Abstract
Background:
Vitamin D deficiency is a proposed risk factor for multiple sclerosis (MS), but its role in progressive MS is not well understood.
Objective:
To examine the association between vitamin D levels and MRI features in primary progressive (PPMS) and secondary progressive MS (SPMS).
Methods:
Serum 25-hydroxyvitamin D (25[OH]D) and 25-hydroxyvitamin D3 (25[OH]D3) levels were obtained from 267 subjects enrolled into the Secondary and Primary Progressive Ibudilast NeuroNEXT Trial in Multiple Sclerosis (SPRINT-MS). Associations between imaging data and vitamin D levels was determined using Pearson or Spearman correlation and multivariate regression analyses.
Results:
267 patients (age 55.6±7.4, 47.2% male, and 51.3% PPMS) were evaluated with quantitative MRI and vitamin D levels. 25(OH)D and 25(OH)D3 were similar between PPMS and SPMS. There was no significant association between vitamin D and T1/2 lesion volume and brain parenchymal fraction. Modest associations were found between 25(OH)D3 and whole brain-magnetization transfer ratio (WB-MTR, r=0.17, p=0.007) and normal appearing grey matter MTR (NAGM-MTR, r=0.15, p=0.02).
Conclusions:
25(OH)D3 levels were not associated with brain volume or lesional measures in progressive MS contrary to what has been described in relapsing remitting MS. An association between WB-MTR and NAGM-MTR suggest higher vitamin D levels may exert a protective role on myelin content in progressive MS.
Keywords: Progressive Multiple Sclerosis, Primary Progressive Multiple Sclerosis, Secondary Progressive Multiple Sclerosis, Vitamin D, MRI, Brain Volume, Lesions
1. INTRODUCTION
There is evidence that low serum levels of vitamin D are a risk factor for developing MS.1–3 The majority of data linking vitamin D and MS comes from relapsing remitting MS (RRMS) studies with relatively little data in progressive MS which includes primary progressive MS (PPMS) and secondary progressive MS (SPMS). The available evidence, however, does suggest vitamin D levels are lower in progressive MS compared to RRMS4 and that vitamin D may predict conversion to SPMS.5 This relationship may be confounded by disability level associated with decreased sun exposure. In RRMS, the number of T2 lesions as well as measures of active inflammation have an inverse relationship with vitamin D levels.6–8 Though the exact mechanism is not clear, vitamin D is an immunomodulator and can produce anti-inflammatory effects in both the innate and adaptive immune systems.9 In MS, vitamin D has also been shown to modulate inflammatory activity showing increased frequency of lesion formation.10–12 Randomized control trials with vitamin D supplementation have presented mixed results though the methodology, supplementation doses and outcome measures vary widely.13–16 Two recent double-blind, placebo-controlled trials of vitamin D supplementation did not meet statistical significance on primary endpoints of disease-free activity but did show a trend toward reduced lesion activity on MRI.17,18 The lack of large, randomized control trials have produced a wide range of recommendations from professional societies without a clear consensus for providers.19,20
The significance of vitamin D levels in fully progressive MS cohorts as related to clinical progression or MRI measures remains unclear. There is evidence in other MS cohorts that vitamin D influences disease progression. For example, low vitamin D levels are associated with higher lesion volume, brain atrophy and disability markers over time.21 Progressive MS presents a challenge for clinicians as patients have persistent accrual of disability independent of relapses with few effective therapies. This makes investigation of modifiable MS risk factors, such as vitamin D, important. The aim of this study was to investigate the association of vitamin D and MRI disease characteristics in progressive MS. We hypothesized that MRI measures including T1/T2 lesion burden, volumetric measurements, axonal integrity and myelin content measures would correlate with vitamin D levels.
2. METHODS
Data was obtained from the screening visit for the NeuroNEXT 102 (NN102)/Secondary and Primary Progressive Ibudilast NeuroNEXT Trial in Multiple Sclerosis (SPRINT-MS). SPRINT-MS was a randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability and activity of ibudilast (MN-166) in progressive MS patients.22 Subjects were enrolled at 28 sites across the United States between November 2013 and June 2015. Bio-banked screening blood samples were obtained for vitamin D analysis from subjects who consented to storage of samples and future research analysis on these samples. Only subjects with complete clinical and MRI data along with available blood samples were included in this analysis. The clinical data included demographics, MS disease history, Expanded Disability Status Scale (EDSS) scores, timed 25-foot walk (T25FW), 9-hole peg test (9HPT), Symbol Digit Modalities Test (SDMT), and 2.5% low contrast visual acuity test (LCVA). The SPRINT-MS trial was approved by the NeuroNEXT Central Institutional Review Board at Massachusetts General Hospital and the secondary analysis in this paper was approved separately by the Cleveland Clinic Institutional Review Board. The data/samples were collected at the screening visit.
2.1. Participants
SPRINT-MS included patients ages 21-65 and a diagnosis of either PPMS or SPMS according to International Panel Criteria.23 Concomitant treatment with glatiramer acetate (GA) or interferon beta (IFNβ-1 a or IFNβ-1b) was allowed. Patients had to be able to walk 25 feet either with or without an assistive device (EDSS 6.5 or lower) along with clinical disease progression over the previous two years as documented by worsening on the EDSS, T25FW, or 9HPT. Vitamin D supplementation data was not available in the SPRINT study database.
2.2. MRI Measures
All MRI studies were conducted using Siemens (Trio or Skyra) or GE (version 12x or higher) 3 tesla systems. To ensure consistent results among the different sites, imaging physicists reviewed the scanning protocols prior to enrollment along with monthly quality assurance assessments thereafter. The image acquisition included 3D spoiled gradient-recalled echo; proton density weighted and T2 weighted 2D turbo/fast spin-echo; 2D T2-weighted FLAIR; 3D spoiled gradient-recalled echo with selective excitation, with and without magnetization transfer pulse; 64-direction high angular resolution diffusion imaging. There was no gadolinium utilized in the study. Conventional MRI measures included T2 lesion volume, T1 lesion volume and brain parenchymal fraction (BPF). Additional MRI measures included whole brain magnetization transfer ratio (WB-MTR), normal appearing brain tissue MTR (NABT-MTR), normal appearing grey matter MTR (NAGM-MTR), corticospinal tract diffusion tensor imaging measures (longitudinal diffusivity [LD] and transverse diffusivity [TD]). MTR is an advanced MRI sequence which measures myelin content and axonal density and even has the potential to distinguish remyelinated lesions.24–26 Diffusion tensor imaging is an imaging technique to measure neuronal micro-structures along with the integrity of white matter tracts. It can identify subtle changes in acute inflammatory lesions which are not seen on more conventional studies.27,28
2.3. Vitamin D Assessments
Serum 25-hydroxyvitamin D2 (25[OH]D2) and 25-hydroxyvitamin D3 (25[OH]D3) were measured from the available screening samples of the SPRINT-MS study. Subjects agreeing to bio-banking had serum samples stored at −80° C at the centralized study laboratory at the University of Rochester. Stored aliquots of 0.5 ml were thawed for 30 minutes at room temperature and 25(OH)D2 and 25(OH)D3 levels were measured by Tandem Mass Spectrometry at the University of Rochester.29,30 Total serum 25-hydroxyvitamin D (25[OH]D) levels represented the sum of 25(OH)D2 and 25(OH)D3.31 A cut-off for 25(OH)D sufficiency was set at 30 ng/ml. Although the definition of adequate 25(OH)D levels is still debated, sources suggest that 30 ng/mL is adequate for the general population.1,32–35 To explore the dichotomized 25(OH)D3 levels were grouped in the following categories based on previous vitamin D research: <30 ng/ml, 30-39 ng/ml, 40-49 ng/ml ≥ 50 ng/ml.36 Deseasonalized vitamin D values were used in the analysis which accounted for geographic and seasonal variation of ultraviolet light, latitude of the study site and the month that the serum sample was obtained.
2.4. Statistical Analysis
Patient demographics, medical history and MRI features were summarized using proportions, means, and median values. The relationship between continuous variables and vitamin D were examined across 25(OH)D3 groups using a mixed model to account for potential unequal variance.37 Due to the skewed distribution, log transformation of T25FW, T1/T2 volume and inverse transformation of 9HPT were used in the analysis. P for trend of change on categorical variables across 25(OH)D3 groups was evaluated using Cochran-Armitage trend test. 25(OH)D and 25(OH)D3 were then compared between PPMS and SPMS using the t test. Associations of MRI features with continuous 25(OH)D3, age and MS performance measures were evaluated using Pearson or Spearman correlation coefficient. In addition, a generalized linear model with an interaction between MS type and 25(OH)D3 was applied to investigate whether the associations between 25(OH)D3 and MRI features were similar between PPMS and SPMS.
Associations between 25(OH)D3 and MRI features were evaluated using multivariate analyses. To account for the potential impact of geographic and seasonal variation of ultraviolet light vitamin D levels, latitude of study site and month of serum obtained were considered in the analyses. Other variables including age, sex, disease duration and variables with P<0.05 in univariate analyses were also included in the potential impact list. Variables from the potential impact list were added to mixed models mentioned above to investigate dichotomized 25(OH)D3 levels on MRI feature. Multivariate regression analysis was performed to determine the relative contribution of 25(OH)D3 on MRI feature among variables of potential impact.
All analyses were performed in SAS version 9.4. P values presented in table or figure are two-sided. Statistical significance was set at P<0.05.
3. RESULTS
3.1. Demographics
A total of 267 of 286 patients (93.4%) enrolled into the SPRINT-MS study had both stored serum and MRI data available for analysis. The overall demographic, clinical, and MRI characteristics are summarized in Table 1. The group was evenly distributed between PPMS and SPMS. Most participants were Caucasian (93.5%) with age ranging from 31.8 - 65.9 years (mean 55.6). Mean 25(OH)D was 43.8 ng/ml (SD 17.8) and 40.7 ng/ml (SD 19.3) for 25(OH)D3. Both 25(OH)D(43.8 vs. 42.9 ng/ml) and 25(OH)D3 (40.7 vs. 39.9 ng/ml) were similar between PPMS and SPMS groups (p = 0.47 & p=0.31).
Table 1:
Patient Characteristics by 25(OH)D3 Categories
| Factor | Overall | 25(OH)D3 group (ng/ml) |
||||
|---|---|---|---|---|---|---|
| <30 | 30-39 | 40-49 | >= 50 | P for trend | ||
| Patient, N (%) | 75 (28.1) | 58 (21.7) | 51 (19.1) | 83 (31.1) | ||
| Mean 25(OH)D3 (CV) | 18.2 (37.6) | 34.8 (8.3) | 44.1 (6.5) | 63.2 (18.9) | ||
| Latitude | 39.7±3.4 | 39.3±4.4 | 40.7±3.2 | 39.3±3.9 | 0.95 | |
| Blood obtained in April-September | 46 (61.3) | 33 (56.9) | 30 (58.8) | 52 (62.6) | 0.80 | |
| Demographics | ||||||
| Age (31.8-65.9 years) | 55.6 ± 7.4 | 53.4±8.8 | 55.0±6.5 | 57.6±6.6 | 56.9±6.4 | 0.001 |
| Sex, Female | 126 (47.2) | 34(45.3) | 26(44.8) | 27(52.9) | 54(65.1) | 0.008 |
| Race, Caucasian | 247 (93.5) | 64(86.5) | 54(93.1) | 48(94.1) | 81(98.8) | 0.003 |
| MS History | ||||||
| MS duration (Years) | 16.4 ± 10.6 | 14.8±9.3 | 14.8±9.9 | 19.5±11.8 | 17.0±11.2 | 0.04 |
| Family MS history | 60 (22.5) | 21(28.4) | 11(20.0) | 9(17.6) | 19(22.9) | 0.42 |
| MS Type | 0.69 | |||||
| PPMS | 137 (51.3) | 37(49.3) | 33(56.9) | 28(54.9) | 39(47.0) | |
| SPMS | 130 (48.7) | 38(50.7) | 25(43.1) | 23(45.1) | 44(53.0) | |
| MS Performance | ||||||
| D-9HPT (Seconds) | 27.5 (22.3,35.6) | 28.4 (22.5,38.2) | 29.2 (23.4,36.5) | 25.4 (21.9,30.6) | 26.6 (22.0,38.3) | 0.20 |
| ND-9HPT (Seconds) | 30.1 (24.9,41.5) | 32.2 (26.8,41.5) | 30.4 (26.1,44.0) | 28.5 (22.4,33.4) | 29.7 (24.8,44.7) | 0.20 |
| T25FW (Seconds) | 9.6 (6.9,16.6) | 10.1 (7.0,16.6) | 9.9 (6.9,13.7) | 9.1 (6.6,12.8) | 9.6 (7.0,20.0) | 0.63 |
| SDMT (No. correct) | 42.6 ± 14.6 | 43.8±14.8 | 39.5±13.2 | 42.7±13.7 | 43.5±15.7 | 0.76 |
| EDSS total score | 13.3 ± 3.8 | 13.5±3.8 | 13.2±3.7 | 13.1±3.4 | 13.5±4.0 | 0.94 |
| EQ5D Index (x103) | 22471.7 ± 5502.8 | 22.3±4.9 | 21.4±4.9 | 23.2±6.3 | 22.9±5.8 | 0.21 |
| MRI Studies | ||||||
| T1 VOLUME | 1.4 (0.50,4.4) | 1.2 (0.42,3.0) | 2.8 (0.54,6.9) | 1.1 (0.28,6.1) | 1.5 (0.53,3.8) | 0.76 |
| T2 VOLUME | 5.8 (2.2,14.8) | 4.6 (2.2,12.3) | 11.6 (3.3,20.1) | 4.7 (1.5,10.4) | 5.0 (2.2,14.5) | 0.62 |
| BPF | 0.80 ± 0.03 | 0.81±0.03 | 0.80±0.03 | 0.80±0.03 | 0.81±0.03 | 0.58 |
| WB-MTR | 0.14 ± 0.26 | 0.08±0.28 | 0.14±0.27 | 0.14±0.24 | 0.20±0.25 | 0.01 |
| NABT-MTR | 0.31 ± 0.27 | 0.28±0.31 | 0.31±0.28 | 0.29±0.25 | 0.36±0.25 | 0.11 |
| NAGM-MTR | −0.28 ± 0.27 | −0.34±0.30 | −0.29±0.27 | −0.31±0.25 | −0.22±0.24 | 0.02 |
| LD | 1.2 ± 0.05 | 1.2±0.06 | 1.3±0.06 | 1.2±0.05 | 1.2±0.05 | 0.87 |
| TD | 0.56 ± 0.04 | 0.55±0.04 | 0.55±0.05 | 0.56±0.05 | 0.56±0.04 | 0.58 |
Frequency and percentage for categorical variables. Mean (CV or Coefficient of variation), mean (SD) or median (IQR) for numerical variables.
25[OH]D3, 25-hydroxyvitamin D3; MS, Multiple Sclerosis; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; D-9HPT, dominant hand 9-hole peg test; ND-9HPT, non-dominant hand 9-hole peg test; T25FW, timed 25 foot walk; EDSS, Expanded Disability Status Scale; SDMT, Symbol Digit Modality Test; BPF, brain parenchymal fraction; WB-MTR, whole brain magnetization transfer ratio; NABT-MTR, normal appearing brain tissue MTR; NAGM-MTR, normal appearing grey matter MTR; corticospinal tract DTI measures (longitudinal diffusivity [LD] and transverse diffusivity [TD])
3.2. Vitamin D Categories and MRI measures
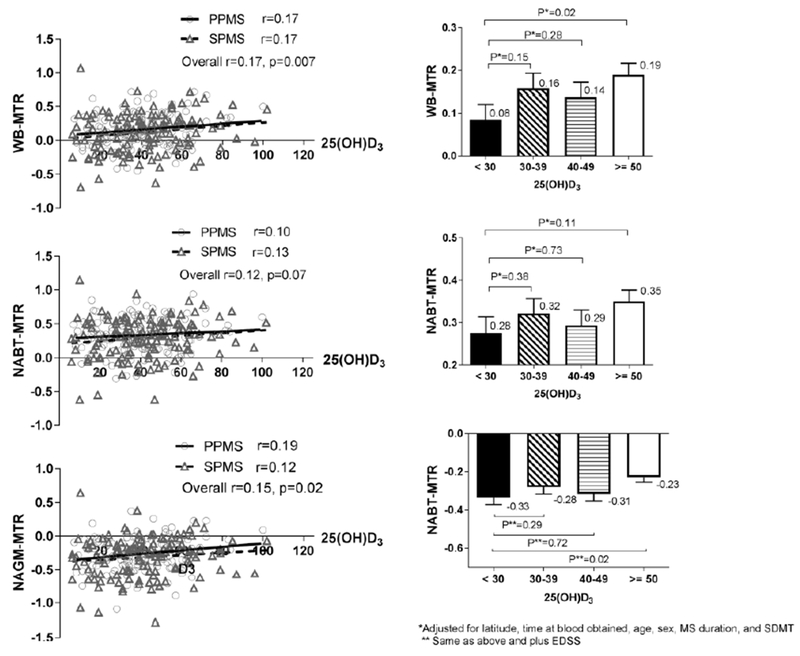
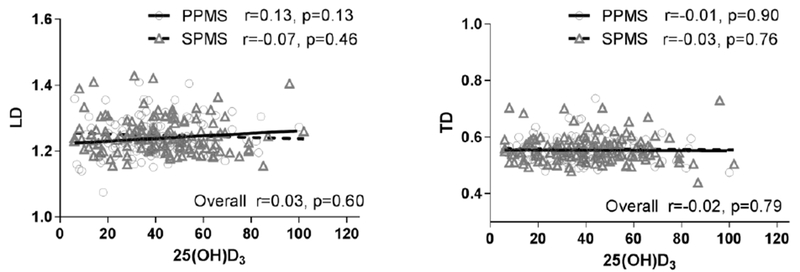
Demographic, clinical, and MRI measures across the categorical forms of 25(OH)D3 (<30, 30-39, 40-49 and >50 ng/ml) are shown in Table 1. Higher vitamin 25(OH)D3 levels were found in older, female patients with a longer disease duration. MS performance measurements including EDSS were similar across all 25(OH)D3 groups. No significant differences were found in any conventional MRI measures. The WB-MTR (p=0.01) and NAGM-MTR (p=0.02) demonstrated a statistically significant trend across all 25(OH)D3 levels. A similar pattern was observed in NABT-MTR (p=0.11) although not statistically significant. When the MTR data were compared between the 25(OH)D3 (≥50) and the 25(OH)D3 (<30) group in the multivariate analysis, WB-MTR (p=0.02) and NABT-MTR (p=0.02) remained statistically significantly between the groups (Figure 1 right panel). There was no association between 25(OH)D3 and either LD or TD in the corticospinal tracts (Figure 2).
Figure 1:
Association between MTR and 25(OH)D3
PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; WB-MTR, whole brain magnetization transfer ratio; NABT-MTR, normal appearing brain tissue MTR; NAGM-MTR, normal appearing grey matter MTR
The left panel is scatterplot of MTR sequences versus 25(OH)D3 level with estimated regression lines for both PPMS and SPMS. Overall Pearson correlation (r, p value) and the correlation within each MS type are presented. The right panel is bar plot of categorical vitamin D levels and MTR sequences. The plot includes mean ± standard error of mean (SEM) of MRT sequences between four 25(OH)D3 categories.
Figure 2:
Scatterplot of DTI including LD and TD versus 25(OH)D3 level with estimated regression lines for both PPMS and SPMS.
Pearson correlation (r, p value) and the correlation within each MS type are presented. PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; DTI, diffusion tensor imaging; LD, longitudinal diffusivity; TD, transverse diffusivity
3.3. Finear Association between Vitamin D and MRI measures
No significant linear correlations were found between conventional MRI measures and 25(OH)D3 levels. Statistically significant correlations were found between 25(OH)D3 with WB-MTR (r=0.17, p=0.007) and NAGM-MTR (r=0.15, p=0.02) (Table 2 and Figure 1 left panel). A similar trend was noted in NABT-MTR (p=0.07).
Table 2:
MRI Associations with 25(OH)D3 and Clinical Features
| T1 Volume r* (P value) |
T2 Volume r* (P value) |
BPF r (P value) |
WB-MTR r (P value) |
NABT-MTR r (P value) |
NAGM-MTR r (P value) |
|
|---|---|---|---|---|---|---|
| 25(OH)D3 | 0.03 (0.59) | 0.01 ( 0.91) | −0.06 (0.31) | 0.17 (0.007) | 0.12 ( 0.07) | 0.15 (0.02) |
| D-9HPT | 0.16 (0.01) | 0.15 (0.01) | 0.02 (0.77) | −0.04 (0.55) | −0.02 (0.69) | −0.02 (0.74) |
| T25FW | 0.07 (0.27) | 0.07 (0.24) | 0 (0.99) | −0.05 (0.41) | −0.04 (0.49) | −0.05 (0.42) |
| LCVA | −0.15 (0.01) | −0.15 (0.02) | 0.15 (0.01) | 0.06 (0.37) | 0.07 (0.29) | 0.06 (0.37) |
| SDMT | −0.31 (<0.001) | −0.31 (<0.001) | 0.38 (<0.001) | 0.20 (0.001) | 0.27 (<0.001) | 0.19 (0.002) |
| EDSS | 0.20 (0.001) | 0.23 (<0.001) | −0.21 (<0.001) | −0.08 (0.20) | −0.09 (0.14) | −0.16 (0.01) |
Spearman correlation coefficient
25[OH]D3, 25-hydroxyvitamin D3; D-9HPT, dominant hand 9-hole peg test; T25FW, timed 25 foot walk; LCVA , 2.5% low contrast visual acuity test; SDMT, Symbol Digit Modality Test; EDSS, Expanded Disability Status Scale; BPF, brain parenchymal fraction; WB-MTR, whole brain magnetization transfer ratio; NABT-MTR, normal appearing brain tissue MTR; NAGM-MTR, normal appearing grey matter MTR
In the multivariate analysis, no significant associations were found between vitamin D levels and conventional MRI metrics which included T1/T2 lesion volume or BPF (Table 3). Associations of 25(OH)D3 with WB-MTR (p=0.01) and NAGM-MTR (p=0.03) remained significant after accounting for age, gender, disease duration, time of serum obtained and latitude of study site. The association with NABT-MTR showed a similar trend but was not statistically significant (p=0.08). 25(OH)D3 (β=0.15) explained more variation than age (β=0.12) and disease duration (β=−0.01) on WB-MTR. 25(OH)D3 (β=0.14) also explained more variation than age (β=0.02) and disease duration (β=0.006) on NAGM-MTR. The same pattern was observed on NABT-MTR.
Table 3:
Multivariate Regression Analysis of 25(OH)D3 and Other Factors explaining MRI Feature
| WB-MTR | NABT-MTR | NAGM-MTR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | b* | β** | P value | b* | β* | P value | b* | β* | P value |
| 25(OH)D3 | 0.0021 | 0.1524 | 0.01 | 0.0016 | 0.1098 | 0.08 | 0.0019 | 0.1377 | 0.03 |
| Month | 0.0091 | 0.1030 | 0.09 | 0.0092 | 0.1005 | 0.10 | 0.0039 | 0.0437 | 0.48 |
| Latitude | −0.0072 | −0.1044 | 0.09 | −0.0076 | −0.1052 | 0.09 | −0.0030 | −0.0421 | 0.50 |
| Age | 0.0042 | 0.1186 | 0.07 | 0.0013 | 0.0340 | 0.60 | 0.0008 | 0.0213 | 0.75 |
| Sex | −0.0269 | −0.0508 | 0.42 | −0.0027 | −0.0050 | 0.94 | 0.0239 | 0.0445 | 0.49 |
| MS duration | −0.0003 | −0.0112 | 0.86 | −0.0007 | −0.0254 | 0.70 | 0.0001 | 0.0060 | 0.93 |
| SDMT | 0.0043 | 0.2367 | 0.0001 | 0.0054 | 0.2900 | <.0001 | 0.0032 | 0.1726 | 0.01 |
| EDSS | - | - | - | - | - | - | −0.0072 | −0.1021 | 0.12 |
Regression coefficient;
Standardized coefficient
25[OH]D3,25-hydroxyvitamin D3; MS, Multiple Sclerosis; SDMT, Symbol Digit Modality Test; EDSS, Expanded Disability Status Scale; WB-MTR, whole brain magnetization transfer ratio; NABT-MTR, normal appearing brain tissue MTR; NAGM-MTR, normal appearing grey matter MTR
4.0. DISCUSSION
This study investigated the association of MRI measures and vitamin D levels in a purely progressive MS cohort. Similar to previous studies, 25(OH)D levels were not found to be different in PPMS and SPMS.4 In the current study, mean 25(OH)D levels were above sufficiency with a little over 70% of patients having 25(OH)D levels greater than 30ng/ml, which is contrary to the results previously reported in progressive MS studies.4,5
The association of vitamin D levels to BPF and lesional volumes, well described in RRMS, were not found in this progressive cohort.6,21,38 It is unclear why there was no association between vitamin D and these MRI measures. Previous studies of vitamin D and MRI measures in MS focus on RRMS populations which make direct comparisons difficult. For example, two longitudinal cohorts with clinically isolated syndrome (CIS)/early RRMS patients illustrated that higher vitamin D levels portended to a lower number of T2 and gadolinium-enhancing lesions but no significant relationship between brain volume measurements.38,39 A recent study investigating patients with CIS also showed a positive association with vitamin D and higher gray matter volume.40 A prospective CIS study also showed a similar relationship between vitamin D and brain volume along with other metrics including worsening EDSS scores and new lesion formation on MRI.21 Our study population was older, had a longer disease duration, only used cross-sectional data, and was not able to evaluate new lesions formation, as contrast was not given. In a retrospective study of a heterogenous, unselected group of MS patients (554 patients: 51.8% RRMS, 34.1% SPMS and 14.0% PPMS) highlighted that vitamin D levels were only associated with the occurrence of relapses in younger MS patients.41 This may suggest that vitamin D has more effect on the early inflammatory portion of the disease which are measured by lesional and atrophy measures and may help explain why there was no correlation in our study.4,39
In our study, there was a positive correlation between 25(OH)D3 levels and measures of myelin content as measured by MTR, suggesting a potential protective role of vitamin D in myelin integrity. The mechanism for this potential myelin protection in progressive MS is not clear, but may be related the proposed mechanisms for vitamin D in RRMS which include immune modulation, downregulation of proinflammatory markers, and antioxidant effects.42–44 We hypothesize that many of these effects may also have a protective role over myelin in progressive MS.
MTR has been proposed as a marker of myelin content and is sensitive to show disability and treatment effects in progressive MS.45–47 Our data demonstrates an association of vitamin D levels with myelin measures in both lesional and non lesional tissue supported by a correlation with higher SDMT and EDSS scores. The positive associations between vitamin 25(OH)D3 and WB/NAGM-MTR values suggest that vitamin D may have a protective role in progressive MS patients. The lack of correlation between T1/T2 measures in our dataset supports the possibility vitamin D has an effect separate for formation of new lesions.
There is much debate about the adequate levels of 25(OH)D and the target for replacement.48,49 Our results showed progressive MS patients with 25(OH)D3 levels greater than 30 ng/ml had preserved WB-MTR and NAGM-MTR when compared to vitamin D deficient patients. More research is needed to validate the ideal target for vitamin D levels, as many have proposed targeting levels above what has previously been considered sufficient.32–34 The ideal target in progressive MS remains unknown.
This study has several limitations. Studying vitamin D levels in a progressive MS cohort with an average disease duration of 16 years poses inherent difficulties with establishing causal links to a disease that evolved over more than a decade. It is also quite possible that a modern MS cohort will be more likely to have vitamin D supplementation as there is a growing awareness about the importance of vitamin D in the clinical settings. Regretfully, use of vitamin D supplementation data was not available in the SPRINT-MS study. There were also no measures of UV exposure (including sunscreen use) available in the dataset which has been independently associated with MS onset and disease progression.50 Other life style elements such as smoking, exercise or dietary intake were not considered in the analysis. An additional aspect to consider is the cross-sectional design of this analysis with the lack of longitudinal vitamin D levels. Some studies have shown vitamin D may have long term effects including levels in utero being a risk factor for the disease during adulthood.51 To truly identify if vitamin D has a myelin protective role would require a more comprehensive, longitudinal prospective study. Other helpful MRI markers for disease activity in progressive MS such as enhancing lesions and cortical thickness were not available for analyses and could have offered further evidence for a role of vitamin D independent of inflammation. This study provides limited support regarding the importance of vitamin D in progressive MS through its observations regarding myelin integrity as indicated by MTR.
Highlights.
Low vitamin D is a risk factor for developing MS and correlates to disease activity
Progressive MS presents unique challenges in regard to management
This article investigates MRI measures and vitamin D levels in progressive MS
Vitamin D levels were similar between subgroups of progressive MS
Vitamin D may have a protective role on myelin content in progressive MS
Acknowledgments
Funding Statements/Sources of Support
-- NINDS (U01NS082329; U01NS077179, U01NS077352)
-- NMSS (PP-1603-08307)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest/Conflicts of Interest
Justin R Abbatemarco: Nothing to disclose
Robert J Fox: Consulting fees from Actelion, Biogen, EMD Serono, Genentech, Novartis, and Teva; advisory committees for Biogen Idee and Novartis; and clinical trial contract and research grant funding from Biogen and Novartis.
Hong Li: Nothing to disclose
Daniel Ontaneda: Grant support from Genzyme, Genentech, Novartis, consulting from Biogen Idee, Genzyme, and Genentech.
AUTHOR DECLARATION
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- 1.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296(23):2832–8. doi: 10.1001/jama.296.23.2832 [DOI] [PubMed] [Google Scholar]
- 2.Soilu-Hanninen M, Airas L, Mononen I, et al. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler 2005;11(3):266–71. doi: 10.1191/1352458505ms1157oa [DOI] [PubMed] [Google Scholar]
- 3.van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 2007;254(5):581–90. doi: 10.1007/s00415-006-0315-8 [DOI] [PubMed] [Google Scholar]
- 4.Smolders J, Menheere P, Kessels A, et al. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008;14(9):1220–4. doi: 10.1177/1352458508094399 [DOI] [PubMed] [Google Scholar]
- 5.Muris AH, Rolf L, Broen K, et al. A low vitamin D status at diagnosis is associated with an early conversion to secondary progressive multiple sclerosis. J Steroid Biochem Mol Biol 2016;164:254–57. doi: 10.1016/j.jsbmb.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 6.Mowry EM, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol 2012;72(2):234–40. doi: 10.1002/ana.23591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson S Jr., Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010;68(2):193–203. doi: 10.1002/ana.22043 [DOI] [PubMed] [Google Scholar]
- 8.Soilu-Hanninen M, Aivo J, Lindstrom BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2012;83(5):565–71. doi: 10.1136/jnnp-2011-301876 [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010;9(6):599–612. doi: 10.1016/S1474-4422(10)70086-7 [published Online First: 2010/05/25] [DOI] [PubMed] [Google Scholar]
- 10.Smolders J, Schuurman KG, van Strien ME, et al. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J Neuropathol Exp Neurol 2013;72(2):91–105. doi: 10.1097/NEN.0b013e31827f4fcc [published Online First: 2013/01/22] [DOI] [PubMed] [Google Scholar]
- 11.Smolders J, Moen SM, Damoiseaux J, et al. Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci 2011;311(1-2):37–43. doi: 10.1016/j.jns.2011.07.033 [published Online First: 2011/08/25] [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre d’Hellencourt C, Montero-Menei CN, Bernard R, et al. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res 2003;71(4):575–82. doi: 10.1002/jnr.10491 [published Online First: 2003/01/28] [DOI] [PubMed] [Google Scholar]
- 13.Burton JM, Kimball S, Vieth R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010;74(23):1852–9. doi: 10.1212/WNL.0b013e3181e1cec2 [published Online First: 2010/04/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosayebi G, Ghazavi A, Ghasami K, et al. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest 2011;40(6):627–39. doi: 10.3109/08820139.2011.573041 [DOI] [PubMed] [Google Scholar]
- 15.Kampman MT, Steffensen LH, Mellgren SI, et al. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler 2012;18(8):1144–51. doi: 10.1177/1352458511434607 [published Online First: 2012/02/23] [DOI] [PubMed] [Google Scholar]
- 16.Loken-Amsrud KI, Holmoy T, Bakke SJ, et al. Vitamin D and disease activity in multiple sclerosis before and during interferon-beta treatment. Neurology 2012;79(3):267–73. doi: 10.1212/WNL.0b013e31825fdf01 [published Online First: 2012/06/16] [DOI] [PubMed] [Google Scholar]
- 17.Smolders JHR, Vieth R, Holmoy T, Marhardt K, Schluep M, Killestein J, Barkof F, Grimaldi LE, Beelke M. High dose cholecalciferol (vitamin D3) oil as add-on therapy in subjects with relapsing-remitting multiple sclerosis receiving subcutaneous interferon1a. ECTRIMS, 2016. [Google Scholar]
- 18.W Camu CP-D, Hautecoeur P, Besserve A, Deleglise A.S Jean, Lehert P, Souberbielle JC. Cholecalciferol supplementation in relapsing multiple sclerosis patients treated with subcutaneous interferon beta-1a: a randomized controlled trial: American Academy of Neurology, 2017. [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96(1):53–8. doi: 10.1210/jc.2010-2704 [published Online First: 2010/12/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385 [published Online First: 2011/06/08] [DOI] [PubMed] [Google Scholar]
- 21.Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014;71(3):306–14. doi: 10.1001/jamaneurol.2013.5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016;50:166–77. doi: 10.1016/j.cct.2016.08.009 [published Online First: 2016/08/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69(2):292–302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahajan KR, Ontaneda D. The Role of Advanced Magnetic Resonance Imaging Techniques in Multiple Sclerosis Clinical Trials. Neurotherapeutics 2017;14(4):905–23. doi: 10.1007/s13311-017-0561-8 [published Online First: 2017/08/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmierer K, Scaravilli F, Altmann DR, et al. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 2004;56(3):407–15. doi: 10.1002/ana.20202 [published Online First: 2004/09/07] [DOI] [PubMed] [Google Scholar]
- 26.Chen JT, Kuhlmann T, Jansen GH, et al. Voxel-based analysis of the evolution of magnetization transfer ratio to quantify remyelination and demyelination with histopathological validation in a multiple sclerosis lesion. Neuroimage 2007;36(4):1152–8. doi: 10.1016/j.neuroimage.2007.03.073 [published Online First: 2007/06/05] [DOI] [PubMed] [Google Scholar]
- 27.Lin TH, Chiang CW, Perez-Torres CJ, et al. Diffusion MRI quantifies early axonal loss in the presence of nerve swelling. J Neuroinflammation 2017;14(1):78. doi: 10.1186/s12974-017-0852-3 [published Online First: 2017/04/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 2011;22(2):185–96, viii. doi: 10.1016/j.nec.2010.12.004 [published Online First: 2011/03/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Ouweland JM, Vogeser M, Bacher S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord 2013;14(2):159–84. doi: 10.1007/s11154-013-9241-0 [published Online First: 2013/03/30] [DOI] [PubMed] [Google Scholar]
- 30.Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82(5):1942–8. doi: 10.1021/ac9026862 [published Online First: 2010/02/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan VF, Gray AR, Peddie MC, et al. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr 2013;109(6):1082–8. doi: 10.1017/S0007114512002851 [published Online First: 2012/11/22] [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int 2005;16(7):713–6. doi: 10.1007/s00198-005-1867-7 [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81(3):353–73. doi: 10.4065/81.3.353 [DOI] [PubMed] [Google Scholar]
- 34.Bhargava P, Cassard S, Steele SU, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials 2014;39(2):288–93. doi: 10.1016/j.cct.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Fjeldstad C, Fjeldstad AS, Weir JP, et al. Association of vitamin D deficiency with RNFL thickness in MS individuals without history of optic neuritis. Mult Scler Relat Disord 2014;3(4):489–93. doi: 10.1016/j.msard.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 36.Park SK, Garland CF, Gorham ED, et al. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS One 2018;13(4):e0193070. doi: 10.1371/journal.pone.0193070 [published Online First: 2018/04/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng HL, Ying. Model selection in linear mixed effect models. Journal of Multivariate Analysis 2010;109 doi: 10.1016/j.jmva.2012.02.005 [DOI] [Google Scholar]
- 38.Fitzgerald KC, Munger KL, Kochert K, et al. Association of Vitamin D Levels With Multiple Sclerosis Activity and Progression in Patients Receiving Interferon Beta-1b. JAMA Neurol 2015;72(12):1458–65. doi: 10.1001/jamaneurol.2015.2742 [published Online First: 2015/10/13] [DOI] [PubMed] [Google Scholar]
- 39.Mowry EM, Azevedo CJ, McCulloch CE, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology 2018;91(24):e2256–e64. doi: 10.1212/WNL.0000000000006644 [published Online First: 2018/11/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mowry EM, Pelletier D, Gao Z, et al. Vitamin D in clinically isolated syndrome: evidence for possible neuroprotection. Eur J Neurol 2016;23(2):327–32. doi: 10.1111/ene.12844 [published Online First: 2015/11/01] [DOI] [PubMed] [Google Scholar]
- 41.Muris AH, Smolders J, Rolf L, et al. Vitamin D Status Does Not Affect Disability Progression of Patients with Multiple Sclerosis over Three Year Follow-Up. PLoS One 2016;11(6):e0156122. doi: 10.1371/journal.pone.0156122 [published Online First: 2016/06/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nataf S, Garcion E, Darcy F, et al. 1,25 Dihydroxyvitamin D3 exerts regional effects in the central nervous system during experimental allergic encephalomyelitis. J Neuropathol Exp Neurol 1996;55(8):904–14. [DOI] [PubMed] [Google Scholar]
- 43.Chen KB, Lin AM, Chiu TH. Systemic vitamin D3 attenuated oxidative injuries in the locus coeruleus of rat brain. Ann N Y Acad Sci 2003;993:313–24; discussion 45-9. [DOI] [PubMed] [Google Scholar]
- 44.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem 1992;49(1):26–31. doi: 10.1002/jcb.240490106 [DOI] [PubMed] [Google Scholar]
- 45.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128(Pt 11):2705–12. doi: 10.1093/brain/awh641 [DOI] [PubMed] [Google Scholar]
- 46.Hayton T, Furby J, Smith KJ, et al. Longitudinal changes in magnetisation transfer ratio in secondary progressive multiple sclerosis: data from a randomised placebo controlled trial of lamotrigine. J Neurol 2012;259(3):505–14. doi: 10.1007/s00415-011-6212-9 [DOI] [PubMed] [Google Scholar]
- 47.Khaleeli Z, Sastre-Garriga J, Ciccarelli O, et al. Magnetisation transfer ratio in the normal appearing white matter predicts progression of disability over 1 year in early primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2007;78(10):1076–82. doi: 10.1136/jnnp.2006.107565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc 2010;85(8):752–7; quiz 57–8. doi: 10.4065/mcp.2010.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes 2008;15(6):489–94. doi: 10.1097/MED.0b013e328317ca6c [DOI] [PubMed] [Google Scholar]
- 50.Lucas RM, Ponsonby AL, Dear K, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011;76(6):540–8. doi: 10.1212/WNL.0b013e31820af93d [published Online First: 2011/02/09] [DOI] [PubMed] [Google Scholar]
- 51.Munger KL, Aivo J, Hongell K, et al. Vitamin D Status During Pregnancy and Risk of Multiple Sclerosis in Offspring of Women in the Finnish Maternity Cohort. JAMA Neurol 2016;73(5):515–9. doi: 10.1001/jamaneurol.2015.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]