Abstract
ILDR2 is a member of the Ig superfamily, which is implicated in tricellular tight junctions, and has a putative role in pancreatic islet health and survival. We recently found a novel role for ILDR2 in delivering inhibitory signals to T cells. In this article, we show that short-term treatment with ILDR2-Fc results in long-term durable beneficial effects in the relapsing-remitting experimental autoimmune encephalomyelitis and NOD type 1 diabetes models. ILDR2-Fc also promotes transplant engraftment in a minor mismatch bone marrow transplantation model. ILDR2-Fc displays a unique mode of action, combining immunomodulation, regulation of immune homeostasis, and re-establishment of Ag-specific immune tolerance via regulatory T cell induction. These findings support the potential of ILDR-Fc to provide a promising therapeutic approach for the treatment of autoimmune diseases.
The immune system uses a variety of inhibitory mechanisms to maintain immune homeostasis (1). Immune check-points are critical components in controlling immune activation following Ag recognition by T cells in the immune synapse; as such, they became attractive targets for cancer immunotherapy, as well as for the treatment of autoimmune diseases (2-4). In autoimmunity, this approach was pioneered using CTLA4-Fc, which blocks the costimulatory signal transmitted by the interaction of CD80/CD86 with CD28, and provided a strong clinical proof-of-concept of this strategy (5, 6). In parallel, enhancement of costimulation by blocking CTLA4 or the PD1/PDL-1 coinhibitory pathways revolutionized cancer immunotherapy (2, 7, 8). Although not all of the recently approved immune modulatory therapies will be functionally efficacious in all disease indications tested, the knowledge gained from the preclinical and clinical trials will advance our scientific understanding of various disease states.
The pathogenesis of autoimmune diseases is associated with loss of immune tolerance to self-antigens, which is manifested by elevated levels of cytokines of the Th1/Th17 cell axis (e.g., IFN-γ and IL-17), as well as other proinflammatory cytokines, such as GM-CSF, IL-6, and TNF-α (9). Another important component in this equation is regulatory T cells (Tregs), which play a pivotal role in limiting the duration and magnitude of the immune response and in maintaining self-tolerance to prevent the development of autoimmunity (10). It is hypothesized that the absence or dysfunction of Tregs is associated with acceleration of autoimmunity, whereas Treg-inducing strategies and adoptive transfer of Tregs have been shown to be promising therapeutic approaches (4, 11). Current therapeutic strategies for autoimmune diseases rely broadly on immunosuppressive treatments or on blockade of the activity of single cytokines (e.g., TNF-α, IL-17, and IL-6) and require life-long treatment (12). Although these treatments have been shown to be beneficial in autoimmunity, they do not target the cause of the disease, and their use is associated with increased vulnerability to opportunistic infections and development of malignancies (13, 14). Thus, re-establishing immune tolerance, specifically to auto-antigens, has become an ultimate goal in developing novel therapies for treatment of autoimmune diseases.
ILDR2 is a member of the Ig domain containing protein superfamily, which has been identified as a component of tricellular tight junctions (tTjs) (15) and has a putative role in pancreatic islet cell health and survival (16). To further study the effect of ILDR2 on human and mouse T cells, as well as to evaluate its efficacy in mouse models of autoimmune diseases, we produced two versions of fusion proteins composed of the extracellular domain (ECD) of human ILDR2 fused to an Fc portion of human IgG1 (ILDR2-hFc) or the ECD of human ILDR2 fused to an Fc portion of mouse IgG2a (ILDR2-mFc). Hecht et al. (17) demonstrated that the use of the human ECD in the mouse version of the fusion protein was possible, because the ECDs of human and mouse homologs share 98% identity.
In this article, we describe the potent and long-lasting immunomodulatory activity of short-term administration of ILDR2-Fc, which rebalances immune homeostasis and re-establishes Ag-specific immune tolerance via the activation of Tregs, leading to an amelioration of autoimmunity in disease models and promotion of bone marrow (BM) transplant engraftment. Collectively, these findings support the potential of ILDR2-Fc to provide promising therapeutic approaches for the treatment of autoimmune diseases.
Materials and Methods
Mice
Female SJL/J (Harlan Labs, Indianapolis, IN), NOD, DO11.10, BALB/c, CD45.1+ C57BL/6 (B6.SJL-Ptprca Pepcb/BoyJ), and C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) mice were housed under specific pathogen–free conditions in the Northwestern University Center for Comparative Medicine.
Production of ILDR2-mFc and ILDR2-hFc
ILDR2-mFc is composed of the full human ECD fused to mouse IgG2a Fc via a TEV linker. Various batches of this protein were produced in HEK293 cells (Compugen) or in CHO-S cells (Catalent Pharma Solutions, Middleton, WI). ILDR2-hFc is composed of the full human ECD fused to human IgG1 Fc carrying a C220S mutation at the hinge domain. Various batches of this protein were produced in CHO-S cells (Catalent Pharma Solutions and Cobra Biologics, Södertälje, Sweden). All proteins were produced using fed-batch technology and purified using a Protein A column.
Active and adoptive relapsing-remitting experimental autoimmune encephalomyelitis
Active relapsing-remitting experimental autoimmune encephalomyelitis (R-EAE) was induced in 6–7-wk-old SJL/J mice by s.c. immunization with 50 μg of proteolipid protein (PLP)139–151/CFA emulsion divided among three sites on the back and flanks on day 0. Mice were treated from the onset of disease remission (i.e., after the peak of the acute phase) with ILDR2-mFc or isotype-matched control (mouse IgG2a [mIgG2a]; clone C1.18.4; Bio X Cell, West Lebanon, NH) via i.p. injection at the indicated doses, three times per week for 2 wk. To neutralize IL-10 or TGF-β, anti–IL-10 (Rat IgG1; clone JESS-2A5; Bio X Cell) or anti–TGF-β (mouse IgG1, clone 1D11.16.8; Bio X Cell) blocking Ab or the respective isotype control (rat IgG1, clone HRPN or mouse IgG1 [MOPC-21]; Bio X Cell) was injected i.p. (100 μg per injection) immediately after ILDR2 or mIgG2a treatment. For Treg inactivation, two i.p. injections (500 μg per injection) of anti-CD25 Ab (clone 7D4; Bio X Cell) were given 2 wk after the last treatment with ILDR2-Fc or mIgG2a. Rat IgM (clone eBRM; eBioscience) was used as control Ig.
For induction of adoptive R-EAE, SJL/J mice were primed with PLP139–151/CFA or PLP178–191, as described above. Inguinal lymph nodes were collected on day 8 postpriming and the total lymph node cells were reactivated in culture in the presence of the priming PLP peptide (20 μg/ml) for 3 d at a density of 8 × 106 cells per milliliter in HL-1 medium. At the end of culture, cells were collected, and 3–5 × 106 blast cells were transferred into naive recipient SJL/J mice. Where indicated, cells were treated with ILDR2-Fc or control Ig during culture reactivation. Mice were followed for disease activity and scored in a blinded manner as follows: 0, no abnormality; 1, limp tail; 2, limp tail and hind limb weakness; 3, hind limb paralysis; 4, hind limb paralysis and forelimb weakness; and 5, moribund.
Delayed-type hypersensitivity assay
On day 45 postdisease induction, mice were anesthetized, and delayed-type hypersensitivity (DTH) responses to inducing and spread myelin epitopes were tested via injection of 10 μg of PLP178–191 in one ear and myelin basic protein (MBP)84–104 into the opposite ear. The level of ear swelling was assayed at 24 h postchallenge using a dial thickness gauge.
Ex vivo recall responses
On the indicated day, spleens were collected, and the total cells were activated ex vivo at 0.5 × 106 cells per well with OVA323–339, PLP139–151, PLP178–191, MBP84–104 (20 μg/ml), or anti-CD3 (1 μg/ml). Two sets of cultures were set up side-by-side. One set was pulsed with 1 μCi of tritiated thymidine at 24 h and harvested at 72 h to determine cell proliferation. In the second set of cultures, supernatants were collected at 72 h, and peptide-specific cytokine production was determined by LiquiChip.
Treg flow cytometry (for anti–TGF-β study)
Spleens were collected at the end of the study, and splenocytes were subjected to FACS analysis for evaluation of CD4+CD25+Foxp3+ Tregs as follows. Cells were washed three times in 1× PBS and resuspended with 100 μl of LIVE/DEAD Fixable Aqua Dead Cell Stain Kit, for 405 nm excitation (Invitrogen) for 20 min on ice, followed by three washes in 1× PBS + 5% FCS. Cells were then resuspended in 100 μl of mouse Fc Block (anti-CD16/32; clone 93; eBioscience) diluted 1:100 in 1× PBS + 5% FCS and incubated at 4°C for 20 min. The cells were washed three times in 1× PBS + 5% FCS and then stained for 30 min in surface staining mixture containing the following Abs in a final volume of 100 μl: anti-CD45 allophycocyanin-Cy7 (clone 30-F1; eBioscience), anti-CD3 PE-Cy7 (clone 145-2C11; eBioscience), anti-CD4 Pacific Blue (eFluor 450) (clone GK1.5; eBioscience), anti-CD25 FITC (Alexa Fluor 488) (clone 7D4; eBioscience), and fluorescence minus one control. Then cells were washed three times in 1× PBS, resuspended in 200 μl of freshly made Fix/Perm solution (eBioscience), and incubated overnight (or 30 min) at 4°C. Following three washes in 1× Permeabilization Buffer, the cells were resuspended in the intracellular staining mixture of anti-Foxp3 PerCPCy5.5 (clone FJK-16s; eBioscience) and in a final volume of 100 μl of Permeabilization Buffer for 30 min at 4°C. Then cells were washed three times with Permeabilization Buffer and two times with 1× PBS + 5% FCS, resuspended in 400 μl of 1× PBS + 5% FCS, and analyzed by flow cytometry using the following gating scheme: singlets (forward scatter [FSC-A] versus FSC-H) → cells (side scatter-A versus FSC-A) → live (cell viability stain–negative cells) → CD45hi → CD3/CD4+ → CD25/Foxp3+.
Type 1 diabetes model
Six-week-old NOD mice were purchased from The Jackson Laboratory. At 10 wk of age, mice were treated with ILDR2-mFc or isotype control via i.p. injections three times per week for 2 wk. Blood glucose levels were monitored weekly from 8 wk of age until the mice reached 26 or 30 wk of age, as detailed below. Mice were considered diabetic upon having two consecutive blood glucose readings > 250 mg/dl. Diabetic mice were sacrificed on the day of the second high glucose reading. Blood was obtained from the tail vein, and the blood glucose level was measured using a OneTouch UltraSmart Blood Glucose Monitoring System (OneTouch; Johnson & Johnson, New Brunswick, NJ) in a blinded manner.
Histocompatibility Y Ag BM transplantation model
Female C57BL/6 recipient mice (CD45.2+) were sublethally irradiated with 200 rad/cGy 24 h before transplantation of 5 × 106 BM cells from female C57BL/6 (CD45.1+) or male C57BL/6 (CD45.1+) mice. Mice were treated i.p. with either control Ig or ILDR2-mIg (300 μg per dose) three times a week starting 1 wk prior to transplantation and continuing for 4 wk following transplantation. Anti-CD40L (clone MR-1; Bio X Cell) was used as a positive control.
Beginning at 2 wk following BM transplantation, blood samples were collected from the tail of each mouse once a week for 7 wk and analyzed for CD45.1+/CD45.2+ chimerism, as follows. Blood (two or three drops) was collected into 200 μl of PBS + EDTA (50 mM), samples were spun down, and RBCs were lysed with ammonium chloride. The cells were washed three times with PBS, stained with the viability dye Aqua Dead Cell Stain (Invitrogen), blocked with Fc Receptor block (anti-mouse CD16/32; eBioscience), and stained using the following Abs: Viability Dye (LIVE/DEAD Fixable Aqua Dead Cell Stain Kit; Invitrogen), anti-CD45.2 FITC (clone 104; eBioscience), anti-CD45.1 allophycocyanin-Cy7 (clone A20; eBioscience), anti-CD3 PerCP (clone 145-2C11; BD Biosciences), anti-CD4 eFluor 450 (clone GK1.5; eBioscience), anti-Foxp3 PE-Cy7 (clone FJK-16s; eBioscience), anti-CD25 allophycocyanin (clone 7D4; eBioscience), and anti-CD44 PE (clone IM7; eBioscience). Chimerism was determined by flow cytometric analysis (on total live cells) of the percentage of CD45.1+ versus CD45.2+ cells present in the blood of female recipient mice. The blood samples were also stained to determine the percentages of CD4+ effector T cells (Teffs) and CD4+ Tregs present within the blood using the cellular markers CD44, CD25, and Foxp3.
Th cell differentiation in mouse and human cells
Naive CD4+ T cells were isolated from 10 DO11.10 mice (OVA323–339-specific TCR-transgenic) via autoMACS sorting (CD4− sort plus and CD25+ isolation, followed by CD62L+ sort) and activated with OVA323–339 peptide (20 μg/ml) and soluble control Ig or ILDR2-mFc in the presence of irradiated APCs in a 1:1 ratio. To promote differentiation, the following cytokine and Ab mixtures were added to the cultures: Th1 cells: 128 U IL-2 (National Cancer Institute), 10 ng/ml IL-12, and 10 μg/ml anti–IL-4; Th2 cells: 128 U IL-2, 10 ng/ml IL-4, 10 μg/ml IL-12, and 10 μg/ml anti–IFN-γ; and Th17 cells: 10 ng/ml TGF-β, 50 ng/ml IL-6, 4 ng/ml IL-23, 10 μg/ml anti–IL-4, and 10 μg/ml anti-IFN-γ (all from eBioscience). Supernatants were harvested at 72 h and analyzed for cytokine production using a LiquiChip (8-plex kit; Millipore).
Naive human CD4+ T cells were isolated from total PBMCs of four healthy human donors using a CD4+ T Cell Isolation Kit II, human (Miltenyi Biotec) and cocultured with autologous irradiated PBMCs at a 1:1 ratio (2.5 × 105 T cells plus 2.5 × 105 irradiated PBMCs per well). T cells were activated with anti-CD3 (0.5 μg/ml) and anti-CD28 (0.5 μg/ml) in the presence of the following Th0-, Th1-, Th2-, Th17-, or inducible Treg (iTreg)-promoting conditions: Th1 cells: 128 U IL-2 (National Cancer Institute), 10 ng/ml anti–IL-12, and 10 μg/ml anti–IL-4; Th2: 128 U IL-2, 10 ng/ml IL-4, 10 μg/ml IL-12, and 10 μg/ml anti–IFN-γ; and Th17 cells: 10 ng/ml TGF-β, 50 ng/ml IL-6, 4 ng/ml IL-23, 10 μg/ml anti–IL-4, and 10 μg/ml anti–IFN-γ (all from eBioscience). ILDR2-hFc or control Ig (human IgG1 [hIgG1] = SYNAGIS) was added at 10 μg/ml. Control Ig was used to adjust the amount of tested protein in each well to a total of 10 μg/ml. Supernatants for cytokine-secretion wells were collected at 96 h. Each sample was tested in duplicates.
In vitro iTreg induction
Untouched CD4+CD25− (obtained by negative selection) T cells were isolated from pooled spleens of BALB/C mice using a T cell isolation kit (Miltenyi Biotec), according to the manufacturer’s instructions. Cells were seeded at 1 × 105 per well onto plates (Sigma) that were precoated with 2 μg/ml anti-mouse CD3 mAb (clone 145-2C11; BD Biosciences), together with IDLR2-mFc or control Ig, as indicated, in complete RPMI 1640 medium with 10% FBS in the presence of soluble anti-CD28 (1 mg/ml; clone CD28.2; eBioscience), recombinant mouse TGF-β (0.1–30 ng/ml; R&D Systems), and recombinant human IL-2 (5 ng/ml; R&D Systems). On day 4 poststimulation, CD4+CD25+Foxp3+ cells (iTregs) were analyzed by flow cytometry using a Mouse Regulatory T Cell Staining Kit (eBioscience), according to the manufacturer’s instructions.
Multiple sclerosis patient PBMC purification and activation
Blood was obtained from relapsing-remitting multiple sclerosis (RR-MS) patients during relapse prior to treatment (BioOptions, Brea, California). The patients were at various times postdiagnosis and were receiving different treatments. However, all RR-MS patient samples were collected from patients who consented to donation during the onset of a disease relapse/exacerbation. Total PBMCs were isolated and cultured in vitro with X-VIVO 15 medium only (“no stimulation”), anti-CD3 (clone OKT3; eBioscience), MBP84–99 peptide, or tetanus toxoid peptide (TT830–843) (Peptides International, Louisville, KY) and purified by HPLC (purity of 96–99%). ILDR2-hFc (0.1–10 μg/ml) or control Ig (10 μg/ml; SYNAGIS) was added at the time of culture initiation. After 5 d in culture, supernatants were collected for cytokine analysis using a LiquiChip (8-plex kit; Millipore), and proliferative responses were measured via [3H] TdR incorporation.
Statistical analyses
Mean disease scores in R-EAE studies were analyzed from the day treatment began. One-way ANOVA with repeated measures was used, followed by a Bonferroni posttest of selected pairs. Because “repeated-measure” analysis cannot process incomplete data, mice that died during the study had to be excluded from the statistical analysis. Such cases are indicated in the figure legend. DTH, recall responses, and cell count data were analyzed using two-way ANOVA, followed by the Bonferroni posttest. Data from the NOD model of type 1 diabetes (T1D) was analyzed by one-way ANOVA and a Tukey multiple-comparison posttest. Transplant engraftment in the BM transplant model was analyzed by one-way ANOVA, followed by the Dunnett posttest. Differences between each treatment group compared with the control Ig–treated group were determined. Single comparisons between two means were carried out using a t test.
Results
Short-term treatment with ILDR2-mFc leads to long-term efficacy in the R-EAE model of multiple sclerosis and the NOD model of T1D
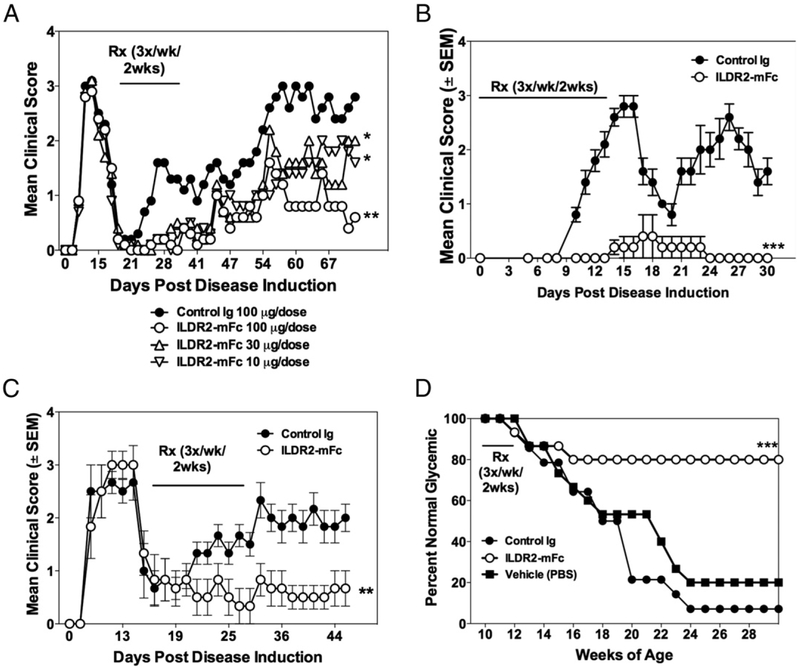
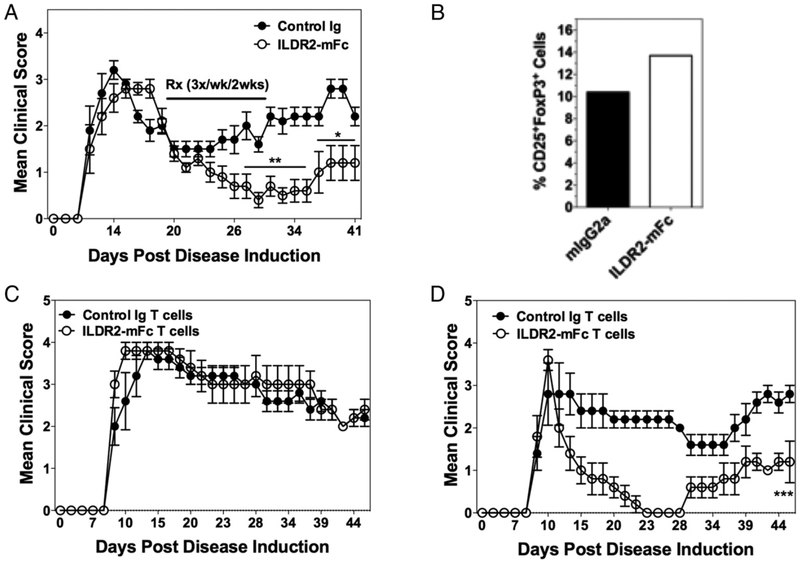
The therapeutic activity of ILDR2-mFc was tested in the PLP139–151/CFA-induced R-EAE model of multiple sclerosis (MS) in SJL/J mice. In this model, ILDR2-mFc or control Ig was administered in a therapeutic mode, starting at the onset of disease remission, three times a week for 2 wk. The results show that a short period of treatment with ILDR2-mFc resulted in a durable and dose-dependent decrease in disease severity and protected the mice from disease progression, as demonstrated by the reduction in relapse frequency at all doses tested (10, 30, and 100 μg) (Fig. 1A). The decrease in disease severity is also reflected in a decrease in the disease relapse frequency (Supplemental Fig. 1A) and the mean cumulative disease score (Supplemental Fig. 1B). This beneficial effect lasted until study termination: 47 d after cessation of treatment. To further determine the ability of ILDR2-mFc to modulate inflammatory CD4+ T cell–mediated autoimmunity, we next assessed the ability of ILDR2-mFc treatment to decrease PLP139–151-induced adoptive R-EAE in SJL/J mice. The data show that ILDR2-mFc treatment significantly decreases the level of disease severity in mice treated at the time of PLP139–151 blast cell transfer (Fig. 1B) or at disease remission (Fig. 1C).
FIGURE 1.
Short-term treatment with ILDR2-mFc induces long-term beneficial effect in active and adoptive models of R-EAE and prevents development of T1D in NOD mice. (A) R-EAE was induced in SJL/J mice using PLP139–151 in CFA. At the onset of remission (day 19), mice were treated i.p. with ILDR2-mFc (10, 30, or 100 μg per dose) or control Ig (mIgG2a; 100 μg per dose). Treatment was given three times a week for 2 wk. Mean clinical scores of 10 mice per group are presented. **p < 0.01 versus control Ig, *p < 0.05 versus ILDR2-mFc (30 μg). (B) Adoptive-transfer EAE was induced by i.v. injection of 5 × 106 PLP139–151 blast cells into recipient SJL/J mice. Starting on the day of cell transfer, mice were treated with ILDR2-mFc or control Ig (mIgG2a) at 100 mg per dose, three times a week for 2 wk. Mean clinical scores of 15 mice per group are presented. ***p < 0.0001 versus control Ig. (C) Adoptive-transfer EAE was induced by i.v. injection of 3 × 106 PLP139–151 blast cells into recipient SJL/J mice. At the onset of disease remission (day 17), mice were treated with ILDR2-mFc or control Ig (mIgG2a) at 100 mg per dose each, three times a week for 2 wk. Mean clinical scores of 10 mice per group are presented. **p < 0.01 versus control Ig. (D) NOD mice were treated with ILDR2-mFc or control Ig (mIgG2a) at 100 μg per dose, three times a week for 2 wk, starting from 10 wk of age. Blood glucose levels were monitored weekly from 8 to 30 wk of age. Presented are the percentage normal glycemic mice (n = 14 or 15 mice per group). One representative experiment of two or three independent experiments is presented for each experimental set. ***p < 0.001 versus control Ig.
The efficacy of ILDR2-mFc was also tested in a model of T1D in NOD mice, which spontaneously develop T1D disease with age. NOD mice were treated three times a week for 2 wk, starting from 10 wk of age, a time point at which blood glucose levels are normal but autoreactive pancreatic disease leading to peri-insulitis due to β-islet cell loss is already ongoing (18, 19). Similar to the effect observed in the R-EAE models, the short period of treatment with ILDR2-mFc resulted in a significant decrease in disease incidence and enabled durable protection from development of T1D in 80% of the mice that lasted until study termination when mice reached 30 wk of age (i.e., 18 wk after cessation of treatment) (Fig. 1D). In contrast, 80–90% of the mice treated with vehicle alone or control Ig developed T1D by 24 wk of age. Therefore, these findings show that short-term treatment with ILDR2-mFc, in mice with established autoimmune disease, results in a dramatic and prolonged decrease in disease incidence and severity.
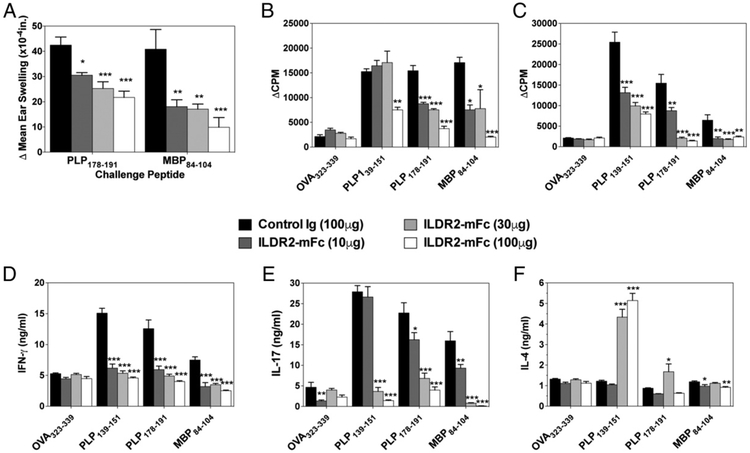
ILDR2-mFc inhibits epitope spreading and restores immune homeostasis in PLP139–151/CFA-induced R-EAE
To explore the long-term efficacy and the mechanisms underlying the beneficial effects of ILDR2-mFc, we next determined recall responses to the disease-inducing and to spread epitopes in PLP139–151/CFA-induced R-EAE in SJL/J mice. Epitope spreading is a phenomenon that underlies the relapsing nature of the disease in the R-EAE model, as well as in RR-MS, which results from activation of new autoreactive T cell clones as a result of chronic damage to the CNS and exposure to new self-epitopes as disease progresses (20-23). Further to the amelioration of disease progression in the active R-EAE model shown in Fig. 1A, the durable effect of ILDR2-mFc treatment was manifested by a dose-dependent inhibition of epitope spreading, as demonstrated by reduced DTH responses (ear swelling) to rechallenge of the mice with the dominant spread epitope, PLP178–191, as well as to the secondary spread epitope, MBP84–104, at the time of study termination (day 76) (Fig. 2A). This dose-dependent inhibition of epitope spreading was also demonstrated by ex vivo recall responses of splenocytes harvested from the treated mice on day 76 (Fig. 2B) or day 45 (Fig. 2C), showing inhibition of cell proliferation in response to disease-inducing (PLP139–151) and spread epitopes. In addition, analysis of cytokine release in these reactivation cultures revealed that ILDR2-mFc treatment leads to an immunomodulatory shift in vivo, which is manifested by inhibiting secretion of Th1 and Th17 cell cytokines (IFN-γ and IL-17, respectively), while increasing secretion of the Th2 cytokine (IL-4) (Fig. 2D-F). Similar findings were also observed using the adoptive-transfer model of R-EAE, whereby ex vivo cultures of total splenocytes showed that ILDR2-mFc treatment inhibited the activation of spread epitope–specific CD4+ T cells (i.e., CD4+ T cells specific for PLP178–191 and MBP84–104) (Supplemental Fig. 2). Furthermore, treatment with ILDR2-mFc skewed the PLP139–151-specific CD4+ T cell response toward the more tolerogenic IL-4/IL-10 phenotype while inhibiting secretion of the proinflammatory cytokines IFN-γ, IL-17, and GM-CSF (Supplemental Fig. 2). OVA323–339 peptide was included in these studies as an irrelevant Ag; as expected, it did not affect cell activation in any of the cultures.
FIGURE 2.
ILDR2-mFc treatment inhibits myelin epitope-specific epitope spreading in the R-EAE model. (A) On day 76 postdisease induction, five mice from each treatment group were analyzed for recall responses to spread epitopes, via injection of 10 μg of PLP178–191 in one ear and MBP84–104 into the opposite ear. The level of ear swelling was assayed 24 h postchallenge. The data are presented as the mean net ear swelling. (B) Recall responses were also carried on splenocytes. On day 76 postdisease induction, total splenocytes were collected from five representative mice from each treatment group and activated in the presence of OVA323–339, PLP139–151, PLP178–191, or MBP84–104 (20 μg/ml). Cells were pulsed with 1 mCi of tritiated thymidine at 24 h and harvested 72 h postculture set up. Cell proliferation in recall responses was also carried out using splenocytes from day-45 mice (C) or IFN-γ, IL-17, and IL-4 secretion was analyzed by LiquiChip following 72 h of culture (D–F). One representative experiment of two or three independent experiments is presented for each experimental set. *p < 0.05, **p < 0.01, ***p < 0.001 versus control Ig.
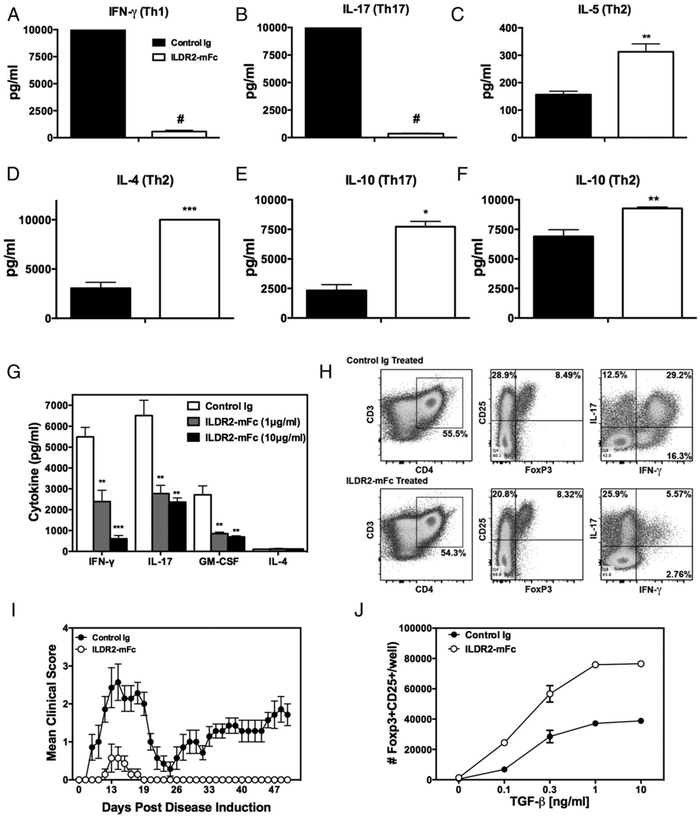
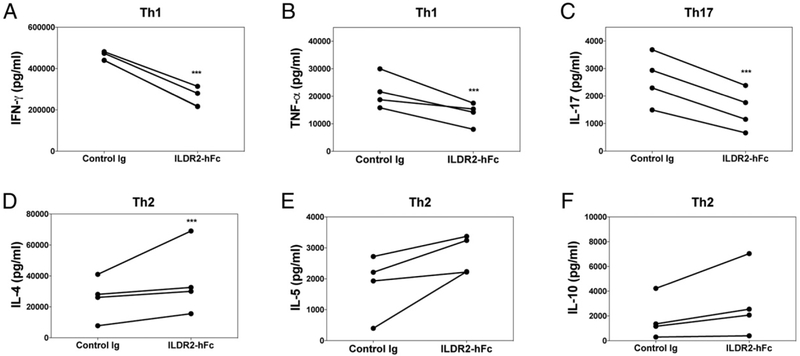
ILDR2-mFc regulates immune homeostasis by inhibition of Th1 and Th17 differentiation and upregulation of Th2 and Treg induction in vitro
The immunoregulatory effect observed in splenocyte recall responses was further investigated by testing the effect of ILDR2-mFc during in vitro differentiation of naive CD4+ T cells from DO11.10 mice toward Th1, Th17, and Th2 cell phenotypes upon antigenic stimulation with OVA323–339 in the presence of irradiated APCs. Addition of ILDR2-mFc to these cultures resulted in inhibition of IFN-γ and IL-17 secretion under Th1- and Th17-driving conditions (Fig. 3A, 3B), respectively, whereas it induced increased levels of the Th2 cytokines, IL-5 and IL-4, under Th2-driving conditions (Fig. 3C, 3D). In addition, secretion of the anti-inflammatory and immunoregulatory cytokine IL-10 was upregulated by ILDR2-mFc under Th2- and Th17-driving conditions (Fig. 3E, 3F).
FIGURE 3.
ILDR2-mFc downregulates Th1 and Th17 differentiation and enhances Th2 and Treg differentiation in vitro. Naive CD4+ T cells isolated from DO11.10 mice were activated with OVA323–339 peptide (20 μg/ml) in the presence of irradiated APCs under Th1-, Th17-, or Th2-driving conditions, as described in Materials and Methods. Soluble ILDR2-mFc or control Ig (mIgG2a) (5 mg/ml) was added to these cultures. (A–F) Supernatants were collected after 72 h and analyzed for cytokine production via LiquiChip. Lymph node cells from PLP139–151/CFA primed mice were reactivated ex vivo with PLP139–151 in the presence of ILDR2-mFc or control Ig. The level of cytokine secretion (G) was assessed in triplicate, and the phenotype of the resultant cells was assessed via flow cytometry (H). These ex vivo-reactivated blasts were transferred to naive SJL/J mice and evaluated for transfer R-EAE induction (I). (J) Freshly isolated CD4+CD25− T cells were activated for 4 d with plate-bound anti-CD3 (2 μg/ml) and coimmobilized with 10 μg/ml ILDR2-mFc or control Ig (mIgG2a), in the presence of soluble anti-CD28 (1 μg/ml), with IL-2 (5 ng/ml) over the indicated range of TGF-β concentrations. The number of CD25+Foxp3+ cells was determined via FACS. Data represent mean ± SD of duplicate wells. One representative experiment of two or three independent experiments is presented for each experimental set. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001 ILDR2-mFc versus control Ig.
Inhibition of proinflammatory cytokine secretion was also observed in ex vivo reactivation cultures of Teffs. In this assay,sensitized blast cells were generated by ex vivo PLP139–151 reactivation of lymph node cells harvested on day 8 from PLP139–151/CFA-primed mice. Addition of ILDR2-mFc to the reactivation cultures resulted in a concentration-dependent decrease in the secretion of IFN-μ, IL-17, and GM-CSF, all of which are highly involved in the pathogenesis of many autoimmune diseases (24-26) (Fig. 3G). No effect was observed on IL-4 secretion in these cultures. Also, addition of ILDR2-mFc to the reactivation culture decreased the percentage of CD4+ T cells that were CD25hiFoxp3− but did not alter the percentage of CD25hiFoxp3+CD4+ T cells, resulting in a net increase in the Treg/Teff ratio. In support of the decrease in the level of secreted IFN-γ, addition of ILDR2-mFc greatly decreased the percentage of IFN-γ+ and IFN-γ+IL-17+CD4+ T cells (Fig. 3H). This is in contrast to the percentage of IL-17+CD4+ T cells that was increased following the addition of ILDR2-mFc. Considering the secreted cytokine data and flow cytometric analysis, addition of ILDR2-mFc decreased the amount of IL-17 secreted per CD4+ T cell. These findings suggest that ILDR2-mFc treatment within the reactivation cultures decreases CD4+ Teff activation, as determined by the decrease in the percentage of CD25hiFoxp3−CD4+ T cells and the decrease in IFN-γ+CD4+ T cells, but it does not alter the percentage of CD25hi Foxp3+CD4+ T cells. Furthermore, adoptive transfer of these cells into naive SJL/J mice, following their reactivation ex vivo in the presence of ILDR2-mFc, failed to induce R-EAE, whereas cells reactivated in the presence of control Ig induced relapsing-remitting disease, as expected (Fig. 3I).
In addition, we investigated the effect of ILDR2-mFc on iTreg differentiation in vitro. Naive CD4+CD25− mouse T cells were activated by anti-CD3 coimmobilized to microplates, together with ILDR2-mFc or control Ig, and in the presence of soluble anti-CD28. Treg differentiation was induced by supplementing the culture with IL-2 in the absence or presence of increasing concentrations of TGF-β. ILDR2-mFc enhanced iTreg differentiation, with an additive effect over that of TGF-β across the wide range of concentrations tested (Fig. 3J). Similar results were obtained using CD4+CD25−CD62L+ naive T cells (data not shown).
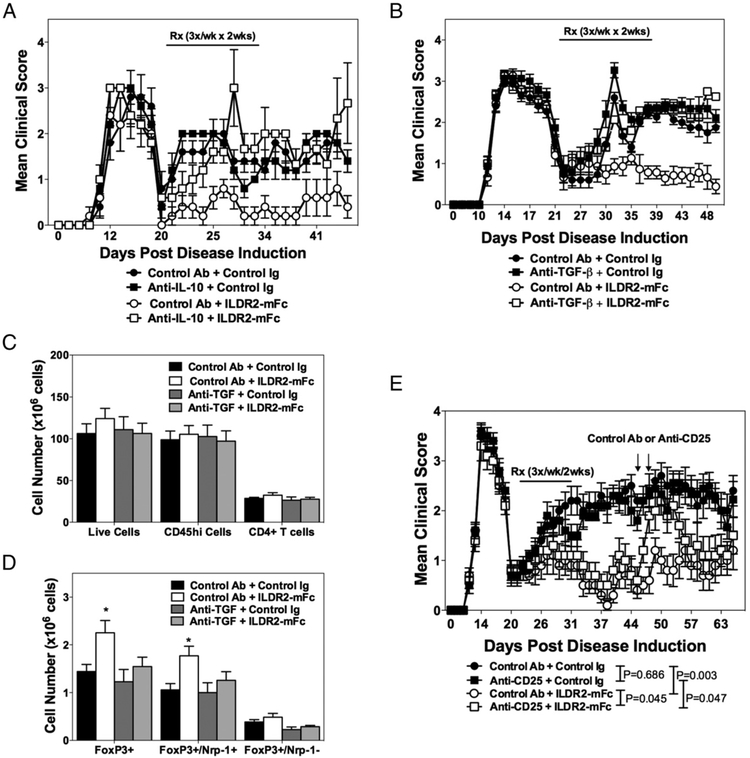
ILDR2-mFc function is mediated by Tregs
The long-term therapeutic effect of ILDR2-mFc, along with its ability to upregulate IL-10, which plays a key role in Treg biology and function, suggested that it may act via induction of Treg-mediated immune tolerance. This hypothesis was supported by abolishment of the therapeutic effect of ILDR2-mFc in mice treated concomitantly with anti–IL-10 blocking Ab (Fig. 4A). Similar abrogation of the therapeutic effect of ILDR2-mFc was also observed upon concomitant blocking of the TGF-β pathway, which is critical for Treg differentiation, using anti–TGF-β blocking Ab (Fig. 4B). In both studies, administration of isotype-matched control Abs did not alter the beneficial effect of ILDR2-mFc or the disease course in the absence of ILDR2-mFc treatment. In correlation with the effect seen following anti–IL-10 and anti–TGF-β blockade on ILDR2-mFc function, a significant increase in the number of splenic Tregs was observed following ILDR2-mFc treatment. Additionally, the ILDR2-mFc–induced increase in the number of Tregs was abolished upon cotreatment with anti–TGF-β, indicating a direct linkage between the induction of Tregs by ILDR2-mFc and its therapeutic benefit (Fig. 4C).
FIGURE 4.
Abolishment of ILDR2-mFc-mediated tolerance by Treg blockade with anti-IL-10 or anti–TGF-β, and transient Treg neutralization with anti-CD25 Abs. SJL/J mice were primed with PLP139–151/CFA. Starting from disease remission, mice were treated with ILDR2-mFc or control Ig (mIgG2a). These treatments were followed by a second injection of anti–IL-10 or control Ab (rat IgG1) (days 20–31; n = 5, experiment completed once) (A) or anti–TGF-β or control Ab (mouse IgG1) (B) (days 20–31; n = 15, experiment completed twice). All treatments were given i.p. at 100 μg per dose, three times a week for 2 wk. In the latter study, splenocytes were analyzed on day 44 for the presence of total live cells, CD45hi immune cells, and total CD4+ T cells (C), and the cell number for different Foxp3+ Treg subsets was determined (D). A few mice died in these studies as a result of disease exacerbation and were not scored further: two mice died on day 28 in the anti–IL-10 + ILDR2-mFc treatment group in the study presented in (A). In the study presented in (B), one mouse died on day 31 in the anti–TGF-β + control Ig group, and one died on day 36 in the control Ab + ILDR2-mFc group; in the anti–TGF-β + ILDR2-mFc treatment group, one mouse died on day 35, and another died on day 36. (E) In another study, transient Treg neutralization was induced 2 wk after the last ILDR2-mFc or control Ig (mIgG2a) administration by two injections of anti-CD25 or control Ab (rat IgM), at 500 μg per dose, on days 46 and 48 (indicated by arrows). Statistical analysis of anti-CD25 effect was carried out starting from the day of anti-CD25 or control Ab administration until day 58, because of the transient effect of the anti-CD25 Ab. One representative experiment of two is presented. *p < 0.05 versus control Ig.
The mechanistic linkage between the durable efficacy of ILDR2-mFc in the R-EAE model and Treg functionality was also evaluated using anti-CD25 Abs, which were previously shown by us (27) and other investigators (28) to transiently inactivate Treg function for a period of 7–14 d. As shown in Fig. 4D, two injections of anti-CD25 Ab given 2 wk after completion of ILDR2-mFc treatment caused a transient disease relapse that was alleviated ~7–9 d after the first anti-CD25 injection, reflecting the expected regain of function by Tregs. Altogether, these studies indicate that ILDR2-mFc induces in vivo differentiation of iTregs whose functionality is crucial for the durable effect observed in the R-EAE model and by which ILDR2-mFc treatment mediates restoration of immune tolerance.
ILDR2-mFc induces donor-specific tolerance and enhances Treg numbers in the histocompatibility Y Ag BM transplantation model
To further evaluate the ability of ILDR2-mFc to induce tolerance, we tested whether ILDR2-mFc treatment allows for minor mismatch BM engraftment. In this model, male BM cells from C57BL/6 (CD45.1+) mice are transplanted into sublethally irradiated female C57BL/6 (CD45.2+) recipient mice and are rejected because of histocompatibility Y Ag (Hya) mismatch, providing a tool for testing the effect of potential drugs on the induction of tolerance toward the grafted cells. Female mice were followed for donor CD45.1+ chimerism within their blood over an 8-wk period following transplantation. Female mice were treated with ILDR2-mFc or control Ig, at 300 μg per dose, three times a week starting 1 wk before BM transplantation for a total of 5 wk. Transplantation of female CD45.1 BM cells into female CD45.2 recipients was included as a positive control group for complete BM engraftment.
As shown in Supplemental Fig. 3A, donor BM cells (male CD45.1+) were detected in the blood of ILDR2-mFc–treated female recipients as early as 2 wk posttransplantation; by week 4, a significant number of CD45.1+ male cells was present in the blood of the recipient females. Furthermore, the presence of these cells was maintained until study termination at 8 wk posttransplantation (4 wk after cessation of treatment). Similar results were observed in the female BM cell transplant group. The decrease in CD45.1+ cell counts observed at week 8 is probably due to the rejection of cells based on the differences in CD45.1 and CD45.2 alleles, as suggested by a similar finding in the group transplanted with female CD45.1 BM cells. In the control Ig treatment group, a substantial number of CD45.1+ cells was not present at any time in the blood of recipient females following BM transplantation, indicating graft rejection. Treg analysis in the spleen of recipient mice revealed a significant increase in the number of CD45.1+CD25+Foxp3+ Tregs in the ILDR2-mFc–treated group, which was >2-fold higher than the levels of Tregs observed in the positive-control group (i.e., female CD45.1+ BM cell transfer) (Supplemental Fig. 3B). In contrast, the percentage of female host Tregs was not significantly different between the treatment groups. These results indicate that ILDR2-mFc is effective in preventing graft rejection and induces a tolerogenic environment toward the Hya minor mismatch Ag, likely by induction of donor-derived Tregs.
ILDR2-mFc–induced tolerance is Ag specific and can be transferred
An important feature of induction of immune tolerance is the ability to prevent disease development by i.v. transfer of Tregs. Thus, we asked whether the tolerogenic activity of ILDR2-mFc is dependent on Tregs, and, as such, whether the transfer of purified CD4+ T cells from ILDR2-mFc–treated mice is able to protect recipient animals from disease development. To answer this question, CD4+ T cells were purified from spleens of mice that were treated with ILDR2-mFc or control Ig from the onset of remission of the acute phase of PLP139–151-induced R-EAE, 10 d after treatment was ended (i.e., on day 42), and transferred to naive mice. The recipient mice received PLP139–151-specific or PLP178–191-specific blast cells to induce transfer R-EAE. As shown in Fig. 5A, ILDR2-mFc treatment from the onset of disease remission resulted in disease inhibition in the donor mice, as previously observed. It is noteworthy that more Tregs were observed within the total splenocytes from ILDR2-mFc–treated mice compared with control Ig–treated mice (Fig. 5B). Interestingly, transfer of CD4+ T cells harvested from ILDR2-mFc–treated mice resulted in protection from R-EAE induction by the PLP178–191 spread epitope–specific blast cells (Fig. 5D), whereas no protection was evident following experimental autoimmune encephalomyelitis (EAE) induction by transfer of blasts specific to the disease-initiating PLP139–151 epitope (Fig. 5C).
FIGURE 5.
ILDR2-mFc induces Ag-specific immune tolerance in the R-EAE model, which can be transferred to naive mice. SJL/J mice were primed with PLP139–151/CFA. Starting from disease remission (day 20), mice (n = 10) were treated i.p. with ILDR2-mFc or control Ig (mIgG2a) at 100 μg per dose, three times a week for 2 wk. (A) Mice were followed for clinical disease. (B) On day 42, 10 d after the final treatment, spleens were harvested and pooled. A sample of pooled splenocytes was analyzed for CD25+Foxp3+ expression via FACS. T cells were sorted from these donor mice, and 5 × 106 splenic CD4+ T cells were transferred to naive recipient SJL/J mice (n = 5) (C and D). Two days later, adoptive-transfer R-EAE was induced in the recipient mice by i.v. injection of 5 × 106 PLP139–151 (C) or PLP178–191 (D) blast cells. Recipients were followed for clinical disease symptoms. One representative experiment of two is presented. *p < 0.05, **p < 0.01, ***p < 0.001 versus control Ig.
These results indicate that ILDR2-mFc promotes induction of a Treg population that can protect naive recipient mice from disease induced by the adoptive transfer of activated myelin peptide–specific blast cells. The specific protection from disease development in response to the spread epitope stems from the dominance of this autoreactive spread epitope during the time of treatment with ILDR2-mFc (21) and indicates that the tolerance induced by ILDR2-mFc is specific to CD4+ T cells that are active during the time of treatment, thus suggesting that ILDR2-mFc induces tolerance in an Ag-specific manner. Importantly, the mechanism of action driving the spread epitope–specific tolerance is Treg mediated and mechanistically different from the inhibitory effects observed in ex vivo–recall splenocyte cultures in response to inducing and spread epitopes, which reflected direct inhibition of Teffs, and a shift from proinflammatory to anti-inflammatory responses. Collectively, these findings point toward a unique mechanism of action of ILDR2-mFc as a regulator of immune homeostasis that downregulates Th1 and Th17 responses and enhances Th2 and IL-10, concomitantly with the re-establishment of Ag-specific immune tolerance through induction of iTregs.
ILDR2-hFc has immunomodulatory effects in human T cells from healthy donors and MS patients
We next tested whether the immunomodulatory activity mediated by the mouse ILDR2 pathway is also valid and functional on human T cells. Total PBMCs were isolated from healthy human donors, and the isolated naive CD4+ T cells were activated in the presence of Th1-, Th17-, and Th17-promoting conditions. For these studies, a fully human ILDR2-Fc fusion protein, ILDR2-hFc, was used. Similar to the effect displayed in vitro and in vivo in mice, ILDR2-hFc displayed immunomodulatory activity in human cells, as demonstrated by inhibited secretion of IFN-γ and TNF-α under Th1-driving conditions and inhibited secretion of IL-17 under Th17-driving conditions, while promoting IL-4, IL-5, and IL-10 under Th2-driving conditions (Fig. 6).
FIGURE 6.
ILDR2-hFc regulates immune human T cell responses by downregulating Th1 and Th17 and upregulating Th2 differentiation. Naive human CD4+ T cells were isolated from total PBMCs from four healthy human donors and cocultured with autologous irradiated PBMCs. T cells were then activated with anti-CD3 (0.5 μg/ml) and soluble anti-CD28 (0.5 μg/ml) in the presence of Th1 (A and B), Th17 (C), or Th2 (D–F) differentiation-promoting conditions, as detailed in Materials and Methods. ILDR2-hFc or control Ig (hIgG1) was added at 10 μg/ml. Supernatants were collected at 96 h, and cytokine levels were evaluated in duplicate. One representative experiment of two is presented. ***p < 0.001 versus control Ig.
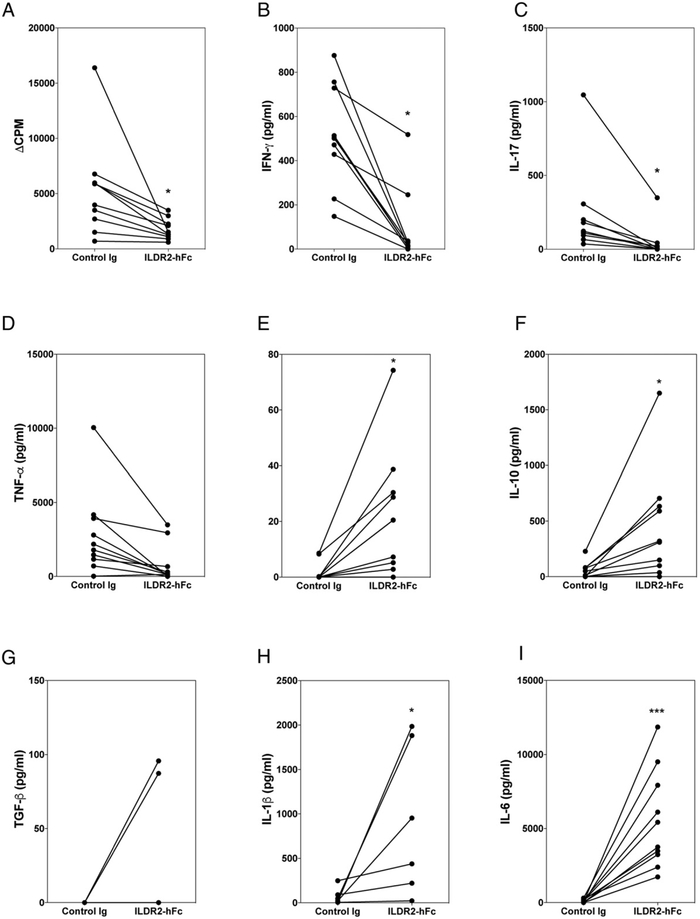
We next evaluated the effect of ILDR2-hFc on peripheral blood cells derived from MS patients isolated during disease relapse. Secretion of a wide range of cytokines was analyzed following stimulation of MS patient PBMCs with MBP84–99 peptide (Fig. 7), which drives MS-specific autoreactive T cell activation in these patients. We also assessed the polyclonal stimulation with anti-CD3, as well as the Ag-specific stimulation with tetanus toxoid peptide (TT830–843) (Supplemental Fig. 4). Addition of ILDR2-hFc to these cultures decreased the level of cellular proliferation and secretion of the proinflammatory cytokines IFN-γ, IL-17, and TNF-α (Fig. 7A-D, Supplemental Fig. 4), whereas secretion of the anti-inflammatory and Treg-related cytokines IL-4, IL-10, and TGF-β was increased (Fig. 7E-G, Supplemental Fig. 4). Of note, IL-12 and IL-23 were also analyzed; however, their levels were within background levels of the detection assay (data not shown). In addition, an unexpected increase in IL-6 and IL-1β was observed under all culture conditions (Fig. 7H, 7I, Supplemental Fig. 4); this was not evident in any of the other in vitro or in vivo experimental set ups in which ILDR2-Fc was tested. Although the presence of TGF-β and IL-6 is expected to promote Th17 differentiation (29), the opposite effect is observed in these cultures, as manifested by the inhibition of IL-17 (Figs. 6C, 7C). Although generally viewed as proinflammatory, IL-6 and IL-1β are also known to have anti-inflammatory properties under certain conditions (30). Further experiments to investigate the source of IL-6 and IL-1β by intracellular staining carried out using PBMCs from healthy individuals indicated that B cells, monocytes, and DCs contribute to the increase in IL-6 and, to a lesser extent, IL-1β (data not shown). These observations suggest that IL-6 and/or IL-1β secretion may reflect a possible off-target effect of ILDR2-hFc via its Fc domain on Fcγ receptor–bearing cells in PBMC cultures. Therefore, the functional significance of the observed increase in IL-6 and IL-1β requires further investigation.
FIGURE 7.
Immunomodulatory effect of ILDR2-hFc on MS patient PBMCs. Total PBMCs were isolated from the blood of MS patients (n = 6–10) and cultured in vitro with MBP84–99 peptide (20 μg/ml) in the presence of ILDR2-hFc or control Ig (hIgG1) (10 μg/ml each), which were added at time of culture initiation. After 5 d in culture, proliferative responses were measured via [3H]TdR incorporation (A), and supernatants were collected for cytokine analysis (B–I). There was no detectible TGF-β for four of six MS patient samples. The data are presented as CPM or specific cytokine level for each MS patient sample tested using control Ig (0 μg/ml) versus ILDR2-hFc (10 μg/ml). *p < 0.05, ***p < 0.001.
Overall, these results demonstrate that ILDR2-hFc modulates the autoreactive T cell responses of MS patients. The outcome of this study suggests the potential therapeutic use of ILDR2-hFc to attenuate autoreactive T cell activation and inflammatory responses and to promote anti-inflammatory and regulatory responses expected to be beneficial to patients with MS and other autoimmune diseases.
Discussion
Tolerance-inducing therapies are viewed as the holy grail for treatment of autoimmune diseases, because they are expected to provide durable effect through specific elimination of the pathogenic immune response and/or activation of Ag-specific regulation, leaving the beneficial function intact. The data presented in this article provide substantial evidence supporting re-establishment of immune tolerance by ILDR2-Fc treatment. First, short-term treatment with ILDR2-mFc provides a significant long-lasting beneficial effect on clinical progression of autoimmune disease in the R-EAE and T1D models. Furthermore, ILDR2-mFc promotes transplant engraftment in a model of Hya minor mismatch BM transplantation that is largely dependent on a Hya-specific CD4+ T cell response (31). These observations point to potential restoration of the immune tolerance breach in autoimmunity and induction of tolerance in transplantation.
The mechanism of action of ILDR2-Fc lies in its ability to favor a proinflammatory to anti-inflammatory switch of the immune response. By downregulating the proinflammatory Th1 and Th17 responses, while upregulating the Th2 response and IL-10 secretion, ILDR2-Fc has the potential to restore immune homeostasis. These effects were evident on immune responses to the disease-inducing epitope, as well as on spread epitopes, which become major targets of the immune response during R-EAE progression (32) as a consequence of ongoing chronic tissue destruction. Our data show that ILDR2-mFc treatment of PLP139–151-sensitized lymph node cells in vitro decreased the level of CD4+ Teff responses. Ligation of the putative ILDR2 receptor on the surface of an activated CD4+ T cell results in modulation of the CD4+ T cell phenotype, whereas if ILDR2-mFc binds to undifferentiated CD4+ T cells, ILDR2-mFc induces an increase in the number of the cells that differentiate into Foxp3+ Tregs. If ILDR2-mFc binds to CD4+ T cells that are producing IFN-γ or IL-17, the signal induced by ILDR2-mFc will cause a decrease in the activation status of CD4+ T cells, involving decreases in CD25, IFN-γ, or IL-17 expression. Although the data show a decrease in the level of IL-17 secreted by PLP139–151-sensitized lymph node cells reactivated in the presence of ILDR2-mFc, the percentage of IL-17+CD4+ T cells increased. Given that IFN-γ inhibits the production of IL-17 (33), the increased percentage of IL-17 may be a consequence of the decreased levels of secreted IFN-γ. However, although the increase in the percentage of IL-17+CD4+ T cells would appear to run counter to the overall functionality of ILDR2-mFc, addition of ILDR2-mFc decreased the level of IL-17 secreted per cell. Additionally, ILDR2-mFc decreased the percentage of activated CD25hiFoxp3−CD4+ Teffs and at the same time increased the percentage of CD25hiFoxp3+ Tregs. Thus, transfer of these cells into recipient SJL/J mice resulted in significantly decreased clinical disease because of an increased overall Treg/Teff ratio. In contrast, binding of ILDR2-mFc to IL-4-producing CD4+ T cells induces increased levels of secreted IL-4, IL-5, and IL-10. This ILDR2-mFc–induced shift from an inflammatory to a regulatory/anti-inflammatory CD4+ T cell phenotype, concomitant with the induction of Tregs, illustrates the multifactorial immune-modulatory functions of ILDR2.
The modulatory activity of ILDR2-Fc was also confirmed using human cell–based assays, reproducing the Th1/Th17 to Th2 shift observed in vitro and in vivo in mouse systems. In addition, translational assays using PBMCs from RR-MS patients, obtained during disease relapse and prior to immunosuppressive treatment, further indicated a similar immunomodulatory effect of ILDR2-Fc upon stimulation of these cells with different stimuli. These findings support the potential of ILDR2-Fc to provide a promising therapeutic approach for MS and other autoimmune diseases.
The mechanism of action of ILDR2-Fc is clearly different from other costimulatory pathway–directed therapeutics, such as CTLA4-Fc (abatacept), which is the only currently approved therapy based solely on the modulation of costimulation (34). Although CTLA4-Fc blocks costimulatory pathways involved in CD4+ T cell priming and activation via the CD80/86-CD28 pathway, ILDR2-Fc appears to activate a peripheral and inducible inhibitory pathway. In addition, accumulating data indicate that CTLA4-Fc does not induce Tregs; rather, it diminishes them and, in accordance with this, does not induce durable immune tolerance (35-38). In contrast, ILDR2-mFc treatment during R-EAE induced a transferable population of CD4+ Tregs. The most profound evidence comes from the R-EAE study in which transfer of CD4+ T cells from mice treated with ILDR2-mFc during remission from PLP139–151-induced R-EAE afforded protection to naive recipient mice against the development of transfer R-EAE induced by activated PLP178–191 cells. These data show that immune tolerance was induced by ILDR2-mFc treatment and could be transferred by cells within the CD4+ T cell compartment (Fig. 5). Interestingly, transfer of these CD4+ T cells from ILDR2-mFc–treated mice in remission from PLP139–151-induced R-EAE was able to modulate disease severity in transfer EAE mediated by T cells specific for the PLP178–191 spread epitope but not to the disease-initiating PLP139–151 epitope. This indicates that ILDR2-mFc induces Tregs specific for the epitope, driving the immune response at the time of drug delivery. The function of Tregs has been ascribed to cell–cell and cell-secreted–mediated mechanisms (39). Published data also show that Ag-specific iTregs are more suppressive in vivo than natural Tregs (40). The data presented in Fig. 5 appear to support this conclusion. Additionally, the mode of action studies in the R-EAE model presented in this article, using blocking Abs for pathways that are central for Treg differentiation, stability, and function (i.e., TGF-β, CD25, and IL-10), demonstrated direct linkage between the durable efficacy mediated by ILDR2-mFc and the presence and functionality of Tregs. Therefore, ILDR2-mFc has a functional activity on activated CD4+ T cells (i.e., disease-inducing CD4+ Teffs) and the spread epitope–specific CD4+ T cells via enhancing iTreg differentiation in vitro and in vivo.
The role of ILDR2 as a regulator of homeostasis and inducer of immune tolerance has not been reported previously. The first publication relating to ILDR2 described its mouse ortholog, Lisch-like, as a candidate modifier of susceptibility to type 2 diabetes in mice (16). Subsequently, ILDR2 was described as an endoplasmic reticulum membrane protein that participates in cellular lipid synthesis and responses to endoplasmic reticulum stress, and it was proposed to mediate hepatic lipid homeostasis (41, 42). More recently, ILDR2 was identified as a protein component of tTJs and is required for recruitment of tricellulin to tTJs, similarly to its close paralogs LSR (ILDR3) and ILDR1 (15, 43). Based on this function, these related proteins have been designated as angulin family proteins (15). Although speculative, the presence of ILDR2 at tTJs may have a biological function in modulating tissue-infiltrating CD4+ T cells in the absence of epithelial breakdown. Although no immune-related function has been reported previously for ILDR2, or any of the other angulin family members, a recent publication describes strong upregulation of ILDR2 mRNA in human DC2 cells, which represents a subpopulation of polarized dendritic cells capable of promoting immune regulation through induction of Th2 differentiation (44). This report is in direct agreement with our findings showing enhancement of Th2 responses by ILDR2-Fc, and it provides additional correlative support for the function of ILDR2 in favoring a Th1/Th17 to Th2 switch. Although the identity of the counterpart receptor molecule (s) for ILDR2, which mediate the above-described responses in T cells, is not yet known, the ILDR2-mFc functional studies presented in this article suggest that the putative ILDR2 receptor is expressed by activated CD4+ T cells.
In summary, our data indicate that ILDR2-Fc acts as a regulator of immune homeostasis and has the capability to induce Ag-specific tolerance when administered during ongoing inflammatory T cell–mediated immune responses involved in Th1/Th17-mediated autoimmune diseases, as well as in a model of BM transplantation involving a minor histocompatibility Ag mismatch. This is mediated via a unique mechanism of action combining immunomodulatory deviation toward less pathogenic Th phenotypes in combination with Treg induction. Further to the beneficial effect of ILDR2-mFc in the MS and T1D models, additional experiments demonstrated efficacy in collagen-induced arthritis models of rheumatoid arthritis and in a humanized skin model of psoriasis (17). Collectively, these data indicate that ILDR2-Fc might provide a promising and safe approach for treatment of autoimmune diseases.
Supplementary Material
Acknowledgments
We thank members of the Miller laboratory for their valuable input.
This work was supported by a grant from Compugen, Ltd.
Abbreviations used in this article:
- BM
bone marrow
- DTH
delayed-type hypersensitivity
- EAE
experimental autoimmune encephalomyelitis
- ECD
extracellular domain
- FSC
forward scatter
- hIgG1
human IgG1
- Hya
histocompatibility Y Ag
- ILDR2-hFc
ECD of human ILDR2 fused to an Fc portion of human IgG1
- ILDR2-mFc
ECD of human ILDR2 fused to an Fc portion of mouse IgG2a
- iTreg
inducible Treg
- MBP
myelin basic protein
- mIgG2a
mouse IgG2a
- MS
multiple sclerosis
- PLP
proteolipid protein
- R-EAE
relapsing-remitting experimental autoimmune encephalomyelitis
- RR-MS
relapsing-remitting multiple sclerosis
- T1D
type 1 diabetes
- Teff
effector T cell
- Treg
regulatory T cell
- tTj
tricellular tight junction
Footnotes
Disclosures
J.R.P. and S.D.M. are grantees of and consultants for Compugen, Ltd. I.H., I.V., I.B., A.N., E.N., and G.R. are employees of Compugen, Ltd. M.-Y.C. has no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Abbas AK, Lohr J, and Knoechel B. 2007. Balancing autoaggressive and protective T cell responses. J. Autoimmun 28: 59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahoney KM, Rennert PD, and Freeman GJ. 2015. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov 14: 561–584. [DOI] [PubMed] [Google Scholar]
- 3.Podojil JR, and Miller SD. 2013. Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs 27: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, and Vignali DA. 2016. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44: 1034–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitale S, and Moots R. 2008. Abatacept: the first T lymphocyte costimulation modulator, for the treatment of rheumatoid arthritis. Expert Opin. Biol. Ther 8: 115–122. [DOI] [PubMed] [Google Scholar]
- 6.Moreland L, Bate G, and Kirkpatrick P. 2006. Abatacept. Nat. Rev. Drug Discov 5: 185–186. [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, Quezada SA, Chambers CA, Korman AJ, and Allison JP. 2009. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med 206: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Wolchok JD, Chan TA, Mellman I, Palucka K, Banchereau J, Rosenberg SA, and Dane Wittrup K. 2015. Immunotherapy: the path to win the war on cancer? Cell 161: 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buc M 2013. Role of regulatory T cells in pathogenesis and biological therapy of multiple sclerosis. Mediators Inflamm. 2013: 963748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Yamaguchi T, Nomura T, and Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 11.Arellano B, Graber DJ, and Sentman CL. 2016. Regulatory T cell-based therapies for autoimmunity. Discov. Med 22: 73–80. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, and Krueger JG. 2017. Highly effective new treatments for psoriasis target the IL-23/type 17 T cell autoimmune axis. Annu. Rev. Med 68: 255–269. [DOI] [PubMed] [Google Scholar]
- 13.Luchetti MM, Balloni A, and Gabrielli A. 2016. Biologic therapy in inflammatory and immunomediated arthritis: safety profile. Curr. Drug Saf 11: 22–34. [DOI] [PubMed] [Google Scholar]
- 14.Bluestone JA, and Bour-Jordan H. 2012. Current and future immunomodulation strategies to restore tolerance in autoimmune diseases. Cold Spring Harb. Perspect. Biol 4: a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, and Furuse M. 2013. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. [Published erratum appears in 2013 J. Cell Sci. 126: 3797.] J. Cell Sci 126: 966–977. [DOI] [PubMed] [Google Scholar]
- 16.Dokmanovic-Chouinard M, Chung WK, Chevre JC, Watson E, Yonan J, Wiegand B, Bromberg Y, Wakae N, Wright CV, Overton J, et al. 2008. Positional cloning of “Lisch-Like”, a candidate modifier of susceptibility to type 2 diabetes in mice. PLoS Genet. 4: e1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht I, Toporik A, Podojil JR, Vaknin I, Cojocaru G, Oren A, Aizman E, Liang SC, Leung L, Dicken Y, et al. 2018. ILDR2 is a novel B7-like protein that negatively regulates T cell responses. J. Immunol 200: 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Charlton B, Shimada A, Dal Canto R, and Fathman CG. 1996. Monoclonal T cells identified in early NOD islet infiltrates. Immunity 4: 189–194. [DOI] [PubMed] [Google Scholar]
- 19.Jimeno R, Gomariz RP, Gutiérrez-Cañas I, Martínez C, Juarranz Y, and Leceta J. 2010. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol. Cell Biol 88: 734–745. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann PV, Forsthuber T, Miller A, and Sercarz EE. 1992. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358: 155–157. [DOI] [PubMed] [Google Scholar]
- 21.McRae BL, Vanderlugt CL, Dal Canto MC, and Miller SD. 1995. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med 182: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintana FJ, Patel B, Yeste A, Nyirenda M, Kenison J, Rahbari R, Fetco D, Hussain M, O’Mahony J, Magalhaes S, et al. ; Canadian Pediatric Demyelinating Disease Network. 2014. Epitope spreading as an early pathogenic event in pediatric multiple sclerosis. Neurology 83: 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson AP, Harp CT, Noronha A, and Miller SD. 2014. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol 122: 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpus WJ, Kennedy KJ, Smith WS, and Miller SD. 1996. Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139-151 peptide. J. Neurosci. Res 45: 410–423. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, and Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 26.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, and Becher B. 2015. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43: 502–514. [DOI] [PubMed] [Google Scholar]
- 27.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, and Miller SD. 2006. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol 176: 3301–3305. [DOI] [PubMed] [Google Scholar]
- 28.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, and Riley EM. 2007. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J. Immunol 178: 4136–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghilardi N, and Ouyang W. 2007. Targeting the development and effector functions of TH17 cells. Semin. Immunol 19: 383–393. [DOI] [PubMed] [Google Scholar]
- 30.Covarrubias AJ, and Horng T. 2014. IL-6 strikes a balance in metabolic inflammation. Cell Metab. 19: 898–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, and Miller SD. 2010. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation. J. Immunol 185: 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderlugt CL, and Miller SD. 2002. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol 2: 85–95. [DOI] [PubMed] [Google Scholar]
- 33.Damsker JM, Hansen AM, and Caspi RR. 2010. Th1 and Th17 cells: adversaries and collaborators. Ann. N. Y. Acad. Sci 1183: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison C 2005. Abatacept as add-on therapy for rheumatoid arthritis. Issues Emerg. Health Technol 73: 1–4. [PubMed] [Google Scholar]
- 35.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, and Chandraker A. 2012. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am. J. Transplant 12: 846–855. [DOI] [PubMed] [Google Scholar]
- 36.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, and Bluestone JA. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12: 431–440. [DOI] [PubMed] [Google Scholar]
- 37.Pieper J, Herrath J, Raghavan S, Muhammad K, Vollenhoven R, and Malmström V. 2013. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol. 14: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patakas A, Ji RR, Weir W, Connolly SE, Benson RA, Nadler SG, Brewer JM, McInnes IB, and Garside P. 2016. Abatacept inhibition of T cell priming in mice by induction of a unique transcriptional profile that reduces their ability to activate antigen-presenting cells. Arthritis Rheumatol. 68: 627–638. [DOI] [PubMed] [Google Scholar]
- 39.Josefowicz SZ, Lu LF, and Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Podojil JR, Chang J, Luo X, and Miller SD. 2010. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J. Immunol 184: 6629–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen FT, Roitel O, Bonnard L, Notet V, Pratte D, Stenger C, Magueur E, and Bihain BE. 2008. Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem 283: 25650–25659. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe K, Watson E, Cremona ML, Millings EJ, Lefkowitch JH, Fischer SG, LeDuc CA, and Leibel RL. 2013. ILDR2: an endoplasmic reticulum resident molecule mediating hepatic lipid homeostasis. PLoS One 8: e67234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwamoto N, Higashi T, and Furuse M. 2014. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct. Funct 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 44.Gueguen C, Bouley J, Moussu H, Luce S, Duchateau M, Chamot-Rooke J, Pallardy M, Lombardi V, Nony E, Baron-Bodo V, et al. 2016. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J. Allergy Clin. Immunol 137: 545–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.