Abstract
Introduction
Postoperative pulmonary complications (PPCs), strongly associated with higher mortality risk, can develop in up to 58% of patients undergoing abdominal surgery. More and more evidence shows that the use of a lung-protective ventilation strategy has a lung protection effect in patients undergoing abdominal surgery, however, the role of positive end-expiratory pressure (PEEP) during the intraoperative period in preventing PPCs for laparoscopic surgery is not clearly defined.
Methods and analysis
A total of 208 patients with a high risk of PPC, undergoing laparoscopic abdominal surgery, will be enrolled and randomised into a standard PEEP (6–8 cm H2O) group and a low PEEP (≤2 cm H2O) group. Both groups will receive a fraction of inspired oxygen of 0.50 and a tidal volume of 8 mL/kg ideal body weight (IBW). Standard perioperative fluid management and analgesic treatments are applied in both groups. The primary end point is PPC within 7 days after surgery. Secondary end points are the modified Clinical Pulmonary Infection Score, postoperative extrapulmonary complications, postoperative surgical complications, intensive care unit length of stay, hospital length of stay, 30-day mortality.
Ethics and dissemination
The study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (People’s Hospital of Hangzhou Medicine College) (registration number KY2018026) on 22 October 2018. The first participant was recruited on 15 April 2019 and the estimated completion date of the study is October 2021. The results of this trial will be submitted to a peer-reviewed journal.
Trial registration number
http://www.chictr.org.cn, ID: ChiCTR1800019865. Registered on 2 December 2018; preresults.
Keywords: positive end-expiratory pressure, postoperative pulmonary complications, laparoscopic surgery
Strengths and limitations of this study.
The intervention is blinded.
Standard perioperative fluid management and analgesic treatments are applied in both groups.
The primary outcome measure is patient-centred.
This is not a multicentre randomised trial.
Background
Every year around the world, approximately 230 million patients require surgery with general anaesthesia and mechanical ventilation.1 Laparoscopic surgery has been widely accepted because it is associated with less blood loss, less postoperative pain and rapid recovery.2 3 The incidence of postoperative pulmonary complications (PPCs) in patients undergoing general surgery is approximately 5%,4 and 12%–58% of patients undergoing abdominal surgery will develop a PPC.4 5 Furthermore, PPCs are strongly associated with prolonged postoperative hospital stays and a higher risk of mortality.6–8
Nearly 30% of surgery patients undergoing general anaesthesia and mechanical ventilation are at intermediate risk to high risk for PPCs according to large cohort studies.5 9 Both alveolar overstretching and atelectasis induce the release of inflammatory mediators, leading to lung and systemic organ damage.10 Lung-protective ventilation including the use of low tidal volumes and positive end-expiratory pressure (PEEP), aims to prevent atelectasis and improve gas exchange.11 12 Furthermore, PEEP has been found to reduce mortality in patients with the acute respiratory distress syndrome (ARDS) and critically ill patients.13
Adopting an appropriate PEEP may prevent PPCs. When high PEEP is applied, the alveolus may be overinflated and pulmonary vascular resistance is likely to increase; however, use of low PEEP may not prevent atelectasis.10 Compared with non-protective mechanical ventilation without PEEP, a number of studies have shown that the use of a lung-protective ventilation strategy has a lung-protective effect in patients with healthy lungs who are undergoing abdominal surgery, reducing the incidence of PPC.14 15 Despite all these studies recommending the use of low tidal volume,10 the appropriate PEEP has not yet been defined. A multicentre observational study has shown that approximately 20% of patients do not receive PEEP during routine anaesthetic practice.16 In the Intraoperative Protective Ventilation Trial that included patients undergoing major abdominal surgery with intermediate risk and high risk of PPCs, compared with a practice of non-protective mechanical ventilation including higher tidal volumes without PEEP, a lung-protective ventilation strategy with lower tidal volumes and PEEP of 6 cm H2O was associated with improved clinical outcomes.14 Furthermore, in another study including patients undergoing abdominal non-laparoscopic surgery lasting more than 2 hours, compared with a standard ventilation strategy, a protective ventilation strategy with 10 cm H2O PEEP improved respiratory function and reduced the modified Clinical Pulmonary Infection Score (mCPIS).15 However, another study has shown that low tidal volume combined with low PEEP (3 cm H2O) ventilation may induce postoperative inflammation and may increase the risk of PPCs during major surgery such as hepatectomy.17 In an international multicentre trial, Protective Ventilation Using High Versus Low PEEP, including patients undergoing open abdominal surgery with high risk for PPCs, compared with low PEEP (≤2 cm H2O), a ventilation strategy of high PEEP (12 cm H2O) did not reduce the incidence of PPCs, but more likely caused haemodynamic instability.18 Therefore, the authors suggested a ventilation strategy of low tidal volume combined with low PEEP (≤2 cm H2O).18
It should also be noted that all these studies included only open surgeries or various types of abdominal surgery; they did not include patients planning to undergo laparoscopic surgery. Some studies have suggested that laparoscopic-assisted gastrectomy was beneficial for postoperative respiratory function recovery. Nevertheless, it is also necessary to consider the effects of pneumoperitoneum (PnP) on airway pressure and pulmonary function. The role of PEEP during the intraoperative period in preventing PPCs for laparoscopic surgery has not been clearly defined. We hypothesised that, when compared with low PEEP, standard PEEP may prevent the incidence of PPCs and may reduce the occurrence of organ dysfunction. These anticipated results may further improve our knowledge regarding the effects of intraoperative PEEP on PPCs, and survival rates and in-hospital stays in patients undergoing laparoscopic surgery.
Methods/design
Objectives of the study
This trial aimed to compare the effects of low tidal volumes combined with standard PEEP (6–8 cm H2O) with those of low PEEP (≤2 cm H2O) in patients at risk for complications undergoing laparoscopic surgery during general anaesthesia in terms of: (1) PPCs. (2) mCPIS, postoperative extrapulmonary complications, changes in chest X-ray findings and oxygenation. (3) Intraoperative complications including hypoxaemia, massive transfusion. (4) Postoperative surgical complications, intensive care unit (ICU) lengths of stay, hospital lengths of stay and 30-day mortality.
Study end points
Primary outcome measure
The primary end point of PPCs is defined according to a previous report19 including any new atelectasis or infiltrates on a chest X-ray, respiratory failure (defined as the need for non-invasive or invasive ventilation) or partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) <300 within 7 days after surgery.
Secondary outcome measures
Secondary outcome variables are any pulmonary complications and extrapulmonary complications as follows:
PPCs within 30 days after surgery. Those PPCs are scored according to a grading scale ranging from 0 to 420 (grade 0 representing no PPCs and grades 1–4 representing gradually worse forms of PPCs) within 7–30 days after surgery (table 1).
Table 1.
Grade scale for postoperative pulmonary complications
| Grade scale | Detailed description |
| Grade 1 |
|
| Grade 2 |
|
| Grade 3 |
|
| Grade 4 | Ventilatory failure: postoperative non-invasive ventilation dependence ≥48 hours, or re-intubation with subsequent period of ventilator dependence ≥48 hours |
-
PPCs will also be analysed separately.
Pneumonia is defined according to Centres for Disease Control criteria21 as follows: patients with altered or new pulmonary opacities on chest X-ray; patients should also meet at least two of the following criteria: (1) Temperature ≥38.5°C or <36°C. (2) Leucocyte count >12×109/L or <4×109/L. (3) Purulent sputum: new cough or difficulty breathing or previous coughing or difficulty breathing is further aggravated.
Postoperative hypoxaemia and severe hypoxaemia:22 hypoxaemia is defined as PaO2 <60 mm Hg or oxygen saturation (SpO2) <90% on room air, but responding to oxygen treatment (hypoventilation should be excluded). Severe hypoxaemia is recorded in cases when the patient requires non-invasive or invasive mechanical ventilation.
Suspected pulmonary infection is described in a previous study:18 the patient takes antibiotics and should meet at least one of the following criteria: (1) Changed or new sputum. (2) Changed or new pulmonary opacities on chest X–ray. (3) Temperature >38.3°C. (4) Leucocyte count >12×109/L.
Pulmonary infiltrate is defined according to consensus guidelines: chest X-ray demonstrating monolateral or bilateral infiltrate.23
ARDS is defined according to the Berlin criteria.25
Suspected pulmonary complications15 are defined in cases where patients display at least three of the following new findings: (1) Cough. (2) Increased secretions. (3) Dyspnoea, (4) Chest pain. (5) Temperature >38°C. (6) Pulse rate >100 beats per minute.
Requirement for postoperative ventilation (respiratory failure that requires non-invasive and/or invasive ventilation) for at any time after surgery according to standard criteria and clinical practice guidelines.20
Table 2.
The definition of modified Clinical Pulmonary Infection Score (mCPIS)
| Items | CPIS Points | ||
| 0 | 1 | 2 | |
| Tracheal secretions | Rare | Abundant | Abundant + purulent |
| Chest X-ray infiltrates | No infiltrate | Diffused | Localised |
| Temperature (°C) | 36.5–38.4 | 38.5–38.9 | ≤36.5 or ≥39.0 |
| Leucocytes count (per mm3) | 4000–11 000 | <4000 or >11 000 | <4000 or >11 000 + band forms ≥500 |
| PaO2/FiO2,(mm Hg) | >240 or ARDS | ≤240 and no evidence of ARDS | |
| Microbiology | Negative | Positive | |
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen.
-
Postoperative extrapulmonary complications within 30 days after surgery:
Systemic inflammatory response syndrome criteria are defined when meeting the following two or more criteria by the most deranged value recorded after surgery:26 (1) Rectal or tympanic temperature >38°C or <36°C (0.5°C will be added to the measured value when oral or other temperatures are used). (2) Heart rate >90 beats/min (excluding those who have a known medical condition or are receiving treatment that would prevent tachycardia). (3) Respiratory rate >20 breaths/min or a PaCO2 <32 mm Hg or requiring mechanical ventilation. Leucocyte count >12 000/mm3 or <4000/mm3 or >10% immature bands.
Sepsis and septic shock:26sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be identified as an acute change in total Sequential (sepsis-related) Organ Failure Assessment Score ≥2 points consequent to the infection. Septic shock is a subset of sepsis in which the underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.
Other extrapulmonary infection including surgical site infection (SSI) and intra-abdominal abscess: SSI27 is defined as SSI within 30 days after surgery; at least the incision has a purulent effluent; the incision drainage fluid or tissue culture results are positive, with pain or tenderness, local swelling, redness or fever.
Need for postoperative blood transfusion.
Postoperative surgical complications: anastomotic leakage and need for surgical reintervention, defined according to consensus criteria.28
Unexpected ICU admission or readmission.
ICU length of stay and hospital length of stay.
Hospital-free days at follow-up day 30.
In-hospital mortality and 30-day mortality (all-cause mortality 30 days after randomisation).
Intraoperative complications: pneumothorax is confirmed by chest X-ray and any other complications.
Study design
This unfunded, parallel-group, double-blinded, prospective, randomised controlled clinical trial was registered at http://www.chictr.org.cn (ChiCTR1800019865) and was conducted at the Department of Anesthesiology and Intensive Care of Zhejiang Provincial People's Hospital. The first participant was recruited on 15 April 2019. This trial protocol is conducted according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (figure 1). The Standard Protocol Items:Recommendations for Interventional Trials 2013 Checklist is given in online additional file 1.
Figure 1.
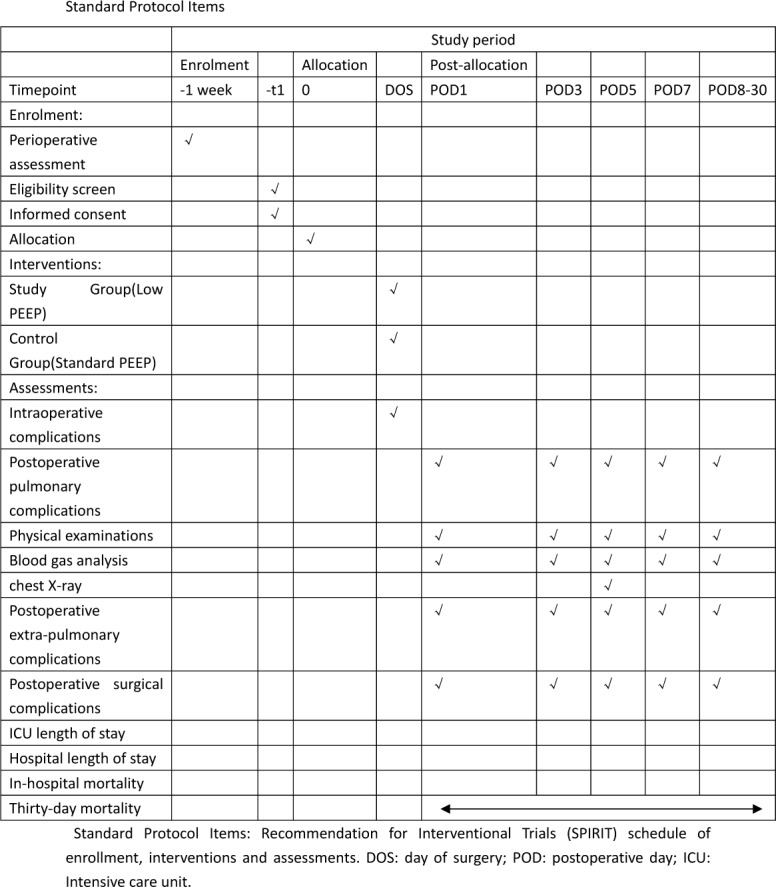
Standard protocol items. PEEP, positive end-expiratory pressure.
bmjopen-2018-028464supp001.pdf (72.6KB, pdf)
Blinding, data collection and randomisation
Researchers will be trained prior to investigation. Study data including patient clinical characteristics, intraoperative respiratory parameters, postoperative outcomes and laboratory test, will be collected onto case report forms (CRFs) (online additional file 2).
bmjopen-2018-028464supp002.pdf (73KB, pdf)
An independent researcher will randomise the participants into the study group (standard PEEP group) and control group (low PEEP group) in a ratio of 1:1. The random sequence will be computer-generated and participants will be allocated in numerical order with sealed opaque envelopes. The attending anaesthesiologist performs anaesthesia strictly according to the research protocol and is also responsible for data during the preoperative, intraoperative and postanaesthesia care unit (PACU) period. The chief surgeon performs the postoperative laboratory testing. An independent researcher will be involved in postoperative follow-up and data collection. Statistical analysis will be performed by a statistician who does not participate in the data collection. Patients, research staff, surgeons, intensive care physicians and the statistician will be unaware of the group allocation. Some preoperative characteristics and laboratory results will automatically be derived from a computer database.
Selection of the participants
Patients scheduled for elective laparoscopic abdominal surgery under general anaesthesia will be screened and recruited during preoperative assessment. Patients meeting inclusion criteria will be required to provide their written informed consent (online additional files 3 and 4). The participant can withdraw from the trial at any time.
Inclusion criteria are patients older than 18 years, American Society of Anesthesiologists (ASA) physical status II or III, body mass index (BMI) between 18 kg/m2 and 35 kg/m2, general anaesthesia expected to last more than 3 hours, an intermediate or high preoperative index for PPC risk by the Assess Respiratory Risk in Surgical Patients in Catalonia Study (ARISCAT Score ≥26, the online additional file 5).
bmjopen-2018-028464supp005.pdf (101.2KB, pdf)
Exclusion criteria are listed as following: emergency surgery or history of previous lung surgery, history of mechanical ventilation within the 2 weeks before recruitment, non-invasive ventilation or oxygen therapy at home, acute respiratory failure (pneumonia, acute lung injury or ARDS), history of chronic obstructive pulmonary disease, persistent haemodynamic instability or severe cardiac disease (New York Heart Association class III or IV, or persistent ventricular tachyarrhythmia’s, or acute coronary syndrome), sepsis or septic shock, need for renal replacement therapy (CRRT), progressive neuromuscular illness, pregnancy, participation in another study or refusal to participate.
Time course of the study
Preoperative admission
Medical history, ASA physical status, BMI, 12-lead ECG, laboratory results, chest X-ray or CT scan, ARISCAT Score and Nutritional Risk Screening (NRS 2002 tool) Score, and the results of echocardiography and spirometry (in cases of history of coronary artery disease or smoking) will be recorded.
Intraoperative care
A central venous catheter and an arterial cannula will be placed before induction of anaesthesia. Peripheral SpO2, arterial blood pressure, heart rate, ECG, end-tidal carbon dioxide tension (EtCO2) and Bispectral Index (BIS) will be monitored continuously. PnP, tidal volume, PEEP, airway pressures including peak pressure and plateau pressure, airway resistance, Vds/Vt, core temperature and arterial blood gas analysis data will be recorded.
Crystalloid (12–15 mL/kg/h) is infused to maintain haemodynamic stability and central venous pressure 5–12 cm H2O. Blood loss and vasodilation are supplemented by colloidal fluid.
Routine anaesthesia is induced with intravenous dexmedetomidine (1 ug/kg) or midazolam (0.05–0.075 mg/kg), cisatracurium (2 mg/kg), propofol (2–3 mg/kg) and fentanyl (1–3 μ/kg) for tracheal intubation. Anaesthesia is maintained with propofol, sevoflurane and remifentanil infusion to maintain BIS at 40–50 until skin suturing is completed. Cisatracurium (1.0–1.5 mg/kg) is administered every hour and the last dose is at least 1 hour before the end of operation.
Ropivacaine is administrated as local incision infiltration anaesthesia before and at the end of the operation, respectively. Fentanyl (1–3 µg/kg) and flurbiprofenaxetil 50 mg are required before remifentanil is stop.
Postoperative care
Patients will be transferred to the PACU after surgery regardless of whether they are still intubated.
Postoperative pain management will be suggested to achieve a Visual Analogue Scale Pain Score of <3/10 using a patient-controlled intravenous analgesia pump including fentanyl (0.3–0.5 µg/kg), flurbiprofenaxetil (100 mg) and palonosetron hydrochloride (0.25 mg) palazidine.
The ICU physician and surgeon will independently monitor clinical progress and all end points by daily physical examinations. Appropriate prophylactic antibiotics and antithrombotic treatments will be administered as required during the postoperative period. Chest X-ray will be performed by an independent, trained radiologist on postoperative day (POD) 5. Arterial blood gas analysis will be performed on POD 1 and POD 3 and other laboratory tests will be performed on POD 1, POD 3, POD 5 and POD 7. The examinations will be repeated and microbiology tests will be performed when the development of pulmonary complications is suspected.
Study arms and intraoperative ventilation protocol
Patients will be randomly assigned to the low PEEP ventilation group (PEEP ≤2 cm H2O) or the standard PEEP group (PEEP=6–8 cm H2O) using a volume-controlled ventilation strategy (Datex Ohmeda S/5 Avance; GE Healthcare, Helsinki, Finland) with a tidal volume of 8 mL/kg IBW, an FiO2 of 0.50 and an inspiratory to expiratory ratio of 1:2. Respiratory rate should be adjusted to maintain ETCO2 between 35 mm Hg and 45 mm Hg and plateau pressure should be no more than 35 cm H2O. IBW is calculated using formulas as follows:14 45.5 + 0.91 × (centimetres of height − 152.4) for women and 50 + 0.91 × (centimetres of height − 152.4) for men. Recruitment manoeuvres (RMs)19 will be performed immediately after tracheal intubation and every time when the ventilator is interrupted until the end of surgery in each group. The compliance of the respiratory system is calculated using the formula VT/(plateau pressure of the respiratory system − PEEP).
RMs will be performed as follows:
1. Pressure support ventilation mode.
2. PEEP set to 30 cm of water.
3. Inspiratory gas flow set to the highest value.
4. Duration of the manoeuvre =30 s.
Rescue therapy will be applied in case of desaturation (defined as a peripheral SpO2 <92%), consisting of increasing FiO2 to 100% in each group and increased PEEP in the low PEEP group (online additional file 6).
bmjopen-2018-028464supp006.pdf (78.7KB, pdf)
From POD 7 to POD 30 (follow-up)
Secondary end points and any mortality will also be evaluated during the follow-up period. The CONSORT flow chart of the trial is shown in figure 2.
Figure 2.
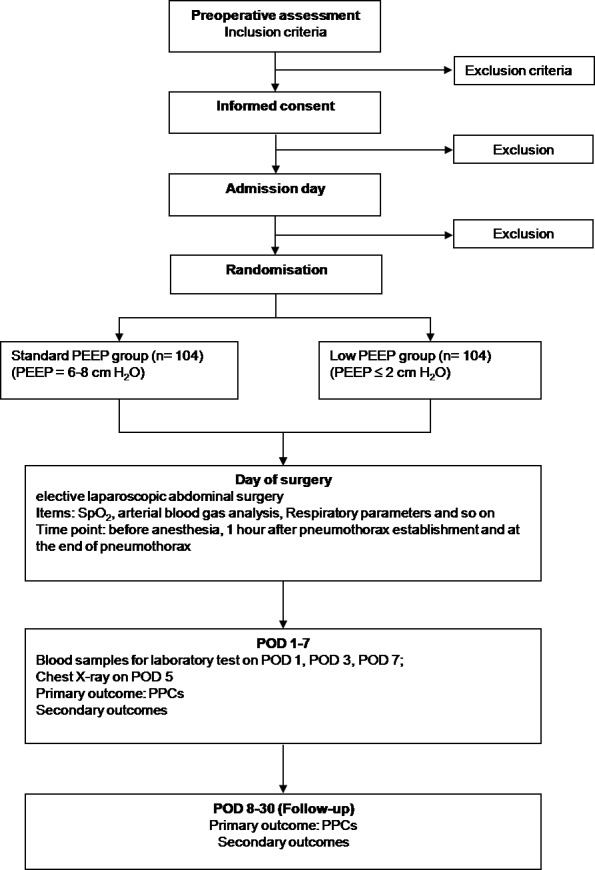
The Consolidated Standards of Reporting Trials (CONSORT) flow chart of the trial. PEEP, positive end-expiratory pressure; POD, postoperative day; PPC, postoperative pulmonary complication; SpO2, oxygen saturation.
Data monitoring and handling of implausible values or missing values
A clinical investigator will identify implausible values. Missing continuous variables should be less than 10% and will be replaced by the mean of all plausible data (both groups) of the respective end point. Data monitoring is managed by an independent investigator who is not involved in the study. The progress of the study will be evaluated and the completeness and accuracy of the data (informed consent forms, source data, CRFs and outcome variables) will be verified.
Statistics
Normally distributed variables will be expressed as the mean±SD and will be compared with Student’s t-test. Categorical variables will be compared using the χ2 test or the Fisher’s exact test. Non-normal continuous variables will be expressed as median (IQR) and evaluated using the Mann-Whitney U-test. The primary and secondary outcomes will all be handled. Intention-to-treat analyses are performed to compare the composite outcome measure at 7 days in the two groups by the χ2 test (or Fisher’s exact test as appropriate) and adjustment using multiple logistic regression analysis will be carried out to identify various risk factors (for the primary outcome and the pulmonary complications at POD 30). A value of p<0.05 will be considered statistically significant and all reported p values will be two-sided. Interim analysis of safety will be conducted after enrolment of the first 104 patients. All analyses will be conducted using SPSS V.18.0 (SPSS, Chicago, Illinois, USA) software.
Sample size calculation
The incidence rate of PPCs was 39% in the low PEEP group.18 A two-tailed χ2 test was performed and we estimated that 188 patients were required to provide 80% power to detect 50% relative difference between the two groups, with a probability of 0.05 for type I error. Assuming 10% loss to follow-up, a total of 208 cases are needed. Analysis is computed using G-Power (V.3.1; Informer Technologies).
Adverse events and interruption of the trial
All patients will be continuously monitored during the study including daily visits during the in-hospital period and daily phone-call visits during the out of hospital follow-up period (until POD 30). All serious adverse, unexpected or possibly related events will be recorded in the CRF and will be reported to the data monitoring and safety committee (DMSC). The DMSC will recommend that the study should be stopped when there is evidence that patient is not safety (a between-group difference in serious adverse events or in 30-day mortality is found).
Ethics and dissemination
Any subsequent protocol and informed consent document amendments must be approved by the responsible ethics committee. All communications between the regulatory authorities and the ethics committee must be recorded.
All recruited patients will be informed of the trial purposes and their duties within the trial before randomisation. Recruited patients can withdraw from the study at any time without providing any specific reason. Patient data will be stored in a separate, safe place, but it may be reviewed by the relevant investigator.
The original data (CRF and relevant records) will be maintained for 5 years and then destroyed according to hospital standards.
Discussion
In this pragmatic, prospective, randomised controlled trial of high-risk patients undergoing laparoscopic surgery, our aim is to assess possible single effects of PEEP levels on major PPCs from those of lower tidal volumes and RMs and to assess relevant clinical parameters associated with alterations in pulmonary function such as chest X-ray, abnormalities, mCPIS, arterial oxygenation/peripheral SpO2 in air and changes in dyspnoea/cough/secretions. Our findings might change the current practice of mechanical ventilation in high-risk patients undergoing laparoscopic surgery.
There are some potential strengths of the present trial protocol. First, the ARISCAT Score of this study will be used to predict PPCs and we will select only the high-risk PPC population that will potentially receive maximum benefit from intraoperative PEEP strategy. Although various scores have been developed for predicting PPC incidence based on various countries and surgical populations, the ARISCAT Score is considered to be the most valuable tool.10 Second, this trial design includes instructions for fluid management standardisation and analgesic treatments during the perioperative period. Third, the included patients will undergo elective abdominal laparoscopic surgical procedures with more than 3 hours of general anaesthesia. Previous studies have reported that both abdominal surgery and longer anaesthesia duration are potential risk factors for PPCs.8
Notably, mechanical ventilation itself is one of the major contributors to PPCs.29 PnP is also an important risk factor for PPCs.30 Intra-abdominal pressure is frequently higher than airway pressure during PnP with carbon dioxide for laparoscopic surgery. This pressure gradient usually causes cephalad displacement of the diaphragm and collapses adjacent pulmonary tissues. PnP also decreases respiratory compliance and arterial oxygenation.31All these influences on PnP finally lead to atelectasis.32
On the other hand, PEEP is thought to prevent the development of atelectasis by keeping the airways open and maintaining adequate gas exchange at the end of the expiratory period during PnP.10 Certainly, the level of PEEP should be adopted according to the patient’s and surgical characteristics as well as the patient’s positioning.
Previous studies reported that very low levels of PEEP were potentially associated with atelectasis by promoting repeated opening and closing of small airways.33 However, higher levels of PEEP may increase mean airway pressure of the respiratory system and likely even impair haemodynamics.
There is an increasing number of highly qualitative randomised controlled trials regarding intraoperative mechanical ventilation and PPCs, but these lack direct assessment of the effect on high-risk patients undergoing laparoscopic surgery. The potential significance of this trial is that it may provide evidence of the effects of intraoperative PEEP on PPCs in high-risk patients undergoing laparoscopic surgery.
bmjopen-2018-028464supp003.pdf (210.7KB, pdf)
bmjopen-2018-028464supp004.pdf (292.6KB, pdf)
Supplementary Material
Acknowledgments
The authors thank all the contributors and collaborators for their support in this study. The authors also thank all the participating patients.
Footnotes
Contributors: Z-fZ and S-fH designed the study protocol and wrote the paper. H-fW and M-zZ designed the statistical method. The work of patient recruitment and data collecting will be done by J-bF, Y-jY, QX, YH and Y-fG. S-fH is the study director and J-bF is the principal investigator of this study. All authors have read the manuscript and approved the final paper submitted.
Funding: This study was supported by the Health Commission of Zhejiang Province (No.2020KY032).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372:139–44. 10.1016/S0140-6736(08)60878-8 [DOI] [PubMed] [Google Scholar]
- 2. Lee CM, Park S. Laparoscopic techniques and strategies for gastrointestinal GISTs. J Vis Surg 2017;3:62 10.21037/jovs.2017.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel B, Leung U, Lee J, et al. Laparoscopic pancreaticoduodenectomy in Brisbane, Australia: an initial experience. ANZ J Surg 2018;88:E440–4. 10.1111/ans.14020 [DOI] [PubMed] [Google Scholar]
- 4. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338–50. 10.1097/ALN.0b013e3181fc6e0a [DOI] [PubMed] [Google Scholar]
- 5. Arozullah AM, Khuri SF, Henderson WG, et al. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001;135:847–57. 10.7326/0003-4819-135-10-200111200-00005 [DOI] [PubMed] [Google Scholar]
- 6. Shander A, Fleisher LA, Barie PS, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety Summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med 2011;39:2163–72. 10.1097/CCM.0b013e31821f0522 [DOI] [PubMed] [Google Scholar]
- 7. Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326–41. discussion 341-3 10.1097/01.sla.0000179621.33268.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smetana GW, Lawrence VA, Cornell JE, et al. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of physicians. Ann Intern Med 2006;144:581–95. 10.7326/0003-4819-144-8-200604180-00009 [DOI] [PubMed] [Google Scholar]
- 9. Arozullah AM, Daley J, Henderson WG, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans administration surgical quality improvement program. Ann Surg 2000;232:242–53. 10.1097/00000658-200008000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Güldner A, Kiss T, Serpa Neto A, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015;123:692–713. 10.1097/ALN.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 11. Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care 2014;18 10.1186/cc13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Futier E, Constantin J-M, Pelosi P, et al. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 2010;113:1310–9. 10.1097/ALN.0b013e3181fc640a [DOI] [PubMed] [Google Scholar]
- 13. Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 14. Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428–37. 10.1056/NEJMoa1301082 [DOI] [PubMed] [Google Scholar]
- 15. Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307–21. 10.1097/ALN.0b013e31829102de [DOI] [PubMed] [Google Scholar]
- 16. Jaber S, Coisel Y, Chanques G, et al. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: tidal volumes and relation to body weight. Anaesthesia 2012;67:999–1008. 10.1111/j.1365-2044.2012.07218.x [DOI] [PubMed] [Google Scholar]
- 17. Sato H, Nakamura K, Baba Y, et al. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BMC Anesthesiol 2016;16:47 10.1186/s12871-016-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hemmes SNT, Gama de Abreu M, Pelosi P, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495–503. 10.1016/S0140-6736(14)60416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruszkai Z, Kiss E, László I, et al. Effects of intraoperative PEEP optimization on postoperative pulmonary complications and the inflammatory response: study protocol for a randomized controlled trial. Trials 2017;18:375 10.1186/s13063-017-2116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hulzebos EHJ, Helders PJM, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006;296:1851–7. 10.1001/jama.296.15.1851 [DOI] [PubMed] [Google Scholar]
- 21. Schumacher M, Wangler M, Wolkewitz M, et al. Attributable mortality due to nosocomial infections. A simple and useful application of multistate models. Methods Inf Med 2007;46:595–600. [PubMed] [Google Scholar]
- 22. Keenan SP, Sinuff T, Burns KEA, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011;183:E195–214. 10.1503/cmaj.100071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The consensus Committee. Intensive Care Med 1994;20:225–32. 10.1007/BF01704707 [DOI] [PubMed] [Google Scholar]
- 24. Fartoukh M, Maitre B, Honoré S, et al. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med 2003;168:173–9. 10.1164/rccm.200212-1449OC [DOI] [PubMed] [Google Scholar]
- 25. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 26. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horan TC, Gaynes RP, Martone WJ, et al. Cdc definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–8. 10.1017/S0195941700015241 [DOI] [PubMed] [Google Scholar]
- 28. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA 2010;304:2620–7. 10.1001/jama.2010.1796 [DOI] [PubMed] [Google Scholar]
- 30. Reinius H, Jonsson L, Gustafsson S, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: a computerized tomography study. Anesthesiology 2009;111:979–87. 10.1097/ALN.0b013e3181b87edb [DOI] [PubMed] [Google Scholar]
- 31. Hazebroek EJ, Haitsma JJ, Lachmann B, et al. Mechanical ventilation with positive end-expiratory pressure preserves arterial oxygenation during prolonged pneumoperitoneum. Surg Endosc 2002;16:685–9. 10.1007/s00464-001-8174-y [DOI] [PubMed] [Google Scholar]
- 32. Park SJ, Kim BG, Oh AH, et al. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surg Endosc 2016;30:4598–606. 10.1007/s00464-016-4797-x [DOI] [PubMed] [Google Scholar]
- 33. Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005;102:838–54. 10.1097/00000542-200504000-00021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028464supp001.pdf (72.6KB, pdf)
bmjopen-2018-028464supp002.pdf (73KB, pdf)
bmjopen-2018-028464supp005.pdf (101.2KB, pdf)
bmjopen-2018-028464supp006.pdf (78.7KB, pdf)
bmjopen-2018-028464supp003.pdf (210.7KB, pdf)
bmjopen-2018-028464supp004.pdf (292.6KB, pdf)


