists SLIGRL, a synthetic peptide that mimics the tethered ligand of the PAR2 receptor, and trypsin protease, which activates PAR2 by cleavage and exposure of the tethered ligand, both caused increases in intracellular calcium in the keratinocytes (P < 0.001) (Figure 1a). PZ-235 significantly reduced the PAR2 calcium responses by at least 50% (P < 0.001) in the differentiated keratinocytes. Incubation of the human keratinocytes with SLIGRL and trypsin for 8 hours caused significantly increased mRNA expression of the early-stage differentiation marker keratin 10 (P < 0.05), an effect that was completely blocked (and suppressed below baseline) by 3 μmol/L PZ-235 (P < 0.001) (Figure 1b). PZ-235 had no detectable effect on keratinocyte cell death/viability at concentrations from 1 to 30 μmol/L (Figure 1c).
Figure 1. PZ-235 pepducin suppresses PAR2 calcium responses and expression of inflammatory mediators of atopic dermatitis in keratinocytes.
(a) The effect of PZ-235 on PAR2 signaling was measured in differentiated normal human epidermal keratinocytes (NHEKs) using a calcium (calcium-4 dye) flux assay. Keratinocytes were stimulated with PAR2 agonist peptide SLIGRL (3 μmol/L) or the PAR2-activating protease trypsin (10 nmol/L) in the presence of PZ-235 (3 μmol/L) or vehicle control (−). (b) PAR2 up-regulates keratinocyte differentiation marker and expression of inflammatory mediators in human keratinocytes which is completely suppressed by PZ-235. Treatment of differentiated NHEKs with PAR2 agonists SLIGRL (3 μmol/L) and trypsin (10 nmol/L) for 8 hours increases expression of the differentiation marker keratin-10 (K10) and atopic dermatitis inflammatory markers NF-κB (p65), Tnfα, and Tslp by quantitative PCR. Quantitative mRNA levels (ΔΔCt) were normalized to Gapdh. (c) NHEK keratinocytes were treated with 0–30 μmol PZ-235 as indicated for 8 hours at 37 °C, and dead cells were quantified by staining with propidium iodide for 15 minutes at room temperature before analysis of fluorescence by flow cytometry in duplicate for each condition. (d) Atopic dermatitis-related markers IL4 and IL13 mRNA were measured in HMC1 mast cells after 24-hour incubation with SLIGRL (3 μmol/L) in the presence or absence of 3 μmol/L PZ-235. (e) HMC1 mast cells were treated with 0–30 μmol/L PZ-235 as indicated for 24 hours at 37 °C, and dead cells were quantified by staining with propidium iodide for 15 minutes at room temperature before analysis of fluorescence by flow cytometry in duplicate for each condition. Means ± standard error of the mean, n = 3 are shown. *P < 0.05, ***P < 0.001 by t test; +P < 0.05, ++P < 0.01 versus individual buffer control by Dunnett test. M, mol/L; RFU, relative fluorescence unit; Tryp, trypsin.
PZ-235 suppresses PAR2-driven expression of proinflammatory mediators in keratinocytes and mast cells
The ability of PZ-235 to suppress the proinflammatory effects of PAR2 was examined in both keratinocytes and mast cells. NF-κB is the major transcription factor that regulates the inflammatory response of keratinocytes, including expression of two key cytokines that are very important in the recruitment and activation of leukocytes, TSLP and TNF-α. Differentiated normal human epidermal keratinocyte cells were treated with PAR2 agonists trypsin and SLIGRL for 8 hours and expression of p65 (NF-κB p65), Tslp, and Tnfα determined by quantitative PCR (Figure 1b). Expression of NF-κBp65 and Tnfα were significantly increased by 4–8-fold in SLIGRL (P < 0.01) and trypsin (P <0.05)-treated keratinocytes, relative to vehicle. PZ-235 (3 μmol/L) inhibited the SLIGRL and trypsin-induced increases in NF-κB and Tnfα expression by 94%–98% (P < 0.001) in the keratinocytes. Tslp was also up-regulated by approximately 2-fold by the PAR2 agonists SLIGRL and trypsin compared with vehicle, although only the SLIGRL-induced increase reached significance (P < 0.05). PZ-235 blocked the PAR2-dependent increases in expression of Tslp by 97%–98% (P < 0.001) in the keratinocytes.
Mast cells have been identified as a main source of both IL-4 and IL-13 in the skin, two inflammatory cytokines found to be key contributors to the pathology of atopic dermatitis and other atopic diseases (Horsmanheimo et al., 1994; Obara et al., 2002). To determine whether PZ-235 could also reduce PAR2-mediated increases in inflammatory mediators from mast cells, cultured HMC1 mast cells were treated with the PAR2 agonist SLIGRL for 24 hours, and expressions of IL4 and IL13 were assessed. Treatment with SLIGRL increased expression of both IL4 (P < 0.01) and IL13 (P = 0.22) relative to buffer control (Figure 1d). PZ-235 (3 μmol/L) completely blocked the PAR2-induced expression of IL4 and IL13 in the mast cells to below baseline levels (P < 0.05). PZ-235 had no significant effect (<1%) on mast cell viability at concentrations from 1 to 30 μmol/L (Figure 1e). These data indicate that PZ-235 is a potent inhibitor of PAR2-mediated increases in key proinflammatory mediators from both keratinocytes and mast cells.
PZ-235 reduces ear and epidermal thickening induced by oxazolone and dust mites in mice
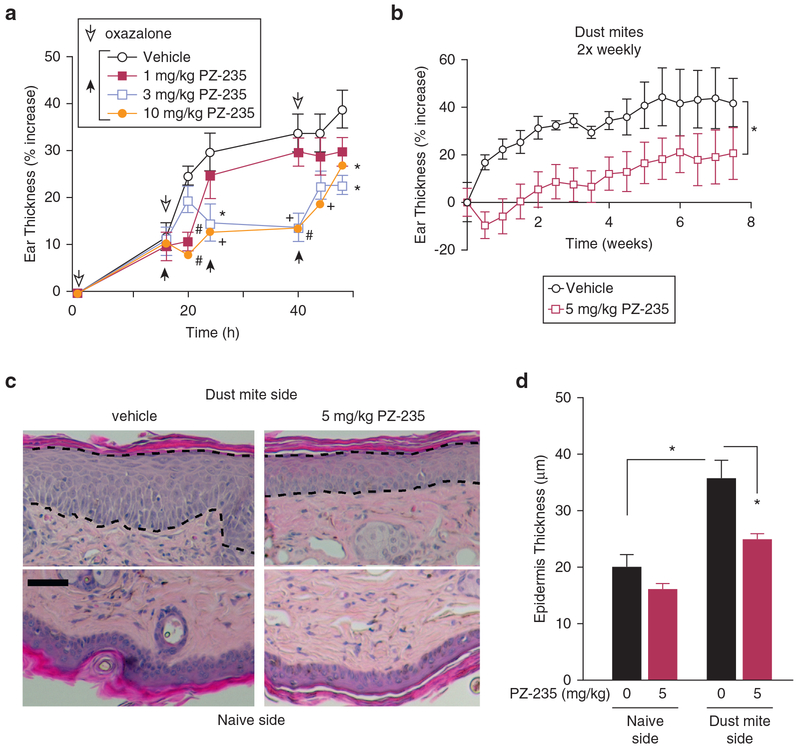
The ability of PZ-235 to reduce acute skin inflammation was evaluated in the oxazolone dermatitis model in mice. Sensitization of the ear to three applications of oxazolone over 48 hours caused acute inflammatory edema, leading to a marked 40% increase in ear skin thickness in vehicle-treated mice (Figure 2a). Delayed subcutaneous (sc) administration of PZ-235 starting at 16 h gave a dose-dependent suppression of the oxazolone-induced swelling (Figure 2a). PZ-235 at 10 mg/kg significantly reduced edema at all time points after PZ-235 administration (P < 0.001–0.05), where the maximum concentration (i.e., Cmax) in mouse plasma reaches 6 μmol/L (Shearer et al., 2016). The intermediate dose of 3 mg/kg showed significantly reduced edema at time points after 20 hours (P < 0.01–0.05), whereas the lowest dose of 1 mg/kg (Cmax in plasma reaches 1.3 μmol/L) (Shearer et al., 2016) reached significance at the 20-hour time point (P < 0.001), showing that PZ-235 can effectively suppress acute cutaneous inflammation induced by a chemical allergen.
Figure 2. PZ-235 inhibits oxazoloneand dust mite-induced ear thickening.
(a) Oxazolone at a dose of 1% in 80% acetone/20% olive oil (20 μl) was initially applied to the mouse ear followed by 16-houredelayed sc injection of PZ-235 (1–10 mg/kg) or vehicle and then additional oxazolone (open arrowheads) and drug/vehicle (black arrowheads) treatments. Means ± standard error of the mean, n = 10 are shown. *P < 0.05, +P < 0.01, #P < 0.001 versus vehicle by Dunnett test. (b–d) Dust mite extract was applied twice weekly for 8 weeks to the ear of flaky tail mice treated daily with sc PZ-235 (5 mg/kg) or vehicle (n = 10), and ear thickness measured using a caliper; *P < 0.05 by repeated measures t test. (c) Dashed lines indicate epidermal borders used to quantify (d) thickness of dust mite-treated and naïve sides on hematoxylin and eosin-stained sections. Scale bar = 25 μm. Means ± standard error of the mean, n = 10 are shown. *P < 0.05 by Dunnett test. h, hour.
Chronic anti-inflammatory effects of PZ-235 were then assessed in the ears of flaky tail mice, a well-validated mouse model of human atopic dermatitis that displays marked deficits in epidermal barrier function due to loss of functional filaggrin (Fallon et al., 2009). These barrier deficits allow permeation of external allergens into the epidermis such as PAR2-activating proteases from house dust mites (Kato et al., 2009; Moniaga et al., 2010). Twice-weekly application of dust mite extract onto the ears of flaky tail mice caused a 40% increase in skin thickening over an 8-week time period (Figure 2b). There was a significant protective effect of daily PZ-235 in suppressing dust mice-induced ear thickening in the flaky tail mice over the 8-week time period (P < 0.05). Histologically, epidermal thickness of the dust mite side of the ears from vehicle-treated flaky tail mice significantly increased by 77% (P < 0.05) compared with the naïve side (Figure 2c and d). This dust mite-induced increase in ear epidermal thickness was significantly reduced by 67% (P < 0.05) by concomitant PZ-235 treatment over the 8-week period in the flaky tail mice. These data indicate that PZ-235 effectively reduces both ear skin thickening and keratinocyte hyperproliferation evoked by chronic application of external dust mite allergens.
Delayed PZ-235 treatment mitigates skin lesion thickness in DNFB-induced chronic dermatitis
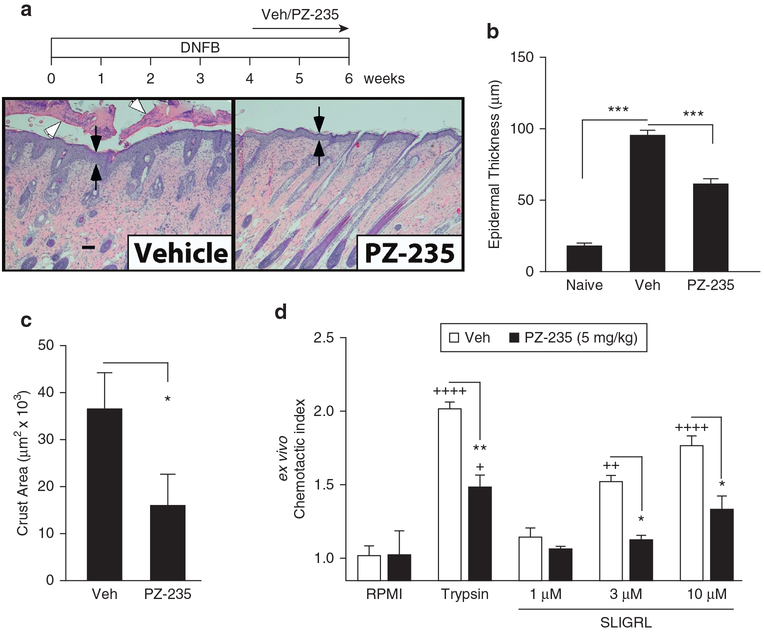
Severe dermatitis lesions were induced in CF1 mice using the hapten DNFB model (Rose et al., 2012). Repeated application of DNFB over 8 weeks to the back skin of vehicle-treated mice resulted in a significant increase in epidermal thickness (P <0.001) by 4-fold (Figure 3a and b). This was due to an increase in the number of keratinocytes making up the live cell layers, indicating increased keratinocyte proliferation. The ability of DNFB to increase epidermal thickness was significantly reduced by 43% (P < 0.001) when PZ-235 was administered during the final 2 weeks (Figure 3b), indicating that the PAR2 pepducin is efficacious as a delayed treatment.
Figure 3. Delayed treatment with PZ-235 limits severity of skin lesions in a DNFB model of dermatitis in mice.
(a–c) Atopic dermatitis-like skin lesions were induced in the backs of CF1 mice by applying DNFB (1% in 80% olive oil/20% acetone) on day 1 and then weekly for 6 weeks at 0.1% with daily subcutaneous injections of PZ-235 (5 mg/kg) or vehicle initiated 4 weeks later during the last 2 weeks(n = 10). Epidermal thickness (black arrows) and skin crusting (open arrow heads) area were measured in hematoxylin and eosin-stained sections. Means ± standard error of the mean are shown. Scale bar = 100 μm. (d) Chemotaxis of neutrophils isolated from whole blood of mice injected subcutaneously with vehicle or PZ-235 (5 mg/kg/day for 21 days) was assessed using anex vivo migration assay to PAR2 agonists, 1–10 μmol/L SLIGRL and 30 nmol/L trypsin. Means ± standard error of the mean, n = 6−10, *P < 0.05, **P < 0.01, ***P < 0.001, versus vehicle by (b) Dunnett test, (c) t test, and (d) two-way analysis of variance and Tukey test; +P < 0.05, ++P <0.01, ++++P < 0.0001 versus RPMI by two-way analysis of variance and Tukey test. M, mol/L; Veh, vehicle.
PZ-235 inhibits skin crusting and ex vivo mouse neutrophil migration to PAR2 agonists
During severe dermatitis or co-infection, large quantities of leukocytes can migrate to the affected site and die as part of the inflammatory response, creating an exudate that dries on the surface of the skin. In this regard, repeated application of DNFB over 8 weeks to the back skin of mice resulted in a marked neutrophilic exocytosis and crusting on the skin surface (Figure 3a). Delayed treatment of PZ-235 (daily sc in weeks 4–6) significantly reduced the skin crusting by 57% (P < 0.05) compared with vehicle-treated mice (Figure 3c). To determine the direct effect of PZ-235 on leukocyte motility in the animals, neutrophils were isolated from mice 4 hours after treatment with PZ-235 or vehicle and tested for ex vivo migration toward gradients of the PAR2 agonists trypsin and SLIGRL. The chemotactic index of neutrophil chemotactic migration toward trypsin and 3–10 μmol/L SLIGRL compared with RPMI media alone, was significantly higher in neutrophils isolated from vehicle-treated mice (Figure 3d) (P < 0.01–0.0001). However, neutrophils isolated from PZ-235–treated mice showed significantly reduced chemotactic migration toward trypsin (30 nmol/L, P < 0.001) and SLIGRL (3 μmol/L and 10 μmol/L, P < 0.05) compared with neutrophils isolated from vehicle-treated mice. This shows that PZ-235 has a direct effect on suppressing PAR2-dependent neutrophil migration in the animals. In addition, dermal mast cells from skin sections from mice treated with DNFB after 6 weeks were identified by toluidine blue staining and morphology, and averages from five fields per mouse (n = 5) were quantified. There was a nonsignificant (P = 0.12) reduction of 30% in average numbers of dermal mast cells per field in the PZ-235–treated mice compared with vehicle control (data not shown).
PZ-235 reduces the severity of dust mite-induced atopic dermatitis lesions in filaggrin-deficient mice
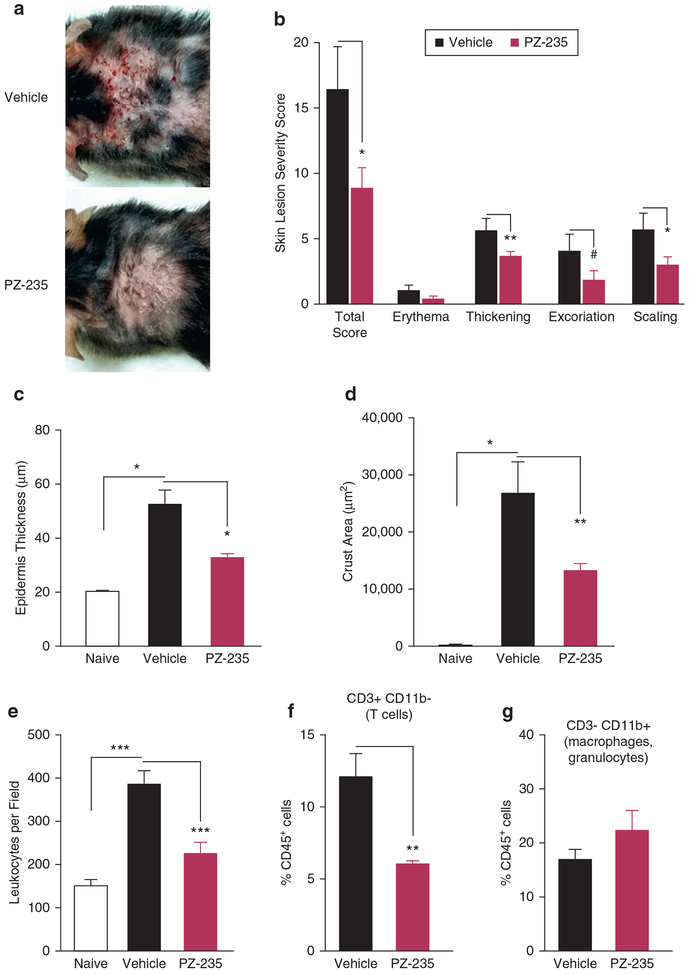
Dust mites are thought to be a major aeroallergen responsible for the outbreak and perpetuation of atopic dermatitis skin lesions in humans (Eichenfield et al., 2017). Dust mite-induced atopic dermatitis skin lesions were elicited in the backs of flaky tail mice by twice-weekly application of dust mite extract over 8 weeks. Skin lesions began to develop within 2 weeks, with evident erythema, skin thickening, excoriation, and lichenification (scaling) appearing by week 4. Skin lesions in PZ-235–treated mice were less severe on average than those in vehicle-treated mice, with obvious differences seen at the 8-week endpoint (Figure 4a). Compared with vehicle-treated mice, PZ-235–treated mice showed a significant 46% reduction in overall lesion severity score (P < 0.05) by the end of week 8 (Figure 4b). PZ-235–treatment also suppressed skin-thickening, excoriation and scaling in the dust mite-treated filaggrin-deficient mice (Figure 4b). By histological analysis, PZ-235 gave a significant 60% protective effect (P < 0.05) against dust mite-induced epidermal thickening (Figure 4c) and 50% reduction (P < 0.05) in leukocyte crusting (Figure 4d). Likewise, histologic quantitation of dermal leukocytes showed that PZ-235 treatment afforded a highly significant (P < 0.001) 68% protective effect in reducing total leukocyte infiltrates in the 8-week chronic dust mite mouse model (Figure 4e). Cell sorting of the CD45-positive cells in the skin samples showed a significant 50% reduction (P < 0.01) in T cells (CD3+CD11b−) but no significant effect on the innate immune cells (macrophages/granulocytes) (CD3−CD11b+) (Figure 4f, g). Together, these data indicate that the PAR2 pepducin was effective in reducing both the symptoms and severe inflammatory skin pathology of chronic dust mite-induced atopic dermatitis, including suppressing the adaptive immune response (T-cell infiltrates) in the flaky tail mice.
Figure 4. PZ-235 limits the severity of atopic dermatitis-like skin lesions in flaky tail mice treated with dust mite extract.
(a) Atopic dermatitis-like back skin lesions were induced in flaky tail mice (n = 6) by applying dust mite extract (40 μl, dose in mineral oil) twice weekly for 8 weeks with concurrent daily subcutaneous injections of PZ-235 (5 mg/kg) or vehicle. (b) Skin lesions were given a severity score based on erythema, skin thickness, scaling/lichenification, and scabbing. (c) Epidermal thickness and (d) skin crusting area were measured histologically in hematoxylin and eosin-stained sections. (e) Total dermal leukocytes in skin sections from flaky tail mice treated for 8 weeks with dust mites in a or naïve mice were identified by hematoxylin and eosin staining and morphology, and average numbers of leukocytes in 8 fields (original magnification × 20) per mouse quantified in a blinded manner. (f–g) CD45+ cells in digested fresh skin samples from dust mite-treated flaky mice in a were identified with CD45-APC-Ab and further stained for CD3-FITC-Ab and CD11b-APC-Cy7-Ab and quantified by FACS. Means ± standard error of the mean, n = 6 are shown. #P = 0.09, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus vehicle by t test (b and f), or Dunnett test (c–e). Ab, antibody; APC, antigen-presenting cell.
PZ-235 reduces itching evoked by mast cell degranulation and dust mites in mice
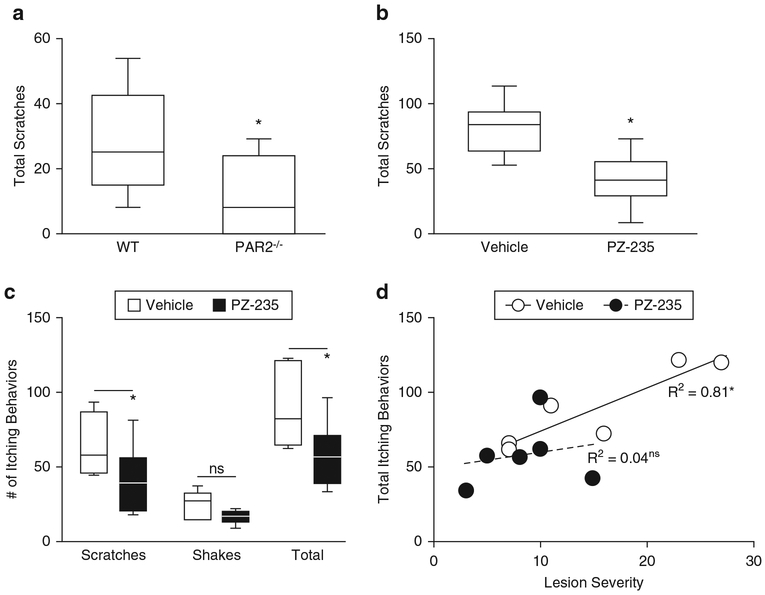
Itch in atopic dermatitis is triggered by mast cell tryptase, which activates PAR2 on cutaneous sensory nerve endings. Release of mast cell tryptase was evoked in mice by wasp venom peptide, mastoparan (MA-1), which stimulates degranulation of mast cells. Intradermal injection of MA-1 resulted in scratching behaviors almost immediately after injection. Mean total scratches after MA-1 injection were significantly lower in PAR2-deficient mice compared with wild-type C57BL6 mice, confirming a role for PAR2 in mast cell-dependent itch (Figure 5a) (P < 0.05). Likewise, total MA1-induced scratches were significantly reduced by 51% (P < 0.05) by PZ-235 in wild-type CF1 mice compared with vehicle (Figure 5b), indicating that the PAR2 pepducin can effectively reduce itch induced by mast cell degranulation.
Figure 5. PZ-235 inhibits itch evoked by mastoparan-1 and dust mite extract.
(a) Mastoparan-1 (MA-1) was injected intradermally (200 μg in 50 μl H2O) into the dorsal skin of PAR2-knockout and C57BL6 wild-type mice or into (b) wild-type CF1 mice 2 hours after sc PZ-235 (10 mg/kg) or vehicle (n = 10) and total scratching behavior assessed for 5 minutes in C57BL6 mice and 30 minutes for CF-1 mice. (c, d) Itch was quantified in flaky tail mice (n = 6) on the last day of dust mite extract treatment (week 8 from Figure 4) and 2 hours after subcutaneous PZ-235 (5 mg/kg) or vehicle and linearly correlated with lesion severity score. Itching was recorded as scratches of the application site with the hindpaw or by biting and “wet-dog” shakes assessed for a period of 5 minutes, beginning 15 minutes after dust mite extract application. Data for a–c are shown as box and whisker plots, Mann-Whitney test, P < 0.05 or in d, significant (*P < 0.05) Pearson R2 correlation or not significant. ns, not significant; WT, wild type.
Finally, dust mite-induced itch was assessed in C57BL6 flaky tail mice after 8 weeks of chronic application of dust mite extract. Dust mite extract caused repetitive scratching and “wet dog” shakes (Figure 5c). Both mean scratches and shakes were reduced in PZ-235–treated flaky tails, but only scratches reached significance (P < 0.05 and P = 0.12, respectively) (Figure 5c). PZ-235 showed a significant inhibition of total itching behaviors (the sum of scratches and shakes) (Figure 5c, P < 0.05). Itch behavior quantified in flaky tail mice on the last day of dust mite extract treatment (week 8 from Figure 4) was found to be significantly linearly correlated with lesion severity score for vehicle-treated mice only, with no correlation for PZ-235 animals (Figure 5d). Together, these data show that PZ-235 can effectively limit itch evoked by external allergens to concomitantly mitigate the resulting epidermal thickening and excoriation/scabbing response in mice.
DISCUSSION
Accumulating evidence points to overactivity of PAR2 in the pathophysiology of atopic dermatitis and ties the receptor to all major symptoms of the disease (Lee et al., 2010). PAR2 has proven to be a difficult receptor to target, likely because of its high constitutive activity and difficulty in blocking the protease-induced ligand activation mechanism (Sevigny et al., 2011b). Small molecule inhibitors have begun to be developed for PAR2; however, they have not yet advanced into the clinical phase because of low efficacy and/or partial agonist effects (Hollenberg et al., 2014; Yau et al., 2013). Here, we show that a therapeutic approach using a PAR2 pepducin, PZ-235, is broadly effective as a potential disease-modifying agent in atopic dermatitis models by mitigating skin thickening, production of inflammatory factors, leukocyte infiltration, skin lesion severity, and itching.
In differentiated human primary keratinocytes, PZ-235 significantly inhibited PAR2-calcium fluxes triggered by peptide ligand and PAR2 protease agonist. Although keratinocytes are considered nonexcitable cells, PAR2 agonists generate robust intracellular calcium fluxes via InsP3-receptors and TRPV1 channels that are required for expression of inflammatory mediators (Gouin et al., 2018). Accordingly, PZ-235 completely suppressed PAR2 induction of differentiation marker keratin 10 and key inflammatory factors NF-κB (p65), TSLP, and TNF-α in primary human keratinocytes. In particular, PAR2 strongly activates NF-κB–dependent expression of the TSLP cytokine (Briot et al., 2009) and the PZ-235 pepducin suppressed the PAR2-TSLP response to below baseline levels. TSLP is a powerful inducer of differentiation of naïve T cells into T helper type 2-type allergic inflammatory cells, associated with large increases in TN-Fα, IL-4, and IL-13 (Liu, 2007). Presumed loss-of-function TSLP variants are associated with diminished severity of atopic dermatitis in patients with filaggrin deficiency (Margolis et al., 2014). Keratinocyte-specific overexpression of TSLP leads to the spontaneous development of an atopic dermatitis-like phenotype in mice with highly elevated TNF-α and IL-4 and mast cell/eosinophil infiltrates (Yoo et al., 2005), and TSLP in skin lesions from AD patients have significant correlations with IL-4 and IL-13 (Sano et al., 2013).
Like keratinocytes, mast cells are a major source of cutaneous proinflammatory mediators thought to underlie the pathology of AD, most importantly IL-4 and IL-13 (Kawakami et al., 2009). Accordingly, dual blockade of IL-4 and IL-13 with dupilumab has shown beneficial effects in patients with moderate to severe atopic dermatitis (Beck et al., 2014). PZ-235 completely blocked the PAR2 activation-dependent expression of IL-4 and IL-13 from human mast cells to below baseline levels, consistent with our previous findings that PAR2 has considerable constitutive activity and plays a key role in mast cell-dependent inflammatory edema (Sevigny et al., 2011b). Acute oxazolone causes a predominantly T helper type 1-driven inflammatory edema that is reduced in PAR2-knockout mice (Kawagoe et al., 2002). PZ-235 was also able to dose-dependently reduce oxazolone-evoked acute inflammatory edema in mice, indicating anti-inflammatory effects on both T cells and mast cells.
Chronic applications of DNFB cause contact hypersensitivity, resulting in epidermal hyperplasia and skin crusting via a T helper type 2-dominated response with increases in cytokines such as TNF-α and IL-4, as seen in human atopic dermatitis (Rose et al., 2012). In the DNFB model, PZ-235 was initiated only in the last 2 weeks of the 6-week DNFB treatment, when lesion development was already well underway, and gave a highly significant reduction in epidermal thickness and leukocyte crusting. Thus, delayed treatment with PZ-235 can halt the progression of severe dermatitis skin lesions in mice, even well after they have developed.
The suppressive effect of PZ-235 in flaky tail mice exposed to dust mites confirms that PAR2 also plays an important role when AD occurs with a genetic deficit in barrier formation. Dust mites are a major aero-allergen that cause robust cutaneous allergic reactions in filaggrin-deficient humans and mice that are much more severe because of their enhanced epidermal permeability (Henderson et al., 2008; Moniaga et al., 2010). As many as 15% of atopic dermatitis patients have been identified as having mutations in fi. As ma that negatively affect epidermal barrier function (Morar et al., 2007). Flaky tail mice have a spontaneous frame shift mutation that results in a truncated and nonfunctional filaggrin protein (Fallon et al., 2009). In chronic models, atopic dermatitis was induced in the ears and back skin of flaky tail mice through the application of dust mite extract, which contains PAR2-activating serine proteases, along with other allergens that can lead to increased release of endogenous proteases by triggering an intense immune reaction. As in the oxazolone and DNFB models, PZ-235 showed significantly reduced epidermal thickness and keratinocyte hyper-proliferation, with inhibitory effects on skin crusting, scaling, and excoriations. In addition, PZ-235 significantly reduced total leukocyte and T-cell infiltration, indicating a suppressive effect on the adaptive immune response in the chronic flaky tail/dust mite model.
In response to dust mite extract application, flaky tail mice displayed prominent itch behaviors. Both scratches and shakes were significantly reduced in flaky tail mice treated with PZ-235, even in this model of advanced skin lesions where animals were exposed to a large amount of allergen. There was also a significant correlation between skin lesion severity and itch in vehicle-treated flaky tail mice, which was absent in PZ-235–treated animals. This relationship between lesion severity and itch intensity is not surprising, because scratching exacerbates lesion severity (Udkoff and Silverberg, 2018), and more severe skin lesions likely reflect a more impaired epidermal barrier that allows enhanced penetration of itch-causing allergens and proteases from the dust mite extract.
Indeed, growing evidence indicates that PAR2 is intimately involved in driving itch during atopic dermatitis (Kempkes et al., 2014; Wilson et al., 2013). PAR2-activating proteases and peptide agonists are known pruritogens in humans and animals, causing itch independent of histamine (Andoh et al., 2012; Tsujii et al., 2009). Itch from cowhage spicules has been attributed to mucunain, which is an exogenous PAR2-activating protease (Reddy et al., 2008, 2010). Both mast cell tryptase and PAR2 receptors are increased in skin lesions from human patients and AD-related animal models, with protease inhibitors and PAR2 antibodies showing antipruritic effects in these models (Steinhoff et al., 2003, 2006; Tsujii et al., 2009). Tryptase, but not histamine, is increased in atopic dermatitis lesions and acts through a distinct subset of TrpV1-positive peptidergic nerve fibers to cause itch (Amadesi et al., 2004; Davidson et al., 2007). Furthermore, antihistamines are not very effective in treating itch in atopic dermatitis patients, even when the itch is triggered by mast cell degranulation (Klein and Clark, 1999; Rukwied et al., 2000). Finally, to determine whether PZ-235 could inhibit itch from activated mast cells, MA-1, a mast cell-degranulating peptide from wasp venom, was used to elicit severe scratching in mice. PZ-235 significantly reduced scratching behaviors due to mast cell degranulation, similar to the effect observed in PAR2-deficient mice.
Given the prominent role of mast cell tryptase in atopic dermatitis-related itch, these observed suppressive effects with PZ-235 bode well for PAR2 blockade as a potential anti-pruritogenic treatment in this disease and for suppressing local skin inflammation and epidermal thickening. In this regard, there is great unmet clinical need for atopic dermatitis treatments that do not cause immune suppression—of particular importance in younger children. Pepducin technology represents a distinct method of specifically targeting recalcitrant or constitutively active receptors such as PAR2 (Covic et al., 2002; Sevigny et al., 2011b) without causing general immune suppression (Kaneider et al., 2005, 2007; O’Callaghan et al., 2012b). Pepducins have advanced into clinical trials with a PAR1-directed pepducin, PZ-128 (Gurbel et al., 2016; Zhang et al., 2012), that has just finished dosing in the TRIP-PCI phase 2 study for a cardiovascular indication. This study extends this approach to show that a PAR2 pepducin may have potential in the effective treatment of patients with the chronic inflammatory skin disease atopic dermatitis.
MATERIALS AND METHODS
Animal models
Male flaky tail (filaggrin-deficient, Flgft) and C57BL/6J (8–12-week-old) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and male CF1 mice (8–12 weeks) from Charles River Laboratories International (Wilmington, MA) in the certified and accredited Division of Laboratory Animal Medicine at Tufts University using International Animal Care and Use Committee-approved protocols. Mice received daily sc injections (100 μl) of vehicle or PZ-235 (P2pal-18S, palmitate-RSSAMDENSEKKRKSAIK-NH2) pepducin antagonist, with injections rotated to a fresh nonlesional site each day. Pepducins were synthesized by Oasis Pharmaceuticals (Lexington, MA) by standard Fmoc solid phase methods and purified to 99.1% purity by reverse-phase high performance liquid chromatography before lyophilization (Sevigny et al., 2011b). Lyophilized dust mite extract (Greer Laboratories, Lenoir, NC) was diluted in mineral oil (2.5 μg/μl) and applied 2 times per week to the back skin between the shoulder blades (40 μl) and/or to the top of the right ear (10 μl). Ear thickness was measured and skin lesion severity was recorded based on four criteria: erythema, lichenification/scaling, skin thickness/edema, and scabbing/crusting. On the last day of dust mite treatment, itch was assessed for a period of 5 minutes, beginning 15 minutes after dust mite extract application. At the 8-week endpoint after death, the right ear and back skin were surgically removed and processed for hematoxylin and eosin staining or for FACS-based sorting of skin leukocytes.
In the DNFB model, 50 μl of the hapten 2,4-dinitrofluorobenzene (Millipore Sigma, Billerica, MA) (1% volume/volume in 20% olive oil/80% acetone) was applied to the shaved back region of male CF1 mice. DNFB was then applied weekly for 6 weeks at a dose of 0.1% volume/volume in 20% olive oil/80% acetone, upon which atopic dermatitis-like lesions due to the contact hypersensitivity reaction were readily evident. During the last 2 weeks of DNFB application, mice began receiving daily sc injections of PZ-235 or vehicle. At the conclusion of the 6-week experiment, DNFB-lesioned skin was dissected from the killed mouse, fixed, and processed for hematoxylin and eosin staining.
In the oxazolone-evoked mouse ear irritation and thickening model, CF1 mice were given 100-μl sc injections of PZ-235 (1, 3, or 10 mg/kg) or vehicle at 16, 24, and 40 hours after oxazolone challenge. Next, 20 μl of oxazolone (Millipore Sigma) (1% in 80% acetone/20% olive oil) was pipetted onto the dorsal ear of mice under isoflurane anesthesia at 0, 16, and 40 hours. Thickness of the ear was sequentially measured at intervals over a period of 48 hours using a digital caliper.
For the wasp venom peptide pruritis model, PZ-235 was injected sc into CF1, Par2−/−, or wild-type C57BL/6J mice 2 hours before administration of 50-μl intradermal injections of 200-μg mast cell-degranulating agent MA-1 (INLKALAALAKKIL-NH2) from wasp venom. In wild-type and Par2−/− C57BL/6J mice, scratches were monitored for 5 minutes, and in CF1 mice, scratch events were recorded for 30 minutes after recovery from anesthesia. The different observation times used resulted from observing peak scratching behavior windows in the CF1 (outbred white mouse) versus C57BL/6J (inbred black mouse) strains. CF1 had a much more delayed scratching response to intradermal mastoparan injections compared with the C57BL/6J.
Cell-based assays
Neonatal normal human epidermal keratinocytes (Lonza, Basel, Switzerland) were cultured with keratinocyte growth media (KGM-Gold, Lonza). HMC1 cells, a human mast cell line (Millipore Sigma), were grown in suspension with Iscove’s medium supplemented with 10% fetal bovine serum, 10 μmol/L monothioglycerol, and 0.5% penicillin/streptomycin. PAR2 calcium responses were measured as previously described (Shearer et al., 2016). Normal human epidermal keratinocytes were cultured until they reached 100% confluency and then switched to media containing 2 mmol/L calcium for 4 days before calcium flux assays. HMC1 mast cells were plated at 106 cells/60-mm dish; the following day, cells were pretreated for 45 minutes with vehicle or PZ-235 and then incubated with PAR2 agonist SLIGRL or vehicle (phosphate buffered saline) for 24 hours. Total RNA was isolated using an RNeasy minikit (Qiagen, Hilden, Germany), and expression of genes of interest was determined by quantitative PCR with 500 nmol/L primers for NF-κB (p65), Tnfα, Tslp, IL4, and IL13 using Lightcycler 480 SYBR Green master mix in 16-μl reactions.
Statistical analyses
Data were analyzed using Statistica 6 (TIBCO Software, Palo Alto, CA) and GraphPad Prism 5 (GraphPad, La Jolla, CA). Comparisons between two independent groups were made using unpaired t tests or Mann-Whitney U tests. Comparisons of multiple groups to a control group were made using one- or two-way analysis of variance followed by Dunnett or Tukey post hoc tests. Linear correlation was assessed using Pearson test.
A detailed Materials and Methods section is available in the Supplemental Materials online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Joe Stevens for assistance in conducting dermatitis models. This study was funded in whole or in part by AR067617 (AK, LC) from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Abbreviations:
- MA-1
mastoparan
- sc
subcutaneous
Footnotes
CONFLICT OF INTEREST
AK and LC report serving as Scientific Founders of Oasis Pharmaceuticals.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2018.08.019.
REFERENCES
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 2004;24:4300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Takayama Y, Yamakoshi T, Lee JB, Sano A, Shimizu T, et al. Involvement of serine protease and proteinase-activated receptor 2 in dermatophyte-associated itch in mice. J Pharmacol Exp Ther 2012;343:91–6. [DOI] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. [DOI] [PubMed] [Google Scholar]
- Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 2009;206:1135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep 2007;7:363–7. [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA 2002;99:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 2007;27:10007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol 2017;139(4S):S49–57. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009;41:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol 2012;13:113–23. [DOI] [PubMed] [Google Scholar]
- Gouin O, L’Herondelle K, Buscaglia P, Le Gall-Ianotto C, Philippe R, Legoux N, et al. Major role for TRPV1 and InsP3R in PAR2-elicited inflammatory mediator production in differentiated human keratinocytes. J Invest Dermatol 2018;138:1564–72. [DOI] [PubMed] [Google Scholar]
- Graham MT, Nadeau KC. Lessons learned from mice and man: mimicking human allergy through mouse models. Clin Immunol 2014;155:1–16. [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Bliden KP, Turner SE, Tantry US, Gesheff MG, Barr TP, et al. Cell-penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscler Thromb Vasc Biol 2016;36:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol 2006;126:2074–86. [DOI] [PubMed] [Google Scholar]
- Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol 2008;121:872–7. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, et al. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol 2014;171:1180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol 1994;131:348–53. [DOI] [PubMed] [Google Scholar]
- Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med 2005;11:661–5. [DOI] [PubMed] [Google Scholar]
- Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol 2007;8:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, et al. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy 2009;64:1366–74. [DOI] [PubMed] [Google Scholar]
- Kawagoe J, Takizawa T, Matsumoto J, Tamiya M, Meek SE, Smith AJ, et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Jpn J Pharmacol 2002;88:77–84. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol 2009;21:666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in neuroimmune communication and itch In: Carstens E, Akiyama T, editors. Itch: mechanisms and treatment. Boca Raton, FL: CRC Press, Taylor and Francis; 2014. [PubMed] [Google Scholar]
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol 1999;135: 1522–5. [DOI] [PubMed] [Google Scholar]
- Lee SE, Jeong SK, Lee SH. (2010) Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J 2010;51:808–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. (2007) Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol 2007;120(2):238–44. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Kim B, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, et al. (2014) Thymic stromal lymphopoietin variation, filaggrin loss of function, and the persistence of atopic dermatitis. JAMA Dermatol 2014;150:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael ES, Kuliopulos A, Covic L, Steer ML, Perides G. (2013) Pharmacological inhibition of PAR2 with the pepducin P2pal-18S protects mice against acute experimental biliary pancreatitis. Am J Physiol Gastrointest Liver Physiol 2013;304:G516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniaga CS, Egawa G, Kawasaki H, Hara-Chikuma M, Honda T, Tanizaki H, et al. Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. Am J Pathol 2010;176:2385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morar N, Cookson WO, Harper JI, Moffatt MF. Filaggrin mutations in children with severe atopic dermatitis. J Invest Dermatol 2007;127:1667–72. [DOI] [PubMed] [Google Scholar]
- O’Callaghan K, Kuliopulos A, Covic L. Turning receptors on and off with intracellular pepducins: new insights into G-protein-coupled receptor drug development. J Biol Chem 2012a;287:12787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan K, Lee L, Nguyen N, Hsieh MY, Kaneider NC, Klein AK, et al. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood 2012b;119:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan GM, Irvine AD. (2010) The role of filaggrin in the atopic diathesis. Clin Exp Allergy 2010;40:965–72. [DOI] [PubMed] [Google Scholar]
- Obara W, Kawa Y, Ra C, Nishioka K, Soma Y, Mizoguchi M. T cells and mast cells as a major source of interleukin-13 in atopic dermatitis. Dermatology 2002;205:11–7. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 2008;28:4331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Shimada SG, Sikand P, Lamotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol 2010;130:1468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Schneider C, Stock C, Zollner TM, Docke WD. Extended DNFB-induced contact hypersensitivity models display characteristics of chronic inflammatory dermatoses. Exp Dermatol 2012;21:25–31. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol 2000;142:1114–20. [DOI] [PubMed] [Google Scholar]
- Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol 2013;171:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley S, Covic L, Jacques SL, Sudmeier J, Baleja JD, Kuliopulos A. Structural basis for thrombin activation of a protease-activated receptor: inhibition of intramolecular liganding. Chem Biol 2003;10:1033–41. [DOI] [PubMed] [Google Scholar]
- Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. (2003) Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J 2003;17:1871–85. [DOI] [PubMed] [Google Scholar]
- Sevigny LM, Austin KM, Zhang P, Kasuda S, Koukos G, Sharifi S, et al. Protease-activated receptor-2 modulates protease-activated receptor-1-driven neointimal hyperplasia. Arterioscler Thromb Vasc Biol 2011a;31(12):e100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny LM, Zhang P, Bohm A, Lazarides K, Perides G, Covic L, et al. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci USA 2011b;108(20):8491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer AM, Rana R, Austin K, Baleja JD, Nguyen N, Bohm A, et al. (2016) Targeting liver fibrosis with a cell-penetrating protease-activated receptor-2 (PAR2) pepducin. J Biol Chem 2016;291:23188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol 2013;132:1132–8. [DOI] [PubMed] [Google Scholar]
- Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol 2008;128:18–25. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. (2006) Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol 2006;126:1705–18. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol 1999;8:282–94. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003;23:6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J Pharmacol Sci 2009;109:388–95. [DOI] [PubMed] [Google Scholar]
- Udkoff J, Silverberg JI. Validation of scratching severity as an objective assessment for itch. J Invest Dermatol 2018;138:1062–8. [DOI] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau MK, Liu L, Fairlie DP. Toward drugs for protease-activated receptor 2 (PAR2). J Med Chem 2013;56:7477–97. [DOI] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. (2005) Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med 2005;202:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Gruber A, Kasuda S, Kimmelstiel C, O’Callaghan K, Cox DH, et al. Suppression of arterial thrombosis without affecting hemostatic parameters with a cell-penetrating PAR1 pepducin. Circulation 2012;126:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Leger AJ, Baleja JD, Rana R, Corlin T, Nguyen N, et al. (2015) Allosteric activation of a G protein-coupled receptor with cell-penetrating receptor mimetics. J Biol Chem 2015;290:15785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.