Abstract
Introduction
Several studies suggest that gut microbiota may play an important role in allergic diseases. The present trial aims to examine effects of the probiotic Enterococcus faecalis on symptoms of allergic rhinitis in patients. Effects of this probiotic on the immune system have been reported by several studies, but the majority of the previous trials were animal studies. In addition, it is well known that symptoms in allergic rhinitis are prone to exhibit high placebo responses. Moreover, recent studies report that even placebos without deception (open-label placebos) are highly effective in reducing symptoms of allergic rhinitis. Our study design combines both new approaches to assess effects on allergic symptoms in patients. The objective of this study is to compare the effects of a probiotic treatment (E. faecalis) with effects seen by open-label placebo, concealed placebo treatment and no treatment control.
Methods and analysis
A total of 120 patients with allergic rhinitis will be randomly assigned to one of four different groups: a double-blind probiotic/placebo group (groups 1 and 2), an open-label placebo group (group 3) and a no-treatment group (group 4) to control for spontaneous variation of symptoms. The primary outcome is the evaluation of allergic symptoms using the Combined Symptoms Medication Score. Furthermore, health-related quality of life is examined (Rhinitis Quality of Life Questionnaire). Secondary outcomes include a visual analogue scale on allergic burden and a second quality of life questionnaire. This report describes the study design of the randomised controlled trial.
Ethics and dissemination
The study design was approved by the ethical committee of the UKT Department of Psychosomatic Medicine and Psychotherapy, Tübingen, Germany. The trial is registered at the German Clinical Trials Register (www.drks.de, DRKS00015804). The trial results will be published in peer-reviewed journals and at conferences.
Trial registration number
German Clinical Trials Register (www.drks.de, DRKS00015804); Pre-results.
Keywords: allergic rhinitis, probiotics, open-label placebos, study protocol
Strengths and limitations of the study.
This is the first randomised controlled trial designed to assess whether the probiotic treatment Enterococcus faecalis has an effect in patients with seasonal allergic rhinitis (previous studies using E. faecalis were predominantly animal studies).
In addition, this study examines the effects of an open-label placebo treatment on symptoms of allergic rhinitis, for the first time comparing effects of an open-label placebo treatment with closed-label (blind) treatment in patients with allergic rhinitis.
The study design includes three control arms, two of which involve placebos, which allows us to compare the effects of the probiotic with concealed and open placebo conditions and with a no-treatment control.
A limitation is the length of recruitment in this study (about 6 months), which may effect spontaneous improvement of allergic rhinitis.
Introduction
Allergic diseases are defined as conditions caused by hypersensitivity of the immune system to something in the environment that in general causes no problems in most people. Allergic diseases such as allergic rhinitis affect up to 20% of all people in the developed world.1 Symptoms of allergic rhinitis include, for example, rhinorrhoea, pruritus, sneezing, nasal congestion, itching, burning or red eyes and scratching feelings in the throat. Allergic rhinitis is known to be an immunoglobulin (Ig)E-mediated disease.2 The main disease-modifying treatment is allergen immunotherapy, which has been shown to be effective for allergic rhinitis with a high level of evidence.3 Furthermore, medical treatments are available for symptomatic relief.
These medications have been proven to be effective for symptomatic relief, but complete symptom resolution is often not achieved.4 Moreover, drug treatment (eg, by histamine antagonists) is often associated with severe adverse events such as fatigue that disables or restricts patients to continue their daily activities, for example, driving cars or working.5 Although last generation histamine antagonists do not show severe adverse events anymore,6 7 current medications for allergic rhinitis may still have some undesirable side effects.8
Recently, it has been suggested that probiotics may be a possible new treatment for allergic rhinitis, in particular, probiotics with low adverse effect profiles such as lactobacillae and bifidobacteria.9 For example, Watts et al reported that probiotics had effects on quality of life and reduced medication use in allergic rhinitis.10 Probiotics are (in general) living microorganisms that can be found in foods such as yoghurt, sauerkraut and pickles. Several studies suggested that gut microbiota may play an important role in immune and allergic diseases.
Effects for probiotics in preventing allergic diseases have been reported in particular when prescribed during the perinatal period.11 When probiotics are administered later, when the allergic disease is already established, studies often report mixed conclusions (eg, 12–16). A recent systematic review and meta-analysis included 22 randomised controlled trials (RCTs). Although there was a high variability among the studies, the results demonstrated significant evidence of beneficial clinical and immunological effects of probiotics in the treatment of allergic rhinitis.15
Effects of the probiotic Enterococcus faecalis have been suggested by several studies. For example, it has been demonstrated that E. faecalis reduces the number of rhinosinusitis episodes.17 Based on similar studies that report beneficial effects of E. faecalis for the immune system,18–23 we hypothesised that this probiotic may also reduce symptoms in seasonal allergic rhinitis.
Symptoms of allergic rhinitis vary depending on seasonal changes of allergic load of the environment as well as on individual sensitivity to environmental allergens. For example, El Hennawi et al showed improved symptoms of allergic rhinitis when stress is controlled by a pharmacological treatment.24 Considering this situation—frequent waxing and waning of symptoms—symptomatic therapies of allergic rhinitis are known to be prone to placebo effects.25–30 While placebo responses may be problematic when testing new therapies, recent studies suggest that the beneficial effects of placebos may directly be used to help patients. We therefore designed a study with three control groups: conventional double-blind, open-label placebo (OLP) and a no-treatment control to adjust for spontaneous symptom variation.
Placebos are composed of inactive ingredients that have no physiological effects on symptoms. Typically, placebos are designed to match active pharmaceuticals in appearance and taste in order to serve as a control condition in double-blind RCTs. In order to do so, placebos are administered in a concealed way.31 Recent studies32 now questioned whether such double-blinded provision of placebo is necessary to elicit placebo effects. For example, RCTs examining the effects of OLPs demonstrated significant improvements for patients with irritable bowel syndrome, episodic migraine attacks, chronic lower back pain, depression and cancer-related fatigue.33–37 In addition, two previous studies showed that OLPs are highly effective in reducing symptoms of allergic rhinitis.38 39 A meta-analysis not only found moderate effects sizes for OLP treatments40 but also sees some methodological limitations that future studies have to address, for example, the need for a closed-label (blinded) placebo condition.32 41
The objective of this study is to test a probiotic treatment (E. faecali s) in patients with allergic rhinitis compared with effects seen by OLP, concealed placebo treatment and no treatment control. The current paper describes the design of this study.
Methods and analysis
The study consists of four arms. The study arms include a double-blind probiotic/placebo group (groups 1 and 2), an OLP group (group 3) and a no-treatment control group (group 4) to control for spontaneous variation of symptoms without treatment (see figure 1). Before and after the treatment (probiotic/placebo, no treatment), we will assess allergic symptoms and health-related quality of life by means of diaries and paper-pencils tests (primary endpoints: Combined Symptoms and Medication Score (CSMS)42; Rhinitis Quality of Life Questionnaire (RQLQ)43). The CSMS is a simple and standardised method that balances symptoms and the need for antiallergic medication. The RQLQ is a disease-specific instrument for evaluating health-related quality of life, including patient’s physical, social and emotional well-being.
Figure 1.
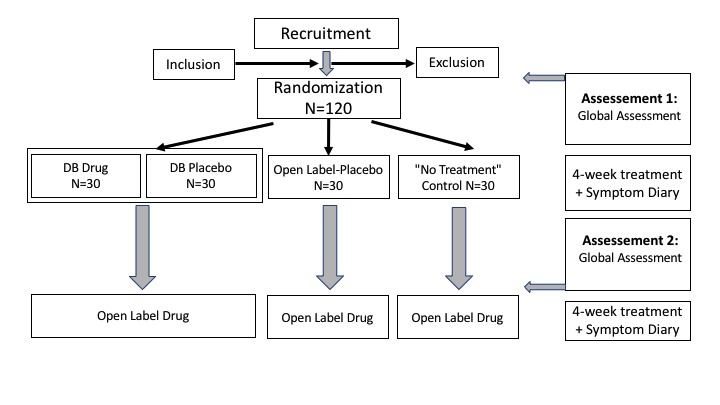
Flow diagram of patient’s enrolment.
Study setting and timeline
The study will be conducted at two sites, the UKT Department of Psychosomatic Medicine and Psychotherapy, Tübingen and Medical School Berlin, Berlin, Germany.
Recruitment of patients will start before the beginning of the spring allergy season (February 2019 and 2020). Start of birch pollen season will be marked. The study will be completed in summer of 2020.
Study duration includes 4 weeks in the treatment phase and another 4 weeks after the end of the experimental phase (open-label probiotic phase). Hence, in total, the length of the study is 8 weeks. Previous studies reported that similar time periods are effective for this type of probiotic in children with rhinosinusitis.17 Pharmacy recommendations also suggest a minimum of 4 weeks of treatment.
Participants: inclusion/exclusion criteria
Patients recruitment will use social media, flyers and in particular the website of the Deutscher Allergie und Asthma Bund (DAAB; German Allergy and Asthma Association).
We will recruit and include 120 participants with a history of allergic rhinitis for at least 2 years. An equal fraction of patients of both sexes is intended, but not enforced. Allergic rhinitis must have been diagnosed by a physician (allergy subspecialist or general physician). Participants need to show test results of IgE sensitisation to aeroallergens (either skin prick test or serum allergen-specific IgE) not older than 24 months prior to the trial.
We will include participants with seasonal allergic rhinitis only (not perennial allergic rhinitis). Further inclusion criterion is an age between 18 and 60 years.
Exclusion criteria are a medical history of diabetes, gastrointestinal diseases, use of antibiotic medication in the last 6 weeks, pregnancy and any known psychiatric or neurological diseases. Furthermore, perennial allergic rhinitis, chronic rhinosinusitis or any other chronic nasal conditions such as anatomical alterations as septum deviation or perforation are excluding criteria. Last, inability to read and understand the study information and insufficient German language skills will exclude from participation in this study.
Study design and interventions
The study design describes a two-centre randomised placebo-controlled four-arm study of a probiotic treatment and its effects on symptoms of allergic rhinitis. The probiotic treatment will be compared with two placebo application modes, a conventional double-blind and OLP application and a no-treatment (untreated group) control arm (see figure 1). After the end of the experimental phase, we will offer the probiotic for another 4 weeks for all patients (open-label probiotic phase).
The probiotic treatment is E. faecalis (DSM 16440), a Gram-positive probiotic species that is constituent of Symbioflor 1 (SymbioPharm, Herborn, Germany) (cells and autolysate of 1.5 to 4.5×107 CFU). It has been demonstrated that E. faecalis stably persists in the human gut when orally administered.44 The probiotic is delivered as drops. The placebo treatment consists of drops containing the carrier solution of the probiotic treatment (lactose monohydrate, glucose monohydrate), but will be indistinguishable in colour, smell and taste from the probiotic.
Study conductance
After signing the informed consent form, patients will complete baseline questionnaires in order to measure the allergic burden (primary endpoints are CSMS, RQLQ). Subsequently, patients are randomised into one of the four arms of the study.
Patients in the first arm receive the probiotic treatment (as drops) for 4 weeks (n=30). In the second arm, the patients receive placebo drops (n=30) indistinguishable from the probiotic in colour and smell/taste. In the third arm, the patients receive the same placebo but are informed that the treatment is a placebo (OLP condition) (n=30). Patients in the fourth arm (n=30) are the no treatment control group; they receive no special therapy but are informed that they are in the untreated group. Patients will be given the supply of the probiotic or placebo, respectively, for 4 weeks (groups 1–3) and are instructed to ingest 30 drops three times a day (groups 1–3), as well as to fill out daily diary forms about their allergic symptoms (all groups). All patients are allowed to continue the usual symptomatic medication of their allergic rhinitis (eg, antihistamines, nasal corticosteroids, etc). Intake of this symptomatic medication will be used as an endpoint using the CSMS.
Patients will then return to the study centre after 4 weeks for a second investigation and questionnaire assessments. At the second visit, we will also ask patients to bring their remaining. A blinded research assistance will then check the amount to evaluate adherence. Furthermore, all patients are offered a 4-week supply of the probiotic treatment in an open-label fashion. If they accept, they are asked to provide questionnaire data on symptoms course (outcome measures) over the 4 weeks, but no further site visit is envisioned.
Measures
Primary endpoint measure is the CSMS, which has been widely used in previous studies to measure allergic symptoms of allergic rhinitis and is recommended by the European Academy of Allergy and Clinical Immunology (EAACI).42 45 The CSMS measures both symptoms of allergic rhinitis such as nasal and eye symptoms and the use of medication. Use of medication will be categorised according to the recommendations of the EAACI with respect to antihistamines, nasal glucocorticoids and oral glucocorticoids.45 Both measures will then build the total symptom score. We will also ask all patients to protocol their allergic burden in a symptom diary on a daily basis during the time of treatment in order to build a daily symptom score based on the CSMS. A second primary endpoint measure is quality of life, measured with the RQLQ. This questionnaire includes 28 questions in seven domains (activity limitations, sleep impairment, non-nasal/eye symptoms, practical problems, nasal symptoms, eye symptoms and emotional problems) and has strong measurement properties.43
Secondary endpoint measures will include visual analogue scales (VAS) to measure the burden of allergic symptoms. VAS have been used to assess the incidence of symptoms or impairment of daily activities.46 Furthermore, we will apply a second questionnaire on quality of life, the Short Form 36 Health Survey Questionnaire (SF-36). This questionnaire is a German version of the health survey developed by Ware and Sherbourne.47 It assesses the quality of life with respect to the perception of health both for patients and healthy people. The instrument includes one multi-item scale that assesses different health concepts such as limitations in physical activities because of health problems, limitations in social activities because of physical or emotional problems, limitations in usual role activities because of physical health problems, bodily pain, general mental health (psychological distress and well-being), limitations in usual role activities because of emotional problems, vitality (energy and fatigue) and general health perceptions. The survey is constructed for self-administration. The SF-36 has also been widely used when measuring effects of allergic rhinitis on everyday life.48
Primary (CSMS and RQLQ) and secondary outcome measures (VAS and SF-36) will be assessed before the trial, after treatment and after follow-up.
Adverse events
The safety of patients will be monitored at each study visit. Participants will receive a study information containing explicit details on whom to contact in case of an adverse event situation. Furthermore, patients will be told to discontinue the study in an adverse event situation.
Data collection: quality management and storage
Researchers will make appointments for the following dates at the end of the first meeting in order to promote participant retention. Data will be collected in an in-person meeting on paper for each measurement and then electronically recorded at the Medical School Berlin. Once recorded, data will be locked to prevent changes. Missing data because of no-show up will be coded as incomplete. Resulting data is then analysed with SPSS V.25. All data collected on paper will be marked with a study identification number to prevent identification of the participant and stored in a locked cabinet. Access to the deidentified data sets will be limited to the study authors.
Analyses
Both primary (CSMS and RQLQ) and secondary endpoints (VAS and SF-36) will be compared between group 1 (probiotic treatment) and group 2 (placebo) for superiority of the probiotic over placebo, between group 2 and group 3 for the size of the OLP effect between open and hidden placebo treatment, and between group 4 and each of the other groups for the contribution of spontaneous variation to the probiotic and (open-label) placebo effects.
We will calculate adjustments for multiple comparisons (post hoc tests).
Power and sample size
Power calculations on the effect of probiotics on our primary outcome parameter, RQLQ, were based on previous studies in allergic rhinitis. Studies on allergic symptoms relative to placebo report effect sizes of d=0.22 or higher.14 49 50 Based on these studies, we used an estimated effect size of f=0.6 (95% CI 1.42 to 0.99) to calculate the sample size, resulting in a required number of participants of n=80. Using analysis of covariance in order to control for baseline scores results in a power at 0.95 to detect a difference in a change from baseline RQLQ, with a 5% level of significance. Given that the difference in change score (means) for this measure is 1.21 and previous studies have shown that mean changes in RQLQ greater than 0.5 can generally be considered as clinically significant, we assume a clinical improvement of the symptoms.43 51
Similar studies investigating the effect of a probiotic mixture (lactobacillus and bifidobacterium) on immune parameters during allergy season calculated that 23 participants per subgroup would be needed to see a difference between probiotic and placebo.52
Furthermore, based on previous studies, we calculated power calculations on the effect of OLPs on symptoms in allergic rhinitis.38 39 Based on a desired power of 0.80, an alpha error probability of 0.05 and an estimated effect size of f=0.5, the required number of participants is a priori set to n=80.
In order to account for dropouts, we aim to include a total of 120 participants.
Blinding and randomisation
After completion of first assessments (first visit), group assignment will be determined by opening an opaque envelope (through a research assistant), revealing the participant’s randomised assignment to one of the four groups. Randomisation is based on a computer-generated random number sequence built by an independent investigator. These researchers will be independent from the members of the study who are responsible for enrolling the participants.
Patients in the probiotics and placebo condition will be blinded (until they finished the study), patients in the OLP condition and in the no treatment control condition will be aware of this assignment. Effective blinding in groups 1 and 2 will be ensured by the company that produces and provides the probiotic and placebo (SymbioPharm GmbH, Herborn, Germany); the group assignment list will be withheld until the final evaluation of the study data. All outcome measurements will be performed by blinded experimenters.
Patient and public involvement
Patient and public representatives will be informed about the study (DAAB). A summary of the findings will be made available to the DAAB.
Ethics and dissemination
The study protocol has been approved by an ethical committee of the University Hospital, Tübingen, Germany and was registered at the German Clinical Trials Register (www.drks.de, DRKS00015804). All participants will give written informed consent prior to entry to the study by a member of the study team and will be made aware that participation is strictly voluntary. Participants may withdraw from the study at any time.
Important protocol modifications will be communicated to the relevant members of the research team.
The eventual trial will be published and subsequently disseminated by the university and social media platforms. The results will also be presented at conferences. Study results will be published in a peer-reviewed journal.
Discussion
The current article describes the methodology of a trial design on effects of a probiotic treatment and OLPs on symptoms of allergic rhinitis.
In his hygiene hypothesis, Strachan suggested a role of microorganisms for allergic reactions.53 In this theory, it is discussed that excessive hygiene may lead to disturbances in the intestinal microbiota. Numerous studies provide support for this assumption. For example, it has been demonstrated that patients with allergies show lower levels of lactobacillus and bacteroides.54 Therefore, using probiotic supplementation in allergic rhinitis might be beneficial.
Several studies suggest that probiotics may have an effect on symptoms of allergic rhinitis. For example, Dennis-Wall et al examined effects of probiotics in individuals with seasonal allergic conditions and found an improvement of rhinoconjunctivitis-specific quality of life.50 Similar effects have been found for a mixture of bifidobacteria treatment in children with seasonal allergic rhinitis and asthma.55 In addition, animal studies found effects of probiotic treatments on pollen-induced allergic nasal symptoms.56 Nevertheless, the effects of probiotics on allergic rhinitis are still not clear and often inconsistent (in particular when the disease is already established).12–16
The mechanisms by which probiotics are thought to be effective in allergic rhinitis are not fully understood. In theory, it is assumed that probiotics exhibit a multitude of mechanisms, ranging from effectively settling its respective mucosal ecological niches (thereby controlling and ousting potentially pathogenic bacteria), via indicating metabolic effects, to stimulating immunological (anti-inflammatory) responses to novel antigen. For allergic rhinitis, it has been suggested that probiotics may activate or inhibit type 1 T helper cells by changing the composition of the gut microbiota.50 57 Probiotics may also stimulate interleukin-10 and thereby inhibiting inflammatory responses.58 Furthermore, probiotics can modify levels of antigen-specific serum IgE levels.59 In addition, Dev et al found that probiotics suppressed histamine signalling.60 Thus, probiotics might change systemic and adaptive immune response and thereby work as immunomodulators. Moreover, it has been shown that probiotics may have a dual effect by improving intestinal as well as central nervous system functions.61 Consuming probiotics may lead to a more balanced intestinal flora in patients with allergic rhinitis, which might constrain damages due to inflammation. In addition, the more balanced intestinal flora may lead to less severe reactions to allergens. However, further research is needed to fully understand the underlying mechanisms.
Since the effects of probiotics on allergic rhinitis are not clear and often inconsistent,12–16 we here aim to examine effects from a probiotic treatment (E. faecalis), compared with two placebo application modes and an untreated group. E. faecalis is part of the normal gastrointestinal flora and along with other lactic acid bacteria often used in food products. Previous studies have already examined E. faecalis, but predominantly in animal studies. To our knowledge, this is the first RCT that investigates effects of E. faecalis in patients with seasonal allergic rhinitis.
Beyond the aim to examine effects of E. faecalis on seasonal allergic symptoms in patients, this study has also a second objective, the possible effects of OLPs. The placebo conditions in this study include a conventional double-blinded placebo and an OLP application. The last application was included because recent studies demonstrate that OLP can result in significant effects on various diseases including allergic rhinitis.40
Although it is well known that symptoms of allergic rhinitis are prone to placebo effects, it is surprising that placebos seem to work even when the patients know that they receive placebos. In traditional randomised controlled studies, placebos are designed to match active pharmaceuticals in order to serve as a control condition. In daily medicine, the placebo effect is often used in a more direct way. For example, a survey of general practioners in Germany reported that 76% administered placebos.62 63 However, it is considered unethical to prescribe placebos with therapeutic purposes because deception is thought to be necessary and would therefore undermine informed consent and trust.64 Hence, many practioners prescribe ‘impure’ placebos, for example, doses of medications, which have no intrinsic pharmacological action on patient’s symptoms. According to a recent national survey of internists and rheumatologists in the USA, only a small number of US physicians used inert placebo pills or injections, but about 50% gave medications that they think to have no specific effect on patients’ conditions.65 Thus, they are prescribed as placebos.
While in the classic understanding it is essential that placebo treatment needs deception of the patient, recent studies report evidence that placebos may work even without concealment or deception. This seems to be very important to profit from beneficial effects of placebos used for a therapeutic purpose in a clear ethical frame. Kaptchuk et al reported a randomised controlled study showing that patients with irritable bowel syndrome symptoms swallowing OLPs had higher mean global improvement scores than a control group.32 Similar studies have been reported on different diseases.34–36
So far it is unclear how OLP exhibit its efficacy. Different mechanisms are discussed and may operate together.40 It has been suggested that the effects of OLPs may be described by classical conditioning. Thus, the effects seen by OLP may be explained by a conditioned expectation. In this view, placebos may retrieve a pharmacological memory.66 This is supported by a recent study on pain perception, which showed that an OLP effect exists in patients who had been conditioned for longer, but not for shorter time periods.67 Embodied cognition is a further way to explain OLP effects. According to this theory, mind and world interact via the body and thereby may influence our cognitions.68 In contrast to the previous explanation, no specific conditioning procedure is necessary. In addition, patient–healthcare provider relations may be important when trying to understand the effects of OLP. It is well known that the social interaction of the patient with the healthcare provider may result in feeling socially supported, which may affect the health system.
However, in order to better understand why OLPs may work, it seems important not only to know if OLPs may result in similar effect sizes than covert placebos but also to compare the effect with other effective or potentially effective treatment options. Unfortunately, to date, there are no OLP studies including a covert placebo condition or an effective other therapy option. The current trial design aims to account for this lack of comparison conditions.
Taken together, the present trial aims to test a probiotic treatment (E. faecalis) in patients with allergic rhinitis compared with effects seen by OLP, double-blinded placebo treatment and no treatment control. With the inclusion of these additional control conditions and endpoints, we hope to determine the effect of the probiotic treatment as well as OLPs on allergic rhinitis.
Supplementary Material
Acknowledgments
We used the SPIRIT checklist when writing this report.
Footnotes
Contributors: PE and MS both drafted and revised the protocol.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PE is a consultant of PrecisionBiotics, Cork, Ireland, as well as of SymbioPharm, Herborn, Germany (the company that provided the probiotic) and Parexel, Durham, North Carolina, USA, companies that produce probiotics. He has also received travel support from Danone, Paris, France and travel support and speaker honorarium from Biocodex, Paris, France; both companies also produce probiotics.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wheatley LM, Togias A. Clinical practice. allergic rhinitis. N Engl J Med 2015;372:456–63. 10.1056/NEJMcp1412282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gould HJ, Sutton BJ, Beavil AJ, et al. The biology of IgE and the basis of allergic disease. Annu Rev Immunol 2003;21:579–628. 10.1146/annurev.immunol.21.120601.141103 [DOI] [PubMed] [Google Scholar]
- 3. Calderon MA, Penagos M, Sheikh A, et al. Sublingual immunotherapy for treating allergic conjunctivitis. Cochrane Database Syst Rev 2011;131 10.1002/14651858.CD007685.pub2 [DOI] [PubMed] [Google Scholar]
- 4. Schatz M. A survey of the burden of allergic rhinitis in the USA. Allergy 2007;62(Suppl 85):9–16. 10.1111/j.1398-9995.2007.01548.x [DOI] [PubMed] [Google Scholar]
- 5. Yanai K, Rogala B, Chugh K, et al. Safety considerations in the management of allergic diseases: focus on antihistamines. Current Med Res Opin 2012;28:623–42. 10.1185/03007995.2012.672405 [DOI] [PubMed] [Google Scholar]
- 6. Novák Z, Yáñez A, Kiss I, et al. Safety and tolerability of bilastine 10 Mg administered for 12 weeks in children with allergic diseases. Pediatr Allergy Immunol 2016;27:493–8. 10.1111/pai.12555 [DOI] [PubMed] [Google Scholar]
- 7. Church MK. Efficacy and tolerability of rupatadine at four times the recommended dose against histamine- and platelet-activating factor-induced flare responses and ex vivo platelet aggregation in healthy males. British Journal of Dermatology 2010;163:1330–2. 10.1111/j.1365-2133.2010.10029.x [DOI] [PubMed] [Google Scholar]
- 8. Horak F, Stubner UP. Comparative tolerability of second generation antihistamines. Drug Safety 1999;20:385–401. 10.2165/00002018-199920050-00001 [DOI] [PubMed] [Google Scholar]
- 9. Kim M, Ku S, Kim S, et al. Safety evaluations of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI. Int J Mol Sci 2018;19:1422 10.3390/ijms19051422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watts AM, West NP, Smith PK, et al. Probiotics and allergic rhinitis: a Simon two-stage design to determine effectiveness. J Altern Complement Med 2016;22:1007–12. 10.1089/acm.2016.0115 [DOI] [PubMed] [Google Scholar]
- 11. Elazab N, Mendy A, Gasana J, et al. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics 2013;132:e666–76. 10.1542/peds.2013-0246 [DOI] [PubMed] [Google Scholar]
- 12. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol 2008;101:570–9. 10.1016/S1081-1206(10)60219-0 [DOI] [PubMed] [Google Scholar]
- 13. Das RR, Singh M, Shafiq N. Probiotics in treatment of allergic rhinitis. World Allergy Organ J 2010;3:239–44. 10.1097/WOX.0b013e3181f234d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol 2015;5:524–32. 10.1002/alr.21492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Güvenç IA, Muluk NB, Mutlu Fezan Şahin, et al. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and Metaanalysis. Am J Rhinol Allergy 2016;30:e157–75. 10.2500/ajra.2016.30.4354 [DOI] [PubMed] [Google Scholar]
- 16. Peng Y, Li A, Yu L, et al. The role of probiotics in prevention and treatment for patients with allergic rhinitis: a systematic review. Am J Rhinol Allergy 2015;29:292–8. 10.2500/ajra.2015.29.4192 [DOI] [PubMed] [Google Scholar]
- 17. Kitz R, Martens U, Zieseniß E, et al. Probiotic E.faecalis – adjuvant therapy in children with recurrent rhinosinusitis. Central European Journal of Medicine 2012;7:362–5. [Google Scholar]
- 18. Castro MS, Molina MA, Azpiroz MB, et al. Probiotic activity of Enterococcus faecalis CECT7121: effects on mucosal immunity and intestinal epithelial cells. J Appl Microbiol 2016;121:1117–29. 10.1111/jam.13226 [DOI] [PubMed] [Google Scholar]
- 19. Castro MS, Azpiroz MB, Molina MA, et al. Preliminary studies on the prevention of the ovalbumin-induced allergic response by Enterococcus faecalis CECT7121 in mice. International Arch Allergy Immunol 2012;157:11–20. 10.1159/000324673 [DOI] [PubMed] [Google Scholar]
- 20. Zhu L, Shimada T, Chen R, et al. Effects of lysed Enterococcus faecalis FK-23 on experimental allergic rhinitis in a murine model. J Biomed Res 2012;26:226–34. 10.7555/JBR.26.20120023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu L-P, Zhang Q-Z, Shimada T, et al. [Anti-allergic effects of the probiotic preparations of enterococcus on experimental allergic rhinitis in mice]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013;48:555–62. [PubMed] [Google Scholar]
- 22. Shimada T, Cheng L, Shi HB, et al. Effect of lysed Enterococcus faecalis FK-23 on allergen-induced immune responses and intestinal microflora in antibiotic-treated weaning mice. J Investig Allergol Clin Immunol 2007;17:70–6. [PubMed] [Google Scholar]
- 23. Shimada T, Zhu L-P, Yin M, et al. Effects of lysed Enterococcus faecalis FK-23 on allergen-induced peritoneal accumulation of eosinophils and serum total IgE concentration in inbred mice. Asian Pac J Allergy Immunol 2008;26:137–41. [PubMed] [Google Scholar]
- 24. El Hennawi DEDM, Ahmed MR, Farid AM. Psychological stress and its relationship with persistent allergic rhinitis. Eur Arch Otorhinolaryngol 2016;273:899–904. 10.1007/s00405-015-3641-6 [DOI] [PubMed] [Google Scholar]
- 25. Horing B, Weimer K, Muth ER, et al. Prediction of placebo responses: a systematic review of the literature. Front Psychol 2014;5 10.3389/fpsyg.2014.01079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 2015;12:472–85. 10.1038/nrgastro.2015.117 [DOI] [PubMed] [Google Scholar]
- 27. Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. The Lancet Psychiatry 2015;2:246–57. 10.1016/S2215-0366(14)00092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abramson HA. Psychic factors in allergy and their treatment. Ann Allergy 1956;14:145–51. [PubMed] [Google Scholar]
- 29. Czubalski K, Zawisza E. The role of psychic factors in patients with allergic rhinitis. Acta Otolaryngol 1976;81:484–8. 10.3109/00016487609119988 [DOI] [PubMed] [Google Scholar]
- 30. del Cuvillo A, Sastre J, Bartra J, et al. Placebo effect in clinical trials involving patients with allergic rhinitis. J Investig Allergol Clin Immunol 2011;21(Suppl 3):40–5. [PubMed] [Google Scholar]
- 31. Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron 2014;84:623–37. 10.1016/j.neuron.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 32. Kaptchuk TJ. Open-Label placebo: reflections on a research agenda. Perspect Biol Med 2018;61:311–34. 10.1353/pbm.2018.0045 [DOI] [PubMed] [Google Scholar]
- 33. Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One 2010;5:e15591 10.1371/journal.pone.0015591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kam-Hansen S, Jakubowski M, Kelley JM, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med 2014;6:218ra5 10.1126/scitranslmed.3006175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carvalho C, Caetano JM, Cunha L, et al. Open-Label placebo treatment in chronic low back pain: a randomized controlled trial. Pain 2016;157:2766–72. 10.1097/j.pain.0000000000000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelley JM, Kaptchuk TJ, Cusin C, et al. Open-Label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom 2012;81:312–4. 10.1159/000337053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoenemeyer TW, Kaptchuk TJ, Mehta TS, et al. Open-label placebo treatment for cancer-related fatigue: a randomized-controlled clinical trial. Sci Rep 2018;8 10.1038/s41598-018-20993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schaefer M, Sahin T, Berstecher B. Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis. PLoS One 2018;13:e0192758 10.1371/journal.pone.0192758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schaefer M, Harke R, Denke C. Open-Label placebos improve symptoms in allergic rhinitis: a randomized controlled trial. Psychother Psychosom 2016;85:373–4. 10.1159/000447242 [DOI] [PubMed] [Google Scholar]
- 40. Charlesworth JEG, Petkovic G, Kelley JM, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta-analysis. J Evid Based Med 2017;10:97–107. 10.1111/jebm.12251 [DOI] [PubMed] [Google Scholar]
- 41. Kaptchuk TJ, Miller FG. Open label placebo: can honestly prescribed placebos evoke meaningful therapeutic benefits? BMJ 2018;363 10.1136/bmj.k3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rondón C, Blanca-López N, Campo P, et al. Specific immunotherapy in local allergic rhinitis: A randomized, double-blind placebo-controlled trial with Phleum pratense subcutaneous allergen immunotherapy. Allergy 2018;73:905–15. 10.1111/all.13350 [DOI] [PubMed] [Google Scholar]
- 43. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 1991;21:77–83. 10.1111/j.1365-2222.1991.tb00807.x [DOI] [PubMed] [Google Scholar]
- 44. Wassenaar TM, Marzorati M, Beimfohr C, et al. Survival of probiotic E coli and ENT. faecalis in the human host after oral intake: results from in vitro and in vivo studies. Biotechnology & Microbiology 2017;2:1–5. [Google Scholar]
- 45. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy 2014;69:854–67. 10.1111/all.12383 [DOI] [PubMed] [Google Scholar]
- 46. Droessaert V, Timmermans M, Dekimpe E, et al. Real-Life study showing better control of allergic rhinitis by immunotherapy than regular pharmacotherapy. Rhin 2017;54:214–20. 10.4193/Rhin14.282 [DOI] [PubMed] [Google Scholar]
- 47. Ware JE, Sherbourne CD. The mos 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 48. Jalali MM, Soleimani R, Alavi Foumani A, et al. Add-on probiotics in patients with persistent allergic rhinitis: a randomized crossover clinical trial. Laryngoscope 2019;129:1744–50. 10.1002/lary.27858 [DOI] [PubMed] [Google Scholar]
- 49. Costa DJ, Marteau P, Amouyal M, et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN study). Eur J Clin Nutr 2014;68:602–7. 10.1038/ejcn.2014.13 [DOI] [PubMed] [Google Scholar]
- 50. Dennis-Wall JC, Culpepper T, Nieves C, et al. Probiotics (Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2) improve rhinoconjunctivitis-specific quality of life in individuals with seasonal allergies: a double-blind, placebo-controlled, randomized trial. Am J Clin Nutr 2017;105:758–67. 10.3945/ajcn.116.140012 [DOI] [PubMed] [Google Scholar]
- 51. Demoly P, Dreyfus I, Dhivert-Donnadieu H, et al. Desloratadine for the treatment of cypress pollen-induced allergic rhinitis. Ann Allergy Asthma Immunol 2009;103:260–6. 10.1016/S1081-1206(10)60191-3 [DOI] [PubMed] [Google Scholar]
- 52. Koyama T, Kirjavainen PV, Fisher C, et al. Development and pilot evaluation of a novel probiotic mixture for the management of seasonal allergic rhinitis. Can J Microbiol 2010;56:730–8. 10.1139/W10-061 [DOI] [PubMed] [Google Scholar]
- 53. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989;299:1259–60. 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Björkstén B, Naaber P, Sepp E, et al. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999;29:342–6. 10.1046/j.1365-2222.1999.00560.x [DOI] [PubMed] [Google Scholar]
- 55. Miraglia Del Giudice M, Indolfi C, Capasso M, et al. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr 2017;43:25 10.1186/s13052-017-0340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsunemine S, Isa Y, Ohno H, et al. Longitudinal study of effects of oral dosage of Bifidobacterium bifidum G9-1 on Japanese cedar pollen-induced allergic nasal symptoms in guinea pigs. Microbiol Immunol 2015;59:690–9. 10.1111/1348-0421.12324 [DOI] [PubMed] [Google Scholar]
- 57. Lyons A, O'Mahony D, O'Brien F, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. ClinExp Allergy 2010;40:811–9. 10.1111/j.1365-2222.2009.03437.x [DOI] [PubMed] [Google Scholar]
- 58. Hoyte FCL, Nelson HS. Recent advances in allergic rhinitis. F1000Research 2018;7 10.12688/f1000research.15367.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shida K, Takahashi R, Iwadate E, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy 2002;32:563–70. 10.1046/j.0954-7894.2002.01354.x [DOI] [PubMed] [Google Scholar]
- 60. Dev S, Mizuguchi H, Das AK, et al. Suppression of histamine signaling by probiotic Lac-B: a possible mechanism of its anti-allergic effect. J Pharmacol Sci 2008;107:159–66. 10.1254/jphs.08028FP [DOI] [PubMed] [Google Scholar]
- 61. Wang H, Lee I-S, Braun C, et al. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil 2016;22:589–605. 10.5056/jnm16018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Linde K, Atmann O, Meissner K, et al. How often do general practitioners use placebos and non-specific interventions? systematic review and meta-analysis of surveys. PLoS One 2018;13:e0202211 10.1371/journal.pone.0202211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meissner K, Höfner L, Fässler M, et al. Widespread use of pure and impure placebo interventions by GPs in Germany. Fam Pract 2012;29:79–85. 10.1093/fampra/cmr045 [DOI] [PubMed] [Google Scholar]
- 64. Blease CR, Bishop FL, Kaptchuk TJ. Informed consent and clinical trials: where is the placebo effect? BMJ 2017;356 10.1136/bmj.j463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tilburt JC, Emanuel EJ, Kaptchuk TJ, et al. Prescribing "placebo treatments": results of national survey of US internists and rheumatologists. BMJ 2008;337 10.1136/bmj.a1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Colloca L, Howick J. Placebos without deception: outcomes, mechanisms, and ethics. Int Rev Neurobiol 2018;138:219–40. 10.1016/bs.irn.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J Pain 2015;16:412–20. 10.1016/j.jpain.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fuchs T, Schlimme JE. Embodiment and psychopathology: a phenomenological perspective. Curr Opin Psychiatry 2009;22:570–5. 10.1097/YCO.0b013e3283318e5c [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


