Abstract
Purpose of Review
Type 1 diabetes (T1D) is an autoimmune disease in which the immune cells selectively destroy the pancreatic beta (β) cells and results in the deficiency of insulin production. The optimal treatment strategy for T1D should be preventing of β-cell destruction in the pancreas. The purpose of this review is to discuss the immunological therapeutic mechanisms that will help to understand the development and control of β-cell destruction. The review also presents a novel method for development of autoantigen (Ag)-specific regulatory T cells (Tregs) for T1D immunotherapy.
Recent Findings
Pancreatic-resident Tregs have the ability to dramatically suppress hyperactive immune cells. Islet cell transplantation is another attractive approach to replace the failed β cells. Due to the limited source of islet cells, research is going on in the use of animal cells and adult stem cells that may be derived from the patient’s own body to produce β cells for transplantation.
Summary
The mechanism behind the pancreatic β-cell destruction is largely unknown. In this review, a novel approach for the generation of tissue-associated Tregs from stem cells is considered. The stem cell-derived tissue-associated Tregs have the ability to home to the damaged pancreas to prevent the destruction. The review also provides new insights on the mechanism on how these suppressive immune cells protect the pancreas from the destruction of autoimmune cells. A novel method to develop functional auto Ag-specific Tregs that are derived from induced pluripotent stem cells (iPSCs), i.e., iPSC-Tregs, is discussed. Adoptive transfer of the iPSC-Tregs can substantially suppress T1D development in a murine model.
Keywords: Pluripotent stem cells, Autoimmune diabetes, Regulatory Tcells, Adoptive transfer, Mice
Introduction
According to the 2015 American Diabetes Association (ADA)’s report, 30.3 million Americans, or 9.4% of the population, had diabetes and there were additional 84 million people with pre-diabetes [1, 2]. Diabetes was the seventh leading cause of death in the United States in 2015. Among individuals with diabetes, approximately 1.25 million children and adults were affected with type 1 diabetes (T1D). T1D is a disease caused by autoimmune destruction of insulin-producing beta (β) cells located in the endocrine pancreas. A number of etiologies have been suggested for the development of T1D. Although genetic predisposition is believed to play a role, T1D is mainly a polygenic disease where genetic factors are controversial [3]. Independently by the etiology, T1D develops because of pathogenic T cell-mediated autoimmune impairment of pancreatic β cells [4, 5]. In fact, T1D is mainly driven by the destruction of insulin-releasing pancreatic β cells, accompanied by cellular invasion by both CD4+ and CD8+ T cells. Lifelong exogenous insulin administration, either using multiple daily injections or by insulin pumps, is currently the only therapeutic option for T1D [6]. While islet or pancreas transplantations are alternative effective approaches to treating T1D, the limited availability of donors, the need of chronic immunosuppression, and the significantly high cost of the procedures are main drawbacks preventing their successful adoption as alternatives to insulin therapy in the majority of individuals with T1D. Consequently, alternative strategies for prevention of the destruction of islet cells by pathogenic T cells assume critical impact, in order to manage the prognosis of the disease. As autoimmune destruction is a continuous process and pathogenic auto-reactive T cells continually destroy the β cell, new approaches should be proposed to prevent the islet cell destruction by suppressing the function of hyperactive pathogenic T cells. Regulatory T cell (Tregs) are known to be suppressive immune cells that have the ability to inhibit the function of over-reactivated T cells and maintain the immune homeostasis. However, the number of Tregs is relatively limited in human that is no sufficient to suppress the function of large numbers of auto-reactive T cells. Consequently, the generation of large numbers of exogenous Tregs and successfully transferring them is essential for such treatment. Therefore, in this review, we precisely describe the in vitro generation of a large number Tregs that can effectively replenish the function of other hyperactive Tregs after adoptive transfer in vivo.
Tregs play a critical role in the maintenance of immune homeostasis, by suppressing the function of hyperactive immune cells. In various animal systems, especially in non-obese murine models, it has been reported that Tregs are highly associated with T1D development. Deficiency of Tregs accelerates the disease prognosis [7•, 8•] underlining the importance of these suppressive immune cells in the pathophysiology of the disease. Tregs suppress hyperactive immunity through several well-established mechanisms, including direct contact of the cell and secretion of suppressive cytokines (e.g., TGF-β and IL-10), as well as regulation of CXCR3 and IL-2 [9–12]. However, in peripheral CD4+ T cell populations, Tregs are only roughly 5–10% in mice and 1–2% in human [13]. Subsequently, in order to suppress hyperactivity of pathogenic immune cells, the development of great numbers of Tregs ex vivo followed by adoptive transfer into the host is needed. Pluripotent stem cells (PSCs) have been demonstrated to induce Treg differentiate that overcomes the limitation of harvesting sufficient numbers of antigen (Ag)-specific Tregs. Consequently, because of the potential ability of PSCs to differentiate into most body’s other cell types, as an alternative approach, PSCs have been used to generate auto Ag-specific Tregs for the treatment of autoimmune diseases [14••]. Nevertheless, the generation of auto Ag-specific PSC-Tregs and the underlying mechanisms have not been optimized and identified. Adoptive transfer of auto Ag-specific PSC-Tregs has the ability to result in Treg accumulation in local inflamed tissues in which Tregs suppress autoimmune responses. This Treg-based on immunotherapy can fundamentally prevent possible general immunosuppression [15–18]. We have demonstrated a development of Ag-specific iPSC-Tregs. In this study, we recently suggested an approach of genetic modification of iPSCs with genes of Ag-specific T cell receptor (TCR) and a transcriptional factor (i.e., FoxP3) following co-culture the gene-transduced iPSCs with the OP9-DL1/DL4 stromal cells. We first revealed that the in vitro-generated PSC-Tregs suppressed autoimmune arthritis in a murine model. In addition, we confirmed that the PSC-Tregs were tissue-specific, functional, and sustainable [19]. Furthermore, we demonstrated that the PSC-Tregs were able to vigorously traffic in the diabetic pancreas and suppress the migration and activity of the pathogenic T cells—the trigger of T1D in a murine model. Of note, we identified an underlying mechanism by which adoptive transfer of the Tregs suppressed T1D development by decreasing the expression of intracellular adhesion molecule-1 (ICAM-1) in the diabetic pancreas and restraining the production of IFN-γ [14••].
Induced PSCs
Induced PSCs (iPSCs) are generated from the somatic cells and have similar characteristics as those of PSCs. The use of human embryonic stem cells (hESCs) is restricted in clinical as well as research settings due to ethical concerns, making it imperative to find effective alternatives. As an alternative of hESCs, iPSCs have been well studied in the recent years, as these cells can be engineered and differentiated to various cell types [20, 21, 22•, 23•]. IPSCs can be created from somatic cells via using transcription factors (e.g., c-Myc, OCT4, Sox2, and KlF4). By using these transcription factors, throughout the past years, mouse and human iPSCs had been generated from somatic cells. By using this approach, T1D-specific iPSCs can be generated from patients with T1D [24, 25]. However, among these transcription factors, c-Myc and KlF4 are oncogenes, indicating the generated iPSCs have a potential to form tumors. To avoid this potential, a new method for generation of human iPSCs was developed, in which only OCT4 and SoX2 were used with a histone deacetylase inhibitor (i.e., valproic acid) [26]. In addition, introducing the transcription factors by using retroviruses or lentiviruses may result in viral integration into the host genome, which has a potential for the risk of tumorigenicity. To avoid this potential, a novel transfection protocol has been used, resulting in plasmid expression in iPSCs deprived of viral integration [27]. Even with the presence of these new developments without the use of oncogenes and viral vectors, the questions of similarity of iPSCs and hESCs still puzzle researchers. In addition, there has been substantial progress made towards elucidating important signaling pathways and regulators controlling cell fate. Such efforts have led to the generation of glucose-responsive and insulin-producing pancreatic progenitor cells, which had been transplanted in mice [28]. Other cell recourses, such as umbilical cord blood, have been also used to generate iPSCs, which have a similar reprogramming efficacy as those of keratinocytes and fibroblasts [29]. There is increasing interest in the development of insulin-producing β cells after the successful generation of iPSCs from various organs. However, the generation of insulin-producing iPSCs still remains a major challenge. In addition, during the period of T1D development, pathogenic immune cells continuously destroy insulin-producing β cells. In one study, it had been demonstrated that iPSC-derived islet-like cell clusters produce insulin and that some cells could differentiate and produce small amounts of C-peptide when stimulating with glucose [30]. These cells were highly glucose-responsive raising the possibility of the high rate of programmed cell death through apoptosis. In another study, human iPSCs derived from skin cells from T1D patients differentiated into β cells, which were able to produce insulin, C-peptide, glucagon, and somatostatin [31•]. The above-mentioned iPSCs were generated by transduction of adult fibroblasts with OCT4, SoX2, and KlF4 [32, 33••]. Nevertheless, the immune system will still target the implanted β cells generated from iPSCs that are created from somatic cells of patients with T1D mellitus. Hence, the use of both iPSC-derived immune cells and β cells for the treatments of T1D may eventually prevent the autoimmune destruction and provide optimal insulin-producing β cells.
Immunological Mechanisms in the Development of T1D and Potential Role of Tregs
The number of T1D patients is gradually increasing. Previously, it was thought that genetic predisposition is one of the main reasons for the increasing T1D; however, genetic predisposition is not solely responsible for increasing the tendency of diseases. Recent advances in this field explained the role of self-reactive immune cells for the development of T1D. The exact mechanism for activating the self-reacting autoimmune cells is still controversial. Some studies suggested that there is an interplay between genetic susceptibility and environmental influences, and this interplay is responsible for activating self-reactive immune cells. T1D also develops due to the interaction between β cells and the components of innate and adaptive immune systems. Hyperactive immune cells become pathogenic and start destroying the β cells through multiple pathways. Therefore, many immunomodulatory strategies have already been proposed for the treatment of T1D. Based on immune therapy, several clinical trials have been carried out by using several molecules, like cyclosporin A, anti-CD3 and anti-CD20 monoclonal antibodies, anti-thymocyte globulin, and IL-1. Results from a large clinical trial with cyclosporin A showed increased T1D remission but only for short duration, and the study reported a progressive increase in daily insulin requirement [34, 35]. Clinical trial with anti-CD3 and anti-CD20 monoclonal antibodies reported that C-peptide level can be maintained only for a transient period by using these two antibodies [36]. Furthermore, anti-thymocyte globulin failed to preserve the β-cell function after 2 years [37]. A randomized controlled trial was also carried out to evaluate the clinical utility of known inhibitors of interleukin-1 (IL-1) and Anakinra, which is known as the human IL-1 receptor antagonist. Both results were safe but not effective as single immunomodulatory drugs for recent-onset T1D [38]. Therefore, the repeated failures observed in clinical trials raised an ultimate need for the use of immunotherapy in patients with T1D.
Tregs are known as suppressive T cells, which can modulate the function of other immune cells like cytotoxic T cells and dendritic cells. Tregs were also utilized for conducting preclinical study in the treatment of T1D which have demonstrated prolonged islet survival and function in mice co-transplanted with islets and Tregs without immune suppression [39]. Tregs were also tested in human clinical trial where these cells were used as a biological alternative for chemical immunosuppression and as a novel approach for modulating immune response in T1D patients undergoing islet transplantation [40]. Clinical trial on newly diagnosed T1D children demonstrated safety and tolerability of autologous expanded Tregs. Tregs also showed the partial control of inflammation and preservation of insulin dosage for the duration of 2 years in diabetic patients [41].
As the number and percentage of Tregs are low and limited in the human body, generation of large numbers of Tregs is essential for the treatment of T1D. One study collected Tregs from T1D patients, then expanded ex vivo, and administered back into the patients in a phase 1 clinical trial. The transferred Tregs survived for up to 1 year in a quarter of patients and preserved C peptide response in all cohorts for up to 1 year and in two cohorts after 2 years [42]. Another phase 1 clinical trial has been performed by the same group by using Tregs in combination with commercially available form of IL-2 called aldesleukin. From all of the clinical trials, none of them was specific to the islet cells. If they are not specific to the targeted cells, it is difficult to demonstrate whether this effect comes from the transferred cells or the endogenous Tregs. Hence, it is essential to home/retain the transferred Tregs into the targeted site and exert their effect specific to the destroyed islet. In this review, we reveal a novel strategy for the development of tissue-associated or Ag-specific Tregs that can particularly migrate to the islet and keep the remaining immune system intact.
Applications of Ag-Specific Tregs in Autoimmune Diseases
Various treatment options and their limitations in autoimmune diseases are illustrated in Table 1. Considering all of the treatment options, Ag-specific Tregs-based immunotherapy is more potent and effective. Tregs can effectively suppress other conventional T cells when activated by cognate Ag [43, 44•]. This is commonly evident when cognate Ag presented on same Ag-presenting cells (APCs) is recognized by the Tregs and the conventional T cells, because of APC-mediated Treg suppressive mechanisms [10, 45•]. Tregs exert their suppression by different mechanisms, including linked suppression, a phenomenon where there is no individual engagement of the antigenic determinant of the targets. By utilizing this pathway, autoimmune-affected tissue can be targeted deprived of engagement of the cognate Ag through aiming a related tissue-associated Ag. It is also conceivable to suppress polyclonal T cell response to a whole organ using tissue- or organ-associated Tregs. Other effector T cells can be “educated” by the Tregs through a process called infectious tolerance, in which Treg-mediated tolerance can be mediated by regulation of dendritic cells and succeeding de novo generation of adaptive Tregs from effector T cells. Furthermore, Tregs function appropriately during normal immune responses without delivering over immunosuppression, which is an endogenous part of the immune system. Therefore, Tregs can be used to reinstate optimal tolerance deprived of interrupting normal immune responses. Finally, Tregs not only suppress conventional T cells, including naive, effector, and memory CD4+ or CD8+ T cells, but also suppress numerous additional immune cells, such as B cells [46, 47•], macrophage, and dendritic cells [48, 49•], as well as monocytes [50, 51••]. Because autoimmune disorders are complicated diseases and numerous immune cells are involved in the process of disease development, both innate and adaptive immune systems need to be controlled in order to restore the tolerance. Consequently, Tregs are the most valuable candidate to provoke a steady and long-term Ag-specific suppression deprived of the possibilities related to overall immunosuppression. The actual role of Tregs involved in the development of autoimmune diseases is still not clear, even though there are some contradictory data that various proportions and numbers of Tregs engage. The reason is the deficiency of appropriate surface markers of Tregs. Previously, Tregs were identified by CD25, which is not suitable because many other cells also express CD25 after stimulation. Recently, this issue has been clarified by utilizing surface markers, such as CD127, with the transcription factor of Foxp3, which are definitive markers for Tregs. Nevertheless, although these markers offer a better solution, they are not perfect. Some studies have already shown that adoptive cell transfer with polyclonal Tregs prevented or slowed the progression of T1D [14••, 52, 53], rheumatoid arthritis (RA) [54, 55••], multiple sclerosis (MS) [56, 57•], and systemic lupus erythematosus (SLE) [58, 59•]. Yet, prevention of autoimmune diseases has not been successfully achieved, because a significant immune response has been usually initiated when disease diagnosis is confirmed. In the settings of T1D and MS, it has been well-established that targeting of disease-associated Ags by Ag-specific Tregs is very effective in regressing existing autoimmune responses. Specifically, high doses of polyclonal Tregs are not able to reverse the ongoing autoimmunity, indicating the importance of Ag specificity. In addition, Ag specificity is needed for Tregs to exert active suppression that requires Tregs to traffic/be accumulated in the suitable organ/tissue. The unique TCRs on the surface control the specificity of T cells, including Tregs. Therefore, in order to reduce the development of an autoimmune disorder, a sufficient quantity of Ag-specific Tregs in the polyclonal Tregs is required to transfer. Even in this case, various additional specificities of the polyclonal Tregs would elicit undesirable suppression of normal immune responses, which were presented in different settings of cancers [60, 61]. Additionally, because Tregs from the patients are usually not sufficient to suppress the induction of disease, it is highly unpredictable for the treatment with these Tregs deprived of amending specificity or function. Consequently, various approaches for generating Ag-specific Tregs are developing, including TCR (T cell receptor)- or CAR (chimeric Ag receptor)-based genetic modification of Tregs, which make Tregs more suitable for adoptive cell transfer based on immunotherapy for autoimmune diseases. Recently, immunotherapy in animals based on Ag-specific Tregs for various autoimmune disorders has been successfully revealed. Yet, because of the difference in Tregs between human and animals (especially mice), how to translate these results into the clinic would be a big challenge. Nevertheless, developing robust and reliable approaches to generate a great number of human Ag-specific Tregs is very desirable.
Table 1.
Various treatment options and their limitations in autoimmune diseases
| Treatment option | Limitation |
|---|---|
| Insulin replacement therapy | Patients become susceptible to severe episodes of hypoglycemia, lifelong dependency on exogenous insulin, insulin resistance, mild obesity, and psychiatric condition. |
| Artificial pancreas | High costs associated with equipment procurement and high sensor replacement costs, buildup of scar tissue due to repeated micro-needle insertion, and premature sensor failure. |
| Immune therapies | Customized, patient-specific and special training needed for the use of this technique. |
| Peptide hormone-based therapies | The impact of peptide-based drugs on long-term glycemic control and on secondary complications still remains to be explored. |
| Xeno-transplantation of islets | Reliable source of islets, developing strategy for immune isolation of xeno-islets, and identifying suitable sites for transplantation. |
| Islet transplantation | The occurrence of instant blood-mediated inflammatory reaction (IBMIR) immediately after transplantation, loss of islet numbers and islet mass due to ischemia, apoptosis of islet cells, and detrimental side effects of immunosuppressive agents. |
| Encapsulation strategy | β-cells are protected by a physical barrier so there are risks for β-cell death due to hypoxia, especially during the early stages post-transplantation. Need consistent engraftment and glucose sensing but will still require sustained immunosuppression for long-term survival and function of encapsulated β-cells. |
| Stem cell-based therapies | Ethical considerations of using embryo-derived stem cells which need to be considered. |
Tregs and the Control of Autoimmune Diabetes
T1D is a complex etiology where immune cells directly attack the specific organ, and inflammation is a critical player during the disease prognosis. Inflammation develops due to the hyperactivity of auto-reactive immune cells. To prevent the continuous inflammation induced by overactive immune cells and retain normoglycemia, in numerous patients with T1D, exogenous supply of insulin is needed. As there is continuous destruction of endogenous auto-reactive T cells, treatment strategies are also designed to be continuous and lifelong, resulting in high morbidity. Replacement of β cells is a process where a large number of β cells have to be recruited to maintain the blood glucose level deprived of constant injection of insulin. However, the β-cell transplantation does not result in adequate results. The primary reason for the unfortunate durable effect is the constant immune devastation of the transplanted islet. Additionally, β-cell transplantation has a big problem, i.e., the shortage of donors, when compared with a large number of patients with T1D. Overall, it is now considered that targeting Tregs is an optimal method to end the hyperactivity of effector immune cells to maintain normal homeostasis. However, the mechanisms by which Tregs regulate the autoimmune responses in vivo and suppress the development of T1D remain to be determined. The scheme that we indicate in this review underlies on the results primarily obtained from murine models; however, associated clinical implications have not been confirmed. Regulating the migration and priming of auto-reactive immune cells in the pancreas are critical to prevent the disease progression. Endogenous Tregs already exist in the pancreatic-draining lymph nodes, and these cells are critical in the modulation of priming of auto-reactive immune cells through restraining cell expansion and differentiation. Remarkably, Tregs play a role in reducing the access of auto-reactive immune cells to dendritic cells and disrupting cell development of T effectors, which induce local inflammation [62, 63•]. By controlling the priming of auto-reactive Tcells in the lymph nodes, Tregs limit the chance of T cells becoming effector cells. Chemokine receptors are other important molecules that attract T effectors into the pancreas. Tregs can suppress the expression of CXC-chemokine receptor 3 (CXCR3) through T helper cells, resulting in subsequent absence of this cell infiltration in the pancreatic islets and ultimately prevent the movement of T effectors [14••, 64]. Additionally, Tregs could suppress autoimmune aggression in the pancreatic islets through regulating the inflammatory reaction (i.e., insulitis) [65, 66••]. Both IL-10 and TGF-β play a crucial role in regulating T1D [67, 68••]. Tregs express IL-10 and TGF-β more than any other immune cells. In the pancreatic islets, during the T1D priming, TGF-β’s transient expression suppressed the disease and stimulated Treg expansion or generation [69, 70•]. IL-10 can modulate APC function, reduce inflammation, and decrease hyperactivity of pathogenic T cells. Moreover, Treg-produced IL-10 could down-regulate the expression of ICAM-1 on various cells, thereby preventing the migration of pathogenic immune cells [14••, 71]. Finally, Tregs can reduce the expression of IFN-γ that can worsen the disease progression.
Generation of Ag/Tissue-Specific iPSC-Tregs
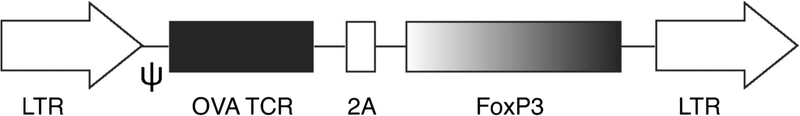
Because autoimmune diabetes is induced by the secretion of autoantigen produced by the islet cells, our method was sufficient to produce numbers of auto Ag-specific Tregs from iPSCs, i.e., iPSC-Tregs, which will be directed to the appropriate site due to their inherent specificity. Hematopoietic stem cells (HSCs) and ESCs can be used to generate T cells. We have previously utilized an in vitro co-culture system to generate Ag-specific iPSC-T cells [15, 19]. In these studies, murine iPSCs were engineered with TCR and co-cultured with the OP9-DL1 cells highly expressing the notch ligand delta-like 1 (DL1). Notch ligand signaling is indispensable for the differentiation of T lymphocytes [72, 73•]. The differentiation of iPSC-Tcells was analyzed for their morphology, expression of cell surface Ag, and function at different days of co-culture. The results indicated T-like differentiation, including the expression of T cell surface markers, such as CD3 and TCR. Significant morphological changes were also observed, including the transformation change from dome-like to grape-like colonies, which remains a representative feature of lymphoid cells. In addition, Nanog and CD117 are the two important identification markers for HSCs and iPSCs. After differentiation, iPSCs do not express stem cell-like markers Nanog and gradually express T cell markers, including CD3, CD4, CD8, and TCR. To determine their functional ability, iPSC-derived T cells were also examined for the secretion of cytokines. These stem cell-derived cells were found to produce IL-2 and IFN-α in a manner similar to mature T cells upon stimulation with anti-CD3 and anti-CD28 antibodies. After the generation of functional iPSC-T cells, we continued to develop auto Ag-specific iPSC-Tregs. Initially, we created a construct of MiDR-TCR-Foxp3-containing genes of ovalbumin (OVA)323–339-specific TCR and Foxp3 (Fig. 1). We retrovirally transduced mouse iPSCs with the MiDR-TCR-FoxP3 construct and then co-cultured the gene-transduced iPSCs on the OP9-DL1-DL4-I-Ab-expressing Notch ligands DL1, DL4, and MHC-II molecule I-Ab in the presence of recombinant cytokines of rIL-7 and rFLt3L. The gene-transduced iPSCs were determined for differentiation through analyzing cell morphological difference. The gene-transduced iPSCs differentiated into mesoderm-like profiles and were related to non-adherent grape-like clusters. At day 22 of co-culture, lymphocyte-like cells fully covered the cell culture plate. From the analyses of cell surface markers by flow cytometry, it can be shown that the iPSC-Tregs markedly expressed CD3- and OVA-specific TCR (Vα2 and Vβ5). The CD3+Vβ5+ cells expressed CD4 but not CD8. The majority of CD3+Vβ5+CD4+ populations expressed CD25, CD127, and CTLA-4—classically expressed on naturally occurring Tregs (nTregs) [74]. Consequently, we determined the function of Ag-specific iPSC-Tregs in vivo. After adoptive transfer, the transferred Ag-specific iPSC-Tregs from the hosts were able to produce suppressive cytokines IL-10 and TGF-β when stimulated with cognate Ag or anti-CD3 plus anti-CD28 antibodies.
Fig. 1.
Generation of MiDR-TCR-FoxP3 retroviral construct. Schematic representation of the retroviral construct MiDR-TCR-FoxP3 containing genes of OVA-specific TCR and FoxP3. Ψ, packaging signal; 2A, picornavirus self-cleaving 2A sequence; LTR, long terminal repeats
Utilization of iPSC-Tregs for the Treatments of T1D
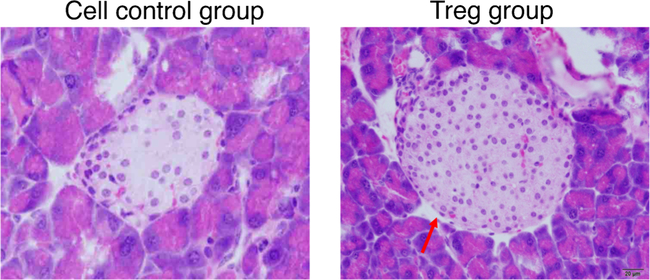
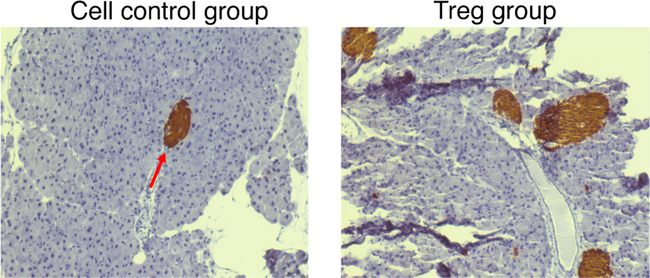
In vitro-differentiated functional iPSC-Tregs were used for the treatment of T1D in an animal model. In this mouse model of T1D, B6 mOVA transgenic (Tg) mice were crossed with OT I TCR Tg mice. For B6 mOVA Tg mice, the pancreatic islet β cells and the renal proximal tubular cells express membrane-bound OVA [75]. After these two strains of mice interbred, the partial F1 generation of mice (B6-mOVA × OT-I double Tg mice) has autoimmune diabetes. In this T1D model, CD8+ T cells from OT-1 TCR Tg mice destroy the OVA+ pancreatic islets, and the F1 mice subsequently start producing low amounts of insulin and develop autoimmune diabetes. The 8-week F1 mice were tested for blood glucose level, and we witnessed that roughly 30% mice had autoimmune diabetes. Consequently, CD8+ T cells from OT I Tg mice were additional activated through inoculating the mice with vaccinia virus that express OVA (VACV-OVA). Following the VACA-OVA administration, all mice showed high blood glucose level and extra urine discharge. Following the successful induction of autoimmune diabetes, we introduced OVA-specific iPSC-Tregs into the mice with autoimmune diabetes. Seven days post-Treg transfer, we examined that > 80% of the diabetic animals had decreased blood glucose level and low urine discharge. The animals were next sacrificed for histological evaluation. Inflammation in the pancreases of mice receiving the Ag-specific iPSC-Tregs was prominently reduced compared with the control mice receiving control cells (Fig. 2). Additional analysis was performed to analyze the islet destruction in both groups of animals. Islet sizes were obviously decreased in the group of mice receiving control cells; conversely, they were relatively usual in mice receiving the iPSC-Tregs (Fig. 3). We further investigated the mechanisms by which the iPSCs-Tregs reduced blood sugar levels and avoided islet damage in the diabetic mice. ICAM-1 is critical in directing pathogenic T cells to the pancreatic islet from the periphery [71]. We identified that ICAM-1 expression was substantially augmented in diabetic mice; conversely, it was prominently decreased in the pancreas of mice having the Ag-specific iPSC-Tregs. We have formerly revealed that Ag-specific iPSC-Tregs secreted suppressive cytokines, such as IL-10 and TGF-β. Consequently, TGF-β, produced from the iPSC-Tregs, shielded the islets from further destruction. Furthermore, IL-10, produced from the iPSC-Tregs, decreased ICAM-1 expression that repressed the accumulation of pathogenic immune cells in the impaired islets and avoided additional damage.
Fig. 2.
Adoptive transfer of Tregs protects the reduction of islet size and number in diabetic mice. Diabetic mice were adoptively transferred with Ag-specific Tregs or cell control. Mice were sacrificed, and their pancreases were prepared for HE staining. Islet size and number were reduced markedly in diabetic mice receiving control cells, which were normal in mice receiving Tregs. Islet is indicated by the red arrow. Scale bar: 20 μm
Fig. 3.
Suppression of destruction of pancreatic β-cells by Ag-specific Tregs. Diabetic mice were adoptively transferred with Ag-specific Tregs or cell control. Mice were sacrificed, and their pancreases were stained with insulin to detect the β-cells. Representative photomicrographs (immunofluorescence staining) of islet destruction (magnification, × 200). Insulin-producing cells are indicated by the red arrow
Strengths and Pitfalls of Treg-Based Therapy
Strengths
Currently, immunosuppressive drugs are the only option for treating immune-mediated diseases. Most of the immunosuppressive drugs are non-Ag-specific and need to be administered throughout the lifetime of the host. Due to their non-specificity, they are not able to distinguish between favorable and devastating immune responses. Beneficial immune cells are also suppressed by the non-specific immunosuppressive drugs, which causes the development of other diseases. As with lifelong treatment with immunosuppressive drugs, disease relapse may happen when stopping the use of the immunosuppressive drugs. In contrast, Tregs are physical cells of the immune system and Treg-based adoptive cell transfer would rebuild the immunological homeostasis destroyed underneath the pathological conditions. Ag-specific Tregs are directed particularly to the site of action and exert their effect on target cells. Other neighboring immune cells are not affected. IL-10 and TGF-β are most important cytokines that exert immunomodulatory function. In vivo transfer of Tregs is the most potent biological source of these two cytokines. It is highly possible that exogenous use of IL-10 and TGF-β as recombinant proteins will not have the similar therapeutic results. Actually, the only use of exogenous IL-10 could not prevent allograft rejection in allogeneic pancreatic islet transplantation; instead, adoptive transfer of Ag-specific Tregs prevented the rejection and induced durable tolerance, because the Tregs produced IL-10 when encountering with Ags. Notably, Treg-based immunotherapy can be personalized to the individual patient and consequently be used to fulfill the precise requests of each patient. Nevertheless, it will be a relatively expensive approach for T1D treatment because individual Tregs cannot be industrial and given effortlessly and naturally.
Pitfalls
At the current stage, the major difficulties that constrain the Treg-based immunotherapy are practical and associate with cell management. It is also a patient-specific immunotherapy thereby limiting its widespread use. The procedure for generation of Ag-specific Tregs involves several steps that include isolation of somatic cells from the patient and introduction of various transcription factors through retroviral transduction or other method to make iPSCs, which are further engineered to become iPSC-Tregs that need to be expanded further by in vitro culture system. Treg separation and management are consequently essential prerequisites for Treg-based immunotherapy. In addition, for the development of Ag-specific Tregs as a therapeutic product, some further considerations should be resolved and various quality controls must be instituted. Good manufacturing practice (GMP) conditions should be used in all procedures, including Treg separation, management, growth, and reinfusion in the patients. Subsequently, this therapeutic method is relatively costly. Undoubtedly, only a small number of institutions or organizations are able to offer entirely the essential infrastructure to perform Treg-based immunotherapy. Yet, since there is a great therapeutic potential, we should predict that not only academic institutions but also pharmaceutical companies would be involved in developing this new therapeutic method. Obviously, an extreme priority is the safety of the manipulated Tregs. The possibilities of unrestrained immune cell increase, overall immunosuppression, and following tumor growth must be wisely censored. Besides, a remarkable advantage of Treg-based immunotherapy over the established treatments must be evidently confirmed.
Conclusion
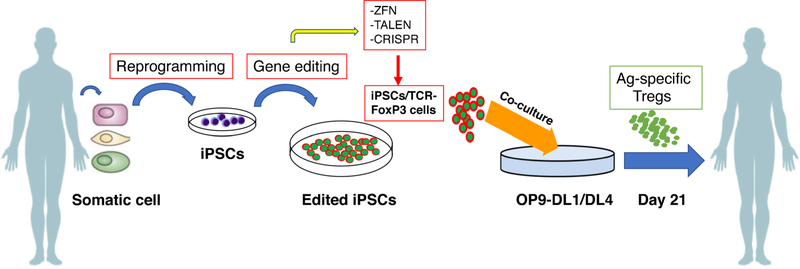
PSCs can differentiate into Ag-specific Tregs that are similar to nTregs. Yet, in autoimmune diabetes, improving the activity of islet cells or inhibiting their devastation from pathogenic immune cells will reduce or prevent the development of disease. Evidently, adoptive transfer of pancreatic tissue-associated iPSC-Tregs in diabetic mice reduced the blood sugar level and restored the islet size. A following work to generate diabetic auto Ag-specific iPSC-Tregs will greatly advance the field of Treg-based immunotherapy of T1D. We have illustrated the design strategy for generation of autoantigen-specific Tregs from human somatic cells (Fig. 4). Because heat-shock proteins (HSPs) are highly expressed in inflamed pancreatic tissues and are associated with the islet cell devastation [60], HSP-specific iPSC-Tregs can be pancreatic tissue-associated Tregs, and adoptive transfer of these Tregs may significantly prevent or suppress T1D. In the past five years, Ag-specific PSC-T cells have been revealed a great potential to be used in adoptive cell transfer based on immunotherapy. It can be expected in the near future that optimal approaches will be developed to generate auto Ag-specific PSC-Tregs in vitro and apply such cells ex vivo for T1D immunotherapy.
Fig. 4.
Step-by-step procedure for generation and treatment with iPSCs derived from patient’s somatic cells. Somatic cells from diabetic patients are transformed to iPSCs, which are engineered according to interest and again administered back to the patient’s body
Funding Information
This work was supported by the National Institutes of Health grants R01AI121180, R01CA221867, and R21AI109239 and by the American Diabetes Association (1–16-IBS-281) to J.S.
Footnotes
Guarantor’s Statement J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Mohammad Haque, Jugal Kishore Das, Xiaofang Xiong, and Jianxun Song declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, et al. 2017 national standards for diabetes self-management education and support. Diabetes Educ. 2019;45(1): 34–49. [DOI] [PubMed] [Google Scholar]
- 2.Rariden C. Prediabetes: a wake-up call. Nursing. 2019;49(4):38–44. [DOI] [PubMed] [Google Scholar]
- 3.Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51(1):R1–13. [DOI] [PubMed] [Google Scholar]
- 4.James EA, Pietropaolo M, Mamula MJ. Immune recognition of beta-cells: neoepitopes as key players in the loss of tolerance. Diabetes. 2018;67(6):1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michels AW, Gottlieb PA. Learning from past failures of oral insulin trials. Diabetes. 2018;67(7):1211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–47. [DOI] [PubMed] [Google Scholar]
- 7.•.Spence A, Purtha W, Tam J, Dong S, Kim Y, Ju CH, et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc Natl Acad Sci U S A. 2018;115(20):5265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study provide a glimpse into the specificities of Tregs in a natural repertoire that are crucial for opposing the progression of autoimmune diabetes.
- 8.•.Yu H, Gagliani N, Ishigame H, Huber S, Zhu S, Esplugues E, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc Natl Acad Sci U S A. 2017;114(39):10443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that modulating gut-associated lymphoid tissue to boost Tr1 cells may be important in type 1 diabetes management.
- 9.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205(9):1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.••.Haque M, Lei F, Xiong X, Das JK, Ren X, Fang D, et al. Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight. 2019;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that the stemcell-derived tissue-associated Tregs can robustly accumulate in the diabetic pancreas, and, through downregulating the expression of ICAM-1 in the local inflamed tissues and inhibiting the production of proinflammatory cytokine IFN-γ, suppress the migration and activity of the pathogenic immune cells that cause T1D.
- 15.Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260(1):1–5. [DOI] [PubMed] [Google Scholar]
- 16.Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, et al. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71(14):4742–7. [DOI] [PubMed] [Google Scholar]
- 17.Lei F, Haque R, Xiong X, Song J. Directed differentiation of induced pluripotent stem cells towards T lymphocytes. J Vis Exp. 2012;63:e3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189(3):1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haque M, Song J, Fino K, Sandhu P, Song X, Lei F, et al. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci Rep. 2016;6:20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. [DOI] [PubMed] [Google Scholar]
- 21.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. [DOI] [PubMed] [Google Scholar]
- 22.•.Lopez-Yrigoyen M, Yang CT, Fidanza A, Cassetta L, Taylor AH, McCahill A, et al. Genetic programming of macrophages generates an in vitro model for the human erythroid island niche. Nat Commun. 2019;10(1):881. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest establishment of an in vitro system to model the human EI niche using macrophages that are derived from human iPSCs, and are also genetically programmed to an EI-like phenotype by inducible activation of the transcription factor, KLF1.
- 23.•.Minagawa A, Yoshikawa T, Yasukawa M, Hotta A, Kunitomo M, Iriguchi S, et al. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23(6):850–8 e4. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that enhancing TCR stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy.
- 24.Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8(2):274–84. [DOI] [PubMed] [Google Scholar]
- 26.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–75. [DOI] [PubMed] [Google Scholar]
- 27.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–53. [DOI] [PubMed] [Google Scholar]
- 28.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. [DOI] [PubMed] [Google Scholar]
- 29.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5(4):353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283(46):31601–7. [DOI] [PubMed] [Google Scholar]
- 31.•.Enderami SE, Mortazavi Y, Soleimani M, Nadri S, Biglari A, Mansour RN. Generation of insulin-producing cells from human-induced pluripotent stem cells using a stepwise differentiation protocol optimized with platelet-rich plasma. J Cell Physiol. 2017;232(10):2878–86. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest a new approach to investigate the role of PRP in pancreatic differentiation protocols and enhance the feasibility of using patient-specific iPSCs and autologous PRP for future beta cells replacement therapies for T1DM.
- 32.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106(37):15768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.••.Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that T1D SC-β cells could potentially be used for the treatment of diabetes, drug screening and the study of β-cell biology.
- 34.Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care. 1992;15(1):66–74. [DOI] [PubMed] [Google Scholar]
- 35.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53(4):614–23. [DOI] [PubMed] [Google Scholar]
- 37.Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59(6):1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemoto N, Konagaya S, Kuwabara R, Iwata H. Coaggregates of regulatory Tcells and islet cells allow long-term graft survival in liver without immunosuppression. Transplantation. 2015;99(5):942–7. [DOI] [PubMed] [Google Scholar]
- 40.Duggleby R, Danby RD, Madrigal JA, Saudemont A. Clinical grade regulatory CD4(+) T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol. 2018;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwinski M, Derkowska I, Zalinska M, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med. 2016;14(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164(1):183–90. [DOI] [PubMed] [Google Scholar]
- 44.•.Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest the use of CD28 based CAR-Tregs for tissue specific immune suppression in the clinic.
- 45.•.Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018;30(10):445–54. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest the new therapies that can reverse immune suppression in the TME and promote anti-tumorimmunity.
- 46.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175(7):4180–3. [DOI] [PubMed] [Google Scholar]
- 47.•.Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that CAR-transduced Tregs are a promising approach for future tolerogenic treatment of hemophilia A patients with inhibitors.
- 48.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. [DOI] [PubMed] [Google Scholar]
- 49.•.Semitekolou M, Morianos I, Banos A, Konstantopoulos D, Adamou-Tzani M, Sparwasser T, et al. Dendritic cells conditioned by activin A-induced regulatory T cells exhibit enhanced tolerogenic properties and protect against experimental asthma. J Allergy Clin Immunol. 2018;141(2):671–84 e7. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that Act-A-iTreg cells instruct the generation of a highly effective immunoregulatory circuit encompassing tolerogenic DCs and forkhead box P3+ Treg cells that could be targeted for the design of novel immunotherapies for allergic disorders.
- 50.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.••.Guipouy D, Gertner-Dardenne J, Pfajfer L, German Y, Belmonte N, Dupre L. Granulysin- and granzyme-dependent elimination of myeloid cells by therapeutic ova-specific type 1 regulatory T cells. Int Immunol. 2019;31(4):239–50. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that ova-Tr1 cells are endowed with a sustained cytotoxic activity that relies on a unique combination of granulysin and granzymes and that preferentially eliminates myeloid target cells in a TCR-independent manner.
- 52.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40. [DOI] [PubMed] [Google Scholar]
- 53.Aiello LP, Group DER. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52(7):2212–21. [DOI] [PubMed] [Google Scholar]
- 55.••.Sun G, Hou Y, Gong W, Liu S, Li J, Yuan Y, et al. Adoptive induced antigen-specific treg cells reverse inflammation in collagen-induced arthritis mouse model. Inflammation. 2018;41(2):485–95. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that adoptive induced antigen-specific Treg cells may have clinical applications for treatment of autoimmunity, including RA and other autoimmune disorders.
- 56.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712–6. [DOI] [PubMed] [Google Scholar]
- 57.•.Wilkinson DS, Ghosh D, Nickle RA, Moorman CD, Mannie MD. Partial CD25 antagonism enables dominance of antigen-inducible CD25(high) FOXP3(+) regulatory T cells as a basis for a regulatory T cell-based adoptive immunotherapy. Front Immunol. 2017;8:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that low IL-2 concentrations coupled with high PC61 concentrations constrained IL-2 signaling to a low-intensity range that enabled dominant stable outgrowth of suppressive CD25high FOXP3+ Tregs. The ability to indefinitely expand stable Treg lines will provide insight into FOXP3+ Treg physiology and will be foundational for Treg-based immunotherapy.
- 58.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177(3):1451–9. [DOI] [PubMed] [Google Scholar]
- 59.•.Yang D, Tian Z, Zhang M, Yang W, Tang J, Wu Y, et al. NKG2D(+)CD4(+) T cells kill regulatory T cells in a NKG2D-NKG2D ligand-dependent manner in systemic lupus erythematosus. Sci Rep. 2017;7(1):1288. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that NKG2D+CD4+ T cells are involved in the pathogenesis of SLE by killing Treg cells in a NKG2D-NKG2DL-dependentmanner. Targeting the NKG2D-NKG2DL interaction might be a potential therapeutic strategy by which Treg cells can be protected from cytolysis in SLE patients.
- 60.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69(20):7895–8. [DOI] [PubMed] [Google Scholar]
- 61.Hutmacher C, Gonzalo Nunez N, Liuzzi AR, Becher B, Neri D. Targeted delivery of IL2 to the tumor stroma potentiates the action of immune checkpoint inhibitors by preferential activation of NK and CD8(+) T cells. Cancer Immunol Res. 2019;7(4):572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.•.Seitz C, Liu S, Klocke K, Joly AL, Czarnewski PV, Tibbitt CA, et al. Multi-faceted inhibition of dendritic cell function by CD4(+)Foxp3(+) regulatory T cells. J Autoimmun. 2019;98:86–94. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that Treg cells induced a specific immunosuppressive program in DCs.
- 64.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173(5):2942–51. [DOI] [PubMed] [Google Scholar]
- 65.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+ CD25+ Treg cells impinge on autoimmune diabetes. J Exp Med. 2005;202(10):1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.••.Zou J, Gao X, Liu T, Liang R, Liu Y, Wang G, et al. Ethylenecarbodiimide-fixed splenocytes carrying whole islet antigens decrease the incidence of diabetes in NOD mice via down-regulation of effector memory T cells and autoantibodies. EndocrJ. 2018;65(9):943–52. [DOI] [PubMed] [Google Scholar]; Findings from this study suggest that ECDI-fixed splenocytes carrying whole isletantigens effectively prevented the onset of T1DM in NOD mice, via suppressing the production of autoantibodies and inducing anergy of autoreactive T cells.
- 67.Roncarolo MG, Battaglia M, Gregori S. The role of interleukin 10 in the control of autoimmunity. J Autoimmun. 2003;20(4):269–72. [DOI] [PubMed] [Google Scholar]
- 68.••.Abdel-Latif M, Abdel-Moneim AA, El-Hefnawy MH, Khalil RG. Comparative and correlative assessments of cytokine, complement and antibody patterns in paediatric type 1 diabetes. Clin Exp Immunol. 2017;190(1):110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that IFN-γ, IL-12 and IL-17 played an essential role in exacerbating EV+-T1D, while C3d, sC5b-9, IL-10 and −20 displayed distinct patterns.
- 69.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101(13):4572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.•.Kuhn C, Rezende RM, da Cunha AP, Valette F, Quintana FJ, Chatenoud L, et al. Mucosal administration of CD3-specific monoclonal antibody inhibits diabetes in NOD mice and in a preclinical mouse model transgenic for the CD3 epsilon chain. J Autoimmun. 2017;76:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that oral CD3-specific mAb has the potential for treating autoimmune diabetes in humans.
- 71.Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55(6):1571–80. [DOI] [PubMed] [Google Scholar]
- 72.Dai B, Wang P. In vitro differentiation of adult bone marrow progenitors into antigen-specific CD4 helper T cells using engineered stromal cells expressing a notch ligand and a major histocompatibility complex class II protein. Stem Cells Dev. 2009;18(2):235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.•.Haque R, Song J, Haque M, Lei F, Sandhu P, Ni B, et al. c-Myc-induced survivin is essential for promoting the notch-dependent T cell differentiation from hematopoietic stem cells. Genes (Basel). 2017;8(3). [Google Scholar]; Findings from this study suggest both c-Myc and survivin as important mediators of the Notch signaling-regulated differentiation of T lymphocytes from hematopoietic stem cells.
- 74.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329(5999):1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184(3):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]