Abstract
Objectives
To compare the use of short implants (≤6 mm) in atrophic posterior maxilla versus longer implants (≥10 mm) with sinus floor elevation.
Design
A systematic review and meta-analysis based on randomised controlled trials (RCTs).
Data sources
Electronic searches were conducted in PubMed, Embase and the Cochrane CENTRAL. Retrospective and prospective hand searches were also performed.
Eligibility criteria
RCTs comparing short implants (≤6 mm) and longer implants (≥10 mm) with sinus floor elevation were included. Outcome measures included implant survival (primary outcome), marginal bone loss (MBL), complications and patient satisfaction.
Data extraction and synthesis
Risks of bias in and across studies were evaluated. Meta-analysis, subgroup analysis and sensitivity analysis were undertaken. Quality of evidence was assessed according to Grading of Recommendations Assessment, Development and Evaluation.
Results
A total of seven RCTs involving 310 participants were included. No significant difference in survival rate was found for 1–3 years follow-up (RR 1.01, 95% CI 0.97 to 1.04, p=0.74, I²=0%, moderate-quality evidence) or for 3 years or longer follow-up (RR 1.00, 95% CI 0.97 to 1.04, p=0.79, I²=0%, moderate-quality evidence). However, short implants (≤6 mm) showed significantly less MBL in 1–3 years follow-up (MD=−0.13 mm, 95% CI −0.21 to 0.05; p=0.001, I²=87%, low-quality evidence) and in 3 years or longer follow-up (MD=−0.25 mm, 95% CI −0.40 to 0.10; p=0.001, I²=0%, moderate-quality evidence). In addition, short implant (≤6 mm) resulted in fewer postsurgery reaction (RR 0.11, 95% CI 0.14 to 0.31, p<0.001, I²=40%, moderate-quality evidence) and sinus perforation or infection (RR 0.11, 95% CI 0.02 to 0.63, p=0.01, I²=0%, moderate-quality evidence).
Conclusions
For atrophic posterior maxilla, short implants (≤6 mm) are a promising alternative to sinus floor elevation, with comparable survival rate, less MBL and postsurgery reactions. Additional high-quality studies are needed to evaluate the long-term effectiveness of short implants (≤6 mm).
Trial registeration number
The protocol has been registered at PROSPERO (CRD42018103531).
Keywords: short dental implant, sinus floor augmentation, systematic review
Strengths and limitations of this study.
Only randomised controlled clinical trials were included.
Participant-unit data were used for syntheses.
Subgroup analyses by follow-up length and categories of complications were performed.
Serious risks of bias were found within and across studies and the quality of evidence was only low to moderate.
Introduction
Dental implants supporting prosthesis are commonly considered a promising method for the rehabilitation of missing teeth.1–3 However, dental implantation in the posterior maxilla is usually challenging due to insufficient vertical bone volume, poor bone quality, limited visibility, reduced interarch space and sinus pneumatisation.4 5 These conditions are exacerbated if patients have a history of wearing removable dentures.6
To achieve sufficient vertical bone volume in the posterior maxilla, sinus floor elevation using the lateral window approach or the osteotomy technique has been introduced and widely used over the past 40 years.7 8 The lateral window approach is commonly used in dental implantation procedures.9 Using these techniques with or without bone grafting, conventional implants can be placed in the elevated sites. The implant success rate is typically greater than 90% in long-term evaluation.10–12 However, sinus floor elevation surgery is usually associated with higher cost, more complicated surgical procedures and a high prevalence of complications such as infection, sinus membrane perforation and graft failure.13–15 In addition, the clinical outcome of sinus floor elevation can also be restricted by extremely insufficient residual bone height, abnormal sinus anatomy, thickening of the sinus membrane, stability of the grafted bone and the number of missing teeth.13 16–18
Short implants with improved implant design and surface properties have been successfully applied as an alternative to sinus floor elevation surgery and have shown good results in posterior maxilla. Implants ≤10 mm,19 ≤8 mm,20 ≤7 mm21 and 6–8 mm22 are reported to have survival rates comparable to those of longer implants. In addition, short implants ≤6 mm in length have been introduced as another alternative in atrophic posterior maxilla.6 23 24 Short implants require a less complicated surgical approach and could be used in cases when sinus floor elevation surgery is not applicable,25 26 especially in cases of maxillary sinusitis, maxillary cyst, large vessels and other cases involving abnormal sinus anatomy. Studies have explored the short-term and long-term survival rates of short implants (≤6 mm).26–30 Unfortunately, the evidence supporting the use of short implants (≤6 mm) in the posterior maxilla is weak, and no guideline statement is currently recommended.
The present systematic review aims to compare the effectiveness of short implants (≤6 mm) and longer implants (≥10 mm) with sinus floor elevation in atrophic posterior maxilla. Our null hypothesis was that the survival rate, patient satisfaction, marginal bone loss (MBL) and surgery-related complications of short implants (≤6 mm) were comparable to longer implants in combination with sinus floor elevation.
Materials and methods
Protocol and registration
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.31
Eligible criteria
Randomised controlled trials (RCTs) meeting the following predetermined inclusion criteria (PICOS format) were included:
Population: Partially edentulous patients in the premolar and molar regions of the maxilla, for whom the residual bone height in the atrophic posterior maxilla was sufficient for the insertion of a short implant (≤6 mm) but insufficient for the insertion of longer implants.
Intervention: One or more short implants (≤6 mm) were placed in the posterior maxilla without sinus floor elevation in the short implant group.
Comparison: One or more longer implants were placed in the posterior maxilla after sinus floor elevation by any technique in the elevation group.
Outcomes: The primary (survival rate) and secondary (MBL, complications and patient satisfaction) outcomes of interest were measured, with a follow-up length of 1 year or longer postloading.
Information sources and search strategies
Two content experts (QY and XW) searched PubMed, Embase and the Cochrane CENTRAL (The Cochrane Central Registration of Controlled Trials) for RCTs, independently and in duplicate. The last search was conducted on 31 May 2018. A methodologist (FH) was consulted to resolve any disagreements. Main search terms included: “dental implant”, “short implant”, “ultrashort”, “alveolar bone loss”, “atrophic maxilla”, “sinus lift”, and “sinus floor elevation”. No restriction was set regarding publication year, publication language or status. The detailed search strategies are listed in the online supplementary file. In addition, retrospective and prospective searches were conducted by checking the reference lists of key articles and studies citing these key articles, using Google Scholar.
bmjopen-2019-029826supp001.pdf (63.8KB, pdf)
Study selection and data collection
Two review authors (QY and XW) conducted the study selection independently and in duplicate. The titles and abstracts of all records were scanned. Full texts of studies were obtained in cases they appeared to meet the inclusion criteria or further information were needed to determine eligibility. Studies excluded at this or subsequent stages were recorded with the reasons for exclusion. All disagreements were resolved by discussion.
Two review authors (QY and XW) extracted the data independently and in duplicate using specifically designed data extraction forms. The extracted data included citation details (year of publication, country of origin, setting and source of funding), details on the participants (demographic characteristics, residual bone height and inclusion criteria), details of intervention (implant length, diameter, brand, surface structure, surgical method, follow-up time, prosthesis type), outcome assessment, sample size calculation and trial registration. Corresponding authors were contacted for missing data or information.
Risk of bias of included studies
Two authors (QY and XW) assessed the risk of bias of each included study independently and in duplicate using the Cochrane risk of bias assessment tool for RCTs.32 Disagreements were resolved through discussion. A third review author (FH) was consulted when necessary. Seven domains were assessed, including sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other bias (factors that had potential influence on outcomes but were not evenly distributed across groups or not clearly reported, such as the manufacturer or diameter of implants). Individual studies were categorised as having low, high or unclear risk of bias. The risk of bias across studies was determined according to the risk of bias in each included study.
Assessment of heterogeneity
Clinical heterogeneity among the included studies was assessed by comparing study design, participant conditions (gender, age, residual bone height), intervention (implant length, diameter, surface structure, surgical method) and outcome measures. Statistical heterogeneity was evaluated using Cochrane’s Q test and the I² statistic. In the Q test, a p<0.1 was considered an indication of significant heterogeneity.
Assessment of publication bias
If at least 10 studies were included in a meta-analysis, We would have used a funnel plot and the Egger’s test33 asymmetry to assess the potential existence of publication bias if at least ten studies were included in a meta-analysis.
Synthesis of results
The unit of analysis was set as participant rather than implant.34 RevMan V.5.3 software was used for data synthesis. Meta-analyses were undertaken only when at least two studies that made similar comparisons reported the same outcomes. The effect measures were risk ratio (RR) for dichotomous outcomes (implant survival and complications) and mean difference (MD) for continuous outcomes (MBL). RR was calculated through Mantel-Haenszel analysis and MD was calculated through inverse variance. P<0.05 was considered statistically significant. The fixed-effect model was used when fewer than four studies were included in a meta-analysis, and the random-effects model was used when four or more studies were included.35–38
Additional analysis
Subgroup analysis by length of follow-up was performed to control for the possibility that function time might influence implant survival.39 In addition, subgroup analysis by categories of complications was performed. Complications were categorised according to into postsurgery reaction (bleeding, swelling and discomfort), biological complications (sinus perforation or infection, implant mobile, peri-implant mucositis and peri-implantitis) and technical complications (complications related to screws and crowns). If risks of bias in some studies were serious, we performed sensitivity analysis by excluding these studies. Considering only three studies were included in the MBL 3 years or longer follow-up but the meta-analysis for MBL included more than three studies, a sensitivity analysis was conducted for MBL by using fixed-effect model.
Summary of findings
Grading of Recommendations Assessment, Development and Evaluation approach40 was adopted to evaluate quality of evidence in this systematic review. A summary of findings table was made with an online tool (cebgrade.mcmaster.ca/gradepro.html). Outcomes were evaluated including survival rate and MBL of one to table was made with an online tool (cebgrade.mcmaster.ca/gradepro.html). Outcomes were evaluated including survival rate and MBL of 1–3 years and 3 years or longer follow-up, and complications. Five domains in quality of evidence were assessed: the overall risk of bias, directness of evidence, consistency of results, precision of estimates, as well as the risk of publication bias. The quality of the body of evidence was classified into four categories: high, moderate, low and very low.
Patient and public involvement
No patient or public was involved in this systematic review.
Results
Study selection
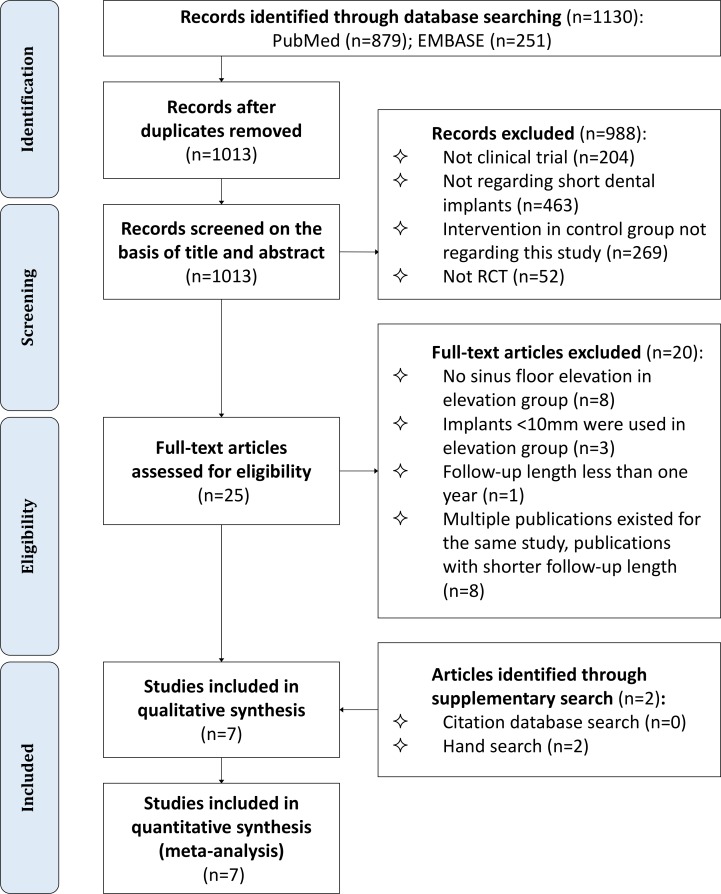
Electronic searches identified a total of 879 titles and abstracts in PubMed and 251 in Embase. After removal of duplicates, the titles and abstracts of 1013 unique items were screened. We then retrieved the full texts of 25 potentially eligible articles, of which 20 were excluded for the reasons described in figure 1. Retrospective and prospective hand searches yielded two more articles. Finally, seven studies29 41–46 met our eligibility criteria and were included in this review (figure 1).
Figure 1.
Flow diagram for study selection. RCT, randomised controlled trial.
Study characteristics
The characteristics of the seven included studies are listed in table 1. One study was a split-mouth trial, and the rest were two-arm parallel RCTs. The length of follow-up ranged from 1 to 3 years. For sinus floor elevation, either osteotomy-mediated sinus floor elevation or the lateral window technique was adopted. In two studies, single crowns were used as the rehabilitation method; in the remaining studies, single crowns or splinted prosthetics were used. The outcome measures used in these studies included implant failure, MBL, complications and patient satisfaction. Overall, 171 participants were included in the short implant groups, and 159 participants were included in the elevation groups.
Table 1.
Characteristics of the included studies
| Study | Design | Subjects | Follow-up period PL | Short implant group | Elevation group | Rehabilitation methods | Outcome measures | |||||||
| Gender (male/female) |
Age | RBH (mm) | Implant (n) | LEN (mm) | DIA (mm) | Implant (n) | LEN (mm) | DIA (mm) | Elevation methods | |||||
| Bolle et al, 201829 | RCT (TAP) | INT 7/13 CON 12/8 |
59.35 (47–73) 63.25 (46–72) |
4–5 | 1 year | 37 | 4 | 4 or 4.5 | 41 | 10, 11.5, 13 | 4 or 4.5 | Osteotomy approach | SG or SP | NIF, MBL, COM |
| Gastaldi et al, 201841 | RCT (TAP) | INT 3/17 CON 7/13 |
58.6 (39–80) 52.8 (42–70) |
4–6 | 3 years | 20 | 5 | 5 | 20 | 10, 11.5, 13, 15 | 5 | Lateral window technique | SG or SP | NIF, MBL, COM |
| Gastaldi et al, 201742 | RCT (TAP) | INT 3/7 CON 5/5 |
53.4 (43–67) 58.6 (48–70) |
5–7 | 3 years | 16 | 5 or 6 | 5 | 18 | 10 | 5 | Osteotomy approach | SG or SP | NIF, MBL, COM, PS |
| Guljé et al, 201443 | RCT (TAP) | INT 7/14 CON 13/7 |
50 (30–71) 48 (29–72) |
6–8 | 1 year | 21 | 6 | 4 | 20 | 11 | 4 | Lateral window technique | SG | NIF, MBL, COM, PS |
| Pohl et al, 201744 | RCT (TAP) | 49/52* | 50.5 (20–75)*– | 5–7 | 3 years | 67 | 6 | 4 | 70 | 11, 13, 15 | 4 | Lateral window technique | SG | NIF, MBL, COM |
| Felice et al, 201845 | RCT (SM) | 11/9 | 57.6 (45–80) | 5–7 | 3 years | 39 | 6 | 4 | 44 | 10, 11.5, 13, 15 | 4 | Lateral window technique | SG or SP | NIF, MBL, COM |
| Bechara et al, 201746 | RCT (TAP) | INT 10/23 CON 9/11 |
47.5±16.2 49.2±13.4 |
≥4 | 3 years | 45 | 6 | 4–8 | 45 | 10, 11.5, 13, 15 | 4–8 | Lateral window technique | SG or SP | NIF, MBL, COM, PS |
*Details for subject information in intervention and control group were not reported.
COM, complications; CON, control; DIA, implant diameter; INT, intervention; LEN, implant length; MBL, marginal bone loss; NIF, number of implant failures; NR, not reported; PL, postloading; PS, patient satisfaction;RBH, residual bone width under sinus floor;RCT, randomised controlled trial; SG, single crowns; SM, split mouth; SP, splinted prosthetics; TAP, two arm parallel.
Risk of bias assessment
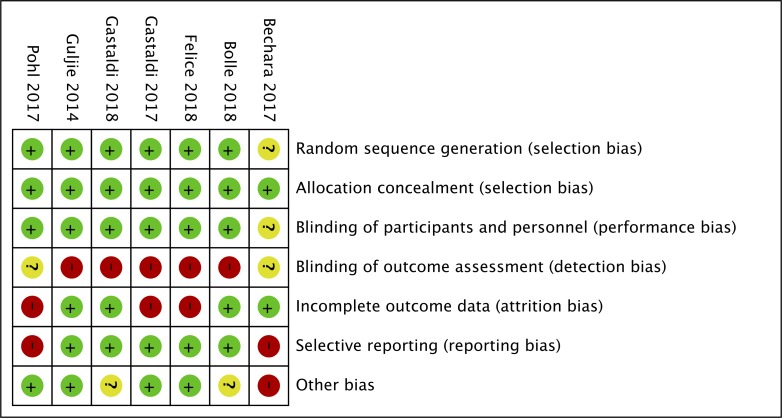
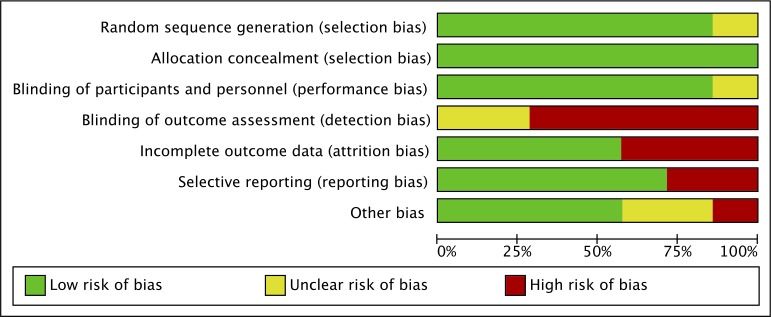
The results of the risks of bias assessment are shown in figures 2 and 3. Selection bias and performance bias were assessed as low in all but one study by Bachara et al 46 due to inadequate description on random sequence generation and blinding of participants. For detection bias, most studies showed high risks because assessors could recognise sites that underwent sinus floor elevation. For attrition bias, three studies42 44 45 was assessed as high. Two studies44 46 showed high risk of reporting bias. Other risks of bias were considered high or unclear in three studies. Overall, all included studies were at high risk of bias for at least one domain (table 2).
Figure 2.
Risk of bias in each included study.
Figure 3.
Risk of bias across included studies.
Table 2.
Details on the risk of bias for each included study
| Study | Random sequence generation | Allocation concealment | Blinding of patients/carers | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other |
| Bolle et al, 201829 | Low risk—quote: ‘a computer-generated restricted randomisation list’ | Low risk-quote: ‘the information on how to treat each patient was enclosed in sequentially numbered, identical, opaque, sealed envelopes.’ | Low risk—quote: ‘treatment allocation was concealed to the investigators in charge of enrolling and treating the patients.’ | High risk—quote: ‘complications were dealt with directly and reported by the responsible clinicians, who were not blinded’; ‘augmented sites could be easily identified on radiographs due to the different implant lengths.’ | Low risk—quote: ‘one patient from the short implant group and one from elevation group dropped out.’ | Low risk—comment; All outcome measure in methods were reported in results | Unclear risk—comment: diameter of implants (4 mm or 4.5 mm) was not controlled |
| Gastaldi et al, 201841 | Low risk—quote: ‘a computer-generated restricted randomisation list’ | Low risk—quote: ‘The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes’ | Low risk—quote: ‘treatment allocation was concealed to the investigators in charge of enrolling and treating the patients.’ | High risk—quote: ‘augmented sites could be easily identified because of the different anatomy of the two sides after the augmentation procedure’ | Low risk—omment: one patient dropped out of the short implant group (1/20), and two patients dropped out of the elevation group (2/20) | Low risk—comment; All outcome measures in methods were reported in results | Unclear risk—comment: information of short implants was not reported |
| Gastaldi et al, 201742 | Low risk—quote: ‘a computer-generated restricted randomisation list’ | Low risk—quote: ‘The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes.’ | Low risk—quote: ‘treatment allocation was concealed to the investigators in charge of enrolling and treating the patients’ | High risk—quote: ‘sinus-lifted sites could be identified on radiographs because they appeared more radio- opaque and implants were longer.’ | High risk—comment: no patients dropped out of the short implant group (0/10); two patients dropped out of the elevation group (2/10) | Low risk—comment; All outcome measures in methods were reported in results | Low risk |
| Guljé et al, 201443 | Low risk—quote: ‘Randomisation was performed using a block randomization sequence to provide equal distribution of subjects.’ | Low risk—quote: ‘A sealed envelope’ | Low risk—quote: ‘A sealed envelope was opened by the surgical assistant at the beginning of the surgical procedure.’ | High risk—quote: ‘blinding was possible in the clinical evaluation but not during analysis of the radiographs.’ | Low risk—comment: no patient dropped out of the short implant group (0/21); one patient in the elevation group died (1/20) | Low risk—omment; All outcome measures in methods were reported in results | Low risk |
| Pohl et al, 201744 | Low risk—quote: ‘A block randomization sequence was used to provide an equal distribution’ | Low risk—quote: ‘A sealed envelope’ | Low risk—quote: ‘After flap elevation, a sealed randomisation envelope was opened to allocate the subject to either one of the two treatment groups.’ | Unclear risk—quote: ‘an independent examiner performed all the radiographic measurements.’ Other information was not reported. | High risk—comment: The reasons for incomplete reporting of MBL were not provided. | High risk—comment: MBL at 3-year follow-up was reported at the implant level rather than at the participant level | Low risk |
| Felice et al, 201845 | Low risk—quote: ‘a computer-generated restricted randomisation list’ | Low risk—quote: ‘The information on how to treat site number one was enclosed in sequentially numbered, identical, opaque, sealed envelopes.’ | Low risk—quote: ‘Treatment allocation was concealed to the investigators in charge of enrolling and treating the patients.’ | High risk—quote: ‘augmented sites could be easily identified because of the different anatomy’ | High risk—comment: it was a split-mouth design study, and two drop-outs (2/20) occurred | Low risk—comment: All outcome measures in methods were reported in results | Low risk |
| Bechara et al, 201746 | Unclear risk—quote: ‘Patients were randomly assigned’ | Low risk—quote: ‘a sequentially numbered sealed envelope’ | Unclear risk—comment: not mentioned | Unclear risk—quote: ‘At each annual inspection, an experienced, calibrated, independent examiner performed a careful clinical examination’, but elevation site can be distinguished | Low risk—comment: one patient dropped out of the short implant group (1/33), and one patient dropped out of the elevation group (1/20) | High risk—comment: MBL was reported at the implant level rather than at the participant level | High risk—comment: diameter of implants was not controlled (4–8 mm) |
MBL, marginal bone loss.
Synthesis of results
Survival rate
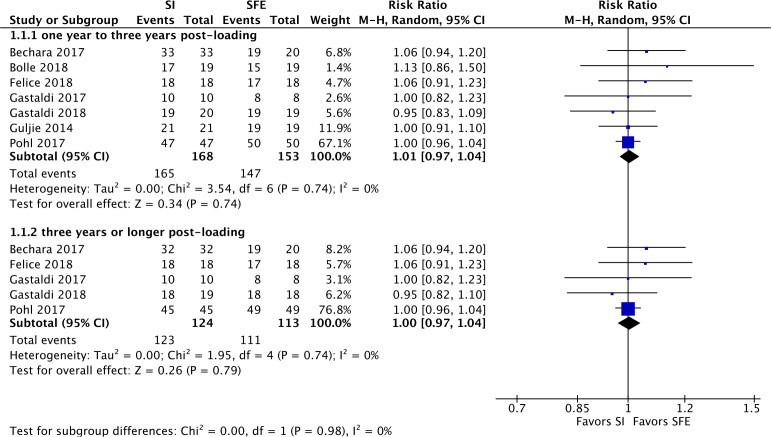
Figure 4 shows the results of a meta-analysis for participant unit implant survival rate with a subgroup analysis based on length of follow-up. Five studies reported 100% survival of short implants (≤6 mm) within the study period. For this outcome, there was no evidence of a difference between the short implant group and the elevation group either 1–3 years postloading (RR 1.01, 95% CI 0.97 to 1.04, p=0.74, I²=0%, seven RCTs, 321participants) or 3 years or longer postloading (RR 1.00, 95% CI 0.97 to 1.04, p=0.79, I²=0%, five RCTs, 237 participants). Further details of the implant failures are summarised in table 3.
Figure 4.
Forest plot for implant survival rate. M-H, Mantel-Haenszel; SI, short implant group; SFE, sinus floor elevation group.
Table 3.
Details of implant failures reported in the included studies
| Study | Short implant group | Elevation group | ||||||
| LEN (mm) | DIA (mm) | PAR/IMP (n) | Details | LEN (mm) | DIA (mm) | PAR/IMP (n) | Details | |
| Bolle et al, 201829 | 4 | 4 or 4.5 | 2/3 | PAR1. One implant was mobile 3 months after placement, and another implant migrated into the sinus 4 months after placement. PAR2. One implant was medially tilted 2 weeks after placement | 10,11.5,13 | 4 or 4.5 | 4/6 | PAR1. One implant was mobile 2 months after placement because of a perforation of the sinus lining at its detachment. Another implant was mobile 2 months later. PAR2. One implant migrated into the sinus 3 months after placement. PAR3. Two implants were mobile 3 months after placement because the patient insisted on wearing her removable denture. PAR4. One implant was mobile, and the patient experienced discomfort when chewing 5 months postloading. |
| Gastaldi et al, 201841 | 5 | 5 | 1/1 | PAR1. One implant failed 3 months postloading. | 10,11.5, 13,15 | 5 | 0 | None |
| Felice et al, 201845 | 6 | 4 | 0 | None | 10,11.5, 13,15 | 4 | 1/2 | PAR1. Two implants failed due to peri-implantitis 2 years postloading. |
| Bechara et al, 201746 | 6 | 4–8 | 0 | None | 10,11.5, 13,15 | 4–8 | 1/2 | PAR1. Two implants were lost caused due to chronic sinus infection with loss of integration/implant stability 2 months after surgery. |
DIA, implant diameter;LEN, implant length; PAR, participant; PAR/IMP, participant/implant.
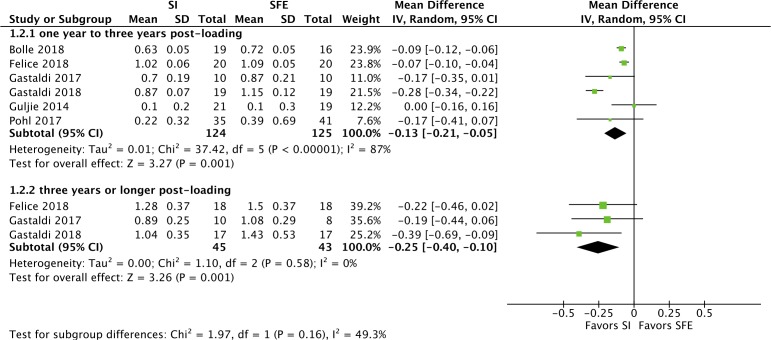
Marginal bone loss
The results of the meta-analysis and subgroup analysis regarding peri-implant MBL are shown in figure 5. A significant difference favouring the short implant group was found for both 1–3 years postloading (MD=−0.13, 95% CI −0.21 to 0.05; p=0.001, I²=87%, six RCTs, 249 participants) and 3 years or longer postloading (MD=−0.25, 95% CI −0.40 to 0.10; p=0.001, I²=0%, three RCTs, 88 participants). In sensitivity analysis by using fixed-effect model, results remained significant for both 1–3 years postloading (MD=−0.11, 95% CI −0.13 to 0.08; p<0.001, I²=87%) and 3 years or longer postloading (MD=−0.25, 95% CI −0.40 to 0.10; p=0.001, I²=0%).
Figure 5.
Forest plot for marginal bone loss. SI, short implant group; SFE, sinus floor elevation group.
Complications
Complications were categorised into postsurgery reaction, biological complications and technical complications (table 4). Short implant group was found with significantly less postsurgery reaction (RR 0.11, 95% CI 0.14 to 0.31, p<0.001, I²=40%, three RCTs, 184 participants) and sinus perforation or infection (RR 0.11, 95% CI 0.02 to 0.63, p=0.01, I²=0%). Only one study29 reported implant migrating into sinus in 5% patients (1/19) in short implant group while in 10.5% patients (2/19) in sinus floor elevation group. No statistically significant difference was found in other complications between short implants (≤6 mm) and longer implants.
Table 4.
Comparisons of complications
| Outcome or subgroup titles | No of studies | No of participants | Statistical methods | Effect size |
| Postsurgery reaction | 3 | 184 | Risk ratio (Fixed, M-H, 95% CI) | 0.11 (0.14 to 0.31)* |
| Biological complications | ||||
| Sinus perforation or infection | 3 | 125 | Risk ratio (Fixed, M-H, 95% CI) | 0.11 (0.02 to 0.63)* |
| Implant mobile | 2 | 132 | Risk ratio (Fixed, M-H, 95% CI) | 0.34 (0.06 to 2.06) |
| Peri-implant mucositis and peri-implantitis | 2 | 54 | Risk ratio (Fixed, M-H, 95% CI) | 0.91 (0.14 to 5.79) |
| Technical complications | Risk ratio (Fixed, M-H, 95% CI) | |||
| Screw loosening | 3 | 169 | Risk ratio (Fixed, M-H, 95% CI) | 2.66 (0.93 to 7.60) |
| Crown loosening, decementation or chipping | 5 | 223 | Risk ratio (Random, M-H, 95% CI) | 1.22 (0.33 to 4.49) |
*Difference between the two groups was significant.
M-H, Mantel-Haenszel.
Patient satisfaction
Three studies42 43 46 reported patient satisfaction. Meta-analysis was not conducted because methods of evaluating patient satisfaction were different. Guljé et al 43 used a questionnaire to evaluate patient satisfaction before surgery and 1 year postloading. Both groups showed improvement of satisfaction after crown placement. Gastaldi et al 42 evaluated patient satisfaction in function and aesthetic aspects. All patients in the short implant group (10) were satisfied with both function and aesthetic aspects. However, three patients in elevation group (3/10) were partially satisfied with function. Bechara et al 46 used a questionnaire evaluating patient satisfaction in function, aesthetic, cleaning of the implant-supported restorations, satisfaction and cost. Significantly more patients in short implant group expressed satisfaction in cost. In the other four aspects, no significant difference was found between short implant group and sinus floor elevation group.
Quality of evidence
For survival rate, the quality of evidence in both subgroups was downgraded by one level (moderate quality evidence) due to serious risks of bias. For short-term MBL (1–3 years follow-up), quality of evidence was downgraded by two levels for serious risks of bias and inconsistency. For long-term MBL (3 years or longer follow-up), quality of evidence was downgraded by one level for serious risks of bias. For complications, the quality of evidence in postsurgery reaction was moderate, downgrading by one level for serious risks of bias. The quality of evidence in other complications was low, downgrading by two levels for serious risks of bias and imprecision. Details are listed in table 5.
Table 5.
Summary of findings
| Short implant (≤6 mm) compared with longer implant (≥10 mm) with sinus floor elevation in atrophic posterior maxilla | ||||||
|
Patient or population: atrophic posterior maxilla Intervention: short implant (≤6 mm) Comparison: longer implant (≥10 mm) with sinus floor elevation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk† (elevation group) | Corresponding risk (short implant group) |
|||||
| Survival rate follow-up: range 1–3 years | 961 per 1000 |
970 per 1000
(932 to 999) |
RR 1.01
(0.97 to 1.04) |
321 (7 RCTs) |
⨁⨁⨁◯ MODERATE‡ |
|
| Survival rate follow-up: range 3 years to longer years | 982 per 1000 |
982 per 1000
(953 to 1000) |
RR 1.00
(0.97 to 1.04) |
237 (5 RCTs) |
⨁⨁⨁◯ MODERATE‡ |
|
| Marginal bone loss follow-up: range 1–3 years | The mean marginal bone loss ranged from 0.1 to 1.15 mm | The mean marginal bone loss in the intervention group was 0.13 mm lower (0.21 lower to 0.05 lower) | – | 249 (6 RCTs) |
⨁⨁◯◯ LOW§ |
|
| Marginal bone loss follow-up: range 3 years to longer years | The mean marginal bone loss ranged from 1.08 to 1.5 mm | The mean marginal bone loss in the intervention group was 0.25 mm lower (0.4 lower to 0.1 lower) | – | 88 (3 RCTs) |
⨁⨁⨁◯ MODERATE‡ |
|
| Postsurgery reaction | 307 per 1000 |
34 per 1000
(12 to 59) |
RR 0.11
(0.04 to 0.31) |
184 (3 RCTs) |
⨁⨁⨁◯ MODERATE‡ |
|
| Biological complications: sinus perforation or infection | 197 per 1000 |
20 per 1000
(4 to 113) |
RR 0.11
(0.02 to 0.63) |
125 (3 RCTs) |
⨁⨁◯◯ LOW¶ |
|
| Biological complications: implant mobile | 59 per 1000 |
20 per 1000
(4 to 121) |
RR 0.34
(0.06 to 2.06) |
132 (2 RCTs) |
⨁⨁◯◯ LOW¶ |
|
| Biological complications: peri-implant mucositis or peri-implantitis | 200 per 1000 |
100 per 1000
(10 to 934) |
RR 0.91
(0.14 to 5.79) |
54 (2 RCTs) |
⨁⨁◯◯ LOW¶ |
|
| Technical complications: screw loosening | 81 per 1000 |
217 per 1000
(76 to 916) |
RR 2.66
(0.93 to 7.60) |
169 (3 RCTs) |
⨁⨁◯◯ LOW¶ |
|
| Technical complications: crown loosening, decementation and chipping | 27 per 1000 |
33 per 1000
(9 to 120) |
RR 1.22
(0.33 to 4.49) |
223 (5 RCTs) |
⨁⨁◯◯ LOW¶ |
|
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Assumed risk is based on the overall event rate in the control groups of the included studies.
‡Downgraded one level due to serious risks of bias.
§Downgraded two levels due to serious risks of bias and serious inconsistency.
¶Downgraded two levels due to serious risks of bias and imprecision.
GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCTs, randomised controlled trials; RR, risk ratio.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to compare the clinical outcome of the use of short implants (≤6 mm) in atrophic posterior maxilla versus longer implants with sinus floor elevation. At 1 year or longer postloading, there is no significant difference in participant unit implant survival rate between the short implant group and the elevation group. The short implant group showed less MBL than the elevation group for 1–3 years follow-up (low-quality evidence) and 3 years or longer follow-up (moderate-quality evidence). In addition, the short implant group showed fewer postsurgery reaction and sinus membrane perforation and infection.
The survival rate in this review was evaluated by participant unit as in a previous Cochrane review.34 In this review, the overall survival rates for the short implant group and the elevation group were 98.21% and 96.08%, respectively, at 1–3 years follow-up and 99.20% and 98.23%, respectively, at longer than 3 years follow-up; no significant difference in survival rate was found. Other studies that assessed survival rate in implant unit had similar outcomes. A retrospective study47 with a follow-up period of 17–48 months reported a 95.12% implant unit survival rate for 5–6 mm short implants. A prospective study6 of 2–3 years reported that 6 mm short implants with microrough surfaces achieved a 100% survival rate in posterior maxilla. Another retrospective study of 5–10 years48 reported a 97% implant unit survival rate for 6 mm short implants supporting single crowns. All these results showed that short implants (≤6 mm) represent a promising rehabilitation method with respect to their short-term and long-term survival rates.
In this review, all of the failed short implants were 4 mm or 5 mm. Although the use of short implants (≤6 mm) could avoid complicated surgical procedures and related early failures, reduced implant length was still the major risk factor in survival rate. The authors of the included studies used wider implants (4–8 mm) to compensate for the short length of the implants. Finite element analyses showed that wider implants had increased functional surface area in cortical bone and decreased stress distribution on the implant neck; these qualities helped improve primary stability, produce a higher survival rate and reduce MBL.49–52 However, it was not determined whether implant length or diameter contributed more to implant failure. Another factor was implant surface structure. Studies53–56 have suggested that the implant surface influences bone-to-implant osseointegration, implant primary stability and MBL. In this review, implants 4 or 5 mm in length had novel surface structures, but they still presented a lower survival rate.
Significantly less MBL was found in the short implant group, and the difference was greater at the longer follow-up period. Additionally, in this review, 5 mm diameter implants tended to induce less MBL than 4 mm diameter implants. Implants 10 mm19 and 8 mm20 were reported to induce MBL similar to that of longer implants, while implants 7 mm57 showed less MBL. These results contradict a previous theory that short implants are more likely to have an extreme crown-to-implant ratio (C/I)58 that induces more peri-implant bone loss and early implant failure.59 60 According to finite element analyses, inappropriate C/I results in adverse occlusal forces such as non-axial forces and overloading.61 Increased C/I was also correlated with more prosthesis complications such as screw loosening, implant or abutment fracture, chipping of the ceramic material and prosthesis fracture.62–65 However, the implants in the studies included in this systematic review had wider diameters (4–8 mm) and different surface structures. These two factors partially compensated for the complications of C/I and contributed to less MBL. Differences in implant diameter and surface structure also introduced heterogeneity among studies with respect to MBL. Short implants tolerated less MBL because of the limited implant length. As a result, less MBL was not necessarily correlated with better clinical outcome. MBL around short implants is still a challenging issue, and much effort should be made to resolve it.
With respect to complications, the use of short implants (≤6 mm) could decrease the incidence of postsurgery reactions and sinus membrane perforation and infection. Sinus membrane perforation was common in the elevation group.14 This was in accordance with a previous study66 that reported more complications in cases involving longer implants with sinus floor elevation and that the surgical procedure made a major contribution to such complications. In this study, incidence of other biological and technical complications was similar between the two groups. Implant migration into the sinus, often with the co-occurrence of sinus infection, had a higher prevalence in the elevation group. When implant migration occurs, implants may be removed, thus leading to implant failure.67 Technical complications, including screw loosening, crown loosening and chipping, were mainly associated with inappropriate loading, which could be resolved by improving supra rehabilitation structure. In addition, for short implants (≤6 mm), risks relating to reduced length could be partially alleviated by improving the design of the implants68 or increasing their diameter. With respect to the prevalence and severity of adverse events, the use of short implants (≤6 mm) was acceptable and was a promising alternative to sinus floor elevation.
The present study has several strengths. First, we conducted a comprehensive literature search, and all included studies were RCTs. Second, participant was used as the unit of analysis to ensure logical statistical syntheses and relevant interpretations. Third, subgroup analysis by follow-up length and categories of complications was performed to reduce bias across studies. However, the evidence included in this systematic review was only of moderate or low quality. Serious risks of bias were found within and across studies. The number of participants and the follow-up period were limited. Due to limited data and methodological heterogeneity among studies, data synthesis for patient satisfaction was not performed. We suggest that researchers in this field carry out more well-designed, long-term and large-scale RCTs to provide high-quality evidence regarding the effects of short implants (≤6 mm).
Conclusions
Within its limitations, the present review suggests that the survival rate of maxillary short implants (≤6 mm) was comparable to that of longer implants (≥10 mm) with sinus floor elevation. However, short implants (≤6 mm) show significantly less MBL and postsurgery reactions. Short implants (≤6 mm) are, therefore, the promising alternative to sinus floor elevation for posterior maxilla with insufficient bone volume. Additional high-quality studies are needed to evaluate the long-term effectiveness and safety of short implants (≤6 mm).
Supplementary Material
Footnotes
QY and XW contributed equally.
Contributors: Conception and design of the review: FH and BS. Methodology: FH. Drafting protocol, performing search strategies, literature searches, literature screening and data extraction: QY and XW. Original draft preparation: XW. Reviewing and editing draft: MS, QY, FH and BS. All authors critically reviewed and revised the manuscript and approved the final version for publication.
Funding: This research was funded by the National Clinical Key Specialties Construction Project ((2013)544), the National Health Commission of China.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: There are no data in this work.
References
- 1. Spitznagel FA, Horvath SD, Gierthmuehlen PC. Prosthetic protocols in implant-based oral rehabilitations: a systematic review on the clinical outcome of monolithic all-ceramic single- and multi-unit prostheses. Eur J Oral Implantol 2017;10 Suppl 1:89–99. [PubMed] [Google Scholar]
- 2. Pjetursson BE, Thoma D, Jung R, et al. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin Oral Implants Res 2012;23 Suppl 6:22–38. 10.1111/j.1600-0501.2012.02546.x [DOI] [PubMed] [Google Scholar]
- 3. Davarpanah M, Martinez H, Etienne D, et al. A prospective multicenter evaluation of 1,583 3i implants: 1- to 5-year data. International Journal of Oral & Maxillofacial Implants 2002;17:820–8. [PubMed] [Google Scholar]
- 4. Esposito M, Felice P, Worthington HV. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. John Wiley & Sons, Ltd, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morand M, Irinakis T. The challenge of implant therapy in the posterior maxilla: providing a rationale for the use of short implants. Journal of Oral Implantology 2007;33:257–66. 10.1563/1548-1336(2007)33[257:TCOITI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 6. Malchiodi L, Caricasulo R, Cucchi A, et al. Evaluation of ultrashort and longer implants with Microrough surfaces: results of a 24- to 36-month prospective study. Int J Oral Maxillofac Implants 2017;32:171–9. 10.11607/jomi.4648 [DOI] [PubMed] [Google Scholar]
- 7. Danesh-Sani SA, Loomer PM, Wallace SS. A comprehensive clinical review of maxillary sinus floor elevation: anatomy, techniques, biomaterials and complications. Br J Oral Maxillofac Surg 2016;54:724–30. 10.1016/j.bjoms.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 8. Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977;16:1–132. [PubMed] [Google Scholar]
- 9. Cha H-S, Kim J-W, Hwang J-H, et al. Frequency of bone graft in implant surgery. Maxillofac Plast Reconstr Surg 2016;38 10.1186/s40902-016-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starch-Jensen T, Aludden H, Hallman M, et al. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int J Oral Maxillofac Surg 2018;47:103–16. 10.1016/j.ijom.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 11. Starch-Jensen T, Schou S. Maxillary sinus membrane elevation with simultaneous installation of implants without the use of a graft material: a systematic review. Implant Dent 2017;26:621–33. 10.1097/ID.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 12. Lee S-A, Lee C-T, Fu MM, et al. Systematic review and meta-analysis of randomized controlled trials for the management of limited vertical height in the posterior region: short implants (5 to 8 mm) vs longer implants (> 8 mm) in vertically augmented sites. Int J Oral Maxillofac Implants 2014;29:1085–97. 10.11607/jomi.3504 [DOI] [PubMed] [Google Scholar]
- 13. Pjetursson BE, Tan WC, Zwahlen M, et al. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol 2008;35:216–40. 10.1111/j.1600-051X.2008.01272.x [DOI] [PubMed] [Google Scholar]
- 14. Tan WC, Lang NP, Zwahlen M, et al. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation Part II: transalveolar technique. J Clin Periodontol 2008;35:241–54. 10.1111/j.1600-051X.2008.01273.x [DOI] [PubMed] [Google Scholar]
- 15. Thoma DS, Zeltner M, Hüsler J, et al. EAO Supplement Working Group 4 - EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: a systematic review. Clinical Oral Implants Res 2015;26:154–69. 10.1111/clr.12615 [DOI] [PubMed] [Google Scholar]
- 16. Lundgren S, Cricchio G, Hallman M, et al. Sinus floor elevation procedures to enable implant placement and integration: techniques, biological aspects and clinical outcomes. Periodontology 2000 2017;73:103–20. 10.1111/prd.12165 [DOI] [PubMed] [Google Scholar]
- 17. Huang H-L, Fuh L-J, Ko C-C, et al. Biomechanical effects of a maxillary implant in the augmented sinus: a three-dimensional finite element analysis. Int J Oral Maxillofac Implants 2009;24:455–62. [PubMed] [Google Scholar]
- 18. Lin Y-H, Yang Y-C, Wen S-C, et al. The influence of sinus membrane thickness upon membrane perforation during lateral window sinus augmentation. Clin Oral Implants Res 2016;27:612–7. 10.1111/clr.12646 [DOI] [PubMed] [Google Scholar]
- 19. Monje A, Suarez F, Galindo-Moreno P, et al. A systematic review on marginal bone loss around short dental implants (<10 mm) for implant-supported fixed prostheses. Clin Oral Implants Res 2014;25:1119–24. 10.1111/clr.12236 [DOI] [PubMed] [Google Scholar]
- 20. Nielsen HB, Schou S, Isidor F, et al. Short implants (≤8mm) compared to standard length implants (>8mm) in conjunction with maxillary sinus floor augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2019;48:239–49. 10.1016/j.ijom.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 21. Uehara PN, Matsubara VH, Igai F, et al. Short Dental Implants (≤7mm) Versus Longer Implants in Augmented Bone Area: A Meta-Analysis of Randomized Controlled Trials. Open Dent J 2018;12:354–65. 10.2174/1874210601812010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al Amri MD, Abduljabbar TS, Al-Johany SS, et al. Comparison of clinical and radiographic parameters around short (6 to 8 mm in length) and long (11 mm in length) dental implants placed in patients with and without type 2 diabetes mellitus: 3-year follow-up results. Clin Oral Implants Res 2017;28:1182–7. 10.1111/clr.12938 [DOI] [PubMed] [Google Scholar]
- 23. Urdaneta RA, Daher S, Leary J, et al. The survival of ultrashort locking-taper implants. Int J Oral Maxillofac Implants 2012;27:644–54. [PubMed] [Google Scholar]
- 24. Markose J, Eshwar S, Srinivas S, et al. Clinical outcomes of ultrashort sloping shoulder implant design: a survival analysis. Clin Implant Dent Relat Res 2018;20:646–52. 10.1111/cid.12608 [DOI] [PubMed] [Google Scholar]
- 25. Fan T, Li Y, Deng W-W, et al. Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta-Analysis of RCTs. Clin Implant Dent Relat Res 2017;19:207–15. 10.1111/cid.12432 [DOI] [PubMed] [Google Scholar]
- 26. Deporter D, Ogiso B, Sohn D-S, et al. Ultrashort sintered porous-surfaced dental implants used to replace posterior teeth. J Periodontol 2008;79:1280–6. 10.1902/jop.2008.070496 [DOI] [PubMed] [Google Scholar]
- 27. Sahrmann P, Naenni N, Jung RE, et al. Success of 6-mm implants with Single-Tooth restorations: a 3-year randomized controlled clinical trial. Journal of Dental Research 2016;95:623–8. [DOI] [PubMed] [Google Scholar]
- 28. Felice P, Checchi L, Barausse C, et al. Posterior jaws rehabilitated with partial prostheses supported by 4.0 X 4.0 mm or by longer implants: one-year post-loading results from a multicenter randomised controlled trial. Eur J Oral Implantol 2016;9:35–45. [PubMed] [Google Scholar]
- 29. Bolle C, Felice P, Barausse C, et al. 4 MM long vs longer implants in augmented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial. Eur J Oral Implantol 2018;11:31–47. [PubMed] [Google Scholar]
- 30. Calvo-Guirado JL, López Torres JA, Dard M, et al. Evaluation of extrashort 4-mm implants in mandibular edentulous patients with reduced bone height in comparison with standard implants: a 12-month results. Clin Oral Implants Res 2016;27:867–74. 10.1111/clr.12704 [DOI] [PubMed] [Google Scholar]
- 31. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JPT GS. Cochrane Handbook for systematic reviews of interventions version 5.1.0: the Cochrane collaboration, 2011. Available: www.cochrane-handbook.org
- 33. Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj British Medical Journal 1997;316:469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esposito M, Ardebili Y, Worthington HV, et al. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst Rev 2014;26 10.1002/14651858.CD003815.pub4 [DOI] [PubMed] [Google Scholar]
- 35. Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Method 2010;1:97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 36. Esposito M, Grusovin MG, Maghaireh H, et al. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database of Systematic Reviews 2013;16 10.1002/14651858.CD003878.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esposito M, Worthington HV, Cochrane Oral Health Group . Interventions for replacing missing teeth: hyperbaric oxygen therapy for irradiated patients who require dental implants. Cochrane Database Syst Rev 2013;43 10.1002/14651858.CD003603.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esposito M, Felice P, Worthington HV, et al. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database of Systematic Reviews 2014;2 10.1002/14651858.CD008397.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tolentino da Rosa de Souza P, Binhame Albini Martini M, Reis Azevedo-Alanis L. Do short implants have similar survival rates compared to standard implants in posterior single crown?: a systematic review and meta-analysis. Clin Implant Dent Relat Res 2018;20:890–901. 10.1111/cid.12634 [DOI] [PubMed] [Google Scholar]
- 40. Andrews J, Guyatt G, Oxman AD, et al. Grade guidelines: 14. going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25. 10.1016/j.jclinepi.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 41. Gastaldi G, Felice P, Pistilli V, et al. Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. 3-year results from a randomised controlled trial. Eur J Oral Implantol [PubMed] [Google Scholar]
- 42. Gastaldi G, Felice P, Pistilli R, et al. Short implants as an alternative to crestal sinus lift: a 3-year multicentre randomised controlled trial. Eur J Oral Implantol 2017;10:391–400. [PubMed] [Google Scholar]
- 43. Guljé FL, Raghoebar GM, Vissink A, et al. Single Crowns in the resorbed posterior maxilla supported by either 6-mm implants or by 11-mm implants combined with sinus floor elevation surgery: a 1-year randomised controlled trial. Eur J Oral Implantol 2014;7:247–55. [PubMed] [Google Scholar]
- 44. Pohl V, Thoma DS, Sporniak-Tutak K, et al. Short dental implants (6 mm) versus long dental implants (11-15 mm) in combination with sinus floor elevation procedures: 3-year results from a multicentre, randomized, controlled clinical trial. J Clin Periodontol 2017;44:438–45. 10.1111/jcpe.12694 [DOI] [PubMed] [Google Scholar]
- 45. Felice P, Barausse C, Pistilli V, et al. Posterior atrophic jaws rehabilitated with prostheses supported by 6 MM long × 4 MM wide implants or by longer implants in augmented bone. 3-year post-loading results from a randomised controlled trial. Eur J Oral Implantol 2018;11:175–87. [PubMed] [Google Scholar]
- 46. Bechara S, Kubilius R, Veronesi G, et al. Short (6-mm) dental implants versus sinus floor elevation and placement of longer (≥10-mm) dental implants: a randomized controlled trial with a 3-year follow-up. Clin Oral Implants Res 2017;28:1097–107. 10.1111/clr.12923 [DOI] [PubMed] [Google Scholar]
- 47. Lombardo G, Pighi J, Marincola M, et al. Cumulative success rate of short and ultrashort implants supporting single Crowns in the posterior maxilla: a 3-year retrospective study. Int J Dent 2017;2017:1–10. 10.1155/2017/8434281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai H-C, Si M-S, Zhuang L-F, et al. Long-term outcomes of short dental implants supporting single crowns in posterior region: a clinical retrospective study of 5-10 years. Clin Oral Implants Res 2013;24:230–7. 10.1111/j.1600-0501.2012.02452.x [DOI] [PubMed] [Google Scholar]
- 49. Himmlová L, Dostálová Tat'jana, Kácovský A, et al. Influence of implant length and diameter on stress distribution: a finite element analysis. J Prosthet Dent 2004;91:20–5. 10.1016/j.prosdent.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 50. Moriwaki H, Yamaguchi S, Nakano T, et al. Influence of implant length and diameter, Bicortical anchorage, and sinus augmentation on bone stress distribution: three-dimensional finite element analysis. Int J Oral Maxillofac Implants 2016;31:e84–91. 10.11607/jomi.4217 [DOI] [PubMed] [Google Scholar]
- 51. Kim S, Jung U-W, Cho K-S, et al. Retrospective radiographic observational study of 1692 Straumann tissue-level dental implants over 10 years: I. implant survival and loss pattern. Clin Implant Dent Relat Res 2018;20:860–6. 10.1111/cid.12659 [DOI] [PubMed] [Google Scholar]
- 52. Lin G, Ye S, Liu F, et al. A retrospective study of 30,959 implants: risk factors associated with early and late implant loss. J Clin Periodontol 2018;45:733–43. 10.1111/jcpe.12898 [DOI] [PubMed] [Google Scholar]
- 53. Tabassum A, Meijer GJ, Wolke JGC, et al. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: a laboratory study. Clin Oral Implants Res 2009;20:327–32. 10.1111/j.1600-0501.2008.01692.x [DOI] [PubMed] [Google Scholar]
- 54. Dos Santos MV, Elias CN, Cavalcanti Lima JH. The effects of superficial roughness and design on the primary stability of dental implants. Clin Implant Dent Relat Res 2011;13:215–23. 10.1111/j.1708-8208.2009.00202.x [DOI] [PubMed] [Google Scholar]
- 55. Falco A, Berardini M, Trisi P. Correlation between implant geometry, implant surface, insertion torque, and primary stability: in vitro biomechanical analysis. Int J Oral Maxillofac Implants 2018;33:824–30. 10.11607/jomi.6285 [DOI] [PubMed] [Google Scholar]
- 56. Jain N, Gulati M, Garg M, et al. Short implants: new horizon in implant dentistry. J Clin Diagn Res 2016;10:Ze14–17. 10.7860/JCDR/2016/21838.8550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Uehara PN, Matsubara VH, Igai F, et al. Short dental implants (≤7mm) versus longer implants in augmented bone area: a meta-analysis of randomized controlled trials. Open Dent J 2018;12:354–65. 10.2174/1874210601812010354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anitua E, Alkhraist MH, Piñas L, et al. Implant survival and crestal bone loss around extra-short implants supporting a fixed denture: the effect of crown height space, crown-to-implant ratio, and offset placement of the prosthesis. Int J Oral Maxillofac Implants 2014;29:682–9. 10.11607/jomi.3404 [DOI] [PubMed] [Google Scholar]
- 59. Quirynen M, Naert I, van Steenberghe D. Fixture design and overload influence marginal bone loss and future success in the BranemarkR system. Clin Oral Implants Res 1992;3:104–11. 10.1034/j.1600-0501.1992.030302.x [DOI] [PubMed] [Google Scholar]
- 60. Blanes RJ. To what extent does the crown-implant ratio affect the survival and complications of implant-supported reconstructions? A systematic review. Clin Oral Implants Res 2009;20:67–72. 10.1111/j.1600-0501.2009.01762.x [DOI] [PubMed] [Google Scholar]
- 61. Verri FR, Batista VEdeS, Santiago JF, et al. Effect of crown-to-implant ratio on peri-implant stress: a finite element analysis. Materials Science and Engineering: C 2014;45:234–40. 10.1016/j.msec.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 62. Quaranta A, Piemontese M, Rappelli G, et al. Technical and biological complications related to crown to implant ratio. Implant Dent 2014;23:180–7. 10.1097/ID.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 63. Urdaneta RA, Rodriguez S, McNeil DC, et al. The effect of increased crown-to-implant ratio on single-tooth locking-taper implants. Int J Oral Maxillofac Implants 2010;25:729–43. [PubMed] [Google Scholar]
- 64. Tawil G, Aboujaoude N, Younan R. Influence of prosthetic parameters on the survival and complication rates of short implants. International Journal of Oral & Maxillofacial Implants 2006;21:275–82. [PubMed] [Google Scholar]
- 65. Schneider D, Witt L, Hämmerle CHF. Influence of the crown-to-implant length ratio on the clinical performance of implants supporting single crown restorations: a cross-sectional retrospective 5-year investigation. Clin Oral Implants Res 2012;23:169–74. 10.1111/j.1600-0501.2011.02230.x [DOI] [PubMed] [Google Scholar]
- 66. Thoma DS, Cha J-K, Jung U-W. Treatment concepts for the posterior maxilla and mandible: short implants versus long implants in augmented bone. J Periodontal Implant Sci 2017;47:2–12. 10.5051/jpis.2017.47.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Manor Y, Anavi Y, Gershonovitch R, et al. Complications and management of implants migrated into the maxillary sinus. Int J Periodontics Restorative Dent 2018;38:e112–8. 10.11607/prd.3328 [DOI] [PubMed] [Google Scholar]
- 68. Mangano F, Frezzato I, Frezzato A, et al. The effect of Crown-to-Implant ratio on the clinical performance of Extra-Short Locking-Taper implants. J Craniofac Surg 2016;27:675–81. 10.1097/SCS.0000000000002562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029826supp001.pdf (63.8KB, pdf)