Abstract
Despite the recent approval of immune-modulatory agents, EGFR inhibition continues to be a cornerstone in the management of SCCHN namely in combination with radiotherapy in the treatment of locoregionally advanced disease as well as in platinum sensitive recurrent or metastatic disease in the first line setting.
Importantly, recent evidence has emerged supporting also an immune-modulatory effect of EGFR inhibition, and interest has now focused on utilizing these effects in the current treatment approaches for SCCHN. In this report we review the rationale and evidence supporting the forging of this new alliance in optimizing the treatment of SCCHN.
Keywords: Immunotherapy and EGFR in head and neck cancer, combination cetuximab and immunotherapy, EGFR monoclonal antibodies and immunotherapy, EGFR and immunotherapy in SCCHN, combination of anti-EGFR and immunotherapy in head and neck cancer Immunotherapy
Rationale for inhibiting EGFR in SCCHN
SCCHN is diagnosed worldwide in more than 500,000 people and is responsible for 380,000 deaths annually [1]. SCCHN is a heterogeneous disease as far as anatomic location and genetic aberrations [2].Despite advances in multimodal therapy, the survival rates and functional outcomes of patients remain limited with an overall 5 year survival rate that is close to 50% [3]. Novel strategies are urgently needed particularly in patients with recurrent or metastatic disease. Abnormal EGFR signaling in a variety of tumors including SCCHN has been correlated with poor prognosis and lower response to therapy [4-8]. More than 80% of SCCHN tumors show EGFR over-expression [7, 9]. Cetuximab is an anti-EGFR receptor antibody contributing to growth suppression and apoptosis of SCCHN [10] and it has been shown to reduce cancer cell proliferation in xenograft models [11-15]. Even though the immunomodulatory effects of cetuximab have been described and well-established earlier, attention to this particular mechanism has surged more recently given the increased interest of this application in SCCHN[16]. Cetuximab in combination with platinum-based chemotherapy has been shown to result in improved overall survival when given as first-line treatment to patients with recurrent/metastatic SCCHN compared with platinum-based chemotherapy alone[17]. In recently reported two phase III trials comparing cetuximab versus cisplatin in combination with radiation for definitive treatment of HPV related oropharyngeal SCC, cisplatin resulted in a superior overall survival compared to cetuximab[18, 19], therefore confirming that cisplatin radiotherapy is the preferred standard of care for patients with HPV-related oropharyngeal SCC
The limitations of anti-EGFR targeting in SCCHN
The human epidermal growth factor receptor (EGFR) family consists of four types of trans-membrane tyrosine kinase receptors, HER1 (EGFR, ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). The general structure of ErbB members includes an extracellular ligand-binding region, an α-helical trans-membrane segment, a cytoplasmic tyrosine-kinase-containing domain, and a C-terminal phosphorylation tail [20, 21]. ErbB members are widely expressed in epithelial, mesenchymal, and neuronal tissues and regulate cell division, proliferation, differentiation, and other normal cellular processes [22, 23].
EGFR is expressed in more than 80% of SCCHN [9]. Ligand binding, resulting in homodimerization or heterodimerization with other HER family members, causes phosphorylation of the tyrosine kinase domain and cell proliferation. The EGFR-specific monoclonal antibody drug cetuximab decreases tumor cell line growth, and increases apoptosis of SCCHN [10]. In vitro and xenograft studies have shown that cetuximab effectively decreases tumor cell proliferation [11-14]. Despite its success in combination therapy, the overall response rate to cetuximab as a single-agent treatment approach in recurrent/metastatic SCCHN does not exceed 13% and is associated with a median response duration of less than 70 days[24]. In combination with chemotherapy, the median progression-free survival for patients receiving it as a first-line therapy is around six months [17]. Furthermore, patients frequently demonstrate primary resistance to EGFR monoclonal antibodies and acquired resistance emerges over time[25, 26], which further stresses the need for mechanistic studies to understand resistance to EGFR inhibition. Analyses performed on tissue samples from large randomized trials have disappointingly revealed that EGFR expression does not seem to be a clinically useful predictive biomarker in SCCHN patients [27]. In addition, EGFR copy number was not predictive of cetuximab efficacy in recurrent/metastatic SCCHN[28], despite being useful in other solid tumors[29, 30]. The survival benefit of chemotherapy plus cetuximab over chemotherapy alone was shown to be independent of tumor p16 and HPV status[31] indicating that resistance mechanisms to these regimens may affect both HPV-positive and –negative subtypes of SCCHN. Despite the argument that single agent anti-EGFR therapy may have a lower clinical activity in HPV-related versus -unrelated disease, and data pointing to an inverse relation between HPV status and EGFR expression[32, 33], no definite evidence exists to distinguish the use of cetuximab based on HPV status. These findings suggest that failure of cetuximab therapy is likely linked to alternative pathways, which may include compensatory signaling from other EGFR family receptors, such as HER2 and HER3, or other downstream resistance mechanisms[15] [34-36].
Resistance mechanisms to EGFR inhibition
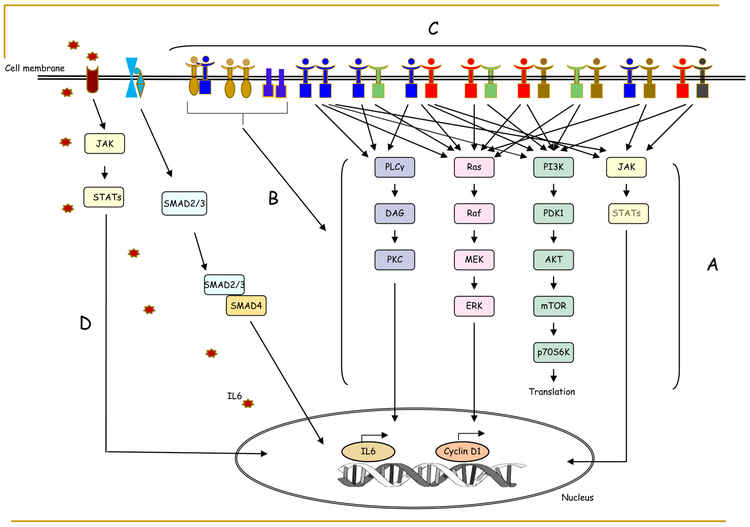
More than 10 mechanisms of resistance to EGFR targeting have been reported in SCCHN[37] (Figure 1). Possible mechanisms for de novo and acquired resistance to EGFR inhibition include mutations in the KRAS, BRAF, NRAS, and PIK3CA genes[25, 38], a secondary mutation (S492R) in the extracellular domain of the EGFR receptor[25, 26], overexpression of the MET proto-oncogene (c-Met)[39], and expression of the in-frame deletion mutation of EGFR variant III, in addition to other possible mechanisms [40, 41]. In other tumor types, genetic alterations in the EGFR-RAS-RAF-MEK signaling pathways are mechanisms of acquired resistance to anti-EGFR antibodies through the possible constitutive activation of intracellular downstream signaling pathways, including RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways[42-47], some of which are rarely observed in SCCHN. Recent evidence has confirmed a possible role of HRAS in EGFR resistance [48-50] and has led to the resurgence of HRAS inhibitors as possible effective therapeutic targets in HRAS mutant SCCHN[51]. Other mechanisms of EGFR resistance may include the dysregulation of EGFR internalization and subcellular localization, including nuclear localization and degradation of EGFR [52-54].
Figure 1.
Resistant mechanisms of EGFR targeted therapy. The following molecular activities will result in bypassing EGFR blockade: (A) Activation mutations or amplification of EGFR downstream signaling effectors; (B) Overexpression of MET proto-oncogene and expression of EGFR variant III; (C) Hetero-dimerization between EGFR family members; and (D) Activation of TGF-beta IL-6 axis
HER3 (ErbB3) is a member of the human EGFR family, which consists of four types of transmembrane tyrosine kinase receptors: HER1 (EGFR, ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4)[15, 20, 21, 45]. Upon binding of HRG1, the physiological HER3 receptor ligand, HER3 dimerizes with other ErbB family members, preferentially HER2[55-57]. High HRG1 expression is associated with activation of HER3 and has been correlated with worse clinical outcome in SCCHN[58]. We and others have demonstrated consistently elevated expression levels of HRG1 in SCCHN in comparison with other solid tumors, such as non-small cell lung and breast cancers[34].
It is of interest that some mechanisms of EGFR resistance are immune-mediated; such an example is resistance to EGFR mediated through the TGF-beta IL6 axis arguing for an interaction between EGFR signaling and the immune-microenvironment[59]. This interaction could potentially be harnessed in future applications focusing on targeting EGFR and the TGF-beta IL-6 axis.
Emergence of immunotherapy as an effective therapeutic modality in SCCHN
Even though the immune-suppressive nature of certain cancers including SCCHN has been long recognized [60], it was not until recently that targeted immunotherapy promoting anti-tumor T-cell activity was demonstrated to induce improved survival and durable objective responses in solid tumors, including advanced melanoma[61]. In addition, preclinical data have suggested a beneficial effect of targeting both the programmed death receptor-1 (PD-1) / PD-ligand 1 (PD-L1) and the cytotoxic T lymphocyte antigen-4 (CTLA4) immune checkpoints in SCCHN [62]. PD-L1 expression has been observed in close to 68% of SCCHN tumors regardless of HPV status [63] and several trials have evaluated the utility of PD-L1:PD-1 blockade for the treatment of recurrent/metastatic SCCHN [64] leading to impressive clinical benefits in heavily pretreated patients.
The anti-PD-1 agents pembrolizumab and nivolumab were approved recently for treatment of recurrent metastatic SCCHN based on the results from phase I, II and III studies[65, 66]. The phase 1b Keynote-012 trial using pembrolizumab showed an unprecedented 1-year survival benefit of 18%, which resulted in FDA approval of the drug for the treatment of platinum-resistant recurrent metastatic SSCHN in August 2016 [67]. The results from Checkmate-141, a phase III trial randomizing patients with recurrent or metastatic, platinum-refractory SCCHN to nivolumab versus investigator’s choice of chemotherapy (weekly cetuximab, docetaxel or methotrexate), demonstrated a doubling of one-year overall survival (OS) (36.0% versus 16.6%, p= 0.0101)[65]. The median OS was 7.5 vs 5.1 months for patients treated with nivolumab versus chemotherapy (HR 0.70, p = 0.01). The median OS by PD-L1 status was 8.7 vs. 4.6 months for patients with tumor PD-L1 expression > 1% vs. PD-L1 < 1%. The 18-month OS rate was 21.5% vs. 8.3% and overall response was 13.3% vs. 5.8%. Nivolumab also doubled the median duration of response versus chemotherapy (9.7 vs 4.0 months). Furthermore, immunotherapy was better tolerated with lower grade 3–4 treatment-related adverse event rates for nivolumab versus chemotherapy (15.3% vs 36.0%). Longer-term follow-up data continued to favor nivolumab with a significant survival benefit (estimated 24-months OS rate 16.7% vs. 6.0%) and better tolerability versus chemotherapy in patients with platinum-refractory disease [68]. The Keynote 040 phase III trial comparing pembrolizumab to docetaxel, methotrexate and cetuximab, supported the results of checkmate 141, showing a clinically meaningful prolongation of overall survival favoring pembrolizumab [69]. In the first line setting Keynote 048 has compared single agent pembrolizumab as well as pembrolizumab in combination with platinum and 5FU to the cetuximab containing Extreme regimen. The results of this trial were reported at the ESMO 2018 meeting and favored both immunotherapy containing versus the cetuximab arm[70]. Though these benefits are groundbreaking in the treatment of recurrent or metastatic SSCHN, there is still a large proportion of the patients who do not benefit from this therapy and might require alternative immune-(re)activation or combination therapies. As immunotherapy continues to be a rapidly evolving field in the treatment of various malignancies including SCCHN, a growing interest in the examination of immune mechanisms to various targeted therapies including EGFR targeting is currently evident. With the move of immunotherapy to the first line setting in SCCHN as well as its application in the definitive treatment setting, exploring methods to combine these agents with EGFR inhibitors and understanding their interactions is strongly warranted.
Immune implications of EGFR targeting
Although EGFR targeting with cetuximab has been approved for more than a decade as an effective treatment modality for SCCHN, recent evidence has emerged showing that immune modulation represents an alternative mechanism by which EGFR inhibition, specifically with cetuximab, elicits clinical activity in SCCHN [71, 72]. Cetuximab and panitumumab, both monoclonal antibodies to EGFR, were shown to activate natural killer (NK) cells, with cetuximab being a more potent activator[73]. Of further interest however, is that myeloid cell-mediated antibody-dependent cellular phagocytosis (ADCP) may differ based on whether the clinical agent is an IgG1 (cetuximab) versus IgG2 (panitumumab) monoclonal antibody, which may have implications on the use of these agents as part of future combinatorial approaches [74].
In addition to blocking EGFR signaling, cetuximab has been shown to increase IFN-γ produced by NK cells via antibody-dependent cellular cytotoxicity (ADCC), which in tumor cells as well as on immune cells within the tumor microenvironment can induce PD-L1 expression [75, 76] arguing for a possible synergistic effect of EGFR and PD-1 inhibition, also since NK cells themselves can express PD-1.[77]. Recently, the intracellular DNA sensor stimulator of interferon genes (STING) has been shown to have a crucial role in the immune response to viruses and tumors by stimulating cytokine production.[78] The evidence also suggesting that cetuximab coupled with the activation of STING enhances the antitumor activity in SCCHN [79]. All these recent findings strongly suggest that EGFR inhibition interacts closely with the tumor microenvironment affecting the cytokine milieu and leading to an immune modulatory effect.
In addition, blocking EGFR might affect mechanisms of resistance to immunotherapy. In fact, EGF was shown to induce overexpression of PD-L1 by increasing the protein levels of STAT1 to enforce the IFNγ-JAK1/2-mediated signaling axis[80]; this could ultimately reduce the response to PD-L1 inhibitors.
Moreover, activation of the EGFR pathway is involved in suppressing the immune response through activation of Tregs or reducing the level of T cell chemo-attractants [81].
Forging the alliance
Given that both EGFR-targeted therapy and immunotherapy are, or are becoming, important cornerstones for treatment of advanced SCCHN and the scientific rationale supporting an immune modulatory effect of EGFR blockade, research focusing on combining these two modalities is clearly warranted and is already underway. Of importance is the observation that an immune-mediated component seems to be a characteristic of monoclonal antibodies to EGFR such as cetuximab, panitumumab, zalutumumab, matuzumab or nimotuzumab rather than small molecule EGFR inhibitors. This increases the interest of forging such an alliance in SCCHN given the noted importance of both classes of agents in this disease. Furthermore, exploration of the biologic interactions between EGFR inhibition and the tumor microenvironment remain highly justified. Of note is that such efforts may have significant implications on the therapeutic approaches in different malignancies given the benefit derived from each of these modalities in different tumor types. is an example of a multicenter clinical trial focusing on the combination of cetuximab and nivolumab, both approved for the treatment of recurrent/metastatic SCCHN (Table1). Patients in this trial will be allowed to have prior exposure of either immune checkpoint inhibitor or cetuximab provided these agents are not administered simultaneously, hence exploring a possible beneficial combinatorial effect of these agents. Another example is the REACH study in locoregionally advanced disease, comparing cisplatin with radiation versus cetuximab, avelumab and radiation, versus cetuximab and radiation () (Table 1). Other trials are exploring similar combinations with radiation therapy in patients who are not eligible to receive cisplatin. Even though these trials will take time to mature, we expect to learn of the clinical benefit of these combinations in a relatively short period of time. An important cell to focus on in this alliance are also the NK cells and their most common inhibitory receptors, since they are key players in the immune-related mechanisms of cetuximab[82]. It has been shown that in SSCHN, NK cells can be highly suppressed in their function due to high presence of inhibitory ligands preventing NK cell infiltration within the tumor [83]. An alternative could be combining cetuximab with NK cell adoptive cell therapy[84]. or targeting other NK cell inhibitory receptors besides PD-1. Recent preclinical work and interim analyses of a phase II clinical study [] showed benefit of blocking the inhibitory receptor NKG2A, on NK cells and T cells, using Monalizumab combined with anti-EGFR targeting in patients with recurrent or metastatic SSNHN, showing 31% partial responses (8/26 patients)[85]. On the other side, combining anti-EGFR-tyrosine kinase inhibitors with immunotherapy did not show a favorable safety profile, with an elevated incidence of interstitial lung disease and an increase of alanine aminotransferase/aspartate aminotransferase levels [86]. Therefore, the anti-EGFR drug choice is crucial to ensure the optimal therapeutic ratio when added to immunotherapy. Recently, also the TLR8 agonist motolimod was shown to recruit circulating EGFR-specific T cells as well as CD8+ T-cells into SCCHN tumors[87]. Despite these promising observations in early phase trials, when motolimod was added to the EXTREME regimen in a large phase II randomized study, it did not seem to impact overall or progression-free survival in patients with recurrent/metastatic SCCHN. Despite these disappointing results, a significant benefit was observed in HPV-positive patients and patients with injection site reactions, suggesting that a subset of patients might benefit from EGFR inhibition combined with TLR8 stimulation[88] (Table 1). More profound biomarker research will have to explore on which criteria this selection should be based. Numerous other therapeutic trials in SCCHN are actively exploring novel combinations of immunotherapy and EGFR inhibition.
Table 1.
Ongoing and published clinical trials combining EGFR monoclonal antibodies with immunotherapeutic agents in SCCHN.
| Design | Title | Trial number | Status |
|---|---|---|---|
| Phase II | A Phase I/II Study of Concurrent Cetuximab and Nivolumab in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma | Recurrent or metastatic | |
| Phase III | Randomized Trial of Avelumab-cetuximab-radiotherapy Versus SOCs in LA SCCHN (REACH) | Loco-regional | |
| Phase III (terminated) | Nivolumab or Nivolumab Plus Cisplatin or Cetuximab, in Combination with Radiotherapy in Patients with Cisplatin-ineligible or Eligible Locally Advanced Squamous Cell Head and Neck Cancer | Locoregional | |
| Phase I | Phase I trial of cetuximab, intensity modulated radiotherapy (IMRT), and the anti-CTLA-4 monoclonal antibody (mAb) ipilimumab in previously untreated, locally advanced head and neck squamous cell carcinoma (PULA HNSCC) | Locoregional | |
| Phase II | Motolimod and Standard Combination Chemotherapy with Cetuximab in Treatment of Patients with Squamous Cell Carcinoma of the Head and Neck: The Active8 Randomized Clinical Trial. | Recurrent and metastatic SCCHN (manuscript published [88]). | |
| Phase II | EACH: Evaluating Avelumab in Combination with Cetuximab in Head and Neck Cancer (EACH) | Locoregional |
Based on the aforementioned it is clear that the new alliance between EGFR and immune targeting approaches has been forged and this development may provide novel additions to the treatment guidelines of advanced SCCHN.
Acknowledgment:
This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com)
NIH funding: This work was supported by an NIH grant P30 CA 138292 to NFS and GZC
Footnotes
Conflict of Interest (COI):
NFS reports receiving compensation for advisory role from BMS, Merck, Lilly, GSK, Roche, J.B.V.: Has had in the last 3 years or has consulting/ advisory relationships with: Amgen, AstraZeneca, Boehringer Ingelheim, Innate Pharma, Merck Serono, Merck Sharp & Dome Corp, PCI Biotech, Synthon Biopharmaceuticals, Debiopharm and Wnt Research and has received lecture fees from Merck-Serono, Sanofi and BMS. PB reports Advisory role for BMS, MSD, Astra Zeneca, Sanofi, Roche, Angelini
MH, reports: Advisory role to Astra Zeneka and Takeda Oncology,
ER, AR, PB, PS, JPR, and GZC report no COI
References
- 1.Bray F, et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018. 68(6): p. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberger NJ and Stabile LP, Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers (Basel), 2017. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, et al. , Cancer statistics, 2014. CA Cancer J Clin, 2014. 64(1): p. 9–29. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, et al. , Correlation of cisplatin sensitivity with differential alteration of EGFR expression in head and neck cancer cells. Anticancer Res, 2000. 20(2A): p. 899–902. [PubMed] [Google Scholar]
- 5.D'Amico TA, et al. , A biologic risk model for stage I lung cancer: immunohistochemical analysis of 408 patients with the use of ten molecular markers. J Thorac Cardiovasc Surg, 1999. 117(4): p. 736–43. [DOI] [PubMed] [Google Scholar]
- 6.Hansen AR and Siu LL, Epidermal growth factor receptor targeting in head and neck cancer: have we been just skimming the surface? J Clin Oncol, 2013. 31(11): p. 1381–3. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Andratschke NH, and M. L, Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys., 2004. March 1;58(3):959–65. [DOI] [PubMed] [Google Scholar]
- 8.Saba NF, Khuri FR, and S. DM, Targeting the epidermal growth factor receptor. Trials in head and neck and lung cancer. Oncology (Williston Park)., 2006. February;20(2):153–61. [PubMed] [Google Scholar]
- 9.Grandis JR FD, Melhem MF, Gooding WE, Drenning SD, Morel PA, Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res, 2000. 6(7): p. 2794–802. [PubMed] [Google Scholar]
- 10.Huang SM BJ, Harari PM, Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res, 1999. 59(8): p. 1935–40. [PubMed] [Google Scholar]
- 11.Kawamoto T, et al. , Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A, 1983. 80(5): p. 1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato JD, et al. , Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med, 1983. 1(5): p. 511–29. [PubMed] [Google Scholar]
- 13.Masui H, et al. , Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res, 1984. 44(3): p. 1002–7. [PubMed] [Google Scholar]
- 14.Gill GN, et al. , Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem, 1984. 259(12): p. 7755–60. [PubMed] [Google Scholar]
- 15.Jiang N, et al. , Combination of anti-HER3 antibody MM-121/SAR256212 and cetuximab inhibits tumor growth in preclinical models of head and neck squamous cell carcinoma Mol Cancer Ther., 2014. July;13(7):1826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szturz P and V. JB, Immunotherapy in head and neck cancer: aiming at EXTREME precision. . BMC Med, 2017. June 2;15(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermorken JB, et al. , Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008. September 11;359(11):1116–27. [DOI] [PubMed] [Google Scholar]
- 18.Gillison ML, et al. , Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial; . Lancet, 2019. January 5;393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehanna H, et al. , Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet, 2019. January 5;393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olayioye MA, et al. , The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J, 2000. 19(13): p. 3159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HH, et al. , Signal transduction by epidermal growth factor and heregulin via the kinase-deficient ErbB3 protein. Biochem J, 1998. 334 (Pt 1): p. 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hynes NE and Lane HA, ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer, 2005. 5(5): p. 341–54. [DOI] [PubMed] [Google Scholar]
- 23.Mendelsohn J and Baselga J, Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol, 2003. 21(14): p. 2787–99. [DOI] [PubMed] [Google Scholar]
- 24.Vermorken JB, et al. , Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol, 2007. 25(16): p. 2171–7. [DOI] [PubMed] [Google Scholar]
- 25.Bardelli A and Janne PA, The road to resistance: EGFR mutation and cetuximab. Nat Med, 2012. 18(2): p. 199–200. [DOI] [PubMed] [Google Scholar]
- 26.Montagut C, et al. , Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med, 2012. 18(2): p. 221–3. [DOI] [PubMed] [Google Scholar]
- 27.Licitra L, et al. , Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer 2013. April;49(6):1161–8. [DOI] [PubMed] [Google Scholar]
- 28.Licitra L, et al. , Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol, 2011. May;22(5):1078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler DL, Dunn EF, and Harari PM, Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol, 2010. 7(9): p. 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moroni M, et al. , Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol, 2005. 6(5): p. 279–86. [DOI] [PubMed] [Google Scholar]
- 31.Vermorken JB, et al. , Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol, 2014. April;25(4):801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szturz P, Seiwert T, and V. JB, How Standard Is Second-Line Cetuximab in Recurrent or Metastatic Head and Neck Cancer in 2017? J Clin Oncol 2017. July 10;35(20):2229–2231. [DOI] [PubMed] [Google Scholar]
- 33.Keck MK, et al. , Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res, 2015. February 15;21(4):870–81. [DOI] [PubMed] [Google Scholar]
- 34.Qian G, et al. , Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. . Cancer 2015. October 15;121(20):3600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Pauw I, et al. , Simultaneous targeting of EGFR, HER2, and HER4 by afatinib overcomes intrinsic and acquired cetuximab resistance in head and neck squamous cell carcinoma cell lines. Mol Oncol 2018. June;12(6):830–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock NI, et al. , Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies. Clin Cancer Res, 2015. October 15;21(20):4597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boeckx C, et al. , Anti-epidermal growth factor receptor therapy in head and neck squamous cell carcinoma: focus on potential molecular mechanisms of drug resistance. Oncologist 2013. 18(7):850–64. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lievre A, et al. , KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res, 2006. 66(8): p. 3992–5. [DOI] [PubMed] [Google Scholar]
- 39.Krumbach R, et al. , Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer, 2011. 47(8): p. 1231–43. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler SE, et al. , Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene, 2010. 29(37): p. 5135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JW, et al. , Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res, 2005. 11(8): p. 2879–82. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias-Bartolome R, Martin D, and Gutkind JS, Exploiting the Head and Neck Cancer Oncogenome: Widespread PI3K-mTOR Pathway Alterations and Novel Molecular Targets. Cancer Discov, 2013. 3(7): p. 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeman RJ, Lui VW, and Grandis JR, STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther, 2006. 6(3): p. 231–41. [DOI] [PubMed] [Google Scholar]
- 44.Sen M, et al. , First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov, 2012. 2(8): p. 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarden Y and Sliwkowski MX, Untangling the ErbB signalling network. Nat Rev Mol Cell Biol, 2001. 2(2): p. 127–37. [DOI] [PubMed] [Google Scholar]
- 46.Van Emburgh BO, et al. , Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol, 2014. May 14 pii: S1574–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rampias T, et al. , RAS/PI3K crosstalk and cetuximab resistance in head and neck squamous cell carcinoma. Clin Cancer Res, 2014. 20(11): p. 2933–46. [DOI] [PubMed] [Google Scholar]
- 48.Hah JH, et al. , HRAS mutations and resistance to the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in head and neck squamous cell carcinoma cells. Head Neck 2014. November;36(11):1547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braig F, et al. , Liquid biopsy monitoring uncovers acquired RAS-mediated resistance to cetuximab in a substantial proportion of patients with head and neck squamous cell carcinoma. Oncotarget 2016. July 12;7(28):42988–42995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bossi P, et al. , Functional Genomics Uncover the Biology behind the Responsiveness of Head and Neck Squamous Cell Cancer Patients to Cetuximab. Clin Cancer Res 2016. August 1;22(15):3961–70. [DOI] [PubMed] [Google Scholar]
- 51.Ho A, et al. , Preliminary results from a phase 2 trial of tipifarnib in Squamous Cell Carcinomas (SCCs) with HRAS mutations. ESMO Congress Presentation, Mjunich, October 2018. 2018. [Google Scholar]
- 52.Wheeler DL, et al. , Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene, 2008. 27(28): p. 3944–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y, et al. , Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res, 2007. 67(17): p. 8240–7. [DOI] [PubMed] [Google Scholar]
- 54.Li C, et al. , Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene, 2009. 28(43): p. 3801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atlas E, et al. , A deletion mutant of heregulin increases the sensitivity of breast cancer cells to chemotherapy without promoting tumorigenicity. Oncogene 2003. May 29;22(22):: p. 3441–51. [DOI] [PubMed] [Google Scholar]
- 56.Tzahar E, et al. , A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol, 1996. October;16(10):: p. 5276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kol A, et al. , HER3, serious partner in crime: therapeutic approaches and potential biomarkers for effect of HER3-targeting. Pharmacol Ther, 2014. 143(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 58.Shames DS, et al. , High heregulin expression is associated with activated HER3 and may define an actionable biomarker in patients with squamous cell carcinomas of the head and neck. PLoS One, 2013. 8(2): p. e56765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao Z, et al. , TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. , 2010. August 31;107(35):15535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young MR, et al. , Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer, 1996. July 29;67(3):333–8. [DOI] [PubMed] [Google Scholar]
- 61.Weber JS, et al. , Phase I/II study of ipilimumab for patients with metastatic J Clin Oncol 2008. December 20;26(36):5950–6. [DOI] [PubMed] [Google Scholar]
- 62.Swanson MS and Sinha UK, Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer—review of current data. Oral Oncology 2015. January;51(1):12–5. [DOI] [PubMed] [Google Scholar]
- 63.Kim HS, et al. , Association Between PD-L1 and HPV Status and the Prognostic Value of PD-L1 in Oropharyngeal Squamous Cell Carcinoma. Cancer Res Treat, 2015. September 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zandberg DP and S. SE, The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2014. July;50(7):627–32. [DOI] [PubMed] [Google Scholar]
- 65.Ferris RL, et al. , Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016. November 10;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bauml J, et al. , Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study J Clin Oncol, 2017. May 10;35(14):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seiwert TY, et al. , Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol, 2016. July;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 68.Ferris RL, et al. , Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol, 2018. June;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen EEW, et al. , Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019. January 12;393(10167):156–167. [DOI] [PubMed] [Google Scholar]
- 70.Burtness B, et al. , Phase 3 study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC); . NCT02358031, ESMO 2019 Abstract and oral presentation 2018. [Google Scholar]
- 71.Concha-Benavente F and Ferris RL, Oncogenic growth factor signaling mediating tumor escape from cellular immunity. Curr Opin Immunol, 2017. 45: p. 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivastava RM, et al. , CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clin Cancer Res, 2017. 23(3): p. 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trivedi S, et al. , Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin Cancer Res, 2016. November 1;22(21):5229–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider-Merck T, et al. , Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol, 2010. January 1;184(1):512–20. [DOI] [PubMed] [Google Scholar]
- 75.Bauman JE and Ferris RL, Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer, 2014. 120(5): p. 624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu J, et al. , Preclinical Evidence That PD1 Blockade Cooperates with Cancer Vaccine TEGVAX to Elicit Regression of Established Tumors. Cancer Res, 2014. 74(15): p. 4042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beldi-Ferchiou A and C.-Z. S, Control of NK Cell Activation by Immune Checkpoint Molecules . Int J Mol Sci, 2017. October 12;18(10). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang CX, et al. , STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ. , 2019. February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu S, et al. , STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncology 2018. March;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng CC, et al. , Epidermal growth factor induces STAT1 expression to exacerbate the IFNr-mediated PD-L1 axis in epidermal growth factor receptor-positive cancers. Mol Carcinog, 2018. November;57(11):1588–1598. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald F and Z. DMW, Front Pharmacol. The Immune System’s Contribution to the Clinical Efficacy of EGFR Antagonist Treatment., 2017. August 24;8:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moy JD, Moskovitz JM, and F. RL, Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer 2017. May;76:152–166. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weil S, et al. , Natural Killer Group 2D Ligand Depletion Reconstitutes Natural Killer Cell Immunosurveillance of Head and Neck Squamous Cell Carcinoma. Front Immunol, 2017. April 10;8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veluchamy JP, et al. , Combination of NK Cells and Cetuximab to Enhance Anti-Tumor Responses in RAS Mutant Metastatic Colorectal Cancer. PLoS One. , 2016. September 7;13(9):e0203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.André P, et al. , Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell, 2018. December 13;175(7):1731–1743.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang H, Liu X, and W. M, Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther, 2018. September 25;11:6189–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shayan G, et al. , Phase Ib Study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 Stimulation in Head and Neck Cancer to Overcome Suppressive Myeloid Signals. Clin Cancer Res 2018. 1;24(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferris RL, et al. , Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck: The Active8 Randomized Clinical Trial. JAMA Oncol, 2018. June 21. doi: 10.1001/jamaoncol.2018.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]