Abstract
Structural links from the nucleus to the cytoskeleton and to the extracellular environment play a role in direct mechanosensing by nuclear factors. Here, we highlight recent studies that illustrate nuclear mechanosensation processes ranging from DNA repair and nuclear protein phospho-modulation to chromatin reorganization, lipase activation by dilation, and reversible rupture with the release of nuclear factors. Recent progresses demonstrate that these mechanosensing processes lead to modulation of gene expression such as those involved in the regulation of cytoskeletal programs and introduce copy number variations. The nuclear lamina protein lamin A has a recurring role, and various biophysical analyses prove helpful in clarifying mechanisms. The various recent observations provide further motivation to understand the regulation of nuclear mechanosensing pathways in both physiological and pathological contexts.
Introduction
The nucleus is the largest membrane-bound structure within eukaryotic cells and was first identified as an organelle in the 18th century, but, by the end of the 19th century, the role of the nucleus in heredity had been recognized (reviewed in ref. [1]). The nucleus harbors most of a cell’s DNA, wrapped up as chromatin, and between the chromatin and the nuclear membrane resides a dense proteinaceous network called the nuclear lamina [2], which confers nuclear stiffness [3,4] and strength against rupture [5–7]. Over the past two decades, an increasing number of lamina-associating proteins have been identified [8–10], including the SUN and KASH proteins that form the LINC complex [11] and link the lamina to the cytoskeleton, which in turn bridges to the extracellular matrix. The lamina and its connections help sculpt nuclear shape [12], which has been seen for decades to correlate with cell shape [13] and which we now understand reflects a dynamic balance between focal adhesion-mediated activation of cytoskeletal contractile forces (i.e. stress fibers) [14] and extracellular matrix softness/stiffness. Since a cell uses its forces to detect extracellular mechanical features, direct nuclear mechanosensing has become a topic of great interest, with many possible consequences for diseases, such as cancer, as well as normal physiological processes including regeneration. Here, we review evidence for nucleus mechanosensing, starting from the modulation of DNA repair to the epigenetic changes in the chromatin, followed with regulation of nuclear proteins and disruption of the nuclear higher structure (Figure 1).
Figure 1. Nucleus mechanosensing.
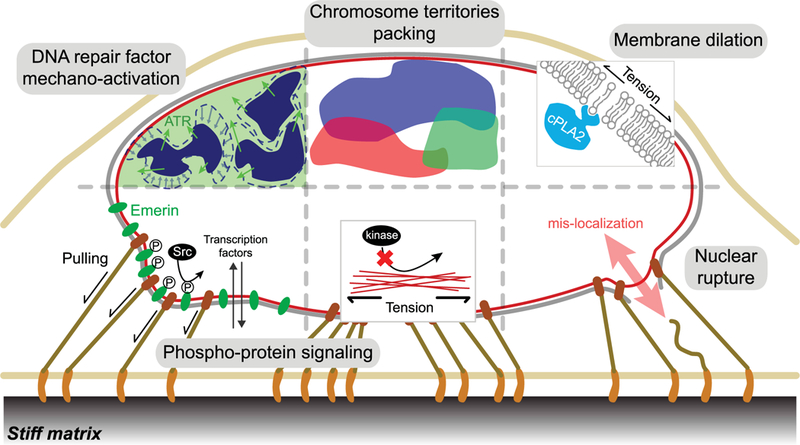
Schematic showing recent evidence of nucleus mechanosensing in factors and processes that range from DNA repair and nuclear protein phosphorylation to chromatin reorganization, nuclear membrane dilation-activated proteins, and reversible rupture with the release of various nuclear factors. Mechanical perturbations and hyperosmotic challenge lead to chromatin condensation, followed by translocation and activation of ATR to the nuclear peripheral region. Tension and compression applied to the nucleus during cell spreading induces intermingling of chromosome territories, which might pack differently in different nuclear shapes. Nuclear membrane stretching upon hypo-osmotic swelling and compression causes cPLA2 to localize to the nuclear membrane, where it activates. Pulling forces on nesprin-1 lead to phosphorylation of emerin, which is essential to the nucleus mechanoresponse and other downstream mechanotransdruction pathways, including transcription co-activator YAP1 localization. Similar to collagen-1 [47], high nuclear tension on the lamin A inhibits the access of kinases, preventing phosphorylation of the lamins, which, in turn, suppress lamin A disassembly and digestion. Migration through constrained spaces and lamin A deficiency cause transient nuclear rupture, which compromises nuclear/cytoplasmic compartmentalization and may thereby inhibit many nuclear processes, including DNA repair.
Nuclear mechanosensing could contribute to regulation of gene expression via various mechanisms (i.e. epigenetic modifications). If transcriptional regulator’s binding interactions with chromatin or another nuclear factor is directly affected by stresses or strains on the nucleus, then such a transcriptional regulator qualifies as a nuclear mechanosensor. In contrast, if a transcriptional regulator localizes into (or out of ) the nucleus only because of mechanosensitive interactions elsewhere ( particularly the cytoskeleton), then such a factor should not be viewed as a nuclear mechanosensor. The latter includes the well-studied transcriptional co-activator YAP1 that translocates into the nucleus in cells growing on stiff extracellular matrix [15], but there are clear examples of regulatory factors that function as nuclear mechanosensors — as reviewed here.
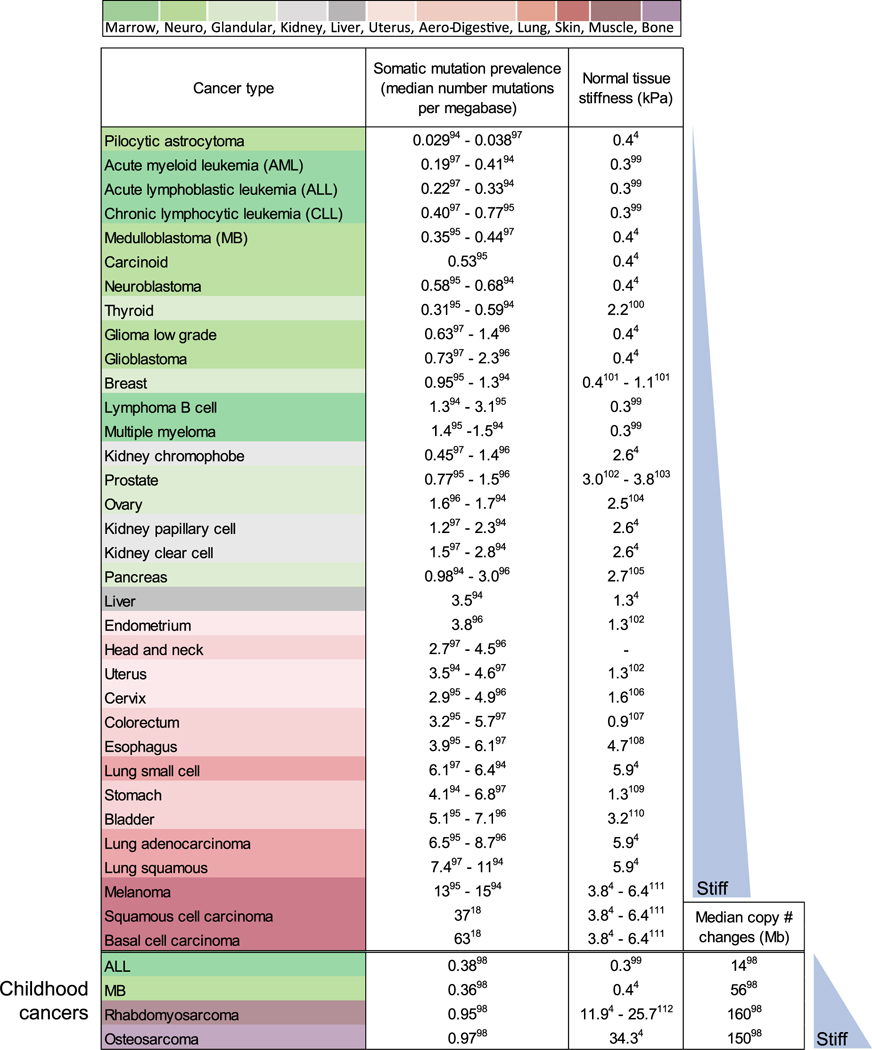
Nuclear mechanosensing by factors that can alter the sequence of the genome — ‘mechanogenomics’ — is now suggested by a diverse set of recent genomic-scale results in cancer. Years ago, genome alterations in cancer might have been inferred from the dysmorphic nuclei that are characteristic of many tumors [16], but today’s high-throughput genome sequencing application is beginning to suggest a direct mechanical link of nuclear mechanosensing to cancer mutations. Solid tumors from stiff tissues — lung, skin, and bone, for example — exhibit more genomic mutation than tumors originating in soft tissues such as brain and marrow (Table 1) [17]. Even for skin cancer where ultraviolet radiation ( particularly with aging) clearly accounts for most mutations [18], changes in chromosome copy numbers strongly associate with tumor stiffness, while point mutations show weak association — with all reaching a maximum for ‘invasive melanoma’ [19]. Epithelial tissues tend to be moderately stiff as part of a barrier function that also exposes them to carcinogens, but carcinogens cannot easily explain the increases in chromosome copy number changes from low rates in soft marrow and brain to elevated rates in stiff muscle and higher rates in rigid bone [18]. Such mutations can result from DNA damage, and so the scaling of mutation rate with tissue stiffness hints at mechano-regulation of key damage-related processes in the nucleus — such as DNA repair.
Table 1.
Genomic variation increases with tissue stiffness across various tumor types.
 |
Mechano-activation of DNA repair factors
DNA breaks occur throughout the cell cycle due to replication and oxidative stress. These breaks are known to significantly affect chromatin mobility both locally [20] and globally [21]. Hence, constant repair of the DNA is essential to maintain genomic integrity. The repair response starts with detection of DNA damage, followed by activation of an ‘upstream’ kinase that initiates a signaling cascade involving phosphorylating of so-called mediator and checkpoint proteins [22]. The ‘upstream’ signaling kinase known as ATR (Ataxia telangiectasia and Rad3-related protein) is essential in the single-strand DNA damage response and appears to be spatially regulated by various mechanical perturbations [23]. Hyperosmotic challenge by both sorbitol and NaCl induces ATR to localize to the nuclear envelope, where it phosphorylates another kinase, Chk1 (checkpoint kinase 1), which indicates ATR activation. The osmotic pressure-induced volume loss occurs because of water extraction through semi-permeable membranes. Cell and nuclear volume change similarly with osmotic pressure (Figure 2A) and fit well to a van der Waals type equation [24]. With extreme hyperosmotic conditions (700 mOsm/kg versus physiological of 300 mOsm/kg), the nucleus volume decreases by ∼30% as the chromatin condenses consistent with a chromatin volume fraction of ∼67% in a typical nucleus [25]. Interchromatin spaces [26] also become increasingly filled with factors such as mechano-activated ATR [23].
Figure 2. Nuclear volume modulation with osmotic challenge and relocalization of mobile nuclear factors.
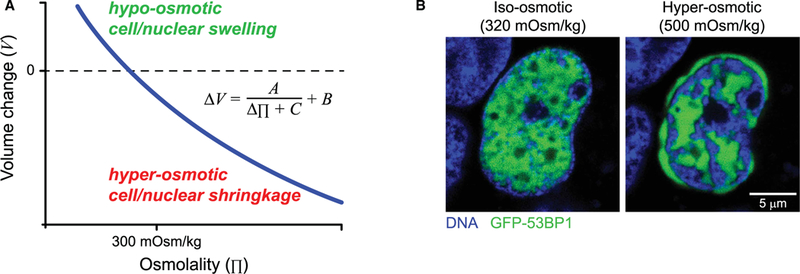
(A) Osmotically induced cell and nuclear changes can be predicted by a standard van der Waals equation that lacks the attraction term [24], where ΔV is the change in cell or nuclear volume and Δπ is the change in osmotic pressure. (B) Resembling previous findings in ATR [23], other nuclear mobile proteins may also translocate after hyperosmotic challenge.
Hyperosmotic challenge likewise creates a gap between the nuclear envelope and the condensed peripheral chromatin [24] into which ATR concentrates. Such hyperosmotically induced relocalization should also apply to other DNA repair proteins (Figure 2B). Segregation of mobile proteins away from the condensed chromatin is consistent with the fact that condensed chromatin is less accessible to DNase [27]. DNA repair should likewise be inhibited by hyperosmotic challenge and thereby favor accumulation of DNA damage. Indeed, multiple studies have demonstrated increased DNA damage under hyperosmotic challenge induced by different osmolytes [28,29], with one study also showing mislocalization of the DNA repair factor Mre11 to the cytoplasm. However, phosphorylation of histone H2AX, a common DNA damage marker, still occurs in hyperosmotic shock [23,30], indicating that kinase access to the condensed chromatin is not entirely blocked. These findings resonate with a much earlier study, which showed that the transcriptional activities of RNA polymerase II tend to be confined to the edge of the condensed chromatin, whereas DNA synthesis, as revealed by BrdU staining, can occur within condensed chromatin [26]. The various observations make it clear that nuclear volume loss in osmotically induced chromatin condensation can segregate mobile nuclear proteins away from chromatin-dense regions.
In another experiment setting, local stretching of the cell membrane with pipettes or by cell compression also leads to chromatin condensation and translocation of ATR [23]. Upon stretching, ATR localizes to the side of the nucleus closest to the pipette, whereas upon compression, ATR again localizes to the nuclear envelope. In both cases, mechanical load is likely to increase the hydrostatic pressure within the cell and nucleus, which squeezes out water and thereby favors chromatin condensation. Interestingly, in contrast with hyperosmotic challenge, ATR is also recruited to the nucleolus after such mechanical perturbations. The liquid-like [31] nucleolus flows under stress [32], and since genotoxic stressors such as UV and radiation disrupt nucleoli [33], a mechanically induced effect on nucleolar stability is an intriguing possibility. These sets of studies suggest that the chromatin, as part of the nucleus, is a sensor against many physical cues like osmotic challenge, tensile, and compressive forces.
Epigenetic changes in response to mechanical stress
Similar to hyperosmotic challenge, cyclic tension [34] and compressive stress [35,36] on nuclei of adherent cells also induce chromatin condensation, followed by various transcriptional changes. The more recent study [36] showed that compressive force induces the translocation of histone deacetylase 3 into the nucleus, which in turn increases the heterochromatin content by initiating removal of acetylation marks on the histone and decreases transcription activities. Such findings suggest a direct epigenetic change in response to mechanical stress. Recently, chromatin condensation state was also shown to dictate nuclear stiffness [37], creating the possibility that chromatin may itself play a role in maintaining nuclear integrity.
The first clue of the role of chromosome dynamic in nuclear mechanosensing comes from studies of embryonic stem (ES) cells. ES cells can differentiate with the proper cues into any cell type via changes in transcription [38]. Nuclei in ES cells are near-spherical and very soft, with little to no lamin A; lamin A turns on in differentiation and stiffens the nucleus [32] as the cells spread and the nucleus flattens. ES cells also have hyperdynamic chromatin, but key proteins such as histones immobilize after differentiation [39]. Inhibition of histone H1 dynamics in ES cells leads to differentiation arrest, which indicates the functional importance of high mobility.
Fluorescence in situ hybridization (FISH) has shown that chromosomes occupy distinct domains in the nucleus in so-called chromosome territories [40]. Although sample preparation for FISH requires fixation and can be harsh on nuclei, the method provides some unique insights. For example, mouse ES cells compared with NIH 3T3 fibroblasts [41] showed chromosome territories in ES cells as relatively random, whereas in NIH 3T3 cells, longer chromosomes, such as chromosome 1, tend to occupy the peripheral region, suggesting more organization and perhaps tighter interactions with the differentiated lamina. Chromosomes in flattened NIH 3T3 nuclei also tend to intermingle or overlap with each other more than in ES nuclei, and such changes in chromosome–chromosome interfaces might regulate transcription [41]. A deeper understanding of causality would perhaps benefit from (i) tracking of chromatin reorganization in the same live cell undergoing large changes in shape and (ii) exerting direct molecular control over chromosome positions. For example, using live-cell DNA-tagging methods (rather than hybridization), stochastic rearrangements of chromosomes [42] and lamina-associated domains [43] have been visualized in cell division from mother to daughter cells. An even more recent example [44] uses a very large, artificial transgene to show locally applied force on the cell surface distends the transgene and increases force-induced transcription, with a dependence on orientation as well as lamin levels. Furthermore, extensive nuclear deformation caused by confined migration through micron-size constriction was reported to also cause extensive stretching of the chromosome [45] another form of chromosome rearrangement. Chromosome territory reorganizations in cell spreading and migration are thus tantalizing pathways for nuclear mechanosensing.
Mechanical regulation of lamin A levels
Most cells cultured on rigid substrates, such as plastic or glass, will spread onto these surfaces, and the stress fibers that assemble to engage adhesions then apply tension and compression to the encaged nucleus, which stretches and squashes to an ellipsoid shape. Cells on soft substrates do not spread or assemble many stress fibers, and they are more rounded as if in suspension, which relieves the adhesion-cytoskeleton forces and leads to a more spherical nuclear shape. Stress on a well-hydrated nucleus is largely sustained by the nuclear lamina proteins, and lamin A level increases with the stress, whereas B-type lamins remain nearly constant [4]. Importantly, the trends agree with the strong positive scaling between nuclear lamin levels and tissue stiffness: lamin A protein levels in the softest tissues such as brain and marrow are on average ∼20-fold lower than levels in stiffer tissues such as cartilage and bone. Cells in stiffer mechanically stressed tissues thus have stiffer and stronger nuclei.
Phosphorylation of lamin A in interphase nuclei is a key part of the underlying mechanosensitive gene circuit [4,46]. Low tension on the nucleus (on soft matrix, or with myosin inhibition, or detachment from plastic) allows access to lamin A of a Ser/Thr kinase(s) in the CDK family (cyclin-dependent kinases). For decades, it has been known that CDKs activated in cell division phosphorylate both the A- and B-type lamins at many sites to solubilize them and uncage the chromatin, and interphase phosphorylation occurs at ∼10-fold lower levels and the phosphorylated lamin A remains in the nucleus. The favored mechanism is that the kinase is constant in concentration and activity, but that the Ser/Thr sites in fibrous assemblies of lamin A become more accessible when the fibers are under low tension (during cell division). This is similar to a mechanism for how proteases degrade collagen I fibers under low versus high tension [47]. Phosphorylated lamin A is mobile in the nucleoplasm and is also degraded faster, based on studies of phosphomimetic constructs of lamin A [46]. Soft matrix thus favors more phosphorylation and more degradation to minimize lamin A levels (Figure 3A,B). Meanwhile, in multiple mouse model studies, lamin C, a splicing variant of the LMNA gene, was shown to be functionally interchangeable with lamin A [48,49]. Although lamin C is less studied, recent findings start to suggest its greater mechanoresponse than lamin A, as evidenced by its stronger correlation with cell stiffness [50] and higher sensitivity to tension-regulated phosphorylation [51]. Further studies on lamin C-specific mechanosensitivity are needed.
Figure 3. Nuclear tension dictates lamin A turnover.

(A) Tension exerted by the stress fibers flattens and smooths the nucleus of cells cultured on stiff substrate. (B) High tension on the lamina inhibits the access of kinases to lamin A, preventing phosphorylation of the lamins, which is essential for lamin A disassembly and turnover. (C) High tension-induced lamin A integrity helps retain SUN2 at the nuclear envelope; this shift of SUN2 from the endoplasmic reticulum into the nucleus also facilitates RARG entry and retention.
Mechano-regulation of lamin A protein can occur in an acute fashion in the time frame of within hours of mechanostimulation, and it eventually feeds back into transcription of the lamin A gene [4]. The promoter region of lamin A (LMNA) harbors multiple retinoic acid response (RAR) elements that bind RAR transcription factors (evident in the ENCODE database), and immunoprecipitation of one of these RAR proteins (RARG) followed by mass spectrometry identified the nuclear envelope protein SUN2 as a potential binding partner. SUN2 is an integral membrane protein that binds lamin A protein, but can also diffuse into the ER (endoplasmic reticulum) contiguous with the envelope. Nuclear entry of RARG was found to be partially regulated by the levels of both SUN2 and lamin A (Figure 3C). This example of a mechanobiological gene circuit is perhaps a first and can be formalized mathematically as follows [4]:
where a, b, c are constants and the degradation term exhibits Michaelis–Menten kinetics with lamin A. Steady-state solutions show that lamin A protein increases as a function of tension or, equivalently, matrix stiffness since tension on the nucleus increases with stiffness of the matrix. Since RAR is, of course, regulated by retinoic acid, which is a membrane-permeable lipophilic molecule derived from vitamin A and essential in development [52], the soluble microenvironment also modulates nuclear mechanosensing of matrix mechanics.
Signaling pathways regulated by lamin A level
Many studies have shown the effect of extracellular mechanical cues on cell biological processes, including the differentiation of mesenchymal stem cells (MSCs) [53] and regulation of transcription factors. The latter include SRF transcription and activation [54,55] as well as nuclear translocation of YAP1 [15], β-catenin [56], NF-κB [57], and NKX2.5 [58]. SRF is especially interesting as it feedbacks and regulates its own expression functions as a master regulator for many other actin-interacting factors, including nonmuscle myosin-IIA [24,46,59,60]. Cell spreading increases SRF transcriptional activity [55] as well as lamin A levels [4]. Importantly, knockdown of lamin A suppresses SRF transcriptional activity [46], supporting a feedback loop between the actomyosin cytoskeleton and the nuclear lamina (Figure 4A,B). The mechanism probably involves nuclear actin that controls nuclear import of megakaryoblastic leukemia 1 (MKL1), which is a co-activator of SRF [60].
Figure 4. Gene circuit of tension-regulated lamin A expression.
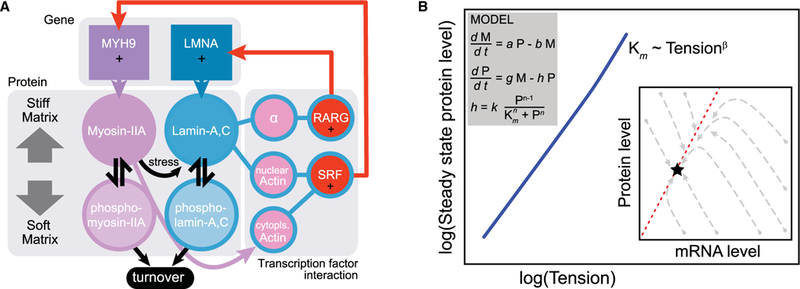
(A) Tension-dependent phosphorylation and turnover feeds into transcriptional regulation. Lamin A,C protein transcriptionally regulates LMNA via the retinoic acid pathway (through SUN2 as a mediator, α) and also MYH9 via the SRF pathway (through nuclear actin). On stiff matrix, non-phosphorylated, contraction-competent myosins positively regulate lamin A,C, favoring assembly and opposing degradation that occurs on soft matrices. (B) A simple model was generated based on the gene circuit: time evolution of LMNA mRNA (M) level is dependent on the LMNA protein level (P), whereas the protein level itself is regulated by a tension-dependent degradation term, h. The model shows that tension-regulated protein turnover can produce steady-state protein levels that scale with cell tension. Trajectories of lamin A message and protein as the model converge from a range of initial conditions to a single steady-state solution appropriate to the tension.
Hippo pathway signaling involving YAP/TAZ is well known to regulate growth in response to key contributions from cell junctions, polarity, and cytoskeleton [61]. In MSCs in 2D culture on stiff mechanical environments, YAP accumulates to regulate differentiation fates even after cells have been transferred to a different mechanical environment (epigenetic memory) [62]. Lamin A overexpression decreases nuclear YAP, which is consistent with decreased levels of YAP in a rigid normal tissue such as bone compared with muscle [4], suggesting that the YAP/TAZ pathway may be downstream of Lamin A.
Mechanosensitive nuclear lamina-associated proteins and nuclear membrane
Physical connections between the nucleus and the extracellular environment also include nuclear envelope interactions with chromatin via proteins such as lamin B receptor LBR [63], LAP2 [64], and emerin [65]. In addition, the lamina-binding SUNs bind nesprins [11] (LINC complex) that physically link to various cytoskeletal components and ultimately to the integrins and extracellular matrix [66]. Indeed, cutting the actin cytoskeleton with laser scissors causes the nucleus to move both laterally and away from the culture substrate [67,68]. Within the nucleus, actin might assemble and have structural roles, as it has been shown recently to stabilize nucleolus positioning (against gravity) in the very large nucleus of a Xenopus oocyte [69].
Even isolated nuclei appear mechanosensitive [70]. In one experiment, by applying magnetic tweezers to nesprin-1 antibody-coated beads, tension was applied to isolated nuclei from a cancer cell line and the nuclei showed a stiffening effect with sequential loads, suggesting an active response. Depletion of lamin A and emerin abolished the stiffening effect, whereas no effects were found after perturbing DNA, histone acetylation states, actin, SUNs, MAN1, or LAP2α. Reducing lamin A levels softened the nucleus, consistent with previous studies [6,32], but lower emerin levels stiffened the nucleus. Importantly, pulling on nesprin-1 led to phosphorylation of emerin at Tyr74 and Tyr95 by Src kinase [70]. Mutation of these sites eliminated the stiffening effects, which reveals the importance of such phosphorylation for nucleus mechano-responses. With intact cells, spreading on stiff substrates leads to emerin phosphorylation, which is abolished by myosin II inhibition (with blebbistatin) and indicates the necessity of actomyosin forces. Mutation of these phosphorylation sites also decreases stress fibers, YAP/TAZ nuclear localization, and SRF transcription, consistent with changes in lamin A knockdown [31]. The reductionist studies with isolated nuclei add to the evidence of nucleus mechanosensing in cellular mechanotransduction.
Although continuity of the outer lipid membrane of the nucleus with the endoplasmic reticulum complicates lipid-based sensing in the nucleus, studies of wound healing in a zebrafish model suggest lipid-mediated nuclear mechanosensing [71]. In response to tissue damage in zebrafish, cytosolic phospholipase A2 (cPLA2) translocates from the nucleoplasm to the nuclear membrane upon hypo-osmotic cellular swelling; there, cPLA2 metabolizes arachidonic acid into chemotactic eicosanoids that ultimately attract leukocytes to the epithelial wound [72]. Hypo-osmotic conditions drive water into a cell, with swelling per the van de Waals equation (Figure 2A). In the zebrafish study, nuclear swelling can also be induced by inhibition of actin polymerization which then also increases cPLA2 activation [73]. Thus, disregard of mechanism of nuclear swelling, cPLA2 will be activated. Depletion of lamin A does not increase hypo-osmotic nucleus swelling, but still enhances cPLA2 activation, supporting a role of lamin A in maintaining membrane integrity [74].
Direct interaction of cPLA2 with membrane lipids was confirmed with giant lipid vesicles that showed cPLA2 recruitment to the membrane upon hypo-osmotic swelling. With HeLa cells, both compression and hypo-osmotic swelling favor cPLA2 enrichment at the membrane. Interestingly, just as with ATR [23], the hypo-osmotic challenge of HeLa cells induced protein relocalization to the nucleolus. With the zebrafish model, only necrotic cells with cPLA2 and nucleus swelling were able to recruit leukocytes. As currently understood, changes in lipid packing in response to nuclear swelling and stretching facilitate the membrane insertion and activation of cPLA2, which supports lipid-based mechanisms to nucleus mechanosensing.
Genomic consequences of nuclear rupture and mislocalization of DNA repair factors
The studies above all involve intact nuclei, but mechanically induced and transient nuclear ruptures might also affect regulated processes. Rupture had been reported for various cancerous cell lines in conventional cultures on rigid plastic [75] and could conceivably affect DNA repair and cancer mutations (Table 1). Indeed, frequent nuclear rupture of lamin A-deficient cells [76] caused transient mislocalization of cytoplasmic and nuclear proteins (including transcription factors) and even the movement of cytoplasmic organelles into the nucleus [77]. Importantly, such rupture can be reduced in frequency by culturing the cells on a soft substrate [5], by blocking actomyosin contractility with blebbistatin [78], or by inhibiting NAT10, which organizes microtubule networks [79]. Matrix stiffness and cytoskeletal forces within cells in 2D culture are thus necessary to induce nuclear rupture.
Migration through rigid narrow channels or small pores in matrix by several cancer lines as well as immortalized epithelial cells (RPE-1) and primary dendritic cells has also been shown to cause nuclear envelope rupture. A first study showed disruption of the nuclear lamina as well as cell death after constricted migration [6]. The nuclear lamina disruption during constricted migration suggested a compromised nuclear structure. Subsequent real-time imaging has since shown that nuclear envelope rupture causes leakage into the cytoplasm of GFP-tagged nuclear localization sequence constructs (GFP-NLS) [7,80]. Rupture tends to occur at the leading edge of the nucleus and more so after lamin A knockdown. One theoretical model concludes that increased pressure within the constriction-deformed nucleus exceeds a critical pressure (at isotonic volume), so that by the Law of Laplace a critical tension causes nuclear rupture, although the role for the ER as a lipid reservoir [81,82] that can dissipate membrane tension appears neglected in such a model. Alternatively, the cytoskeleton that pulls the nucleus during migration [83] might tear the membrane. Regardless of rupture mechanism, these studies also reported local enrichment of overexpressed GFP-53BP1, a protein involved in the DNA damage response, suggesting an increase in DNA damage during confined cell migration. Indeed, such migration-induced DNA damage was confirmed in another study by the use of more direct orthogonal measures of DNA damage (gH2AX and comet assay) [84], and shown to be independent of cell cycle phase [85]. One possible mechanism behind this increase in DNA damage is the depletion of DNA repair factors from the nucleus, which can be driven by (i) the nucleus to the cytoplasm mislocalization of the nuclear factors as the nucleus rupture [84,86] and (ii) segregation of the nuclear factors away from the chromatin that are compacted by the constriction [87], similar process as liquid squeezing out of a compressed sponge, which is consistent with the segregation observed in the hyperosmotically compacted chromatin (Figure 2B). Both nuclear rupture and chromatin compaction are direct physical consequences of the mechanical cues exerted during constricted migration and hence can be referred as a mechanosensation by the nucleus. In addition, for collagenous matrix, pore size and pore stiffness are inversely related [88]. As such, more DNA damage might be expected in migration through the denser collagen matrix of stiffer tissues (Table 1), which could explain the higher mutational load in tumors of stiff tissues. Indeed, increased genomic aberrations after constricted migration were reported [84]. However, the mechanistic link between the upstream DNA repair factor depletion and the downstream genomic aberrations is still missing, which will be an important follow-up of these recent studies.
Conclusion
Nuclear mechanosensing can occur through a diversity of mechanisms that add to the abundance of mechanotransduction processes described in the past for cytoplasmic and plasma membrane factors. The various recent studies on nucleus mechanosensing often converge onto the chromatin and nuclear lamina. Both of these nuclear components are crucial for nuclear mechanical response [37]. Furthermore, lamin A, which is clearly a mechanosensitive protein, is also a highly mutated protein in humans giving rise to many diseases [89,90], and the lamins are often dysregulated in cancer [91–93]. Nonetheless, mechanosensing by lamina proteins might be merely representative of many other key biological processes in the nucleus that range from DNA repair to chromatin reorganization and nuclear integrity, which can all broadly affect the overall state and regulation of this defining organelle of eukaryotes.
Summary.
Nuclear mechanosensing can occur through a diversity of mechanisms that add to the abundance of mechanotransduction processes described in the past for cytoplasmic and plasma membrane factors.
The various recent studies on nucleus mechanosensing often converge onto the chromatin and nuclear lamina.
Both of these nuclear components are crucial for nuclear mechanical response. Furthermore, lamin A, which is clearly a mechanosensitive protein, is also a highly mutated protein in humans giving rise to many diseases, and the lamins are often dysregulated in cancer.
Nonetheless, mechanosensing by lamina proteins might be merely representative of many other key biological processes in the nucleus that range from DNA repair to chromatin reorganization and nuclear integrity, which can all broadly affect the overall state and regulation of this defining organelle of eukaryotes.
Abbreviations
- ATR
ataxia telangiectasia and Rad3-related protein
- CDK
cyclin-dependent kinases
- cPLA2
cytosolic phospholipase A2
- ER
endoplasmic reticulum
- ES
embryonic stem
- FISH
fluorescence in situ hybridization
- MSCs
mesenchymal stem cells
- RAR
retinoic acid response
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Satzinger H (2008) Theodor and Marcella Boveri: chromosomes and cytoplasm in heredity and development. Nat. Rev. Genet 9, 231–238 10.1038/nrg2311 [DOI] [PubMed] [Google Scholar]
- 2.Gerace L and Blobel G (1980) The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 19, 277–287 10.1016/0092-8674(80)90409-2 [DOI] [PubMed] [Google Scholar]
- 3.Dahl KN, Kahn SM, Wilson KL and Discher DE (2004) The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci 117(Pt20), 4779–4786 10.1242/jcs.01357 [DOI] [PubMed] [Google Scholar]
- 4.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J et al. (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamiello C, Kamps MA, van den Wijngaard A, Verstraeten VL, Baaijens FP, Broers JL et al. (2013) Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus 4, 61–73 10.4161/nucl.23388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A et al. (2014) Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol 204, 669–682 10.1083/jcb.201308029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raab M, Gentili M, de Belly H, Thiam H-R, Vargas P, Jimenez AJ et al. (2016) ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 10.1126/science.aad7611 [DOI] [PubMed] [Google Scholar]
- 8.Schirmer EC, Florens L, Guan T, Yates JR III, and Gerace L, (2003) Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301, 1380–1382 10.1126/science.1088176 [DOI] [PubMed] [Google Scholar]
- 9.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B et al. (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol 172, 41–53 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon DN, Zastrow MS and Wilson KL (2010) Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 1, 264–272 10.4161/nucl.11799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr DA and Fridolfsson HN (2010) Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol 26, 421–444 10.1146/annurev-cellbio-100109-104037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK and Spann TP (2002) Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16, 533–547 10.1101/gad.960502 [DOI] [PubMed] [Google Scholar]
- 13.Weiss P and Garber B (1952) Shape and movement of mesenchyme cells as functions of the physical structure of the medium: contributions to a quantitative morphology. Proc. Natl Acad. Sci. U.S.A 38, 264–280 10.1073/pnas.38.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham RJ and Wang Y-L (1997) Cell locomotion and focal adhesions are regulated by substrate fiexibility. Proc. Natl Acad. Sci. U.S.A 94, 13661–13665 10.1073/pnas.94.25.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 16.Zink D, Fischer AH and Nickerson JA (2004) Nuclear structure in cancer cells. Nat. Rev. Cancer 4, 677–687 10.1038/nrc1430 [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer CR, Alvey CM, Irianto J and Discher DE (2017) Genome variation across cancers scales with tissue stiffness — an invasion-mutation mechanism and implications for immune cell infiltration. Curr. Opin. Syst. Biol 2, 103–114 10.1016/j.coisb.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S et al. (2015) Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 10.1126/science.aaa6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A et al. (2015) The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med 373, 1926–1936 10.1056/NEJMoa1502583 [DOI] [PubMed] [Google Scholar]
- 20.Miné-Hattab J and Rothstein R (2013) DNA in motion during double-strand break repair. Trends Cell Biol 23, 529–536 10.1016/j.tcb.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zidovska A, Weitz DA and Mitchison TJ (2013) Micron-scale coherence in interphase chromatin dynamics. Proc. Natl Acad. Sci. U.S.A 110, 15555–15560 10.1073/pnas.1220313110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polo SE and Jackson SP (2011) Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev 25, 409–433 10.1101/gad.2021311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Mazzanti M, Mistrik M, Kosar M, Beznoussenko GV, Mironov AA et al. (2014) ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158, 633–646 10.1016/j.cell.2014.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irianto J, Swift J, Martins RP, McPhail GD, Knight MM, Discher DE et al. (2013) Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys. J 104, 759–769 10.1016/j.bpj.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bancaud A, Huet S, Daigle N, Mozziconacci J, Beaudouin J and Ellenberg J (2009) Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J 28, 3785–3798 10.1038/emboj.2009.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albiez H, Cremer M, Tiberi C, Vecchio L, Schermelleh L, Dittrich S et al. (2006) Chromatin domains and the interchromatin compartment form structurally defined and functionally interacting nuclear networks. Chromosome Res 14, 707–733 10.1007/s10577-006-1086-x [DOI] [PubMed] [Google Scholar]
- 27.Martins RP, Platts AE and Krawetz SA (2007) Tracking chromatin states using controlled DNase I treatment and real-time PCR. Cell. Mol. Biol. Lett 12, 545–555 10.2478/s11658-007-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dmitrieva NI, Cai Q and Burg MB (2004) Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc. Natl Acad. Sci. U.S.A 101, 2317–2322 10.1073/pnas.0308463100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kultz D and Chakravarty D (2001) Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl Acad. Sci. U.S.A 98, 1999–2004 10.1073/pnas.98.4.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmitrieva NI and Burg MB (2008) Analysis of DNA breaks, DNA damage response, and apoptosis produced by high NaCl. Am. J. Physiol. Renal. Physiol 295, F1678–F1688 10.1152/ajprenal.90424.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM et al. (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ and Discher DE (2007) Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. U.S.A 104, 15619–15624 10.1073/pnas.0702576104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulon S, Westman BJ, Hutten S, Boisvert FM and Lamond AI (2010) The nucleolus under stress. Mol. Cell 40, 216–227 10.1016/j.molcel.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo S-J, Thorpe SD, Driscoll TP, Duncan RL, Lee DA and Mauck RL (2015) Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci. Rep 5, 16895 10.1038/srep16895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Berre M, Aubertin J and Piel M (2012) Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr. Biol 4, 1406–1414 10.1039/c2ib20056b [DOI] [PubMed] [Google Scholar]
- 36.Damodaran K, Venkatachalapathy S, Alisafaei F, Radhakrishnan AV, Sharma Jokhun D, Shenoy VB et al. (2018) Compressive force induces reversible chromatin condensation and cell geometry dependent transcriptional response. Mol. Biol. Cell mbcE18040256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens AD, Banigan EJ, Adam SA, Goldman RD and Marko JF (2017) Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 28, 1984–1996 10.1091/mbc.e16-09-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 39.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT and Misteli T (2006) Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 10.1016/j.devcel.2005.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C et al. (2005) Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 3, e157 10.1371/journal.pbio.0030157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maharana S, Iyer KV, Jain N, Nagarajan M, Wang Y and Shivashankar GV (2016) Chromosome intermingling — the physical basis of chromosome organization in differentiated cells. Nucleic Acids Res 44, 5148–5160 10.1093/nar/gkw131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strickfaden H, Zunhammer A, van Koningsbruggen S, Kohler D and Cremer T (2010) 4D chromatin dynamics in cycling cells Theodor Boveri’s hypotheses revisited. Nucleus 1, 284–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H et al. (2013) Single-cell dynamics of genome-nuclear lamina interactions. Cell 153, 178–192 10.1016/j.cell.2013.02.028 [DOI] [PubMed] [Google Scholar]
- 44.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W et al. (2016) Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater 15, 1287–1296 10.1038/nmat4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irianto J, Xia Y, Pfeifer Charlotte R, Greenberg Roger A and Discher Dennis E (2017) As a nucleus enters a small pore, chromatin stretches and maintains integrity, even with DNA breaks. Biophys. J 112, 446–449 10.1016/j.bpj.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A et al. (2014) Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol 24, 1909–1917 10.1016/j.cub.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruberti JW and Hallab NJ (2005) Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem. Biophys. Res. Commun 336, 483–489 10.1016/j.bbrc.2005.08.128 [DOI] [PubMed] [Google Scholar]
- 48.Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Cote N et al. (2006) Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest 116, 743–752 10.1172/JCI27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coffinier C, Jung H-J, Li Z, Nobumori C, Yun UJ, Farber EA et al. (2010) Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J. Biol. Chem 285, 20818–20826 10.1074/jbc.M110.128835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González-Cruz RD, Sadick JS, Fonseca VC and Darling EM (2018) Nuclear lamin protein C is linked to lineage-specific, whole-cell mechanical properties. Cell. Mol. Bioeng 11, 131–142 10.1007/s12195-018-0518-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho S, Abbas A, Irianto J, Ivanovska IL, Xia Y, Tewari M et al. (2018) Progerin phosphorylation in interphase is lower and less mechanosensitive than lamin-A,C in iPS-derived mesenchymal stem cells. Nucleus 9, 230–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhinn M and Dolle P (2012) Retinoic acid signalling during development. Development 139, 843–858 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- 53.Engler AJ, Sen S, Sweeney HL and Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 54.Miralles F, Posern G, Zaromytidou A-I and Treisman R (2003) Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342 10.1016/S0092-8674(03)00278-2 [DOI] [PubMed] [Google Scholar]
- 55.Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS et al. (2010) Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol 12, 711–718 10.1038/ncb2074 [DOI] [PubMed] [Google Scholar]
- 56.Farge E (2003) Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr. Biol 13, 1365–1377 10.1016/S0960-9822(03)00576-1 [DOI] [PubMed] [Google Scholar]
- 57.Khachigian LM, Resnick N, Gimbrone MA and Collins T (1995) Nuclear factor-kappa-B interacts functionally with the platelet-derived growth-factor B-chain shear-stress response element in vascular endothelial-cells exposed to fiuid shear-stress. J. Clin. Invest 96, 1169–1175 10.1172/JCI118106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J et al. (2015) Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater 14, 951–960 10.1038/nmat4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vartiainen MK, Guettler S, Larijani B and Treisman R (2007) Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 10.1126/science.1141084 [DOI] [PubMed] [Google Scholar]
- 60.Ho CY, Jaalouk DE, Vartiainen MK and Lammerding J (2013) Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511 10.1038/nature12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boggiano JC and Fehon RG (2012) Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev. Cell 22, 695–702 10.1016/j.devcel.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C, Tibbitt MW, Basta L and Anseth KS (2014) Mechanical memory and dosing infiuence stem cell fate. Nat. Mater 13, 645–652 10.1038/nmat3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyrpasopoulou A, Meier J, Maison C, Simos G and Georgatos SD (1996) The lamin B receptor (LBR) provides essential chromatin docking sites at the nuclear envelope. EMBO J 15, 7108–7119 10.1002/j.1460-2075.1996.tb01102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martins S, Eikvar S, Furukawa K and Collas P (2003) HA95 and LAP2 beta mediate a novel chromatin-nuclear envelope interaction implicated in initiation of DNA replication. J. Cell Biol 160, 177–188 10.1083/jcb.200210026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manilal S, Nguyen TM, Sewry CA and Morris GE (1996) The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet 5, 801–808 10.1093/hmg/5.6.801 [DOI] [PubMed] [Google Scholar]
- 66.Humphries JD, Byron A and Humphries MJ (2006) Integrin ligands at a glance. J. Cell Sci 119, 3901–3903 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagayama K, Yahiro Y and Matsumoto T (2011) Stress fibers stabilize the position of intranuclear DNA through mechanical connection with the nucleus in vascular smooth muscle cells. FEBS Lett 585, 3992–3997 10.1016/j.febslet.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 68.Mazumder A and Shivashankar GV (2010) Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. J. R. Soc. Interface 7, S321–SS30 10.1098/rsif.2010.0039.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feric M and Brangwynne CP (2013) A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat. Cell Biol 15, 1253–1259 10.1038/ncb2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R et al. (2014) Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol 16, 376–381 10.1038/ncb2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enyedi B, Jelcic M and Niethammer P (2016) The cell nucleus serves as a mechanotransducer of tissue damage-induced infiammation. Cell 165, 1160–1170 10.1016/j.cell.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enyedi B, Kala S, Nikolich-Zugich T and Niethammer P (2013) Tissue damage detection by osmotic surveillance. Nat. Cell Biol 15, 1123–1130 10.1038/ncb2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Irianto J, Kazun S, Wang W and Knight MM (2015) The rate of hypo-osmotic challenge infiuences regulatory volume decrease (RVD) and mechanical properties of articular chondrocytes. Osteoarthritis Cartilage 23, 289–299 10.1016/j.joca.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 74.King MC and Lusk CP (2016) A model for coordinating nuclear mechanics and membrane remodeling to support nuclear integrity. Curr. Opin. Cell Biol 41, 9–17 10.1016/j.ceb.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vargas JD, Hatch EM, Anderson DJ and Hetzer MW (2012) Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 3, 88–100 10.4161/nucl.18954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K et al. (1999) Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol 147, 913–920 10.1083/jcb.147.5.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J et al. (2011) Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum. Mol. Genet 20, 4175–4186 10.1093/hmg/ddr344 [DOI] [PubMed] [Google Scholar]
- 78.Robijns J, Molenberghs F, Sieprath T, Corne TD, Verschuuren M and De Vos WH (2016) In silico synchronization reveals regulators of nuclear ruptures in lamin A/C deficient model cells. Sci. Rep 6, 30325 10.1038/srep30325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larrieu D, Britton S, Demir M, Rodriguez R and Jackson SP (2014) Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344, 527–532 10.1126/science.1252651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B et al. (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 10.1126/science.aad7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L, Guan T and Gerace L (1997) Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J. Cell Biol 137, 1199–1210 10.1083/jcb.137.6.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ et al. (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol 138, 1193–1206 10.1083/jcb.138.6.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petrie RJ, Koo H and Yamada KM (2014) Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 10.1126/science.1256965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irianto J, Xia Y, Pfeifer CR, Athirasala A, Ji J, Alvey C et al. (2017) DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol 27, 210–223 10.1016/j.cub.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfeifer CR, Xia Y, Zhu K, Liu D, Irianto J, Garcia VMM et al. (2018) Constricted migration increases DNA damage and independently represses cell cycle. Mol. Biol. Cell mbcE18020079 29, 1948–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia Y, Ivanovska IL, Zhu K, Smith L, Irianto J, Pfeifer CR et al. (2018) Nuclear rupture at sites of high curvature compromises retention of DNA repair factors. J. Cell Biol 217, 3796–3808 10.1083/jcb.201711161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Irianto J, Pfeifer CR, Bennett RR, Xia Y, Ivanovska IL, Liu AJ et al. (2016) Nuclear constriction segregates mobile nuclear proteins away from chromatin. Mol. Biol. Cell 27, 4011–4020 10.1091/mbc.e16-06-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irianto J, Pfeifer CR, Xia Y and Discher DE (2016) Snapshot: mechanosensing matrix. Cell 165, 1820–e1 10.1016/j.cell.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Worman HJ and Bonne G (2007) ‘Laminopathies’: a wide spectrum of human diseases. Exp. Cell Res 313, 2121–2133 10.1016/j.yexcr.2007.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Capell BC and Collins FS (2006) Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet 7, 940–952 10.1038/nrg1906 [DOI] [PubMed] [Google Scholar]
- 91.Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS and Ramaekers FC (1993) Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am. J. Pathol 143, 211–220 [PMC free article] [PubMed] [Google Scholar]
- 92.Willis ND, Cox TR, Rahman-Casañs SF, Smits K, Przyborski SA, van den Brandt P et al. (2008) Lamin A/C is a risk biomarker in colorectal cancer. PLoS ONE 3, e2988 10.1371/journal.pone.0002988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Irianto J, Pfeifer CR, Ivanovska IL, Swift J and Discher DE (2016) Nuclear lamins in cancer. Cell. Mol. Bioeng 9, 258–267 10.1007/s12195-016-0437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV et al. (2013) Signatures of mutational processes in human cancer. Nature 500, 415–421 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A et al. (2013) Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martincorena I and Campbell PJ (2015) Somatic mutation in cancer and normal cells. Science 349, 1483–1489 10.1126/science.aab4082 [DOI] [PubMed] [Google Scholar]
- 97.Schumacher TN and Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348, 69–74 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 98.Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E et al. (2014) Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep 7, 104–112 10.1016/j.celrep.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin J-W, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA et al. (2014) Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. CellStemCell 14, 81–93 10.1016/j.stem.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prabhune M, Belge G, Dotzauer A, Bullerdiek J and Radmacher M (2012) Comparison of mechanical properties of normal and malignant thyroid cells. Micron 43, 1267–1272 10.1016/j.micron.2012.03.023 [DOI] [PubMed] [Google Scholar]
- 101.Lopez JI, Kang I, You W-K, McDonald DM and Weaver VM (2011) In situ force mapping of mammary gland transformation. Integr. Biol 3, 910–921 10.1039/c1ib00043h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lekka M, Gil D, Pogoda K, Dulińka-Litewka J, Jach R, Gostek J et al. (2012) Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys 518, 151–156 10.1016/j.abb.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 103.Hoyt K, Castaneda B, Zhang M, Nigwekar P, di Sant’agnese PA, Joseph JV et al. (2008) Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark 4, 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu W, Mezencev R, Kim B, Wang L, McDonald J and Sulchek T (2012) Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7, e46609 10.1371/journal.pone.0046609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cross SE, Jin Y-S, Lu Q-Y, Rao JY and Gimzewski JK (2011) Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology 22, 215101 10.1088/0957-4484/22/21/215101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guz N, Dokukin M, Kalaparthi V and Sokolov I (2014) If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys. J 107, 564–575 10.1016/j.bpj.2014.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawano S, Kojima M, Higuchi Y, Sugimoto M, Ikeda K, Sakuyama N et al. (2015) Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci 106, 1232–1239 10.1111/cas.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fuhrmann A, Staunton JR, Nandakumar V, Banyai N, Davies PC and Ros R (2011) AFM stiffness nanotomography of normal, metaplastic and dysplastic human esophageal cells. Phys. Biol 8, 015007 10.1088/1478-3975/8/1/015007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim Y-J, Deo D, Singh TP, Jones DB and De S (2009) In situ measurement and modeling of biomechanical response of human cadaveric soft tissues for physics-based surgical simulation. Surg. Endosc 23, 1298–1307 10.1007/s00464-008-0154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J et al. (2012) Cancer cell recognition--mechanical phenotype. Micron 43, 1259–1266 10.1016/j.micron.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 111.Petrie RJ, Gavara N, Chadwick RS and Yamada KM (2012) Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol 197, 439–455 10.1083/jcb.201201124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathur AB, Collinsworth AM, Reichert WM, Kraus WE and Truskey GA (2001) Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech 34, 1545–1553 10.1016/S0021-9290(01)00149-X [DOI] [PubMed] [Google Scholar]


