Abstract
Objectives
Modifying lifestyle can prevent the progression of chronic kidney disease (CKD) but the specific elements which lead to favourable behaviour change are not well understood. We aimed to identify and evaluate behaviour change techniques and functions in lifestyle interventions for preventing the progression of CKD.
Design
Systematic review.
Data sources
MEDLINE, EMBASE, CINAHL and PsycINFO.
Eligibility criteria
Trials of lifestyle behaviour change interventions (including diet, physical activity, smoking and/or alcohol) published to September 2018 in adults with CKD stages 1–5.
Data extraction and synthesis
Trial characteristics including population, sample size, study setting, intervention, comparator, outcomes and study duration, were extracted. Study quality was independently assessed by two reviewers using the Cochrane risk of bias tool. The Behaviour Change Technique Taxonomy v1 was used to identify behaviour change techniques (eg, goal setting) and the Health Behaviour Change Wheel was used to identify intervention functions (eg, education). Both were independently assessed by three reviewers.
Results
In total, 26 studies involving 4263 participants were included. Risk of bias was high or unclear in most studies. Interventions involved diet (11), physical activity (8) or general lifestyle (7). Education was the most frequently used function (21 interventions), followed by enablement (18), training (12), persuasion (4), environmental restructuring (4), modelling (2) and incentivisation (2). The most common behaviour change techniques were behavioural instruction (23 interventions), social support (16), behavioural demonstration (13), feedback on behaviour (12) and behavioural practice/rehearsal (12). Eighteen studies (69%) showed a significant improvement in at least one primary outcome, all of which included education, persuasion, modelling and incentivisation.
Conclusion
Lifestyle behaviour change interventions for CKD patients frequently used education, goal setting, feedback, monitoring and social support. The most promising interventions included education and used a variety of intervention functions (persuasion, modelling and incentivisation).
PROSPERO registration number
CRD42019106053.
Keywords: chronic kidney disease (CKD), lifestyle, diet, exercise, behaviour change techniques, health behaviour change wheel, Behaviour Change Technique Taxonomy v1, systematic review
Strengths and limitations of this study.
We used comprehensive, evidence-based frameworks to identify and describe behaviour change techniques and intervention functions in lifestyle behavioural interventions for patients with chronic kidney disease.
Coding of behaviour change techniques and intervention functions was systematically and independently conducted by three researchers, and risk of bias was assessed.
Summary estimates could not be ascertained due to the heterogeneity of interventions and outcome measures.
Introduction
Preventing the progression of chronic kidney disease (CKD) is a high priority for patients and clinicians, to reduce the requirement for dialysis.1–3 Lifestyle interventions which modify behavioural risk factors such as poor diet and low physical activity can prevent progression of CKD and life-threatening complications and improve quality of life and survival.4–6 Addressing behaviour change is particularly relevant in CKD as lifestyle modification can be challenging. Poor adherence to diet, medication and other treatments is common in CKD.7 Barriers to modifying lifestyle include low health literacy, conflicts with cultural norms, complicated nutritional requirements and safety concerns.7–11
Guidelines recommend the explicit use of behaviour change for addressing lifestyle risk factors when designing and reporting interventions for patients with CKD.12 13 However, it is uncertain which aspects of lifestyle behaviour change interventions are the most effective, and reporting of behavioural components is often unclear, making implementation in practice problematic.
The Behaviour Change Technique Taxonomy v1 was developed to provide a comprehensive framework that integrates behaviour change techniques used in interventions.14 The Taxonomy was further synthesised into a framework, the Health Behaviour Change Wheel which describes the intervention functions necessary to change health behaviours.15 The Health Behaviour Change Wheel provides a broad, overarching framework in which to characterise behaviour change interventions while the Taxonomy identifies specific techniques related to individual behaviours. The intervention functions described in the Health Behaviour Change Wheel can be delivered by a variety of behaviour change techniques. For example, the intervention function, ‘education’, outlined in the Wheel, can include the behaviour change techniques ‘instruction on how to perform the behaviour’ and ‘information about antecedents’, detailed in the Taxonomy. Similarly, the intervention function ‘incentivisation’ can incorporate techniques such as ‘feedback on behaviour’ and ‘rewards’.
Behaviour change interventions using the Wheel and the Taxonomy can effectively change lifestyle behaviours. For example, a text-messaging and pedometer programme improved physical activity in people at high risk of type 2 diabetes,16 a digital healthy eating programme increased consumption of fruit and vegetables and sustained this over a 6-month period17 and a digital behaviour change programme achieved significant weight loss results in individuals at risk of type 2 diabetes.18 The Taxonomy and the Wheel are recommended approaches to modify lifestyle risk factors for chronic disease prevention.12 16 18 However, these frameworks have not been used in designing and reporting behaviour change strategies in lifestyle interventions for patients with CKD.
We aimed to identify and evaluate behaviour change techniques and intervention functions used in lifestyle interventions for preventing the progression of CKD. This may inform the development of effective and replicable behaviour change interventions for the prevention of CKD, leading to improvements in patient outcomes.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement19 and checklist to report this systematic review (online supplementary file S1).
bmjopen-2019-031625supp001.pdf (64.7KB, pdf)
Selection criteria
We included randomised trials of lifestyle behaviour change interventions (including, but not restricted to diet, physical activity, smoking and alcohol consumption) in adult patients (aged over 18 years) with CKD stages 1–5 and not requiring renal replacement therapy. We did not apply restrictions based on outcomes or language. Studies including a combination of pharmacological therapy and lifestyle were included but trials involving only pharmacological therapies were excluded.
Literature search
A comprehensive search was conducted in MEDLINE (1946 to 20 September 2018), EMBASE (1996 to 20 September 2018), CINAHL (1982 to 20 September 2018) and PsycINFO (1806 to 20 September 2018) using Medical Subject Heading (MeSH) terms relating to CKD, and lifestyle behaviour change interventions (online supplementary file S2), and reference lists of relevant articles and reviews. Author NE screened the studies by title and abstract and assessed full-text articles for eligibility. Those that did not meet the inclusion criteria were excluded.
bmjopen-2019-031625supp002.pdf (48.9KB, pdf)
Data extraction and critical appraisal
The trial characteristics relevant to the population, sample size and study setting as well as intervention (type, mode of delivery, use of theory, intervention functions (as described in the Health Behaviour Change Wheel15 and behaviour change techniques (as described in the Behaviour Change Technique Taxonomy v114)), comparator, outcomes and study duration, were extracted and tabulated. We assessed the risk of bias using the Cochrane tool for randomised studies.20 NE and KM assessed the risk of bias in each study independently and any differences were resolved by discussion.
We contacted the authors of the studies when it was necessary to gather additional information. Supplemental data was available in 12 of the 26 studies. In six studies with no supplemental data, sufficient information was available in the published article. Therefore, we contacted eight authors to request further information and received responses from two authors.
Analysis of intervention functions and behaviour change techniques
The Behaviour Change Technique Taxonomy v1 (the ‘Taxonomy’) and Health Behaviour Change Wheel (the ‘Wheel’) are comprehensive tools for identifying behavioural components in interventions and how frequently they occur.14 15 The two frameworks are complementary and in addition to designing interventions, they have been used as a method for identifying behavioural components in public health interventions and clinical trials.21 The tools have been used in previous systematic reviews to identify behaviour change techniques and functions in health interventions.22–28
Behaviour change techniques
The Behaviour Change Technique Taxonomy consists of 93 behaviour change techniques, such as goal-setting, self-monitoring, social support and re-structuring the physical environment (see online supplementary table S1 for the full taxonomy). The techniques are grouped into 16 domains: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, comparison of outcomes, reward and threat, regulation, antecedents, identity, scheduled consequences, self-belief and covert learning.
bmjopen-2019-031625supp003.pdf (301.9KB, pdf)
Intervention functions
There are nine intervention functions in the Wheel: education, persuasion, incentivisation, coercion, training, enablement, modelling, environmental restructuring and restrictions.15 These are activities designed to change behaviours and include one or more behaviour change techniques. Definitions of each intervention function have been described by Michie et al and were used to inform decisions about what functions were present in each study.15
Authors NE and KM completed online training for interpreting the Wheel and the Taxonomy to ensure consistency and reliability of coding.29 N.E, KM and VS independently read intervention descriptions line-by-line to locate text matching a definition of an intervention function15 and the description of behaviour change techniques from the BCTTv1 coding frame (online supplementary table S1). Each of the 93 behaviour change techniques were indicated as either present or absent in a standardised data extraction form. A behaviour change technique had to be explicitly described to be coded and included in the analysis. The authors compared the codes and discussed discrepancies to reach consensus.
Patient and public involvement
No patient involved.
Results
Literature search and study characteristics
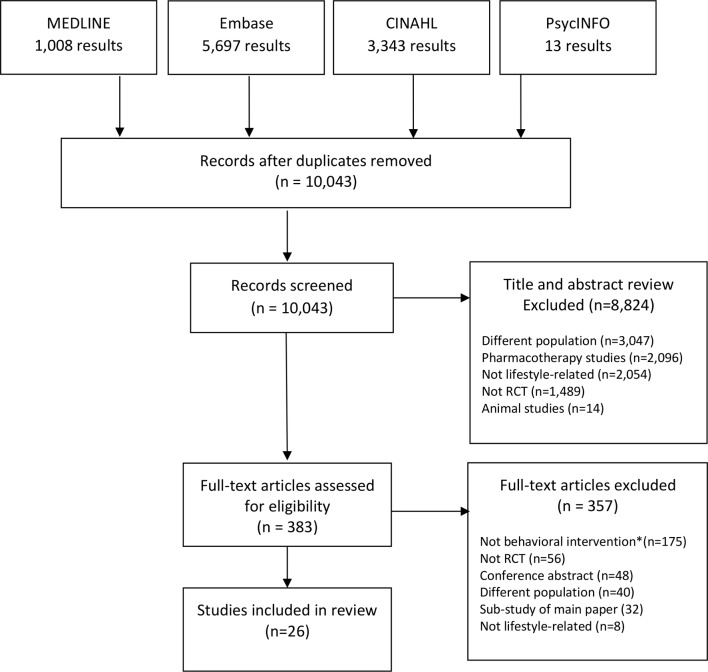
The literature search yielded 10 043 citations from which 26 studies (n=4263 participants) were eligible and included in the review (figure 1). Study characteristics are shown in table 1. The studies were conducted in 15 countries.
Figure 1.
PRISMA flowchart of included/excluded studies. *A behavioural intervention explicitly describes a behaviour change technique which can be coded using the Behavior Change Technique Taxonomy v1. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Table 1.
Characteristics of included studies
| Study | N | CKD Stage | Age (years) | Country | Intervention | Comparator | Primary Outcomes | Study duration (months) |
| Dietary interventions | ||||||||
| Campbell et al 38 | 56 | CKD4–5 | >18 | Australia | Individualised nutritional counselling and regular follow-up | Usual care | Body composition | 3 |
| Clark et al 47 | 590 | CKD3 | 18–80 | Canada | Coaching to increase water intake (drinking containers and water vouchers also provided) | Coaching to maintain usual fluid intake | Change in eGFR | 12 |
| De Brito-Ashurst et al 37 | 56 | eGFR <60 mL and BP >130/80 or taking BP medication; Bangladeshi origin | 18–74 | United Kingdom | Community cooking education sessions facilitated by Bengali workers | Usual care | Reduction in systolic/diastolic BP | 6 |
| Dussol et al 61 | 63 | Type I/II diabetic nephropathy, eGFR60-100 mL | 40–72 | France | Low-protein diet with telephone calls every 6 weeks to help change dietary habits | Usual-protein diet | Decline GFR and 24-hour albumin excretion rate | 24 |
| MDRD Study (1995)* | 840 | eGFR 13–55 mL | 18–70 | United States | Low protein diet with dietician support | Moderate, low and very low protein diets compared | Decline eGFR, dietary satisfaction | 45 |
| Mekki et al 62 | 40 | eGFR 60–90 mL | 47–75 | Algeria | Nutritional advice based on Mediterranean diet | Usual care | Dyslipidaemia | 3 |
| Meuleman et al 32 | 138 | eGFR ≥20 mL | ≥18 | The Netherlands | Sodium restricted diet with self-management, education, motivational interviewing and self-monitoring | Usual care | Sodium excretion & BP | 3 |
| Paes-Barreto et al 46 | 89 | CKD3–5 | ≥18 | Brazil | Intense counselling/education on low protein diet | Standard counselling | Change in protein intake | 4 |
| Pisani et al 42 | 57 | CKD3b–5 | >18 | Italy | Low protein, phosphate and sodium diet, ‘6-tips diet’ checklist | Non-individualised, moderately low protein diet | Protein intake, metabolic parameters and adherence | 6 |
| Rosman e t al 63 | 247 | CrCl 10–60 mL/min | 15–73 | The Netherlands | Dietary protein restriction and dietician visits every 3 months | Usual care | Adherence | 24 |
| Saran e t al 64 | 58 | CKD3–4 | >18 | United States | Dietary sodium restriction (<2 g sodium per day) | Usual diet | Change in hydration status | 1 |
| Physical activity interventions | ||||||||
| Aoike et al 59 | 29 | CKD3–4 | 18–70 | Brazil | Home-based moderate-intensity aerobic exercise programme | Usual care | Cardiopulmonary/functional, BP, CrCl, eGFR | 3 |
| Barcellos et al 65 | 150 | CKD2–4 | >18 | Brazil | Aerobic and resistance training | Usual care | Change in eGFR | 4 |
| Greenwood et al 43 | 20 | CKD3–4 | 18–80 | United Kingdom | Resistance and aerobic training (3 days per week) | Usual care | Change in eGFR | 12 |
| Kao et al 30 | 94 | eGFR ≥15 mL | ≥39 | Taiwan | Group education lecture; individual exercise programme Trans-Theoretical Model | Not specified | Exercise behaviour, depression, fatigue | 3 |
| Leehey et al 66 | 32 | CKD2–4 | 49–81 | United States | Aerobic & resistance training, home exercise (plus dietary management) | Dietary management | Urine protein to creatinine ratio | 12 |
| Rossi et al 45 | 107 | CKD3–4 | ≥18 | United States | Guided exercise twice a week plus usual care | Usual care | Physical function, quality of life | 3 |
| Tang et al 49 | 90 | CKD1–3 | 18–70 | China | Individualised exercise programme (education and home-based aerobic exercise) | Usual care | Physical function, self-efficacy, anxiety, depression, quality of life | 3 |
| Van Craenenbroeck et al 34 | 40 | CKD3–4 | ≥18 | Belgium | Home-based aerobic training programme (four daily cycling sessions, 10 min each) | Usual care | Peripheral endothelial function | 3 |
| Lifestyle interventions | ||||||||
| Flesher et al 39 | 40 | CKD3–4 | 18–80 | Canada | Individual dietary counselling, group nutrition and cooking classes, exercise programme | Usual care | Composite eGFR, TC, urinary sodium, urinary protein and BP | 12 |
| Howden et al 40 | 83 | CKD3–4 | 18–75 | Australia | Multi-disciplinary care, lifestyle and aerobic/resistance training | Usual care | Change in CRF | 12 |
| Ishani et al 41 | 601 | eGFR <60 | >18 | USA | Care by a multi-disciplinary team using a telehealth device | Usual care | Composite death, hospitalisation, emergency visits and admission to a nursing facility | 20 |
| Jiamjariyapon et al 67 | 442 | CKD3–4 | 18–70 | Thailand | Integrated care by multi-disciplinary team and community care workers. Group counselling, home visits | Usual care | Change in eGFR | 24 |
| Joboshi and Oka44 | 65 | Overt proteinuria and clinically diagnosed CKD | 38–86 | Japan | Self-management programme | Standard education | Self-efficacy and self-management behaviour | 3 |
| Patil et al 68 | 76 | Diabetic nephropathy | 30–70 | India | Low-calorie diet, physical activity and behaviour | ACE inhibitor therapy | 24-hour urine protein BMI | 6 |
| Teng et al 31 | 160 | eGFR ≥30 mL/min/1.73 m2 | ≥20 | Taiwan | Lifestyle modification programme based on Trans-Theoretical Model | Standard education | Health behaviours, knowledge, physical function | 12 |
BMI, Body Mass Index; BP, blood pressure;CKD, chronic kidney disease; CrCl, creatinine clearance; CRF, cardiorespiratory fitness; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease study; TC, total cholesterol.
Risk of bias assessment
Overall, the reporting of studies was relatively incomplete, particularly for the blinding of participants and personnel which was missing or unclear in every study (figure 2). Allocation concealment was unclear or at high risk of bias in 20 (77%) studies. Blinding of outcome assessment was also poorly reported with 19 studies showing high or unclear risk of bias for this domain. Domains that performed better were selective reporting with low risk of bias in 21 studies, random sequence generation with low risk of bias in 17 studies and incomplete outcome data showing low risk of bias in 13 studies.
Figure 2.
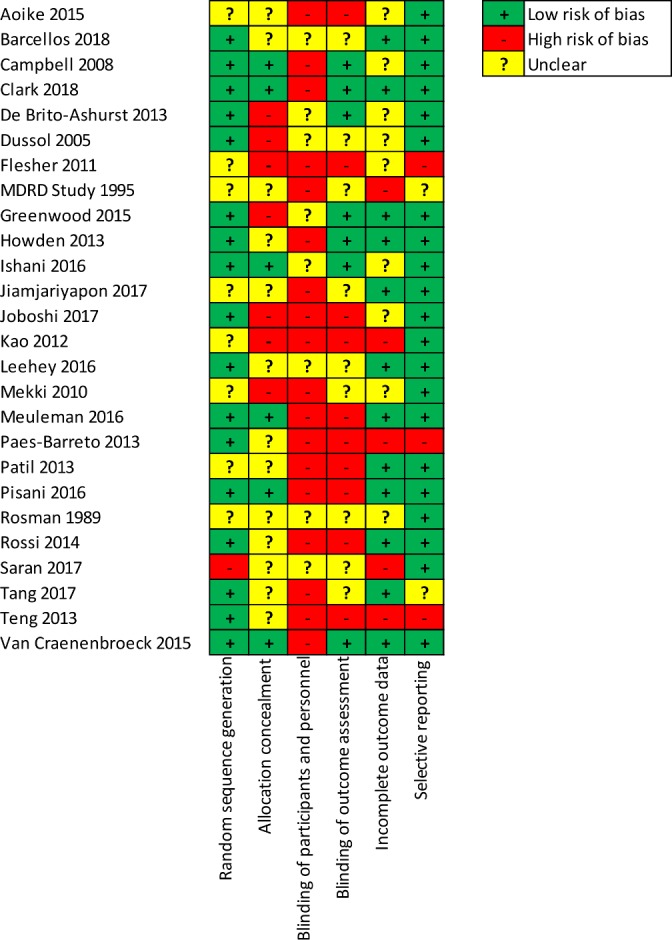
Risk of bias for individual studies (n=26). MDRD, Modification of Diet in Renal Disease study.
Characteristics of the interventions
Across the interventions assessed in the 26 studies included, 11 were dietary interventions, 8 involved physical activity and 7 used any combination of diet, physical activity, weight reduction and/or smoking cessation (lifestyle).
Five studies were informed by theory, three used the Trans-Theoretical Model,30 31 one used self-regulation theory32 and another was informed by contemporary behavioural theory, in particular the self-management approach.33 Two studies used Motivational Interviewing,34 35 a counselling approach which involves behaviour change strategies.36
Only three studies included family members, friends or partners in the intervention to facilitate participant’s behaviour change (online supplementary table S2).31 37
bmjopen-2019-031625supp004.pdf (64.8KB, pdf)
Behaviour change techniques
Table 2 outlines the number of behaviour change techniques present in each lifestyle behaviour change intervention. The number of behaviour change techniques used across interventions ranged from two to 20.
Table 2.
Cross matrix of behaviour change techniques and lifestyle behaviour change trials
| Meuleman et al 32 | MDRD Study (1995)* | De Brito-Ashurst et al 37 | Paes-Barreto et al 46 | Campbell et al 38 | Rosman et al 63 | Dussol et al 61 | Pisani et al 42 | Saran et al 64 | Clark et al 47 | Mekki et al 62 | Tang et al 49 | Kao et al 30 | Greenwood et al 43 | Rossi et al 45 | Aoike et al 59 | Barcellos e t al 65 | Van Craenenbroeck et al 34 | Leehey et al 66 | Howden et al 40 | Ishani et al 41 | Joboshi and Oka44 | Teng et al 31 | Flesher et al 39 | Jiamjariyaponet al 67 | Patil et al 68 | |
| Diet | Physical Activity | Lifestyle | ||||||||||||||||||||||||
| 1.Goals and planning | ||||||||||||||||||||||||||
| 1.1. Goal setting (behaviour) | ||||||||||||||||||||||||||
| 1.2. Problem solving | ||||||||||||||||||||||||||
| 1.3. Goal setting (outcome) | ||||||||||||||||||||||||||
| 1.4. Action planning | ||||||||||||||||||||||||||
| 1.5. Review behaviour goal(s) | ||||||||||||||||||||||||||
| 1.7. Review outcome goal(s) | ||||||||||||||||||||||||||
| 1.8. Behavioural contract | ||||||||||||||||||||||||||
| 1.9. Commitment | ||||||||||||||||||||||||||
| 2.Feedback and monitoring | ||||||||||||||||||||||||||
| 2.1. Monitoring of behaviour by others without feedback | ||||||||||||||||||||||||||
| 2.2. Feedback on behaviour | ||||||||||||||||||||||||||
| 2.3. Self-monitoring of behaviour | ||||||||||||||||||||||||||
| 2.4. Self-monitoring of outcome(s) of behaviour | ||||||||||||||||||||||||||
| 2.6. Biofeedback | ||||||||||||||||||||||||||
| 2.7. Feedback on outcome(s) of behaviour | ||||||||||||||||||||||||||
| 3. Social support | ||||||||||||||||||||||||||
| 3.1. Social support (unspecified) | ||||||||||||||||||||||||||
| 3.2. Social support (practical) | ||||||||||||||||||||||||||
| 3.3. Social support (emotional) | ||||||||||||||||||||||||||
| 4. Shaping knowledge | ||||||||||||||||||||||||||
| 4.1. Instruction on behaviour | ||||||||||||||||||||||||||
| 4.4. Behavioural experiments | ||||||||||||||||||||||||||
| 5.Natural consequences | ||||||||||||||||||||||||||
| 5.1. Information about health consequences | ||||||||||||||||||||||||||
| 5.2. Salience of consequences | ||||||||||||||||||||||||||
| 5.4. Monitoring of emotional consequences | ||||||||||||||||||||||||||
| 6.Comparison of behaviour | ||||||||||||||||||||||||||
| 6.1. Demonstration of the behaviour | ||||||||||||||||||||||||||
| 6.2. Social comparison | ||||||||||||||||||||||||||
| 7.Associations | ||||||||||||||||||||||||||
| 7.1. Prompts/cues | ||||||||||||||||||||||||||
| 8.Repetition and substitution | ||||||||||||||||||||||||||
| 8.1. Behavioural practice/rehearsal | ||||||||||||||||||||||||||
| 8.2. Behaviour substitution | ||||||||||||||||||||||||||
| 8.4. Habit reversal | ||||||||||||||||||||||||||
| 8.6. Generalisation of target behaviour | ||||||||||||||||||||||||||
| 8.7. Graded tasks | ||||||||||||||||||||||||||
| 9.Comparison of outcomes | ||||||||||||||||||||||||||
| 9.2. Pros and cons | ||||||||||||||||||||||||||
| 10.Reward and threat | ||||||||||||||||||||||||||
| 10.3. Non-specific reward | ||||||||||||||||||||||||||
| 10.4. Social reward | ||||||||||||||||||||||||||
| 10.10. Reward (outcome) | ||||||||||||||||||||||||||
| 11.Regulation | ||||||||||||||||||||||||||
| 11.2. Reduce negative emotions | ||||||||||||||||||||||||||
| 11.3. Conserving mental resources | ||||||||||||||||||||||||||
| 12.Antecedents | ||||||||||||||||||||||||||
| 12.5. Adding objects to the environment | ||||||||||||||||||||||||||
| 15.Self-belief | ||||||||||||||||||||||||||
| 15.1. Verbal persuasion capability | ||||||||||||||||||||||||||
| 15.3. Focus on past success | ||||||||||||||||||||||||||
| Number of BCTs | 20 | 18 | 12 | 9 | 7 | 6 | 4 | 4 | 4 | 2 | 2 | 14 | 11 | 9 | 7 | 6 | 6 | 4 | 2 | 9 | 7 | 7 | 7 | 6 | 4 | 4 |
*MDRD study described in two main articles: Gillis et al 33 and Coyne et al 48.
BCT, Behaviour Change Technique.
The top five most frequently observed behaviour change techniques were instruction on how to perform the behaviour (23 interventions, 88%), social support (16, 62%), demonstration of the behaviour (13, 50%), feedback on behaviour (12, 46%) and behavioural practice/rehearsal (12, 46%). Of the 93 possible behaviour change techniques that could have been used, 12 techniques were used in more than 20% of trials, 27 were used at least once and 54 were never used. The mean number of behaviour change techniques was 5, the median was four and the range 2–20.
The two studies with the highest number of behaviour change techniques (20 and 18 in each study) were both informed by theory, with a particular focus on self-regulation and self-management.32 33
Intervention functions
Table 3 lists the intervention functions present in each study (education, enablement, training, persuasion, modelling, incentivisation, environmental restructuring, coercion and restrictions). The number of functions used across interventions ranged from one to seven.
Table 3.
Cross matrix of intervention functions and lifestyle behaviour change trials
| Intervention functions | ||||||||
| Studies | Type of intervention | Education | Enablement | Training | Persuasion |
Environmental
restructuring |
Modelling | Incentivisation |
| Campbell et al 38 | Diet | |||||||
| Clark et al 47 | ||||||||
| De Brito-Ashurst et al 37 | ||||||||
| Dussol et al 61 | ||||||||
| MDRD Study (1995)* | ||||||||
| Mekki et al 62 | ||||||||
| Meuleman et al 32 | ||||||||
| Paes-Barreto et al 46 | ||||||||
| Pisani et al 42 | ||||||||
| Rosman et al 63 | ||||||||
| Saran et al 64 | ||||||||
| Aoike et al 59 |
Physical
Activity |
|||||||
| Barcellos et al 65 | ||||||||
| Greenwood et al 43 | ||||||||
| Kao et al 30 | ||||||||
| Leehey et al 66 | ||||||||
| Rossi et al 45 | ||||||||
| Tang et al 49 | ||||||||
| Van Craenenbroeck et al 34 | ||||||||
| Flesher et al 39 | Lifestyle | |||||||
| Howden et al 40 | ||||||||
| Ishani et al 41 | ||||||||
| Jiamjariyapon et al 67 | ||||||||
| Joboshi44 | ||||||||
| Patil et al 68 | ||||||||
| Teng et al 31 | ||||||||
| Total | 21 | 18 | 12 | 4 | 4 | 2 | 2 | |
Education
Education was used most frequently as an intervention function, present in 21 (81%) interventions (table 3). Examples of educational strategies were: nutritional label reading,38 39 a resistance training booklet for home-based exercise,40 a lecture/workshop about exercise recommendations with demonstrations,30 online education modules on lifestyle modification41 and a written ‘six-tip diet’ checklist.42
Enablement
Eighteen (69%) interventions used enablement. Examples include Motivational Interviewing to improve self-management of diet, lifestyle and physical activity,32 43 supportive telephone calls matching stages of behaviour change,30 self-management techniques to foster self-efficacy38 39 44 and arranging support from friends and family members and ‘buddy’ visits.31 33 Four interventions were specifically designed using a self-management approach and assessed self-efficacy as an outcome.32 33 39 44
Training
Twelve (46%) interventions included training as an intervention function. Training was used in every intervention targeting physical activity but only used in two dietary interventions and two lifestyle interventions. Examples of training include home-based exercise training, guided exercise training in a gym,40 physical therapy or cardiac rehabilitation facility45 or hospital34 and interactive cooking classes.39
Persuasion
Four (15%) interventions used persuasion as an intervention function. A dietary intervention aimed to persuade participants about dietary salt intake by displaying test tubes of salt content alongside a range of high-salt food items.46 In another dietary intervention, positive thinking was applied to participant’s goals and dieticians praised progress and focused on positive results.33 Similarly, a lifestyle intervention used positive reinforcement to increase confidence and celebrate successes related to behaviour change and also discussed lack of exercise, poor dietary habits, risks of not exercising and associated consequences.31 Only one physical activity intervention used persuasion in designing and displaying printed health messages to promote exercise.30
Environmental re-structuring
Four (15%) interventions used environmental restructuring. Two involved placing exercise equipment in the home environment (exercise bicycle, Theraband, weights and Swiss ball)40 43 and two included adding food products and equipment into the home environment (low sodium/protein meals and water bottles).33 47
Modelling
Two (8%) dietary interventions incorporated modelling as an intervention function. Educators used food models and household measuring utensils to model appropriate food portion sizes46 and food tastings provided an example of low protein meals.33
Incentivisation
Two (8%) studies used incentivisation, one in the form of ‘appreciation gifts’ including certificates and mugs33 and another included ‘self-rewards’ chosen by participants.32
Coercion and restrictions
These functions were not used in any of the interventions.
Outcomes
A description of primary outcomes and results reported in studies is included in table 4. Primary outcomes of studies in this review were diverse and were mainly physiological metrics (for example, eGFR, blood pressure, peak VO2 and sodium or albumin excretion). Only six studies included patient-reported and/or behavioural primary outcomes such as quality of life, fatigue, knowledge, self-efficacy, self-management, exercise and health behaviours.30 31 44 45 48 49
Table 4.
Effects of the behaviour change interventions on the primary outcome(s)
| Study | Primary outcome/s | Measures | Intervention (n) | Control (n) | Intervention* | Control* | Mean difference (95% CI) | P value |
| Dietary interventions | ||||||||
| Campbell et al 38 | Body composition | Body cell mass, % | 29 | 27 | 2.0 (1.9 to 5.9)† | 1.5 (5.5 to 2.5)† | 3.5 (2.1 to 9.1) | 0.2 |
| Body cell mass, kg | 0.5 (1.8 to 0.8)† | 0.5 (0.7 to 1.8)† | 1.1 (0.7 to 2.9) | 0.2 | ||||
| Clark et al 47 | Change in eGFR | Change eGFR, mL/min/1.73 m2 | 311 | 308 | −2.2 (−3.3 to −1.1)† | −1.9 (−2.9 to −0.9)† | −0.3 (−1.8 to 1.2) | 0.74 |
| De Brito-Ashurst et al 37 | Change in BP | Reduction systolic/diastolic BP | 25 | 23 | – | – | −8 mm Hg (−11 to −5)/2 (−4 to −2) | <0.001 |
| Dussol et al 61 | Decrease in eGFR | Decrease eGFR, mL/min/1.73 m2 | 25 | 22 | −7±11 | −5±15 | – | – |
| 24-hour albumin excretion rate | Microalbuminuria, mg/d | +114±364 | +156±486 | – | – | |||
| MDRD‡ Study 1 (1995) | Dietary satisfaction (Study A: GFR 25–55 mL/min. 1.73m2) | Dietary satisfaction score | 220 | 221 | 3.6±1.0 | 3.8±1.0 | – | <0.05 |
| Dietary satisfaction (Study B: GFR 13–24 mL/min. 1.73m2 | Dietary satisfaction score | 65 | 59 | 3.1±0.9 | 3.6±0.9 | – | <0.01 | |
| MDRD‡ Study 2 (1996) | Decline eGFR (Study A: GFR 25–55 mL/min. 1.73m2) | Decline eGFR, baseline to 3 years | 291 | 394 | – | – | 3.8 (4.2)§ | – |
| Decline eGFR (Study B: GFR 13–24 mL/min. 1.73m2) | Decline eGFR, baseline to 3 years | 126 | 129 | – | – | 4.0 (3.1)§ | – | |
| Mekki et al 62 | Total cholesterol (TC) | TC/mmol L-1 | 20 | 20 | 4.1±0.5 | 5.4±0.4 | – | <0.05 |
| Triacylglycerols (TG) | TG/mmol L-1 | 2.9±0.1 | 3.9±0.1 | – | <0.05 | |||
| Meuleman et al 32 | Blood pressure | Office systolic BP, mmHg | 67 | 71 | – | – | −7.3 (−12.7 to −1.9)¶ | <0.01 |
| Office diastolic BP, mmHg | – | – | −3.8 (-6.9 to -0.6)¶ | <0.05 | ||||
| Sodium excretion | Sodium excretion rate, mmol/24 hours | – | – | 2.9 (−21.6 to 27.3)¶ | ||||
| Paes-Barreto et al 46 | Change in protein intake | Change protein intake, g/day | 43 | 46 | −20.7 (−30.9%)†† | −10.5 (−15.1%)¶†† | – | 0.04 |
| Pisani et al 42 | Protein intake | Change protein intake, g/kg/day | 27 | 27 | −0.1 (−0.17 to −0.03)† | −0.2 (−0.28 to −0.13)† | – | 0.04 |
| UUN excretion | Change UUN, g/day | −1.3 (−2.1 to −0.5)† | −2.8 (−3.6 to −2)† | – | 0.008 | |||
| SUN | Change SUN, mg/dL | 2.96 (−7.71 to 13.64)† | −16.63 (−27.3 to −5.96)† | – | 0.012 | |||
| Urinary phosphate excretion | Change phosphate excretion, mg/day | −27.6 (−93.7 to 38.4)† | −165.3 (−231.3 to −99.2)† | – | 0.005 | |||
| Serum phosphate concentration | Change serum phosphate, mg/dL | 0.2 (0 to 0.4)† | −0.1 (−0.3 to 0.2)† | – | 0.093 | |||
| Adherence | Met criteria, n, % | 19 (70%)‡‡ | 11 (44%)‡‡ | – | – | |||
| Rosman et al 63 | Adherence (Group A1 & B: CrCl >30) | Median 24-hour urea excretion mmol/24 hours | 45 | 47 | – | – | – | <0.01 |
| Adherence (Group A2 & C: CrCl ≤30) | Median 24-hour urea excretion mmol/24 hours | 23 | 17 | – | – | – | <0.01 | |
| Saran et al64 | Change hydration status | Extracellular Volume, L | 29 | 29 | – | – | −1.02 (−1.48 to 0.56) | <0.001 |
| Intracellular Volume, L | – | – | −0.06 (−0.12 to 0.01) | 0.02 | ||||
| Physical activity interventions | ||||||||
| Aoike et al 59 | Cardiopulmonary | Maximal ventilation, L/min | 14 | 15 | 90.7±28.1 | 76.6±23.3 | – | 0.003 |
| parameters | Ventilatory threshold, VO2 peak, ml/kg/min | 26.1±7.0 | 24.2±7.1 | – | 0.302 | |||
| VO2 in respiratory compensation point, ml/kg/min | 21.7±5.5 | 19.0±5.6 | – | 0.073 | ||||
| Speed in respiratory compensation point, Km/h | 6.8±1.1 | 5.8±1.0 | – | <0.001 | ||||
| Functional capacity | 6MWT, minutes | 583.1±85.2 | 561.2±91.2 | – | 0.028 | |||
| Time up/go test, seconds | 5.82±1.39 | 6.42±1.11 | – | 0.001 | ||||
| Arm curl test, repetitions | 22.8±4.8 | 18.1±3.1 | – | <0.001 | ||||
| STST, repetitions | 24.0±7.1 | 18.3±4.8 | – | <0.001 | ||||
| 2-minute step test, steps | 219.3±36.7 | 179.9±36.3 | – | <0.001 | ||||
| Back scratch test, cm | 6.4±6.6 | 12.6±9.9 | – | 0.05 | ||||
| Systolic and diastolic BP | Systolic BP, mm Hg | 118.7±7.3 | 126.8±6.7 | – | 0.012 | |||
| Diastolic BP, mm HgP | 76.1±4.4 | 81.0±3.7 | – | 0.038 | ||||
| Renal function | Serum creatinine, mg/dL | 2.6±1.1 | 3.2±1.4 | – | 0.215 | |||
| eGFR, mL/min/1.73 m2 | 31.9±13.7 | 23.9±12.2 | – | 0.046 | ||||
| Barcellos et al 65 | Mean change in eGFR | Change eGFR, mL/min/1.73 m2 | 76 | 74 | 61.5 (57.0 to 66.1)† | 59.0 (54.2 to 63.8)† | 0.7 (−4.0 to 5.4) | – |
| Greenwood et al 43 | Mean change in eGFR | Change eGFR, mL/min/1.73 m2 | 8 | 10 | −3.8±2.8 | −8.5±6.4 | 7.8±3.0 (1.1 to 13.5) | 0.02 |
| Kao et al 30 | Depression | Change depression (Beck Depression Inventory-II scale) | 45 | 49 | −3.71§§ | 1.33§§ | – | <0.01 |
| Fatigue | Change fatigue | −4.74§§ | 1.91§§ | – | <0.001 | |||
| Exercise behaviour | Change weekly exercise | 4.28§§ | −1.24§§ | – | <0.001 | |||
| Leehey et al66 | UPCR ratio | UPCR (mg/g) at 52 wks | 14 | 18 | 405 (225 to 1038)††† | 618 (323 to 1155)††† | – | 0.39 |
| Rossi et al 45 | Physical function | 6MWT, minutes | 59 | 48 | 210.4±266 | −10±219.9 | – | <0.001 |
| STST, seconds | 26.9%±27% age prediction*** | 0.7%±12.1% age prediction*** | – | <0.001 | ||||
| Gait speed, cm | 9.5 (−36.4 to 34)††† | 0 (−9 to 13)††† | – | 0.76 | ||||
| QoL (RAND SF-36), | Role functioning/physical | 19.0±31.7 | −8.9±38.4 | – | <0.001 | |||
| mean change from | Physical functioning | 11.1±19.3 | −0.7±18.7 | – | 0.004 | |||
| baseline | Energy/fatigue | 9.8±17.6 | 0.5±18.0 | – | 0.01 | |||
| General health | 4.9±15.3 | −1.2±11.5 | – | 0.03 | ||||
| Pain | 5.7±20.0 | −3.8±24.4 | – | 0.04 | ||||
| Emotional well-being | 4.2±16.9 | −0.4±17.1 | – | 0.2 | ||||
| Social functioning | 4.2±20.8 | 1.6±22.6 | – | 0.57 | ||||
| Role functioning/emotional | 6.9±24.5 | 1.9±29.2 | – | 0.38 | ||||
| Tang et al 49 | Physical function | Change 6MWT, minutes | 42 | 42 | 41.93±14.57 | −5.05±14.81 | – | <0.001 |
| Change STST, seconds | −2.68±1.95 | 0.49±2.07 | – | <0.001 | ||||
| Self-efficacy | Change self-efficacy score | 6.64±6.92 | −3.72±6.80 | – | <0.001 | |||
| Anxiety | Change HAD-A score | −1.02±1.47 | 0.21±2.17 | – | 0.003 | |||
| Depression | Change HAD-D score | −0.76±1.32 | 0.31±1.84 | – | 0.003 | |||
| QoL (KDQOL-SF), | Symptom/problem list | 2.49±4.81 | 0.38±6.97 | – | 0.007 | |||
| mean change from | Effects of kidney disease | 1.90±5.22 | −1.56±9.64 | – | 0.005 | |||
| baseline | Burden of kidney disease | −0.45±15.27 | −15.3±18.11 | – | <0.001 | |||
| SF-12 PCS | 1.08±3.60 | −0.74±4.55 | – | 0.045 | ||||
| SF-12 MCS | 1.87±5.69 | −0.73±4.53 | – | 0.002 | ||||
| Van Craenenbroeck et al 34 | Peripheral endothelial function | Flow mediated dilation of brachial artery | 19 | 21 | 4.6±3.0 | 5.3±3.1 | 0.32 (−1.88 to 2.53) | 0.9 |
| Lifestyle interventions | ||||||||
| Flesher et al 39 | Composite of eGFR, TC, US, UP, BP | Number of improved endpoints | 23 | 17 | 83 | 30 | 0.028 | |
| Howden et al 40 | Change in CRF | VO2, ml/kg/min | 36 | 36 | 2.8±0.7 | 0.3±0.9 | – | 0.004 |
| Ishani et al 41 | Composite death, hospitalisation, emergency visits, admission nursing facility | Occurrence of primary outcome/HR | 451 | 150 | 208 (46.2%) | 70 (46.7%) | – | 0.9 |
| Jiamjariyapon et al 67 | Mean change in eGFR | Change eGFR, mL/min/1.73 m2 | 234 | 208 | 42.4±1.5 | 39.9±2.8 | 2.74 (0.60 to 4.50) | 0.009 |
| Joboshi and Oka44 | Perceived behaviour | Self-efficacy | 32 | 29 | r=0.27, U=318.5** | – | – | 0.035 |
| Self-management | r=0.29, U=310.0** | – | – | 0.026 | ||||
| Patil et al 68 | 24-hour urine protein | 24-hour urine protein, g/d | 23 (B) | 22 (A),31 (C) | 1284.74±1079.94 | A: 1079.27±1269.20; C: 1187.61±756.92 | – | – |
| BMI | Change in BMI (paired t-test) | −1.95±1.10 | A: −0.15±0.38 (p=0.069); C: −2.56±0.68 (p=0.000) | – | 0.000 | |||
| Teng et al 31 | Health-promotion lifestyle | Stress management | 45 | 45 | – | – | 2.76 | 0.10 |
| behaviours (HPLP-IIC) | Interpersonal relations | – | – | 3.88 | 0.05 | |||
| Health responsibility | – | – | 13.63 | 0.001 | ||||
| Physical activity | – | – | 7.50 | 0.01 | ||||
| Spiritual growth | – | – | 2.79 | 0.10 | ||||
| Nutrition | – | – | 2.62 | 0.11 | ||||
| Renal function protection knowledge | Knowledge renal function, Chinese herbs and CKD diet | – | – | No data | 0.001 | |||
| Physical function | 6MWT, minutes | 45 | 45 | 420.4±81.2 | 368.5±99.7 | – | 0.04 | |
*Unless otherwise indicated, values are shown as mean+/-SD.
†Mean change (95% CI).
§Mean decline +/-SD.
¶Mean change from baseline after 6 months.
**Effect size (r) Median, Mann-Whitney's U Test.
††Mean change and % reduction from baseline values.
‡‡ Number of participants who met adherence criteria (n,%).
§§Paired T test.
¶¶ p-value calculated as p<0.05 x group interaction (Aoike 2015).
***STST results standardized as a percentage of age-predicted value using prediction formulas (Rossi 2014).
†††Median (IQR)
BMI, Body Mass Index; BP, blood pressure;CrCl, Creatinine clearance; CRF, Cardiorespiratory fitness; eGFR, estimated glomerular filtration rate; HAD-A/HAD-D, Hospital Anxiety & Depression Scale; HPLP-IIC, Health Promoting Lifestyle Profile-II Chinese version (questionnaire); KDQOL-SF, Kidney Disease & Quality of Life Short Form; 6MWT, 6 min Walk Test; SF-12 PCS/MCS, Physical and Mental Health Composite Scores; QoL, Quality of life; RAND SF-36, 36-Item Short Form Survey; STST, Sit to Stand Test; SUN, Serum urea nitrogen; UP, Urinary protein; UPCR, Urine protein to creatinine ratio; US, Urinary sodium; UUN, urinary urea nitrogen.
Eighteen studies (69%) showed a significant improvement in at least one primary outcome and all of these studies included education, persuasion, modelling and incentivisation as an intervention function (see online supplementary table S3). A meta-analysis of the data was not possible due to heterogeneity of outcome measures across the included studies. The heterogeneity of outcomes also meant we could not link outcomes with specific behaviour change techniques. Many studies are likely to be underpowered to detect modest effects, and so the absence of a statistically significant effect should not be regarded as evidence of no effect.
bmjopen-2019-031625supp005.pdf (20.8KB, pdf)
Discussion
Behaviour change interventions in trials in patients with CKD mostly focused on diet and physical activity. The primary outcomes of the trials were diverse and most were biochemical outcomes (eg, eGFR, blood pressure, peak VO2 and sodium or albumin excretion), with few clinical or patient-reported and/or behavioural outcomes such as quality of life, fatigue, knowledge, self-efficacy and self-management.30 31 38 39 44 45 Only five interventions were underpinned by theory. The most frequently used intervention function was education, followed by enablement and training. Persuasion, environmental restructuring, modelling and incentivisation were used less frequently. Coercion and restrictions (which includes regulation) were not used in any of the studies. The top five most common behaviour change techniques were instruction on how to perform the behaviour, social support, demonstration of the behaviour, feedback on behaviour and behavioural practice/rehearsal. Identity, scheduled consequences and covert learning were not used in any of the studies. No association between frequency of functions or behaviour change techniques and the effect of interventions on outcomes could be identified.
The use of multiple behaviour change techniques does not necessarily lead to better outcomes and some evidence suggests that fewer techniques and the right combinations of techniques suited to the context are more effective.50–52 Education was the most frequent intervention function used across the studies, which may be because it has been consistently shown that patients with CKD lack awareness about lifestyle risk factors and have low health literacy.10 11 53 Specifically, the behaviour change technique, ‘instruction on how to perform the behaviour’, was the most frequently reported technique, used in all interventions except two. We suggest this is highly applicable because dietary interventions can involve complex dietary restrictions of sodium, protein, potassium and phosphate. Patients have sought practical advice about how to implement these restrictions.54 However, most educational strategies used a didactic approach, with health professionals verbally conveying information or providing written materials. Patients with CKD prefer multiple problem-solving and collaborative approaches, in partnership with health professionals.54 Also, written materials for patients with CKD have a reading grade of 9 (age 14–15 years), which is higher than the recommended level (grade 5).10
The intervention function ‘training’ was used in every study targeting physical activity but was only used in two dietary interventions. Patients with CKD are overwhelmed by dietary information which can be complex, restrictive and insensitive to cultural norms.54 A recent review of educational interventions for CKD patients found that including practical skills and workshops was associated with better outcomes.55 For example, a low-salt programme for Bangladeshi patients with CKD in the United Kingdom included cooking and educational sessions facilitated by Bengali workers in a community kitchen. It targeted both patients and family members who cooked their own low-salt version of Bangladeshi recipes and led to a reduction in salt intake and reduced blood pressure for participants.37 Approaches to enabling and training patients for behaviour change incorporating hands-on training may be more effective.
Our findings are similar to recent reviews of behavioural interventions for other conditions (cardiovascular disease, obesity, rheumatoid arthritis, prostate cancer and diabetes), which also found that behavioural interventions are not well-reported, not informed by theory and have diverse outcomes and modes of delivery.25–27 51 56 The behaviour change techniques associated with goals and planning, feedback and monitoring and social support have also been frequently used in behaviour changes interventions in patients with other chronic conditions. These techniques are proven strategies for behaviour change and in line with evidence-based recommendations for lifestyle modification.12 13 57
We identified and described the behaviour change techniques and intervention functions in lifestyle behavioural interventions for patients with CKD with comprehensive evidence-based frameworks. Coding of behaviour change techniques and intervention functions was systematically and independently conducted by three researchers, and risk of bias was assessed. Potential limitations relate to poor reporting. Some interventions may have used behaviour change techniques or intervention functions in their study but did not report them, or details of techniques were unclear. We contacted authors and examined all associated supplementary materials and papers to collect more information.
Lifestyle behaviour change interventions for patients with CKD appear to integrate recommended and proven behaviour change techniques and intervention functions. These techniques such as goals and planning and self-monitoring are important but focus on individual agency rather than external factors. Interventions could be improved by considering the context of behaviour change and the social and physical environment of participants. For example, most of the interventions for physical activity focused on structured exercise programme and a reliance on equipment (eg, exercise bikes). Patients with CKD need to be able to integrate physical activity in to their daily lifestyle.58 However, only one intervention for physical activity gave instructions on how to incorporate physical activity to fit in with daily activities and in environments easily accessible to patients, without the use of equipment.59 This study reported improvements in cardiopulmonary and functional capacities of overweight patients with CKD.
Optimising the social environment and arranging support from friends, family and the community may also improve lifestyle behaviour change interventions for patients with CKD. Family support was used rarely in interventions in this review and only included in two studies.31 37 However, informal caregivers play an important role in the management of CKD and are often required to change their own lifestyle behaviours to support patients with CKD.60 Characteristics of effective educational interventions for patients with CKD involved the patient’s family.55
The quality of the design and reporting of lifestyle behaviour change interventions for patients with CKD requires explicit description of behavioural strategies to ensure interventions are generalisable and replicable. There are numerous evidence-based guidelines that recommend the explicit use of behaviour change techniques for addressing lifestyle risk factors in chronic disease prevention and these may be better used when designing and reporting interventions for patients with CKD. Recently the National Institute of Health and Care Excellence in the UK published comprehensive guidelines specific to behavioural interventions and lifestyle modification.12 The WHO’s recommendations on behaviour change support this and further reinforce the need to consider the social and environmental determinants of health in changing lifestyle behaviours.57
Conclusion
Lifestyle interventions in trials conducted in patients with CKD mostly focus on goals and planning, feedback and monitoring and education. However, we suggest that interventions may be improved by using interactive and tailored training, and strategies to help patients incorporate lifestyle modification in their daily activities, and physical and social environments. Explicit application of behaviour change taxonomies may help to increase the effect of lifestyle behaviour change interventions for improved health outcomes in patients with CKD.
Supplementary Material
Footnotes
Contributors: Research idea and study design: NE, AT, JC; data acquisition: NE, KM, VS; data analysis/interpretation: NE, AT, JC, AB, KM, VS; statistical analysis: NE; supervision or mentorship: AT, JC, AB.
Funding: This work was supported by a Postgraduate Research Scholarship and a National Health and Medical Research Grant (NHMRC: 1098815).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Hemmelgarn BR, Pannu N, Ahmed SB, et al. . Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant 2017;32:847–54. 10.1093/ndt/gfw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tong A, Crowe S, Chando S, et al. . Research priorities in CKD: report of a national workshop conducted in Australia. Am J Kidney Dis 2015;66:212–22. 10.1053/j.ajkd.2015.02.341 [DOI] [PubMed] [Google Scholar]
- 3. Urquhart-Secord R, Craig JC, Hemmelgarn B, et al. . Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis 2016;68:444–54. 10.1053/j.ajkd.2016.02.037 [DOI] [PubMed] [Google Scholar]
- 4. Couser WG, Remuzzi G, Mendis S, et al. . The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–70. 10.1038/ki.2011.368 [DOI] [PubMed] [Google Scholar]
- 5. Dunkler D, Kohl M, Teo KK, et al. . Population-Attributable Fractions of Modifiable Lifestyle Factors for CKD and Mortality in Individuals With Type 2 Diabetes: A Cohort Study. Am J Kidney Dis 2016;68:29–40. 10.1053/j.ajkd.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 6. Ricardo AC, Anderson CA, Yang W, et al. . Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2015;65:412–24. 10.1053/j.ajkd.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beto JA, Schury KA, Bansal VK. Strategies to promote adherence to nutritional advice in patients with chronic kidney disease: a narrative review and commentary. Int J Nephrol Renovasc Dis 2016;9:21–33. 10.2147/IJNRD.S76831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke AL, Young HML, Hull KL, et al. . Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant 2015;30:1885–92. 10.1093/ndt/gfv208 [DOI] [PubMed] [Google Scholar]
- 9. de Brito-Ashurst I, Perry L, Sanders TAB, et al. . Barriers and facilitators of dietary sodium restriction amongst Bangladeshi chronic kidney disease patients. J Hum Nutr Diet 2011;24:86–95. 10.1111/j.1365-277X.2010.01129.x [DOI] [PubMed] [Google Scholar]
- 10. Morony S, Flynn M, McCaffery KJ, et al. . Readability of written materials for CKD patients: a systematic review. Am J Kidney Dis 2015;65:842–50. 10.1053/j.ajkd.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 11. Taylor DM, Fraser SDS, Bradley JA, et al. . A systematic review of the prevalence and associations of limited health literacy in CKD. Clin J Am Soc Nephrol 2017;12:1070–84. 10.2215/CJN.12921216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence Behaviour change: individual approaches. NICE guidelines [PH49], 2014. Available: https://www.nice.org.uk/guidance/ph49 [Accessed 26 Aug 2018].
- 13. The Royal Australian College of General Practitioners Guidelines for preventive activities in general practice. 9th edn, 2016. https://www.racgp.org.au/your-practice/guidelines/redbook/ [Google Scholar]
- 14. Michie S, Richardson M, Johnston M, et al. . The behavior change technique taxonomy (V1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 15. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Sci 2011;6:1–12. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morton K, Sutton S, Hardeman W, et al. . A Text-Messaging and Pedometer program to promote physical activity in people at high risk of type 2 diabetes: the development of the propels follow-on support program. JMIR Mhealth Uhealth 2015;3:e105 10.2196/mhealth.5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tapper K, Jiga-Boy G, Maio GR, et al. . Development and preliminary evaluation of an Internet-based healthy eating program: randomized controlled trial. J Med Internet Res 2014;16:e231 10.2196/jmir.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hampton J, Allen E, Edson C. Service evaluation of a digital behavioural change programme. Future Hosp J 2017;4:173–7. 10.7861/futurehosp.4-3-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Altman DG, Gøtzsche PC, et al. . The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallis A, Bunten A, Bonus A, et al. . The effectiveness of an enhanced invitation letter on uptake of national health service health checks in primary care: a pragmatic quasi-randomised controlled trial. BMC Fam Pract 2016;17:1–8. 10.1186/s12875-016-0426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garnett CV, Crane D, Brown J, et al. . Behavior change techniques used in digital behavior change interventions to reduce excessive alcohol consumption: a meta-regression. Ann Behav Med 2018;52:530–43. 10.1093/abm/kax029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alageel S, Gulliford MC, McDermott L, et al. . Multiple health behaviour change interventions for primary prevention of cardiovascular disease in primary care: systematic review and meta-analysis. BMJ Open 2017;7:e015375 10.1136/bmjopen-2016-015375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardner B, Smith L, Lorencatto F, et al. . How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev 2016;10:89–112. 10.1080/17437199.2015.1082146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hallward L, Patel N, Duncan LR. Behaviour change techniques in physical activity interventions for men with prostate cancer: a systematic review. J Health Psychol 2018;1359105318756501. [DOI] [PubMed] [Google Scholar]
- 26. Heron N, Kee F, Donnelly M, et al. . Behaviour change techniques in home-based cardiac rehabilitation: a systematic review. Br J Gen Pract 2016;66:e747–57. 10.3399/bjgp16X686617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larkin L, Gallagher S, Cramp F, et al. . Behaviour change interventions to promote physical activity in rheumatoid arthritis: a systematic review. Rheumatol Int 2015;35:1631–40. 10.1007/s00296-015-3292-3 [DOI] [PubMed] [Google Scholar]
- 28. Laba T-L, Bleasel J, Brien J-A, et al. . Strategies to improve adherence to medications for cardiovascular diseases in socioeconomically disadvantaged populations: a systematic review. Int J Cardiol 2013;167:2430–40. 10.1016/j.ijcard.2013.01.049 [DOI] [PubMed] [Google Scholar]
- 29. University College London Centre for Behaviour Change BCT taxonomy V1 online training, 2018. Available: http://www.bct-taxonomy.com/ [Accessed 5 Aug 2018].
- 30. Kao Yu‐Hsiu, Huang Yi‐Ching, Chen Pei‐Ying, et al. . The effects of exercise education intervention on the exercise behaviour, depression, and fatigue status of chronic kidney disease patients. Health Educ 2012;112:472–84. 10.1108/09654281211275827 [DOI] [Google Scholar]
- 31. Teng H-L, Yen M, Fetzer S, et al. . Effects of targeted interventions on lifestyle modifications of chronic kidney disease patients: randomized controlled trial. West J Nurs Res 2013;35:1107–27. 10.1177/0193945913486202 [DOI] [PubMed] [Google Scholar]
- 32. Meuleman Y, Hoekstra T, Dekker FW, et al. . Sodium restriction in patients with CKD: a randomized controlled trial of self-management support. Am J Kidney Dis 2017;69:576–86. 10.1053/j.ajkd.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 33. Gillis BP, Caggiula AW, Chiavacci AT, et al. . Nutrition intervention program of the modification of diet in renal disease study: a self-management approach. J Am Diet Assoc 1995;95:1288–94. 10.1016/S0002-8223(95)00338-X [DOI] [PubMed] [Google Scholar]
- 34. Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, et al. . Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3-4: a randomized controlled trial. Am J Kidney Dis 2015;66:285–96. 10.1053/j.ajkd.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 35. van Zuilen AD, Bots ML, Dulger A, et al. . Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int 2012;82:710–7. 10.1038/ki.2012.137 [DOI] [PubMed] [Google Scholar]
- 36. Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press, 1991. [Google Scholar]
- 37. de Brito-Ashurst I, Perry L, Sanders TAB, et al. . The role of salt intake and salt sensitivity in the management of hypertension in South Asian people with chronic kidney disease: a randomised controlled trial. Heart 2013;99:1256–60. 10.1136/heartjnl-2013-303688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campbell KL, Ash S, Davies PSW, et al. . Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am J Kidney Dis 2008;51:748–58. 10.1053/j.ajkd.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 39. Flesher M, Woo P, Chiu A, et al. . Self-Management and biomedical outcomes of a cooking, and exercise program for patients with chronic kidney disease. J Ren Nutr 2011;21:188–95. 10.1053/j.jrn.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 40. Howden EJ, Leano R, Petchey W, et al. . Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 2013;8:1494–501. 10.2215/CJN.10141012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ishani A, Christopher J, Palmer D, et al. . Telehealth by an Interprofessional Team in Patients With CKD: A Randomized Controlled Trial. Am J Kidney Dis 2016;68:41–9. 10.1053/j.ajkd.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 42. Pisani A, Riccio E, Bellizzi V, et al. . 6-tips diet: a simplified dietary approach in patients with chronic renal disease. A clinical randomized trial. Clin Exp Nephrol 2016;20:433–42. 10.1007/s10157-015-1172-5 [DOI] [PubMed] [Google Scholar]
- 43. Greenwood SA, Koufaki P, Mercer TH, et al. . Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis 2015;65:425–34. 10.1053/j.ajkd.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 44. Joboshi H, Oka M. Effectiveness of an educational intervention (the encourage autonomous Self-Enrichment program) in patients with chronic kidney disease: a randomized controlled trial. Int J Nurs Stud 2017;67:51–8. 10.1016/j.ijnurstu.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 45. Rossi AP, Burris DD, Lucas FL, et al. . Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol 2014;9:2052–8. 10.2215/CJN.11791113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paes-Barreto JG, Silva MIB, Qureshi AR, et al. . Can renal nutrition education improve adherence to a low-protein diet in patients with stages 3 to 5 chronic kidney disease? J Ren Nutr 2013;23:164–71. 10.1053/j.jrn.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 47. Clark WF, Sontrop JM, Huang S-H, et al. . Effect of coaching to increase water intake on kidney function decline in adults with chronic kidney disease: the CKD wit randomized clinical trial. JAMA 2018;319:1870–9. 10.1001/jama.2018.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coyne T, Olson M, Bradham K, et al. . Dietary satisfaction correlated with adherence in the modification of diet in renal disease study. J Am Diet Assoc 1995;95:1301–6. 10.1016/S0002-8223(95)00341-X [DOI] [PubMed] [Google Scholar]
- 49. Tang Q, Yang B, Fan F, et al. . Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: a randomized controlled trial in China. Int J Nurs Pract 2017;23:e12519 10.1111/ijn.12519 [DOI] [PubMed] [Google Scholar]
- 50. Berdal G, Bo I, Dager TN, et al. . Structured goal planning and supportive telephone followup in rheumatology care: results from a pragmatic stepped-wedge cluster-randomized trial. Arthritis Care Res 2018. [DOI] [PubMed] [Google Scholar]
- 51. Dombrowski SU, Sniehotta FF, Avenell A, et al. . Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev 2012;6:7–32. 10.1080/17437199.2010.513298 [DOI] [Google Scholar]
- 52. Michie S, Abraham C, Whittington C, et al. . Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690–701. 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 53. Lopez-Vargas PA, Tong A, Howell M, et al. . Patient awareness and beliefs about the risk factors and comorbidities associated with chronic kidney disease : A mixed-methods study. Nephrology 2017;22:374–81. 10.1111/nep.12829 [DOI] [PubMed] [Google Scholar]
- 54. Palmer SC, Hanson CS, Craig JC, et al. . Dietary and fluid restrictions in CKD: a thematic synthesis of patient views from qualitative studies. Am J Kidney Dis 2015;65:559–73. 10.1053/j.ajkd.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 55. Lopez-Vargas PA, Tong A, Howell M, et al. . Educational Interventions for Patients With CKD: A Systematic Review. Am J Kidney Dis 2016;68:353–70. 10.1053/j.ajkd.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 56. Cradock KA, ÓLaighin G, Finucane FM, et al. . Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 2017;14 10.1186/s12966-016-0436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. World Health Organisation, World Health Organisation . Behaviour change strategies and health: the role of health systems. Paper presented at Regional Committee for Europe: Fifty-eighth session Georgia, 2008. [Google Scholar]
- 58. Tong A, Sainsbury P, Carter SM, et al. . Patients' priorities for health research: focus group study of patients with chronic kidney disease. Nephrology Dialysis Transplantation 2008;23:3206–14. 10.1093/ndt/gfn207 [DOI] [PubMed] [Google Scholar]
- 59. Aoike DT, Baria F, Kamimura MA, et al. . Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol 2015;47:359–67. 10.1007/s11255-014-0894-8 [DOI] [PubMed] [Google Scholar]
- 60. Tong A, Sainsbury P, Craig JC. Support interventions for caregivers of people with chronic kidney disease: a systematic review. Nephrology Dialysis Transplantation 2008;23:3960–5. 10.1093/ndt/gfn415 [DOI] [PubMed] [Google Scholar]
- 61. Dussol B, Iovanna C, Raccah D, et al. . A randomized trial of low-protein diet in type 1 and in type 2 diabetes mellitus patients with incipient and overt nephropathy. J Ren Nutr 2005;15:398–406. [DOI] [PubMed] [Google Scholar]
- 62. Mekki K, Bouzidi-bekada N, Kaddous A, et al. . Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct 2010;1:110–5. [DOI] [PubMed] [Google Scholar]
- 63. Rosman JB, Langer K, Brandl M, et al. . Protein-restricted diets in chronic renal failure: a four year follow-up shows limited indications. Kidney Int Suppl 1989;27:S96–102. [PubMed] [Google Scholar]
- 64. Saran R, Padilla RL, Gillespie BW, et al. . A randomized crossover trial of dietary sodium restriction in stage 3-4 CKD. Clin J Am Soc Nephrol 2017;12:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barcellos FC, Del Vecchio FB, Reges A, et al. . Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens 2018;32:397–407. [DOI] [PubMed] [Google Scholar]
- 66. Leehey DJ, Collins E, Kramer HJ, et al. . Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol 2016;44:54–62. [DOI] [PubMed] [Google Scholar]
- 67. Jiamjariyapon T, Ingsathit A, Pongpirul K, et al. . Effectiveness of integrated care on delaying progression of stage 3-4 chronic kidney disease in rural communities of Thailand (escort study): a cluster randomized controlled trial. BMC Nephrol 2017;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patil MR, Mishra A, Jain N, et al. . Weight loss for reduction of proteinuria in diabetic nephropathy: comparison with angiotensin-converting enzyme inhibitor therapy. Indian J Nephrol 2013;23:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031625supp001.pdf (64.7KB, pdf)
bmjopen-2019-031625supp002.pdf (48.9KB, pdf)
bmjopen-2019-031625supp003.pdf (301.9KB, pdf)
bmjopen-2019-031625supp004.pdf (64.8KB, pdf)
bmjopen-2019-031625supp005.pdf (20.8KB, pdf)