Abstract
Rationale
B-1 cell-derived natural IgM antibodies against oxidation-specific epitopes (OSE) on low-density lipoprotein are anti-inflammatory and atheroprotective. Bone marrow (BM) B-1a cells contribute abundantly to IgM production, yet the unique repertoire of IgM antibodies generated by BM B-1a, and the factors maintaining the BM B-1a population remain unexplored. The chemokine receptor CXCR4 has been implicated in human CVD and B cell homeostasis, yet the role of B-1 cell CXCR4 in regulating atheroprotective IgM levels and human CVD is unknown.
Objective
To characterize the BM B-1a IgM repertoire and to determine whether CXCR4 regulates B-1 production of atheroprotective IgM in mice and humans.
Methods and Results
Single-cell sequencing demonstrated that BM B-1a cells from aged ApoE−/− mice with established atherosclerosis express a unique repertoire of IgM antibodies containing increased N-additions and a greater frequency of unique CDR-H3 sequences compared to peritoneal (PerC) B-1a cells. Some CDR-H3 sequences were common to both compartments suggesting B-1a migration between compartments. Indeed, mature PerC B-1a cells migrated to BM in a CXCR4-dependent manner. Furthermore, BM production of anti-OSE IgM and plasma IgM levels were reduced in ApoE−/− mice with B cell-specific knockout of CXCR4, and overexpression of CXCR4 on B-1a cells increased bone marrow localization and plasma anti-OSE IgM, including IgM against malondialdehyde(MDA)-modified LDL. Finally, in a 50-subject human cohort, we find that CXCR4 expression on circulating human B-1 cells positively associates with plasma levels of anti-MDA-LDL IgM antibodies and inversely associates with human coronary artery plaque burden and necrosis.
Conclusions
These data provide the first report of a unique BM B-1a cell IgM repertoire and identifies CXCR4 expression as a critical factor selectively governing BM B-1a localization and anti-OSE IgM production. That CXCR4 expression on human B-1 cells was greater in humans with low coronary artery plaque burden suggests a potential targeted approach for immune modulation to limit atherosclerosis.
Keywords: B-1 cells, IgM antibodies, oxidation-specific epitopes, atherosclerosis, CXCR4, antibody, inflammation, chemokine, Mechanisms, Translational studies
Graphical Abstract
INTRODUCTION
A wealth of evidence in murine models demonstrates an atheroprotective role for B-1 cells, primarily through their ability to produce anti-inflammatory IgM antibodies against oxidation-specific epitopes (OSE), such as malondialdehyde (MDA)1–6. Studies in human cardiovascular disease (CVD) patients have demonstrated that increased amounts of circulating IgM antibodies specific for MDA-modified-LDL are associated with less coronary artery disease and fewer cardiovascular events7, 8, yet the factors regulating production of IgM to MDA-LDL in humans are unknown. A putative human equivalent of the murine B-1 cell was identified by Rothstein and colleagues as a CD20+CD27+CD43+ subset present in peripheral blood that demonstrated key functional hallmarks of murine B-1 cells, including spontaneous T cell-independent IgM production9. However, functional and phenotypic heterogeneity in the CD20+CD27+CD43+ B-1 subset suggests additional markers may further refine the phenotype of anti-OSE IgM producing human B-1 cells10–12.
In mice, B-1 cells can be further divided into phenotypically distinct B-1a and B-1b subsets based on expression of the surface marker CD5, and both subsets secrete anti-OSE IgM and protect against atherosclerosis 3, 5. While peritoneal cavity (PerC) B-1 cells constitutively secrete a small amount of IgM, B-1 cells in the bone marrow (BM) and spleen secrete larger amounts of antibody per cell and significantly contribute to plasma IgM titers13–15. However, the factors that regulate B-1 cell number at sites of high antibody production like the BM, both at steady-state and in the context of chronic inflammation, remain largely unexplored.
Further heterogeneity in the B-1 population occurs at the level of the B cell receptor, which consists of heavy and light chains containing variable regions that determine the specificity of IgM antibodies. The variable region of the immunoglobulin heavy chain (IgH V) is formed by combinatorial joining of germline VH, D, and JH family gene segments through a process called VDJ recombination, and additional heterogeneity is introduced through non-template-encoded N nucleotides (N-region additions) at the junctions between V-D and D-J gene segments. Antigen binding occurs at and is determined by hypervariable regions of IgH V called the complementarity determining regions (CDRs), the most variable of which is CDR-H3, which extends across both V-D and D-J junctions. Analyses of IgH V sequences to date demonstrate that PerC B-1a cells express a restricted VDJ repertoire containing a paucity of N-additions, consistent with their origins from primarily fetal liver precursors that lack expression of Tdt, the enzyme mediating N-additions16–18. However, the prevalence of N-additions increases in PerC and splenic B-1a cells with age, which may arise from the contribution of postnatally developed B-1a cells, from the contribution of B-1 cells from other compartments such as the BM, or from selective antigen-induced pressures that are specific to a particular microenvironmental niche18–21. While BM B-1a cells significantly contribute to IgM production, IgH V analysis of the mature BM B-1a cell population remains undefined. Moreover, given the potential for postnatal diversification and selection, defining the BM B-1a IgM repertoire in the context of atherosclerosis risk factors such as age and hyperlipidemia is of key importance.
Abundant IgM and specifically IgM to OSE are present in human and mouse atherosclerotic lesions 1, 22, and administration or overexpression of anti-OSE IgM antibodies in vivo can inhibit oxidized LDL-induced activation of inflammatory pathways and reduce lesion area 23, 24. However, targeted B-1 cell-specific strategies to increase IgM antibody production in vivo have been limited, likely due to an incomplete understanding of factors that regulate B-1 production of atheroprotective IgM in the setting of hyperlipidemia.
The chemokine receptor CXCR4 regulates cell trafficking and localization25–27. Genome-wide association studies have implicated CXCR4 and its ligand CXCL12 in human CVD 28–31, although results demonstrate conflicting effects, likely due to the broad expression of CXCR4 on a myriad of cell types with both pro- and anti-inflammatory functions. Prior studies have demonstrated that CXCR4 mediates IgM responses to acute immunization with the T-independent antigen NP-Ficoll32, suggesting a role for CXCR4 on B-1 cells. Whether CXCR4 regulates B-1 cell production of anti-OSE IgM in the setting of hyperlipidemia, and the mechanisms underlying this regulation are unknown. Moreover, whether B-1 cell CXCR4 expression is linked to circulating anti-OSE IgM levels or CVD in humans has not been explored.
The present study provides novel characterization of the the BM B-1a IgH V repertoire in aged mice with hyperlipidemia and examines the factors maintaining B-1a number and IgM production within the BM. We demonstrate that the BM B-1a IgH V repertoire in aged ApoE−/− mice is distinct from the PerC B-1a repertoire, containing increased N-additions and greater frequency of unique CDR-H3 sequences. Using adoptive transfer studies, we find that the BM B-1a population is replenished by trafficking of mature B-1a cells from the periphery to the BM in a CXCR4-dependent manner. Furthermore, B cell-specific loss of CXCR4 decreases B-1a number and IgM production specifically within the BM, resulting in decreased plasma IgM. Conversely, B-1a cell-specific overexpression of CXCR4 in vivo associates with increased B-1a localization to the bone marrow and increased plasma anti-OSE IgM. Finally, in a 50-subject human cohort, CXCR4 expression on the circulating human CD20+CD27+CD43+ B-1 subset significantly positively associates with the amount of plasma anti-MDA-LDL IgM, and inversely associates with plaque burden and necrotic area in human coronary arteries. Overall these data indicate that BM B-1a cells uniquely contribute to the IgM antibody repertoire, and that their maintenance is governed by CXCR4, a novel marker associating with protection in human CVD.
METHODS
The authors declare that all study materials and analytic methods are available within the article and its online supplementary files. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Human subjects
50 human subjects were recruited for study through the Cardiac Catheterization laboratory at the University of Virginia as previously described33. All participants provided written informed consent prior to enrollment, and the study was approved by the Human IRB Committee at UVA.
Intravascular ultrasound (IVUS)
One-vessel IVUS was performed on coronary arteries of 50 participants in accordance with the American College of Cardiology standards for image acquisition on a non-infarct related artery, and atheroma burden, maximum stenosis, and plaque composition indices (fibrous, necrotic, or calcified areas) were quantified as previously described33.
Mice
All animal protocols were approved by the Animal Care and Use Committee at the University of Virginia. Mice were purchased from Jackson Laboratory or provided by Drs. Timothy Bender or Gary Owens (University of Virginia) as described in detailed methods. Mice were fed a standard chow diet (Tekland 7012) or a Western diet (TD.88137, 42% kcal from fat).
Cell preparations for murine and human flow cytometry
Bone marrow, spleen, peritoneal cavity, and peripheral blood cells were processed for flow cytometry as previously described 5, 34. Isolation of human peripheral blood mononuclear cells was performed as previously described5. Clone and fluorophore information for flow cytometry antibodies used to FAC-sort or immunophenotype murine B cell subsets are given in Online Table VI, and those used to immunophenotype human B cell subsets are given in Online Table VII.
Single-cell sorting and sequencing of the immunoglobulin heavy chain
B-1a cells were single-cell sorted from bone marrow and peritoneal cavity of five 100-week-old chow-fed ApoE−/− mice. cDNA was generated using RT-PCR, then the variable region of the immunoglobulin heavy chain was amplified using the MsVHE, MsCμE, and MsCμN primers using previously described methods (Online Table VIII;35). PCR products were sequenced (Genewiz) using the MsVHE primer. Sequences were analyzed using an online sequence analysis tool, IMGT/HighV-Quest36.
ELISPOT
MDA-LDL was either provided by Dr. Sotirios Tsimikas (UCSD), or freshly generated by modifying human LDL (Kalen Biomedical, LLC). MDA-LDL was used to coat ELISPOT plates for quantification of anti-MDA-LDL IgM antibody-secreting cells (ASC). ELISPOT to measure total IgM or MDA-LDL-specific IgM ASC among spleen and bone marrow single-cell suspensions was performed as described previously5.
ELISA for quantification of total and anti-OSE IgM or IgG isotypes in mice and humans
Total IgM or IgG subtypes in mouse serum or plasma were measured using colorimetric ELISA as described previously5. Levels of IgM antibodies specific for MDA-LDL, AB1–2 (anti-idiotypic antibody recognizing E06/T15 specificity), and α-1,3-dextran in mouse serum or plasma were determined as previously described5, 37, 38. Levels of IgM or IgG against MDA-LDL in human plasma were measured by chemiluminescent ELISA as previously described 39.
Real-time polymerase chain reaction
Semi-quantitative real-time PCR was performed on a CFX96 Real-Time System (BioRad). Data were calculated by the ΔΔCt method and normalized to 18s ribosomal RNA levels. Primer sequences are listed in Online Table VIII.
Retrovirus production
CXCR4-GFP retrovirus was generated using the pMigR1 retroviral vector (provided by Dr. Timothy Bender, UVA), into which mouse CXCR4 was subcloned. The MigR1 (Ctl-GFP) or CXCR4-GFP retroviruses were generated using calcium phosphate transfection in 293T cells. Viral titer was determined by quantifying the frequency of GFP+ cells 24 hours after transduction of 3T3 cells.
Retroviral overexpression of CXCR4 on mouse B cells
Mouse peritoneal B cells were enriched using depletion with Miltenyi MACS columns (details in Online Table IX), and stimulated with TLR9 agonist CpG ODN 1668 (Invivogen) to induce cell proliferation required for retroviral transduction. Cells were transduced at a 20:1 multiplicity of infection with Ctl-GFP or CXCR4-GFP retroviral particles. Cells were FACS-sorted for GFP+ B-1a cells to be used for adoptive transfer. Approximately 30–40% of B-1a cells were successfully transduced by this method.
Adoptive transfer
Non-transduced B-1 cells, or Ctl-GFP transduced B-1a cells, or CXCR4-GFP transduced B-1a cells were FAC-sorted to better than 99% purity from their parent gate then adoptively transferred into Rag1−/− ApoE−/− or CD45.1 ApoE−/− host mice either intraperitoneally, or intravenously via retro-orbital injection.
Statistics
Statistics were performed using GraphPad Prism Version 7.0a (GraphPad Software, Inc) or SAS 9.4. Results from all replicated experiments are displayed and bar graphs display mean ± standard error of the mean.
RESULTS
IgM of mature bone marrow B-1a cells is distinct from IgM of peritoneal B-1a cells in atherosclerotic mice
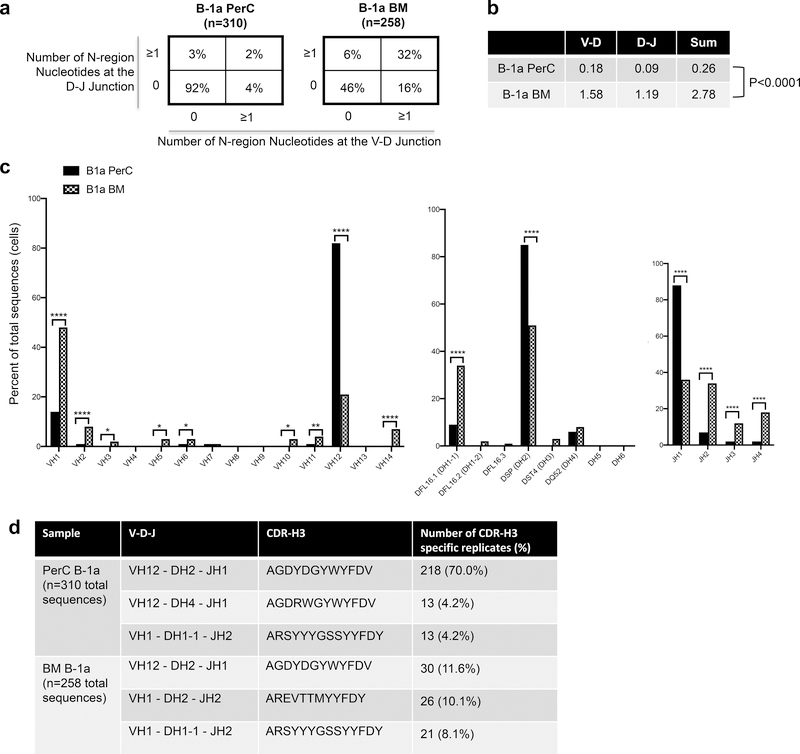
To characterize the immunoglobulin repertoire of the mature bone marrow B-1a cell population in the context of atherosclerosis, we utilized 100-week-old chow-fed ApoE−/− mice. B-1a cells from BM and PerC were single-cell sorted and the variable region of the immunoglobulin heavy chain was sequenced. BM B-1a cells displayed increased diversity at the V-D and D-J junctions, as evidenced by an increase in N-additions, with 32% of sequences containing ≥1 N-additions at both junctions, compared to 2% of PerC B-1a sequences (P<0.0001 by 2X4 χ2 analysis, df=3; Figure 1a). Moreover, the average number of N-additions at the V-D and D-J junctions, as well as the average sum of N-additions at both junctions was significantly greater in BM B-1a cells (Figure 1b). Together, these data indicate increased diversity and a shift away from germline in the BM B-1a immunoglobulin heavy chain repertoire of aged ApoE−/− mice as compared to PerC B-1a cells.
Figure 1. The bone marrow B-1a cell IgH V repertoire is distinct from the peritoneal B-1a cell IgH V repertoire.
(a) Percentage of total sequences with 0 N-additions at both V-D and D-J junctions (lower left), 1 or more N-additions at both junctions (upper right), or one or more N-addition at either the V-D or D-J junction (upper left or lower right) in the variable region of the immunoglobulin heavy chain of sorted bone marrow or perC B-1a cells. (b) Average number of N-additions at either the V-D junction, the D-J junction, or the average sum of N-additions at both junctions in sorted bone marrow and peritoneal cavity B-1a cells. P<0.0001 by Mann-Whitney test. (c) Frequency of total sequences utilizing the given VH, D, and JH gene segments in the variable region of the immunoglobulin heavy chain from single cell-sorted B-1a cells from peritoneal cavity (black bars) or bone marrow (checkered bars) of 100-week-old chow-fed ApoE−/− mice. ****P<0.0001, **P<0.01, or *P<0.05 by 2×2 χ2 analysis, df=1. (d) VDJ usage, CDR-H3 amino acid sequence, and number and frequency of replicates for the top three most frequent replicate sequences found in sorted PerC or BM B-1a cells.
Comparison of VH, D, and JH gene segment usage revealed marked differences between the BM and PerC B-1a repertoire, with increased VH1, VH2, VH3, VH5, VH6, VH10, VH11, VH14, DH1–1, JH2, JH3, and JH4 gene family usage in BM B-1a cells compared to PerC B-1a (Figure 1c). In contrast, PerC B-1a cells showed increased usage of VH12, DH2, and JH1 family genes.
Importantly, sequence comparison between PerC and BM B-1a cells revealed that a high frequency of PerC B-1a cells (92%) and to a lesser extent BM B-1a cells (65%) were replicate sequences present in more than one B-1a cell in these compartments. Notably, the sequence of the most common CDR-H3 in the BM, AGDYDGYWYFDV, was also the most common CDR-H3 sequence in the PerC (Fig. 1d) and other sequences were also common to both BM and PerC (Online Table I), suggesting that B-1a migration between compartments could be occuring.
Intriguingly, AGDYDGYWYFDV accounted for 70% of total PerC B-1a sequences, while it only accounted for 11.6% of total BM B-1a sequences (Fig. 1d), suggesting that selection pressure driving the presence or survival of B-1a cells expressing AGDYDGYWYFDV is stronger in the PerC than the BM in aged ApoE−/− mice. Additionally, the presence of more unique CDR-H3 sequences in the BM also suggests that the BM B-1a microenvironment is more permissive for IgH V diversification.
Mature PerC-derived B-1a cells migrate to the bone marrow and the bone marrow B-1 population does not rely on the spleen
To examine the factors that maintain BM B-1 cells, we first determined whether the BM B-1 population can be replenished by B-1 cells from the circulation. Short-term intravenous transfer of CD45.2 ApoE−/− PerC B-1 cells into CD45.1 ApoE−/− hosts revealed that a small fraction of the total B-1 population in PerC and spleen were emigrated CD45.2+ donor cells, as expected in immunocompetent hosts with an endogenous B cell compartment (Online Fig. I). Importantly, CD45.2+ B-1 cells were also present in the bone marrow, indicating that mature PerC B-1 cells can migrate from the periphery to the BM (Online Fig. I).
The adult PerC B-1 cell population has previously been shown to depend upon the spleen3, 40. To determine whether the adult BM B-1 cell population relies upon splenic migrants, we performed sham or splenectomy surgery on ApoE−/− mice and quantified BM B-1 cell populations after 4 weeks of Western diet feeding (Online Fig. IIa). Bone marrow B-1a and B-1b cell numbers were not altered by absence of the spleen, suggesting that B-1a migrants to the BM may come from locations other than the spleen (Online Fig. IIb).
CXCR4 facilitates mature B-1a migration to the bone marrow
CXCR4 has a well-established role in mediating progenitor cell retention in the BM27, yet whether CXCR4 is needed for mature B-1a cell homing to BM is unknown. To examine the role of B cell CXCR4 on B-1a cell BM migration, we developed a B cell-specific CXCR4 knockout mouse model on the atherosclerosis-prone ApoE−/− background. This is a Cre-Lox model in which Cre recombinase is under control of the CD19 promoter, thereby deleting floxed CXCR4 alleles in all B cells (B-1 and B-2). ApoE−/− mice lacking CXCR4 in B cells (CXCR4BKOApoE−/−) display efficient knockdown of CXCR4 mRNA and protein expression in B cells, and a defect in migratory capacity towards its ligand CXCL12 relative to littermate control mice that retain expression of CXCR4 (CXCR4WT ApoE−/−) (Online Fig. III).
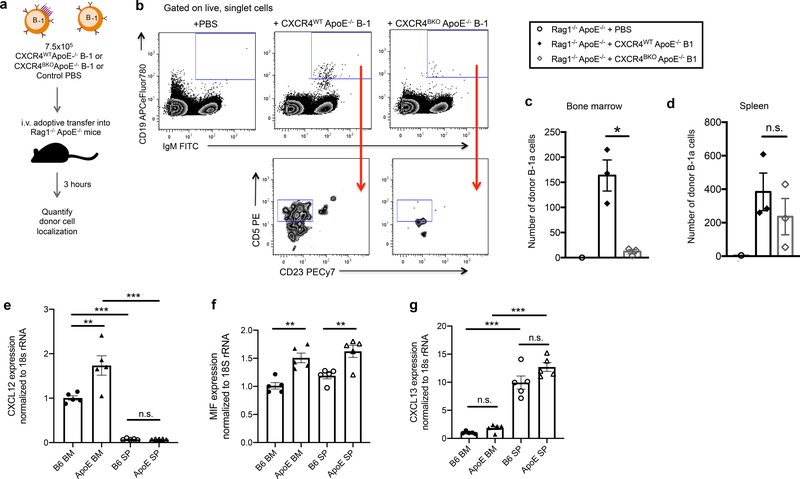
Adoptive transfer of PerC B-1 cells from CXCR4WT ApoE−/− or CXCR4BKOApoE−/− mice into T- and B cell-deficient Rag1−/− ApoE−/− hosts (Fig. 2a) revealed that B-1a cells migrated to both the BM and spleen, however only BM homing was CXCR4-dependent (Fig. 2b–d). To examine why CXCR4 might preferentially facilitate B-1a migration to the BM, and how that is altered in an inflammatory state, we compared mRNA expression of the CXCR4 ligands, CXCL12 and MIF, as well as another important B cell chemokine, CXCL13, in spleen and BM of C57BL/6J and ApoE−/− mice. Results demonstrate greater expression of CXCL12 in BM of C57BL/6J and ApoE−/− mice compared to spleen, and increased CXCL12 expression in ApoE−/− BM compared to C57BL/6J BM (Fig. 2e). MIF expression was not significantly different between BM and spleen, but was increased in ApoE−/− mice compared to C57BL/6J (Fig. 2f). CXCL13 expression was significantly higher in spleen than BM, and was not significantly different between ApoE−/− and C57BL/6J mice (Fig. 2g). These results provide evidence for differential chemokine expression in spleen and BM at baseline and in hyperlipidemic states, providing a likely mechanism for CXCR4-dependent B-1a migration preferentially to the BM over the spleen.
Figure 2. B-1a cells traffic to the bone marrow in a CXCR4-dependent manner.
(a) Experimental schematic for short-term adoptive transfer. (b) Representative gating strategy from one bone marrow sample per transfer condition for quantification of adoptively transferred B-1 cells in Rag1−/−ApoE−/− mice receiving control PBS (n=1) or 7.5×105 CD19+ B220lo IgM+ CD23− peritoneal cavity B-1 cells from CXCR4WT ApoE−/− (n=3) or CXCR4BKO ApoE−/− (n=3) donor mice. Quantification of the number of donor B-1a cells (CD19+IgM+CD23−CD5+) recovered in bone marrow (c) and spleen (d) of Rag1−/− ApoE−/− recipient mice 3 hours post-intravenous transfer. *P<0.05 or n.s. indicates non-significant p-value by Kruskal-Wallis test. Quantification of CXCL12 (e), MIF (f), and CXCL13 (g) mRNA expression normalized to 18s ribosomal RNA expression in total bone marrow and spleen single cell suspensions isolated from C57BL/6 mice (n=5) or ApoE−/− mice (n=5) ***P<0.001, **P<0.01, *P<0.05 or n.s. indicates non-significant p-value by one-way ANOVA with Tukey’s multiple comparisons test. Error bars represent mean ± s.e.m.
CXCR4 is critical for maintaining bone marrow B-1a cell number, bone marrow IgM ASC, and circulating IgM levels
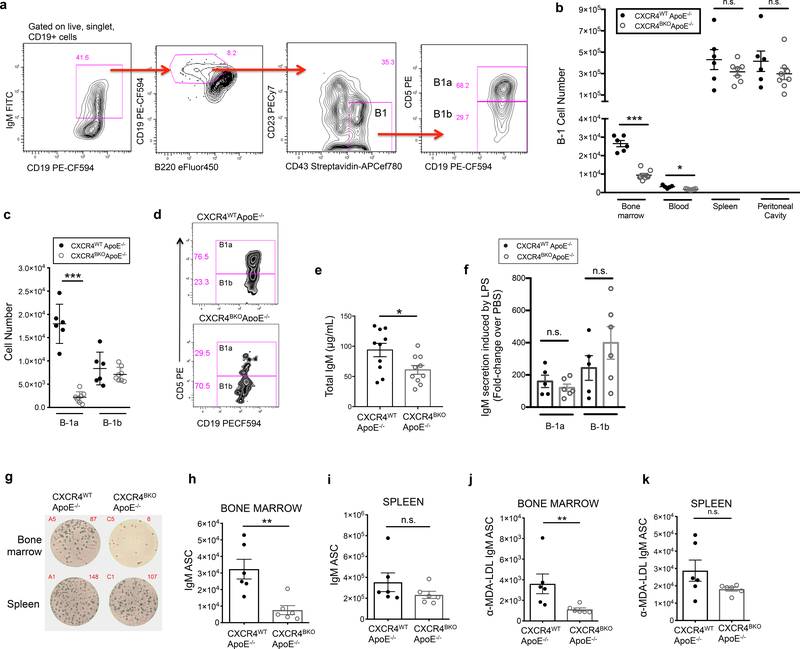
To determine if B cell-specific loss of CXCR4 impacts the endogenous BM B-1a population, we quantified BM B-1 cells in CXCR4BKOApoE−/− mice by flow cytometry. We identified B-1 cells as live CD19+ IgM+ B220mid-lo CD23− CD43+ cells in the bone marrow and further classified into B-1a and B-1b subsets based on expression of CD5 (Fig. 3a). CXCR4BKOApoE−/−mice harbor a significant reduction in total B-1 cells in the bone marrow and blood, but not spleen or peritoneal cavity (Fig. 3b). Importantly, the reduction in B-1 number in the bone marrow was attributable to a loss specifically in the B-1a subset, while B-1b numbers were equal between genotypes (Fig. 3c,d). Loss of CXCR4 additionally reduced the number of CD19+B220hiCD23+CD43− B-2 cells in the bone marrow, but did not impact B-2 cell numbers in blood, spleen, or perC (Online Fig. IVa). There were no significant differences in other immune cell types, including CD3+ T cells, CD115+ monocytes, and Ly6G+ neutrophils in the spleen, bone marrow, and blood between CXCR4WT ApoE−/− and CXCR4BKOApoE−/− mice (Online Fig. V).
Figure 3. CXCR4 is critical for maintaining bone marrow B-1a cell number, bone marrow IgM ASC, and circulating IgM levels in ApoE−/− mice.
(a) Representative gating strategy for quantification of bone marrow B-1 cells by flow cytometry. (b) Quantification of B-1 cells from the bone marrow, blood, spleen, and peritoneal cavity of 8-week-old CXCR4WT ApoE−/− (n=6) or CXCR4BKO ApoE−/− (n=8) mice using flow cytometry. (c) Quantification of B-1a and B-1b cell number in bone marrow of CXCR4WT ApoE−/− (n=6) and CXCR4BKO ApoE−/− (n=8) mice. (d) Representative flow plots of B-1a and B-1b gating from bone marrow of CXCR4WT ApoE−/− or CXCR4BKO ApoE−/− mice. (e) Calculated concentration of total IgM in sera of 8-week-old CXCR4WT ApoE−/− (n=10) or CXCR4BKO ApoE−/− (n=10) mice. (f) Fold change in concentration of IgM in supernatants of cultured peritoneal B-1a or B-1b cells from CXCR4WT ApoE−/− (n=5) or CXCR4BKO ApoE−/− (n=6) mice in response to 50 μg/mL LPS over the amount secreted in response to control PBS. (g) Representative ELISPOT wells depicting IgM antibody-secreting cells (ASC) from bone marrow and spleen of 8-week-old CXCR4WT ApoE−/− or CXCR4BKO ApoE−/− mice. Calculated numbers of IgM ASC in bone marrow (h) and spleen (i) of 8-week-old CXCR4WT ApoE−/− (n=6) or CXCR4BKO ApoE−/− (n=6) mice. Calculated numbers of anti-MDA-LDL IgM ASC in bone marrow (j) and spleen (k) of 8-week-old CXCR4WT ApoE−/− (n=6) or CXCR4BKO ApoE−/− (n=6) mice. Error bars represent mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001, or n.s. indicating non-significant p-value by Mann-Whitney test.
Consistent with fewer BM B-1a cells, total circulating IgM in CXCR4BKOApoE−/−mice was significantly reduced compared to CXCR4WT ApoE−/− littermate controls (Fig. 3e). Sort-purified B-1a and B-1b cells from CXCR4WT ApoE−/− and CXCR4BKOApoE−/− mice secreted equivalent amounts of IgM after stimulation with LPS, a TLR4 agonist which induces B-1 cell IgM production (Fig. 3f), indicating that loss of CXCR4 does not reduce the ability of B-1 cells to produce and secrete IgM. Instead, B cell-specific loss of CXCR4 resulted in reduced numbers of IgM antibody-secreting cells (ASC) and MDA-LDL-specific IgM ASC in the bone marrow but not in the spleen (Fig. 3g–k). Titers of IgG1, IgG2b, IgG2c, and IgG3 were not significantly different between CXCR4WT ApoE−/− and CXCR4BKOApoE−/− mice (Online Fig. IVb–e). Taken together, these data provide evidence that CXCR4 regulates plasma IgM titers by maintaining the number of IgM antibody-secreting B-1a cells specifically within the bone marrow.
CXCR4 overexpression associates with increased B-1a localization to the bone marrow and increased plasma anti-OSE IgM levels
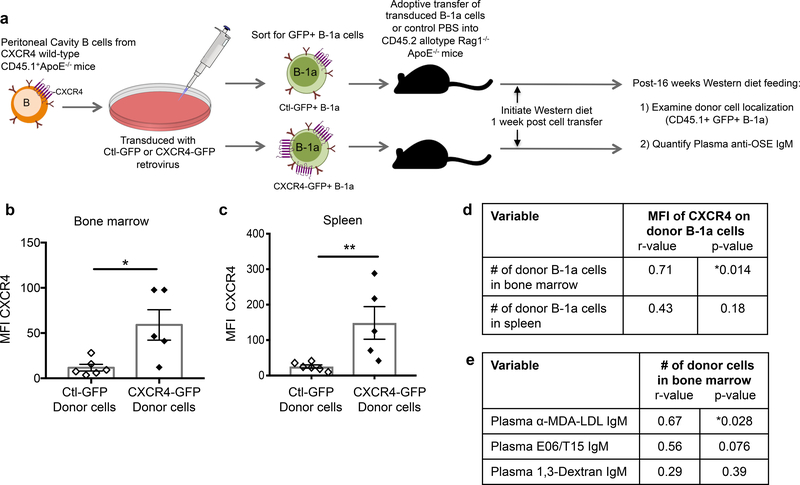
To determine if increasing CXCR4 expression in wild-type B-1a cells could increase localization to the bone marrow and boost IgM production, CXCR4 was overexpressed in B-1a cells using a retroviral approach. The generated CXCR4-GFP retrovirus was able to effectively increase B-1 cell surface CXCR4 expression and functional migration towards CXCL12 compared to a control (Ctl-GFP) retrovirus (Online Fig. VIa–c), without affecting B cell viability (Online Fig. VId–e). Rag1−/−ApoE−/− recipient mice were adoptively transferred with Ctl-GFP+ B-1a cells, CXCR4-GFP+ B-1a cells, or control PBS and fed Western diet for 16 weeks (Fig. 4a). Donor B-1a cells recovered in the bone marrow and spleen of recipients given CXCR4-GFP+ B-1a cells maintained increased CXCR4 expression after 16 weeks (Fig. 4b,c). However, the degree of CXCR4 overexpression varied between mice. Therefore, correlative analysis of the full experimental cohort was performed to determine the effect of CXCR4 overexpression on donor B-1a cell localization. A positive association was identified with the MFI of CXCR4 on donor B-1a cells and the number of donor B-1a cells in the bone marrow but not spleen (Fig. 4d). These data provide further evidence that CXCR4 expression preferentially mediates B-1a migration to the bone marrow. Furthermore, the number of donor B-1a cells in the bone marrow positively associated with circulating levels of IgM against MDA-LDL and a trend towards increased IgM against phosphocholine (plasma E06/T15 IgM), but not with IgM against α-1,3-Dextran, a classic T-independent bacterial surface antigen used as a non-OSE type antigen control (Fig. 4e).
Figure 4. Overexpression of CXCR4 in murine B-1a cells associates with increased B-1a cell localization to the bone marrow and increased plasma anti-OSE IgM levels.
(a) Experimental setup: Peritoneal B cells from CXCR4 wild-type CD45.1 allotype ApoE−/− mice were transduced with CXCR4-GFP or Ctl-GFP retrovirus and 1×105 successfully transduced B-1a cells were intravenously transferred into Rag1−/−ApoE−/− hosts, which were fed 16 weeks of Western diet. Quantification of the mean fluorescence intensity (MFI) of CXCR4 on recovered CD45.1+GFP+ donor B-1a cells in bone marrow (b) or spleen (c) of Rag1−/−ApoE−/− mice receiving CXCR4-GFP+ B-1a cells (n=5) or Ctl-GFP+ B-1a cells (n=6) after 16 weeks Western diet feeding. *P<0.05 or **P<0.01 by Mann-Whitney test. (d) The MFI of CXCR4 on donor B-1a cells correlated with the number of donor B-1a cells in bone marrow or spleen of Rag1−/−ApoE−/−recipient mice after 16 weeks Western diet feeding. N=11 mice. (e) The number of donor B-1a cells in bone marrow correlated with circulating levels of anti-MDA-LDL IgM, E06/T15 IgM specific for phosphocholine, or anti-1,3-Dextran IgM after 16 weeks Western diet feeding. Data presented as correlation coefficient (r) and statistical significance (p).
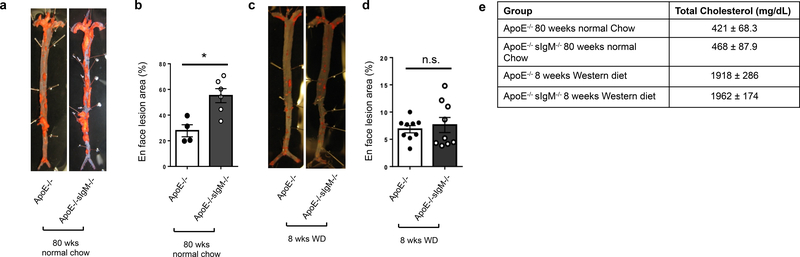
Loss of secreted IgM enhances atherosclerosis in aged ApoE−/− mice with modest cholesterol levels
To determine if secreted IgM impacts atherogenesis in a setting relevant to human disease, we examined atherosclerosis by en face analysis in 80-week-old chow-fed ApoE−/− mice lacking secreted IgM (sIgM−/−). As age has been shown to accelerate atherogenesis41, one might consider this model more comparable to a 70-year-old human with hypercholesterolemia. Aged chow-fed ApoE−/− sIgM−/− mice had significantly increased atherosclerosis compared to chow-fed ApoE−/− controls (Figure 5a,b) and had cholesterol levels of 421–468 mg/dL (Figure 5e). In contrast, in a typical model of murine atherosclerosis (young mice fed Western diet for 8 weeks), atherosclerosis did not differ between genotypes (Fig. 5c,d). Notably, this model is comparable to a much younger human and has total cholesterol levels approaching 2000 mg/dL (Fig. 5e), representing an extreme atherogenic pressure not encountered in human CVD. These findings suggest that typical murine models of atherosclerosis may not be appropriate for determining if CXCR4 mediates B-1 cell production of anti-OSE IgM and atheroprotection.
Figure 5. Loss of secreted IgM enhances atherosclerosis in aged ApoE−/− mice with modest cholesterol levels.
Representative images (a) and quantification (b) of Sudan-IV+ lesion area in aortas from 80-week-old ApoE−/− (n=4) and sIgM−/−ApoE−/− (n=6) mice fed normal chow diet. Representative images (c) and quantification (d) of Sudan-IV+ lesion area in aortas from 16-week-old ApoE−/− (n=9) and sIgM−/−ApoE−/− (n=9) mice fed 8 weeks of Western diet. *P<0.05 or non-significant (n.s.) by Mann-Whitney test. (e) Total cholesterol levels in ApoE−/− and sIgM−/−ApoE−/− mice fed 8 weeks of Western diet or 80 weeks normal chow diet.
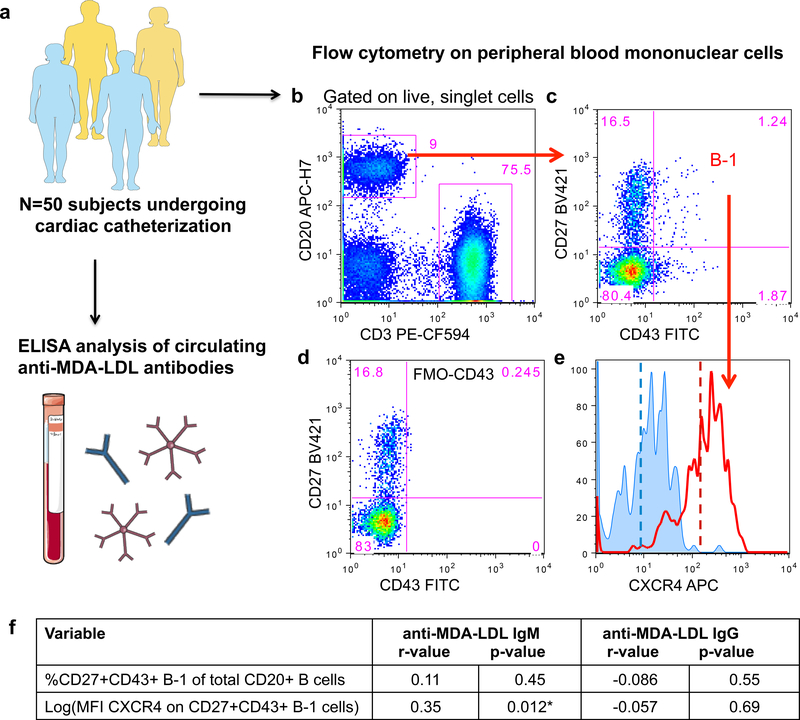
CXCR4 expression on human peripheral B-1 cells associates with increased amount of plasma anti-MDA-LDL IgM antibodies
Accordingly, we turned to a human atherosclerosis cohort to test for association between B-1 cell CXCR4 expression and anti-OSE IgM and coronary artery disease. We analyzed a cohort of 50 human subjects presenting to the cardiac catheterization laboratory at the University of Virginia. We quantified circulating levels of IgM and IgG antibodies specific for MDA-LDL and CXCR4 expression on circulating B-1 cells (Fig. 6a,b). We used the flow cytometry strategy originally defined by Rothstein and colleagues 9 when quantifying the B-1 population. In this strategy, and for all findings in this report, human B-1 cells are defined as live, singlet, CD20+CD3−CD27+CD43+ cells. Representative flow cytometry plots of circulating immune cells from one subject (Fig. 6b,c) reveal a diffuse population of human B-1 cells, when gated based on an FMO-CD43 control (Fig. 6d). Cell surface expression of CXCR4 was measured as mean fluorescence intensity (MFI) of CXCR4 on B-1 cells (Fig. 6e). As the MFI of CXCR4 on B-1 cells was a skewed variable, we performed log transformation to approximate normality for use in correlative analysis. The frequency of CD20+CD3−CD27+CD43+ B-1 cells within the total CD20+ B cell population displayed no significant association with plasma levels of anti-MDA-LDL IgM (Fig. 6f). Notably, the addition of cell surface expression of CXCR4 to the analysis of B-1 cells significantly improved the positive association with amount of plasma anti-MDA-LDL IgM (Fig. 6f). No associations were identified between anti-MDA-LDL IgG levels and CXCR4 expression on B-1 cells (Fig. 6f) or anti-MDA-LDL IgG or IgM levels and CXCR4 expression on other peripheral B cell subsets including naïve CD20+CD3−CD27-CD43- B cells and memory CD20+CD3−CD27+CD43− B cells (Online Table II).
Figure 6. CXCR4 expression on peripheral human B-1 cells associates with increased circulating amounts of anti-MDA-LDL IgM antibodies.
(a), A 50-subject human cohort was analyzed for circulating anti-MDA-LDL IgM or IgG antibody levels and CXCR4 expression on peripheral blood B cell subsets. (b) Representative flow cytometry of peripheral blood mononuclear cells subset into CD20+ B cells and CD3+ T cells. (c) CD20+ B cells further subset into CD27+CD43+ B-1 cells. (d) FMO minus CD43 control used to set CD43 positivity. (e) Representative histograms depicting CXCR4 expression on the CD27+CD43+ B-1 subset from an FMO minus CXCR4 control (blue histogram) or a representative patient sample (red histogram). Dashed lines indicate mean fluorescence intensity (MFI) of CXCR4. (f) The percentage of CD20+CD3−CD27+CD43+ B-1 cells of total CD20+ B cells, or CXCR4 expression on B-1 cells (log(MFI CXCR4)) correlated with circulating amounts of anti-MDA-LDL IgM or IgG antibodies in 50 subjects. Correlation data presented as correlation coefficient (r) and statistical significance (p).
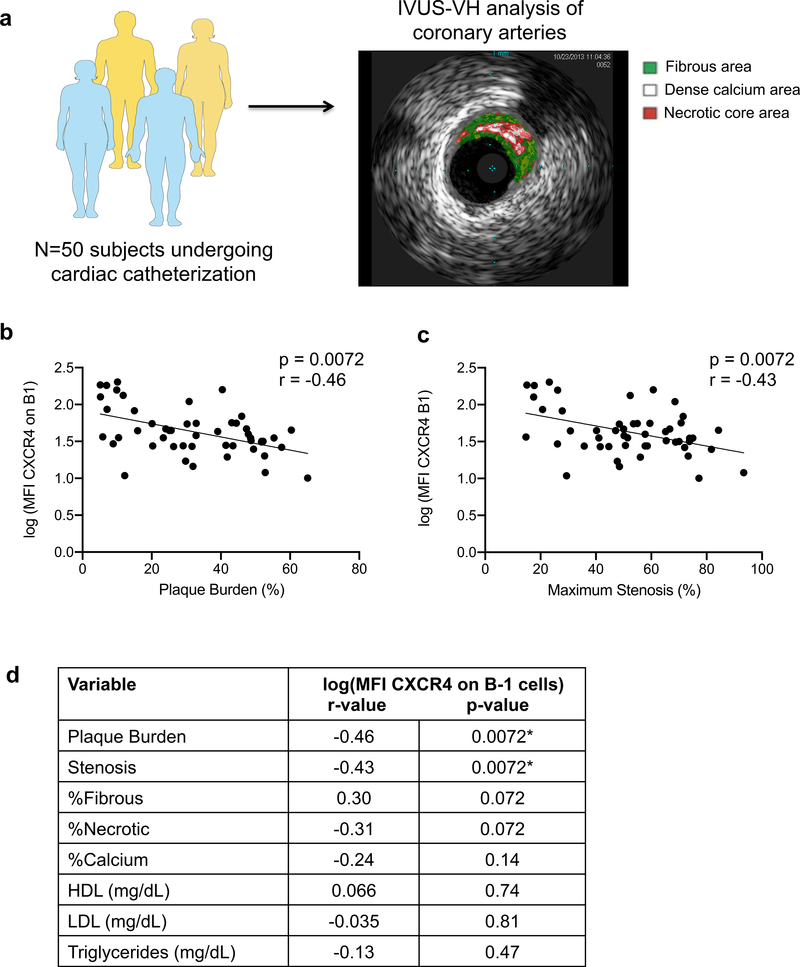
CXCR4 expression on human peripheral B-1 cells inversely associates with plaque volume and stenosis and indices of plaque instability
Next, we analyzed the relationship between human B-1 CXCR4 expression and plaque burden and composition in human coronary arteries, as quantified by intravascular ultrasound and virtual histology (IVUS-VH) (Fig. 7a). Clinical and laboratory characteristics of this patient cohort are provided in Online Table III. Consistent with the positive association of CXCR4 expression on human B-1 cells with circulating levels of IgM to MDA-LDL, human B-1 CXCR4 expression showed an inverse association with plaque burden and stenosis (Fig. 7b,c). Furthermore, while the frequency of circulating B-1 cells was not significantly associated with plaque burden, stenosis, or plaque characteristics (Online Table IV), CXCR4 expression on these B-1 cells demonstrated a trending positive association with the percentage of fibrous area in plaque and a trending inverse association with the percentage of necrotic area in plaque (Fig. 7d), indicative of a more stable plaque phenotype. Additionally, there were no significant associations of B-1 CXCR4 expression and circulating levels of HDL, LDL, or triglycerides (Fig. 7d). Consistent with these findings, CXCR4 expression was not different on B cell subsets between age-matched C57BL/6 mice and hypercholesterolemic ApoE−/− mice fed chow or Western diet (Online Fig. VII). Multivariate analysis indicated that CXCR4 expression on human B-1 cells predicted plaque burden independently of LDL cholesterol, age, or gender in this 50-subject cohort (β coefficient= −11.33; standard error 3.04; P-value=0.0005). Finally, no significant associations were seen between human B-1 CXCR4 expression and other CAD risk factors such as hsCRP, BMI, HbA1c%, or creatinine (Online Table V).
Figure 7. CXCR4 expression on peripheral human B-1 cells associates with decreased plaque volume and protected plaque phenotype.
(a) Plaque burden, stenosis, and composition were measured in human coronary arteries by intravascular ultrasound (IVUS) and virtual histology (VH). (b) Correlation plot of plaque burden with CXCR4 expression on B-1 cells (log(MFI CXCR4)) in 50 subjects. (c) Correlation plot of maximum stenosis with CXCR4 expression on B-1 cells (log(MFI CXCR4)) in 50 subjects. (d) Associations between CXCR4 expression on peripheral human B-1 cells and plaque burden, stenosis, plaque characteristics, or plasma cholesterol levels in 50 subjects. Data presented as correlation coefficient (r) and False Discovery Rate (FDR)-corrected statistical significance (p). *P<0.05 for FDR-corrected p-values.
DISCUSSION
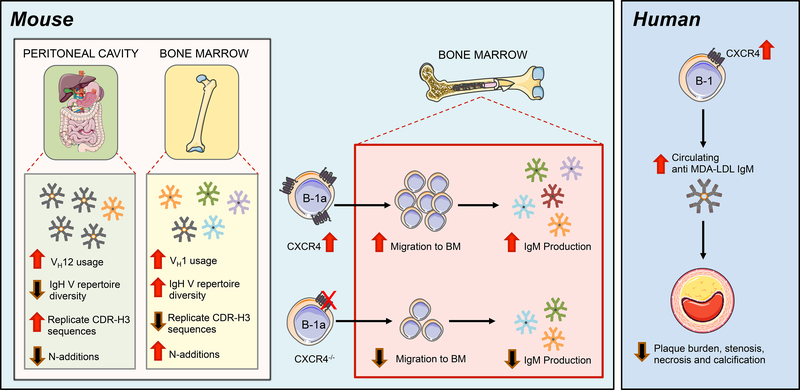
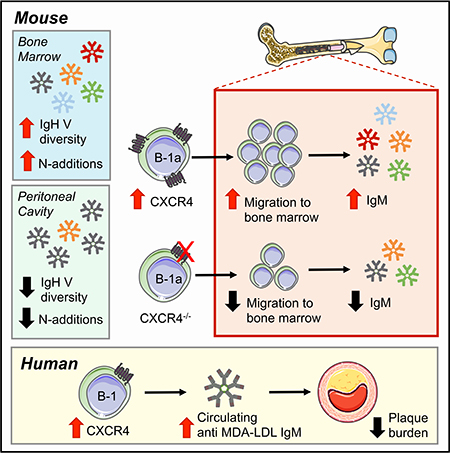
As B-1 cell-derived anti-OSE IgM antibodies have been demonstrated to be atheroprotective, identifying the factors that regulate their production has potential therapeutic implications. BM B-1a cells are known to be major contributors to circulating IgM levels13. While comparisons in immunoglobulin repertoire and circulatory patterns between splenic and peritoneal B-1a populations in non-atherosclerotic mice have been explored16–20, 42–45, little is known about what maintains the BM B-1a population or their IgM repertoire, particularly in the setting of atherosclerosis. Here we characterize, for the first time, the immunoglobulin heavy chain repertoire of BM B-1a cells in the context of atherosclerosis, and find that it is distinct from that of PerC B-1a cells. Moreover, we identify CXCR4 as a critical factor regulating BM B-1a number, BM IgM ASC number, and plasma levels of anti-OSE IgM in mice, and further as a novel marker associating with atheroprotection in humans (Fig. 8).
Figure 8. CXCR4 mediates atheroprotective IgM production in the bone marrow, a niche permissive for a unique repertoire of IgM antibodies.
In mice (left), the BM IgH V repertoire is distinct and diversified compared to B-1a cells in the peritoneal cavity. The IgH V repertoire displayed by BM B-1a cells contains increased VH1 gene segment usage, more N-additions, and a greater frequency of unique CDR-H3 sequences, resulting in greater IgH V repertoire diversity. The BM B-1a compartment is maintained by CXCR4, as loss of CXCR4 results in reduced B-1a migration to the BM and decreased anti-OSE IgM production in the BM. Conversely, overexpression of CXCR4 results in increased B-1a migration to the BM and increased anti-OSE IgM levels. In humans (right) CXCR4 expression on the human B-1 cell subset associates with increased circulating IgM, decreased atherosclerotic plaque burden, and a protected plaque phenotype in coronary arteries.
Previous studies comparing the IgH V region of single cell-sorted or bulk-sorted PerC B cell subsets from non-immune C57BL/6 or BALB/c-ByJ mice have demonstrated that PerC B-1a cells have a restricted heavy chain VDJ repertoire, and lose diversity in VDJ rearrangements and gain N-additions with age16, 18, 19. In line with these studies, we found that the PerC B-1a repertoire from 100-week aged ApoE−/− mice was very limited. Some CDR-H3 sequences in our dataset were previously observed on deep sequencing of bulk peritoneal B-1a cells from young C57BL/6 mice16, supporting the conservation of B-1a sequences. Notably in aged ApoE−/− mice, we found that 70% of PerC B-1a sequences utilized one identical VH12-DH2-JH1 nucleotide sequence that encoded one identical CDR-H3 amino acid sequence, a significantly higher frequency than previously reported in C57BL/6 mice, in which two CDR-H3 sequences comprised 43% of total PerC B-1a sequences16. Strikingly, we also found that N-additions in aged ApoE−/− PerC B-1a cells were very limited, with 92% of sequences containing 0 N-additions at both V-D and D-J junctions. These data contrast with previous studies, which showed that PerC B-1a from 7–24 month old BALB/c-ByJ mice contained 28% of sequences with 0 N-additions19, and from 12-week-old C57BL/6 mice that contained ~60% of sequences with no N-additions16. These differences may be due to differences in mouse background, vivarium conditions, or perhaps the heightened inflammatory microenvironment of the 100-weeks-aged ApoE−/− mouse, which may drive preferential selection for germline sequences in the PerC.
In contrast, our novel BM B-1a repertoire data in aged ApoE−/− mice reveals significantly more N-additions and fewer replicate sequences, indicating a shift away from germline and increased antibody diversity compared to PerC B-1a. Despite this diversity, some CDR-H3 sequences were common to both BM and PerC B-1a cells. It remains undetermined whether the adult BM B-1a population is maintained by circulating B-1a cells from PerC or elsewhere, or from resident adult BM B-1 cell progenitors, or both. On one hand, the commonalities seen between PerC and BM B-1a sequences raise the intriguing hypothesis that certain B-1 cell clones preferentially migrate to the BM for antibody production. Consistent with this hypothesis, our data utilizing adoptive transfer of B-1a cells either overexpressing or deficient in CXCR4 demonstrate that PerC-derived mature B-1a cells traffic to the BM in a CXCR4-dependent manner. Alternatively, the differences observed between PerC and BM B-1a IgH V sequences suggest that niche-specific signals within the BM may also permit antibody repertoire diversification.
The most frequently utilized CDR-H3 in both BM and Perc B-1a was AGDYDGYWYFDV, a sequence previously observed to be associated with binding to phosphatidylcholine(PtC)18. Prior studies by Witztum and colleages have demonstrated that another PtC-binding antibody binds oxidized LDL and specifically, the phosphocholine (PC) head group present on PtC and oxidized phospholipids16. Our finding of high prevalence of AGDYDGYWYFDV in PerC and BM B-1a cells of aged ApoE−/− mice, coupled with the known abundance of PC-containing oxidized phospholipids in atherosclerosis23 leads us to speculate that the hyperlipidemic conditions in ApoE−/− mice may drive preferential selection and expansion of IgM antibodies against PC and perhaps other OSE.
We additionally provide evidence that B-1a cell trafficking to the BM likely does not depend on B-1a cell migration from the spleen, as splenectomy does not alter B-1 cell numbers within the BM. Instead, the preferential expression of CXCL12 in the BM and CXCL13 expression in the spleen may guide B-1 cells with varying expression of CXCR4 or CXCR5 (receptor for CXCL13), to the BM or spleen, respectively. We found that CXCR4 expression on B cell subsets remains constant between C57BL/6, ApoE−/− chow-fed and ApoE−/− Western diet-fed mice. Future analysis of CXCR5 expression, or the ratio of CXCR4:CXCR5 on B-1 cells, may further help delineate additional trafficking patterns of B-1 cells.
To examine the relationship between B-1 CXCR4 expression and atherosclerosis, we utilized a human CVD cohort. We chose this approach due to significant caveats of available murine models, including the lack of B-1a-specificity in the CXCR4BKOApoE−/− model, and the marked hyperlipidemia and immunodeficient setting of the Western diet-fed Rag1−/− ApoE−/− model. While a protective role for B-1 cells in murine atherosclerosis is well established, translating these findings to humans has been challenging. The human equivalent of the murine B-1 cell has remained elusive due to its distinct cell surface phenotype and low frequency in peripheral blood 46. Since its initial discovery, controversy has existed on the difference between human B-1 cells and plasmablasts (PB) or pre-plasmablasts (pre-PB) 47, though Rothstein and colleagues have since demonstrated that the CD20+CD27+CD43+ B-1 subset differs from pre-PB and PB based on CD38 expression, transcriptional profiling, antibody repertoire, and other characteristics 12. Additionally, differential expression of CD11b on the CD27+CD43+ B-1 population has been shown to associate either with increased IgM production (CD11b- human B-1) or increased IL-10 production and capacity to modulate T cells (CD11b+ human B-1)11. This suggests that a deeper heterogeneity within the CD27+CD43+ B-1 subset exists, and additional surface markers may correlate with functional differences. We found in a 50-subject human cohort that B-1 CXCR4 expression significantly positively associated with plasma levels of IgM to MDA-LDL, echoing our murine findings that CXCR4 is critical for maintaining circulating anti-OSE IgM levels.
Advances in coronary artery imaging, such as the use of IVUS-VH, have also improved our abilities to translate murine discoveries to humans. By utilizing IVUS-VH to quantify coronary artery plaque volume and characteristics, we provide the first evidence that B-1 CXCR4 expression inversely associates with plaque burden and stenosis, and positively associates with a more stable plaque phenotype. These correlations support a hypothesis in which CXCR4 directs B-1 migration to sites of IgM antibody production like the bone marrow, thereby increasing circulating anti-OSE IgM levels that can modify plaque phenotype and reduce plaque burden.
In sum, this report provides novel findings demonstrating a distinct IgM repertoire between PerC and BM B-1a cells in the setting of atherosclerosis, and niche-specific differences in CXCR4-dependent B-1a cell maintenance and IgM production. Furthermore, our initial proof-of-concept study overexpressing CXCR4 on B-1a cells in vivo provides a rationale for future studies investigating whether modulating chemokine receptor expression in a targeted manner can have therapeutic potential. Our initial findings in a 50-subject cohort, coupled with our novel murine findings that production of circulating IgM to OSE and B-1a homeostasis depends on CXCR4, underscore the need for larger studies examining the association between human B-1 CXCR4 expression and plaque characteristics.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
IgM antibodies against oxidation-specific epitopes (OSE), like malondialdehyde(MDA)-modified LDL are associated with atheroprotection in mouse models and in human patients with cardiovascular disease (CVD).
Bone marrow (BM) B-1a cells contribute significantly to amount of plasma IgM.
The putative human B-1 cell subset was previously identified as a CD20+CD3−CD27+CD43+ population in the circulation.
What New Information Does This Article Contribute?
The repertoire of IgM expressed by BM B-1a cells is unique and displays greater heterogeneity compared to B-1a cells in the peritoneal cavity, where B-1 cells are found in abundance but where IgM secretion is low.
The chemokine receptor CXCR4 regulates BM B-1a cell number in mice through mediating B-1a trafficking to the BM, and consequently sustains IgM production in the bone marrow.
CXCR4 expression on human B-1 cells associates with increased amount of plasma anti-MDA-LDL IgM antibodies and decreased atherosclerosis in human coronary arteries.
B-1 cells in mice and humans produce atheroprotective anti-OSE IgM antibodies. While BM B-1a cells contribute significantly to overall plasma IgM titers, little is known about what mechanisms maintain the BM B-1a population. Here we show that CXCR4 is necessary for maintaining B-1a and IgM secreting-cell number in the BM, and consequently plasma IgM levels. Moreover, we find that the repertoire of IgM expressed by BM B-1a cells during atherosclerosis is unique and displays greater heterogeneity than that of peritoneal cavity B-1a cells. Certain IgM sequences were commonly expressed by both peritoneal cavity and BM B-1a cells, suggesting that B-1a migration between compartments may be occurring. We show that, indeed, CXCR4 mediates the migration of peritoneal B-1a cells to the BM. Finally, in 50 human CVD subjects, CXCR4 expression on the putative human B-1 subset associated with increased levels of plasma anti-MDA-LDL IgM antibodies and less coronary artery plaque burden. These findings highlight a previously underappreciated diversity in human and murine B-1 cell populations and identify CXCR4 as a novel biomarker associating with protection in human CVD. Moreover, these results suggest that a targeted approach for limiting atherosclerosis via increasing B-1 CXCR4 expression may be relevant to humans.
ACKNOWLEDGEMENTS
We thank Joanne Lannigan, Mike Solga, and Claude Chew from the UVa flow cytometry core, and Naomi Tsuji and Alexander Ludlow from Western Michigan University Homer Stryker M.D. School of Medicine for their excellent technical assistance. We thank Fabrizio Drago from University of Virginia for his assistance with statistical analysis. We thank Thomas Prohaska at University of California San Diego for assistance in comparative analysis to previously identified sequences.
SOURCES OF FUNDING
This work was supported by 1R01 HL107490, 1R01 HL136098, Project 3 of P01 HL055798 and P01 HL136275-01 (CAM), Core B of P01 HL055798 and P01 HL136275-01 (CAM and AMT), and R01AI142004. AU was supported by AHA Predoctoral fellowship 16PRE30300002 and 5T32AI007496-20.
Nonstandard abbreviations and Acronyms
- ASC
Antibody-secreting cell
- BM
Bone marrow
- CDR-H3
Complementarity determining region 3 of the heavy chain
- CXCR4BKOApoE−/−
ApoE knockout mice with B cell-specific knockout of CXCR4
- CXCR4WT ApoE−/−
Wild-type littermates to CXCR4BKOApoE−/− mice
- CVD
Cardiovascular disease
- FACS/MACS
Fluorescence or Magnetic Activated Cell Sorting
- FMO
Fluorescence minus one control
- IgH V
Variable region of the immunoglobulin heavy chain
- IVUS-VH
Intravascular ultrasound and virtual histology
- LPS
Lipopolysaccharide
- MDA-LDL
Malondialdehyde-modified low density lipoprotein
- N-additions
Non-template-encoded nucleotide additions
- OSE
Oxidation-specific epitope
- PC
Phosphocholine
- PerC
Peritoneal cavity
- PtC
Phosphatidylcholine
Footnotes
DISCLOSURES
Drs. Tsimikas and Witztum are co-inventors and receive royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins, and are co-founders of Oxitope, Inc. Dr. Tsimikas currently has a dual appointment with UCSD and is an employee of Ionis Pharmaceuticals. Dr. Witztum is a consultant to Ionis Pharmaceuticals.
REFERENCES
- 1.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL and Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. The Journal of clinical investigation. 2009;119:1335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A and Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PloS one. 2012;7:e29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A and Toh B-H. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circulation Research. 2011;109:830–840. [DOI] [PubMed] [Google Scholar]
- 4.Perry HM, Bender TP and McNamara CA. B cell subsets in atherosclerosis. Frontiers in Immunology. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL and McNamara CA. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsiantoulas D, Gruber S and Binder CJ. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front Immunol. 2012;3:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS and Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. Journal of Lipid Research. 2007;48:425–433. [DOI] [PubMed] [Google Scholar]
- 8.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL and Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. Journal of the American College of Cardiology. 2012;60:2218–29. [DOI] [PubMed] [Google Scholar]
- 9.Griffin DO, Holodick NE and Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20(+)CD27(+)CD43(+)CD70(−). The Journal of Experimental Medicine. 2011;208:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descatoire M, Weill JC, Reynaud CA and Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin DO and Rothstein TL. Human “orchestrator” CD11b(+) B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity. Mol Med. 2012;18:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quach TD, Rodriguez-Zhurbenko N, Hopkins TJ, Guo X, Hernandez AM, Li W and Rothstein TL. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. Journal of immunology. 2016;196:1060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi YS, Dieter JA, Rothaeusler K, Luo Z and Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. European journal of immunology. 2012;42:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holodick NE, Tumang JR and Rothstein TL. Immunoglobulin secretion by B1 cells: Differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. European Journal of Immunology. 2010;40:3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holodick NE, Vizconde T and Rothstein TL. Splenic B-1a Cells Expressing CD138 Spontaneously Secrete Large Amounts of Immunoglobulin in Naive Mice. Front Immunol. 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prohaska TA, Que X, Diehl CJ, Hendrikx S, Chang MW, Jepsen K, Glass CK, Benner C and Witztum JL. Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. Journal of immunology. 2018;200:1702–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vale AM, Tanner JM, Schelonka RL, Zhuang Y, Zemlin M, Gartland GL and Schroeder HW, Jr. The peritoneal cavity B-2 antibody repertoire appears to reflect many of the same selective pressures that shape the B-1a and B-1b repertoires. Journal of immunology. 2010;185:6085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J and Herzenberg LA. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 2015;4:e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holodick NE, Vizconde T, Hopkins TJ and Rothstein TL. Age-Related Decline in Natural IgM Function: Diversification and Selection of the B-1a Cell Pool with Age. The Journal of Immunology. 2016:1600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holodick NE, Vizconde T and Rothstein TL. B-1a cell diversity: nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. Journal of immunology. 2014;192:2432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgarth N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol. 2016;7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP and Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–9. [DOI] [PubMed] [Google Scholar]
- 23.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Maatta A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Yla-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S and Witztum JL. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou MY, Pattison J, Torzewski M, Sollors J, Friedmann T, Lai NC, Hammond HK, Getz GS, Reardon CA, Li AC, Banka CL and Witztum JL. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. Journal of the American College of Cardiology. 2011;58:1715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A and Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). The Journal of Experimental Medicine. 1996;184:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foussat A, Balabanian K, Amara A, Bouchet-Delbos L, Durand-Gasselin I, Baleux F, Couderc J, Galanaud P and Emilie D. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur J Immunol. 2001;31:350–9. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q, Jones D and Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. [DOI] [PubMed] [Google Scholar]
- 28.Doring Y, Pawig L, Weber C and Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Frontiers in physiology. 2014;5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiechl S, Laxton RC, Xiao Q, Hernesniemi JA, Raitakari OT, Kahonen M, Mayosi BM, Jula A, Moilanen L, Willeit J, Watkins H, Samani NJ, Lehtimaki TJ, Keavney B, Xu Q and Ye S. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2678–83. [DOI] [PubMed] [Google Scholar]
- 30.Mehta NN, Li M, William D, Khera AV, DerOhannessian S, Qu L, Ferguson JF, McLaughlin C, Shaikh LH, Shah R, Patel PN, Bradfield JP, He J, Stylianou IM, Hakonarson H, Rader DJ and Reilly MP. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur Heart J. 2011;32:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Runmin G, Jiamei J, Zhiliang J, Yonghua C, Zhizhou S, Guizhou T and Shuguang L. Genetic variation of CXCR4 and risk of coronary artery disease: epidemiological study and functional validation of CRISPR/Cas9 system. Oncotarget. 2018;9:14077–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nie Y, Waite J, Brewer F, Sunshine M-J, Littman DR and Zou Y-R. The Role of CXCR4 in Maintaining Peripheral B Cell Compartments and Humoral Immunity. The Journal of Experimental Medicine. 2004;200:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari H, Nguyen AT, Yang X, Hisada Y, Tsimikas S, Mackman N, Taylor A and McNamara CA. Association of D-dimer with Plaque Characteristics and Plasma Biomarkers of Oxidation-Specific Epitopes in Stable Subjects with Coronary Artery Disease. J Cardiovasc Transl Res. 2018. [DOI] [PubMed] [Google Scholar]
- 34.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP and McNamara CA. B-Cell Aortic Homing and Atheroprotection Depend on Id3. Circulation Research. 2012;110:e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantor AB, Merrill CE, Herzenberg LA and Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. Journal of immunology. 1997;158:1175–86. [PubMed] [Google Scholar]
- 36.Lefranc M-P, Giudicelli V, Duroux P, Jabado-Michaloud J, Folch G, Aouinti S, Carillon E, Duvergey H, Houles A, Paysan-Lafosse T, Hadi-Saljoqi S, Sasorith S, Lefranc G and Kossida S. IMGT®, the international ImMunoGeneTics information system® 25 years on. Nucleic Acids Res. 2015;43:D413–D422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL and Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–43. [DOI] [PubMed] [Google Scholar]
- 38.Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, Chou M-Y, Wiesner P, Tsiantoulas D, Corr M, VanNieuwenhze MS, Tsimikas S, Binder CJ, Witztum JL and Hartvigsen K. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. Journal of Lipid Research. 2014;55:2137–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJP, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw K-T and Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. Journal of Lipid Research. 2011;52:1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardemann H, Boehm T, Dear N and Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JC and Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. [DOI] [PubMed] [Google Scholar]
- 42.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T and Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawahara T, Ohdan H, Zhao G, Yang YG and Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. Journal of immunology. 2003;171:5406–14. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Shin DM, Abbasi S, Jain S, Kovalchuk AL, Beaty N, Chen S, Gonzalez-Garcia I and Morse HC 3rd. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Tung JW, Ghosn EE, Herzenberg LA and Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin DO and Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol. 2012;3:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covens K, Verbinnen B, Geukens N, Meyts I, Schuit F, Van Lommel L, Jacquemin M and Bossuyt X. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121:5176–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.