Abstract
Cardiac myxomas are the most common benign cardiac tumor. We investigated the immunohistochemical properties of 11 surgically excised cardiac myxomas, in order to analyze the correlation between macrophages and mast cell populations and clinical parameters. CD68+/CD163−/iNOS− (M0) cells represent the most abundant macrophage phenotype; however, CD68+/CD163+ cells (M2) were also frequent. CD68+/iNOS+ (M1) elements were rare. Mast cells, defined as a population of c-kit (CD117)+ and/or tryptase+ cells were also detected. Statistical analysis showed significant correlations between c-kit (CD117)+ and tryptase, CD68 and erythrocyte sedimentation rate (ESR), ESR and red blood cell count (RBC), and prothrombin time and platelet count. The inverse correlation between RBCs in peripheral blood and ESR suggested that anemia associated with chronic inflammatory disease is a noncasual event in patients suffering from cardiac myxoma. Mechanical hemolysis may be only a minor component of anemia, according to the lack of correlation between echographic surface and RBCs. Moreover, tumor size did not correlate with ESR, showing that inflammatory state may depend from both tumor cells population and inflammatory infiltrate. In the future, modulation of macrophage polarization in cardiac myxomas might represent important therapeutic target.
Keywords: Cardiac myxomas, erythrosedimentation rate, innate immunity, macrophages, mast cells
Introduction
Cardiac myxoma is the most common benign cardiac tumor and is currently considered a neoplasm. The origin and histopathogenesis of cardiac myxoma are debated; however, two main hypotheses suggest an origin from multipotent mesenchymal cells or from endocardial tissue. Many authors have shown that myxoma cells display mesenchymal and endothelial properties, suggesting the involvement of endocardial–mesenchymal transition of the endocardial cushion.1 The histology of cardiac myxoma has been described in detail.2 Myxoma cells are stellate or fusiform with eosinophilic cytoplasm, may be binucleated and even display syncytial features. Myxoma cells may form rings, cords and nests, which are often closely associated with capillaries. Moreover, vascular channels often appear to develop from myxomatous structures.1 Associated features consist of hemorrhage, extramedullary hematopoiesis, inflammatory infiltrates and hemosiderin deposits. Dystrophic calcifications are also possible.2
Inflammatory infiltrates in cardiac myxoma comprise granulocytes, mast cells, lymphocytes, macrophages and hemosiderin-laden macrophages. In a previous study, we showed a significant correlation between angiogenesis and the number of mast cells present in cardiac myxomas.3 The presence of a strong macrophage infiltrate in cardiac myxoma has been also described.4,5 Macrophages are an essential component of innate immunity and play a central role in inflammation, tissue repair, infection, allergy, metabolism and cancer.6 In response to various signals, macrophages may undergo classical M1 activation (pro-inflammatory macrophages) or alternative M2 activation (immunoregulatory macrophages), which have been elegantly described as ‘extremes of a continuum in a universe of activation states’.6 Functional polarization of macrophages has been observed under both physiological and pathological conditions.7–9 A recent study revealed abundant extravascular macrophages in the healthy heart, located in direct contact with endothelial cells.10 Healthy heart-associated macrophages exhibited an M2 phenotype; however, the expression of inflammatory genes, including IL-1β, has been also reported suggesting that a definitive classification as M1 or M2 phenotype is difficult.11 In tumors, the so-called ‘tumor-associated macrophages’ (TAMs) display anti-inflammatory and proangiogenic activity, thereby promoting the growth of the neoplasia. Although phenotypic polarization of macrophages has been described in malignant neoplastic diseases, little is known about the polarizing potential of TAMs in many benign tumors, including cardiac myxoma.
Most patients affected by cardiac myxoma display inflammatory and immune features, recognized as nonspecific constitutional symptoms, which may be associated with elevated serum levels of inflammatory cytokines and growth factors.4,12–14 Despite intense research in the field of the molecular basis of cardiac myxoma has been carried out, the relationship between histopathological properties and clinical aspects are far from being elucidated.
We therefore investigated the state of macrophage polarization and mast cells count and the possible correlation with clinical and laboratory parameters in 11 surgically excised cardiac myxomas.
Materials and methods
Patients and tissue specimens
We performed a retrospective analysis on 11 sporadic cardiac myxomas (four women and seven males, mean age 52 yr, range 43–67 yr) previously diagnosed on formalin-fixed and paraffin-embedded 4 -µm thick tissue sections. Patients with concurrent infective or autoimmune diseases and other cardiac pathologies were excluded from the study. Diagnosis were confirmed on hematoxylin and eosin slides by two pathologists (GD and CM), and hemorrhagic areas were found in cases 7 and 10 (Table 1).
Table 1.
Number of positive cells in myxomas and laboratory data.
| Patient | CD117 | CD68 | CD163 | iNOS | Triptase | EM (mm2) | ESR (× 106/µl) | PT | aPTT | RBC | PLT (× 103/µl) | WBC (× 103/µl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 184 | 22 | 9 | 21 | 15.70 | 13 | 12.9 | 28.5 | 3.45 | 237 | 12.7 |
| 2 | 22 | 222 | 58 | 38 | 16 | 10,29 | 119 | 14.7 | 34.7 | 3.11 | 280 | 23.4 |
| 3 | 7 | 58 | 73 | 19 | 12 | 4.24 | 12 | 11 | 28 | 4.1 | 137 | 6.8 |
| 4 | 32 | 235 | 167 | 20 | 29 | 5.27 | NA | 15.2 | 24 | 4.63 | 274 | 33.4 |
| 5 | 43 | 66 | 26 | 19 | 63 | 8.32 | 40 | 12.3 | 26.4 | 4.53 | 186 | 9.7 |
| 6 | 4 | 25 | 36 | 16 | 4 | 10.68 | 11 | 13 | 33.2 | 4.58 | 356 | 7.2 |
| 7 | 25 | 237 | 278 | 42 | 16 | 3.11 | 58 | 12.9 | 34.1 | 3.5 | 252 | 5 |
| 8 | 1 | 99 | 78 | 4 | 6 | 10.68 | 67 | 12.2 | 29 | 2.93 | 144 | 18.7 |
| 9 | 3 | 80 | 23 | 19 | 7 | 2.47 | 11 | 13.4 | 29.3 | 5.28 | 155 | 5.6 |
| 10 | 12 | 41 | 68 | 28 | 15 | 3.14 | 8 | 11.5 | 30.7 | 5.03 | 205 | 8 |
| 11 | 30 | 59 | 72 | 22 | 22 | 14.69 | 7 | 15.6 | 37 | 5.17 | 219 | 9.6 |
EM: echocardiographic measures; NA: not available.
Laboratory parameters analyzed were echocardiographic dimensions (mm2 area), erythrocyte sedimentation rate (ESR), red blood cell count (RBC), activated partial thromboplastin time (aPTT), prothrombin time (PT), platelet count (PLT) and white blood cell count (WBC).
Immunohistochemistry
All cases were tested for CD68 (mouse, 1:1500; Dako, Glostrup, Denmark), inducible NO synthase (iNOS) (mouse, 1:100; Sigma, St. Louis, MO, USA) and CD163 (mouse, 1:200; Leica Microsystems, Wetzlar, Germany) by means of an Autostainer Link 48 (Dako). We consider CD68 as a pan-macrophage marker, CD163 as a marker for M2-polarized macrophages and iNOS as a marker for M1-polarized macrophages. In this work CD68+/CD163−/iNOS− cells were considered as M0-polarized macrophages, CD68+/CD163+ cells were recognized as M2-polarized macrophages, whereas CD68+/iNOS+ cells were considered typical M1-polarized macrophages, when a consistent morphology was also displayed.15–18 Mast cells in all cases were then immunohistochemically stained for tryptase (monoclonal mouse, clone 10D11, 1:150 dilution; Leica, Mannheim, Germany) and c-kit (CD117) (polyclonal rabbit, 1:400; Dako).19,20
The extent of infiltrating tryptase+ cells, c-kit (CD117)+ cells, CD68+ cells, iNOS+ cells and CD163+ cells was evaluated in 10 different areas. A direct cell count was performed by using the cell count function in Image J 1.42 software,21 on 10 fields for all the cases involved in this study. Each field consisted of a photo obtained at 400× magnification.
For confocal microscopy, sections were deparaffinized before endogenous peroxidase quenching and heat-induced epitope retrieval. In order to characterize the distribution pattern of M1/M2-polarized macrophage, two distinct double stainings to detect CD163/iNOS and CD68 were performed. After permeabilization and blocking with 100 µl 0.5% saponin and 10% BSA in PBS 1× for 30 min, sections were incubated (1–2 h) with primary reagents in the same buffer used for permeabilization. Slides were extensively washed with PBS 1×, then incubated with secondary reagents (FITC-conjugated anti-mouse; 1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and washed again. Abs directed to CD163 and iNOS were directly added to slices, whereas for immunostaining of CD68 (rabbit, 1:100; Abcam, Cambridge, UK), we used an amplification biotin/streptavidin-based method. In this case, after permeabilization, the cells were sequentially incubated with streptavidin and biotin using reagents of the avidin/biotin blocking kit from Vector, and then processed for immunofluorescence with secondary biotinylated Ab (anti-rabbit; 1:1000; Sigma) and Alexa-Fluor 555-conjugated streptavidin (1:400; Santa Cruz Biotechnology). The cells were counterstained with DAPI (2 µg/ml; Santa Cruz Biotechnology), mounted using an antifade mounting medium (Life Technologies, Monza, Italy) and observed with Laser confocal scanning microscopy (SP2 LSCM; Leica Microsystems). Single staining for CD68, CD163 and iNOS, as well as negative controls, were also carried out. The double immunofluorescence staining procedure was repeated three times.
Statistical analysis
The Spearman’s rho correlation coefficient was used as a measure of the strength and direction of the linear relationship between variables. P < 0.05 was considered to indicate a statistically significant difference. Data were analyzed with SPSS 20.0 software (IBM, Armonk, NY, USA).
Results
Immunostaining for CD117 and tryptase
c-Kit (CD117)+ and tryptase+ cells were considered mast cells. Statistical analysis confirmed a positive correlation between CD117+cells and tryptase+ cells, suggesting that no active degranulation process was underway. Mast cells appeared to be scattered throughout the tissue, as previously shown.3
Immunostaining for CD68, CD163 and iNOS
Immunohistochemistry for CD68, iNOS and CD163 showed single-positive cells in all cases analyzed. Numerous CD68+ and CD163+ cells were observed in cases of myxoma displaying mesenchymal histological type. Macrophages appeared to be scattered throughout the tissue and concentrated near areas with hemorrhage. iNOS+ cells compatible with macrophagic morphology were rare.
The numbers of CD68+ macrophages (25–237, median 118.73 cells/field) were higher than the numbers of CD163+ macrophages (22–278, median 81.91 cells/field). The number of CD163+ cells was higher than the number of CD68+ cells in haemorrhagic cases (cases 7 and 10).
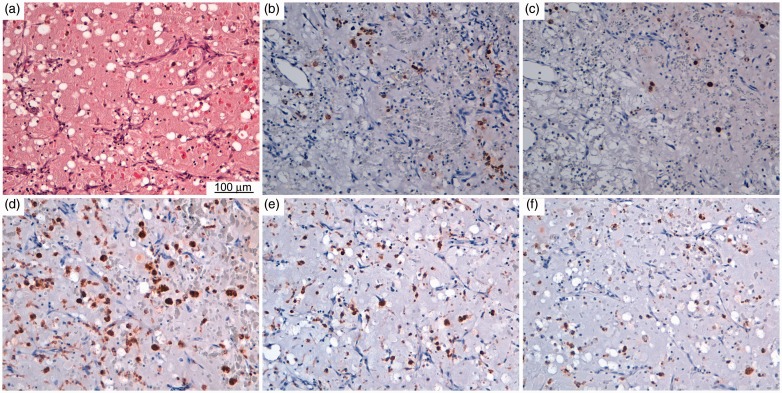
Such data (shown in Table 1) suggest that the most abundant macrophage phenotype was a CD68+ macrophage; however, CD163+ cells corresponding to M2-polarized macrophages were also frequent (Figure 1).
Figure 1.
(a) Histology and immunohistochemistry of mast cells and macrophages in myxoma samples. Histological slice stained with hematoxylin and eosin. Representative light microscopy images showing immunohistochemical detection (brown staining) of (b) c-kit (CD117), (c) tryptase, (d) CD68, (e) CD163 and (f) iNOS.
Confocal microscopy
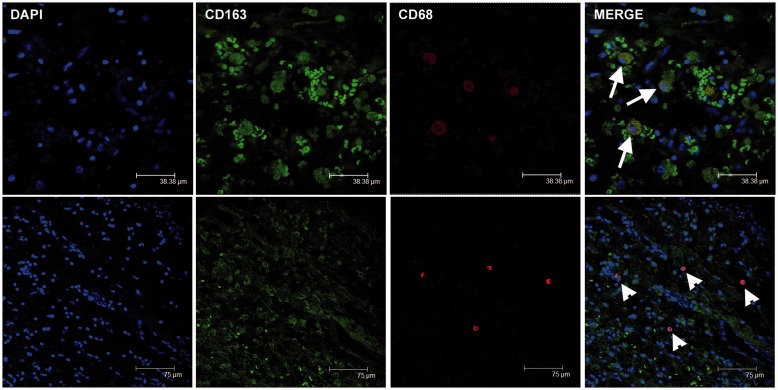
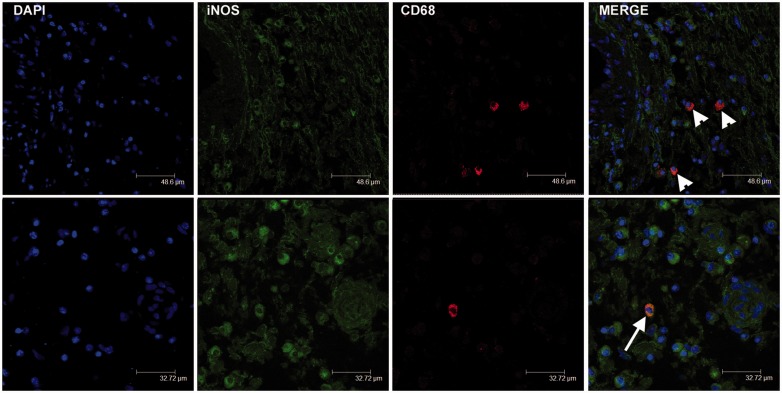
Double-immunofluorescence staining was used in order to evaluate the co-expression of CD68 and CD163, as well as CD68 and iNOS. Confocal microscopy confirmed immunohistochemical data, i.e. that the number of cells with double staining corresponded to the lower count between CD68+ macrophages and CD163+ macrophages (Figure 2). The number of CD163+ cells was higher than the number of CD68+ cells in haemorrhagic tumors (Table 1). iNOS+ cells considered as M1-polarized macrophages were CD68 positive (Figure 3). A complete evaluation of macrophagic population was biased in confocal microscopy owing to the different intensity and diffusion of the signals in the single cell, so that merge cannot always show the co-expression.
Figure 2.
Double-immunofluorescence staining for CD68 and CD163 in cardiac myxomas. Composite images showing cells double-labeled for CD68 and CD163 (arrows; yellow overlay). CD68+/CD163− macrophages were also evident (arrowheads).
Figure 3.
Double-immunofluorescence staining for CD68 and iNOS in cardiac myxomas. Composite images showing rare cells double-labeled for CD68 and iNOS (arrow; yellow overlay). CD68+/iNOS− macrophages were widely distributed (arrowheads).
Laboratory data and statistical analysis
Laboratory data are shown in Table 1. Statistical analysis showed the following significant correlations: c-kit and tryptase (r = 0.943, P < 0.0001); CD68 and ESR (r = 0.742, P = 0.014); ESR and RBCs (r = −0.875, P = 0.001); PT and PLT (r = 0.606, P = 0.048). Conversely, no correlations have been reported between CD68+, CD163+ or iNOS+ macrophages and tumor size or other laboratory parameters.
Discussion
Tumors are characterized by a mixture of cells of different lineages, including malignant cells, and also innate and adaptive immune cells, fibroblasts, endothelial cells and others. This heterogeneous cell population together with stroma and cell free molecules, generally indicated as the microenvironment, represent ‘a fertile soil for cancer cells’, as postulated by Paget. Cardiac myxoma has been described as a benign neoplasm, largely made up by numerous thin-walled blood vessels together with lepidic cells within an amorphous mucopolysaccharide-rich matrix.22 In the last decade, attention has been directed to the role of innate immunity in cardiac myxoma progression, and, in particular, to the involvement of lepidic cell- and macrophages-derived cytokines/growth factors in angiogenesis.4,13,14 Moreover, the potential correlation of these factors with clinicopathologic features of the patients has been rarely investigated.23,24
To the best of our knowledge, this is the first report to investigate the pattern of macrophage polarization in cardiac myxoma. Zhang et al. investigated macrophage population in cardiac myxoma by CD68 staining, which is considered a pan-macrophage marker.4,25 Gaumann et al. compared TAM populations in benign and malignant cardiovascular neoplasms; in particular, they reported a significantly higher infiltration of TAM in cardiac myxoma when compared with sarcomas of the pulmonary artery.5 In their work, TAMs were counted via the evaluation of two different markers, CD68 and CD163; interestingly, the expression of either CD163 alone or in combination with CD68 appears indicative of M2 differentiation.26 However, they did not evaluate the M1-polarized macrophage population. In our work, we have reported that the majority of macrophages are in steady state (M0-polarized macrophages), as confirmed by CD68 immunoreactivity. CD68+/iNOS+ M1-polarized macrophages and CD68+/CD163+ M2-polarized macrophages have been also scarcely represented.
Interestingly, the CD68+ macrophage count correlates with ESR in patients with cardiac myxoma. Conversely, no correlations have been reported between CD68+, CD163+, iNOS+ macrophages and tumor size, or other laboratory parameters. Our data well fit with the results reported by Zhang et al.,4 who showed no correlation between CD68+ macrophage count and tumor size or microvessel density.
In our series, red cell count in peripheral blood was inversely related to ESR, suggesting that anemia associated with chronic inflammatory disease was a noncasual event in patients suffering from such cardiac neoplasms.27 Anemia of inflammation is a spectrum of acute and chronic forms of anemia whose common pathophysiological denominator is their occurrence as a result of immune activation,28 including the production of pro-inflammatory cytokines such as IL-6 or TNF-α by M1-polarized macrophages.29 The fact that M1-polarized macrophages were almost absent in our series could suggest that lepidic cells are the predominant source of IL-6 reported in the serum of patients. However, we showed that tumor size did not correlate with ESR, suggesting that inflammatory state may depend on both, tumor cells population and inflammatory infiltrates. As a matter of the fact, CD68+ macrophages in cardiac myxoma expressed high levels of monocyte chemotactic protein-1, CC chemokine receptor-2 and thymidine phosphorylase, recognized as factors involved in angiogenesis.4 The life span of circulating RBCs may be negatively affected by inflammatory mediators such as TNF-α, and by mechanical stress caused by the tumor mass in the atrial cavity. Both these mechanisms may contribute to cause decreasing in count of RBC in cardiac myxomas.28,30
The distribution of c-kit+ elements were nearly coincident with that of tryptase+ cells, suggesting the specificity of these Abs as mast cells markers. Such elements did not influence general symptomatology of patient with cardiac myxomas, and mechanical hemolysis may be only a minor component of anemia, according to the lack of correlation between echographic surface and RBCs.
Thrombocytopenia has been previously reported to be associated with cardiac tumors.31,32 Even though the mechanism by which intracardiac tumor leads to thrombocytopenia remains unclear, the mechanical shear stress caused by tumor-induced flow obstruction has been suggested to be responsible for the increased breakdown of platelets.33 Obviously, in our analysis we reported a significant correlation between aPTT and PLT.
In summary, macrophages in cardiac myxoma display a M0-polarized phenotype. Few M2-polarized macrophages and rare M1-polarized macrophage have been also observed. CD68+ macrophages, CD163+ macrophages, iNOS+ macrophages and mast cell count did not correlate with tumor growth, suggesting that many different sources of cytokines and growth factor contribute to tumor development and proliferation. The significant correlation between CD68+ macrophages count and ESR indicate that both tumor infiltrates and cancer cells could contribute to nonspecific constitutional signs and symptoms occurring in 90% of cases in patients with cardiac myxoma. Moreover, according to the inverse correlation between RBC and ESR, the inflammatory state may play a role in the genesis of anemia observed in patients with cardiac myxoma.
Based on the fact that macrophages-derived factors regulate angiogenesis, we speculate that M0-polarized and M2-polarized macrophages in cardiac myxoma may play a role in angiogenesis events, as previously suggested for mast cells.3 However, a direct cross-talk between mast cells and M2-polarized macrophages can be excluded, according to recent evidence suggesting a direct interaction of mast cells with M1-polarized macrophages in aneurysm formation and rupture.34
In the future, modulation of macrophage activation state in cardiac myxomas will represent important targets for therapy.35
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Di Vito A, Mignogna C, Donato G. The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology. Histopathology 2015; 66: 321–332. [DOI] [PubMed] [Google Scholar]
- 2.Bartoloni G, Pucci A. Cardiac myxoma. In: Basso C, Valente ML, Thiene G. (eds). Cardiac tumor pathology, New York: Humana Press, 2013, pp. 31–44. [Google Scholar]
- 3.Donato G, Conforti F, Camastra C. The role of mast cell tryptases in cardiac myxoma: histogenesis and development of a challenging tumor. Oncol Lett 2014; 8: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang T, Koide N, Wada Y, et al. Significance of monocyte chemotactic protein-1 and thymidine phosphorylase in angiogenesis of human cardiac myxoma. Circ J 2003; 67: 54–60. [DOI] [PubMed] [Google Scholar]
- 5.Gaumann A, Strubel G, Bode-Lesniewska B, et al. The role of tumor vascularisation in benign and malignant cardiovascular neoplasms: a comparison of cardiac myxoma and sarcomas of the pulmonary artery. Oncol Rep 2008; 20: 309–318. [PubMed] [Google Scholar]
- 6.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2014; 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 2017; 10: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YH, He M, Wang Y, et al. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol 2017; 8: 120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal 2014; 26: 192–197. [DOI] [PubMed] [Google Scholar]
- 10.Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLOS ONE 2012; 7: e36814–e36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 2014; 102: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano T, Taga T, Yasukawa K, et al. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A 1987; 84: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto H, Sakamaki T, Kanda T, et al. Immunosuppressive drugs inhibit the production of interleukin-6 and interleukin-8 in cultured cardiac myxoma cells. Res Commun Mol Pathol Pharmacol 1997; 97: 60–66. [PubMed] [Google Scholar]
- 14.Sakamoto H, Sakamaki T, Sumino H, et al. Production of endothelin-1 and big endothelin-1 by human cardiac myxoma cells—implications of the origin of myxomas. Circ J 2004; 68: 1230–1232. [DOI] [PubMed] [Google Scholar]
- 15.Renne M, Conforti F, Camastra C, et al. Macrophage activation and patterns of inflammation in obese and non-obese women with breast carcinoma. Eur J Inflamm 2014; 12: 197–200. [Google Scholar]
- 16.Mignogna C, Signorelli F, Vismara MF, et al. A reappraisal of macrophage polarization in glioblastoma: Histopathological and immunohistochemical findings and review of the literature. Pathol Res Pract 2016; 212: 491–499. [DOI] [PubMed] [Google Scholar]
- 17.Perrotta I, Carito V, Russo E, et al. Macrophage autophagy and oxidative stress: an ultrastructural and immunoelectron microscopical study. Oxid Med Cell Longev 2011; 2011: 282739–282739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrotta I, Brunelli E, Sciangula A, et al. iNOS induction and PARP-1 activation in human atherosclerotic lesions: an immunohistochemical and ultrastructural approach. Cardiovasc Pathol 2011; 20: 195–203. [DOI] [PubMed] [Google Scholar]
- 19.Ammendola M, Sacco R, Sammarco G, et al. Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol Res Pract 2014; 2014: 951957–951957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ammendola M, Sacco R, Sammarco G, et al. Mast cells positive to tryptase and c-kit receptor expressing cells correlates with angiogenesis in gastric cancer patients surgically treated. Gastroenterol Res Pract 2013; 2013: 703163–703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins TJ. ImageJ for microscopy. Biotechniques 2007; 43: 25–30. [DOI] [PubMed] [Google Scholar]
- 22.Burke AP, Virmani R. Cardiac myxoma. A clinicopathologic study. Am J Clin Pathol 1993; 100: 671–680. [DOI] [PubMed] [Google Scholar]
- 23.Parissis JT, Mentzikof D, Georgopoulou M, et al. Correlation of interleukin-6 gene expression to immunologic features in patients with cardiac myxomas. J Interferon Cytokine Res 1996; 16: 589–593. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza CE, Rosado MF, Bernal L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex Heart Inst J 2001; 28: 3–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Medrek C, Pontén F, Jirström K, et al. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012; 12: 306–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev 2014; 262: 153–166. [DOI] [PubMed] [Google Scholar]
- 27.Ekström M, Svenarud P. Atrial myxoma: a rare but well-described cause of increased erythrocyte sedimentation rate and anaemia. BMJ Case Rep 2015; pii: bcr2015209820. [DOI] [PMC free article] [PubMed]
- 28.Nairz M, Theurl I, Wolf D, et al. Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wien Med Wochenschr 2016; 166: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak TW, Saunders ME, Jett BD. Primer to the immune response, Amsterdam: Elsevier, 2014, pp. 73–73. [Google Scholar]
- 30.Moldawer LL, Marano MA, Wei H, et al. Cachectin/tumor necrosis factor-alpha alters red blood cell kinetics and induces anemia in vivo. FASEB J 1989; 3: 1637–1643. [DOI] [PubMed] [Google Scholar]
- 31.Kucharski W, Kosmala W, Silber M, et al. Thrombocytopenia and disseminated intravascular coagulation in a patient with left atrial myxoma: a case report. Kardiol Pol 2003; 59: 421–424. [PubMed] [Google Scholar]
- 32.Semino A, Danova M, Perlini S, et al. Unusual manifestations of disseminated neoplasia at presentation: right-sided heart failure due to a massive cardiac metastasis and autoimmune thrombocytopenia in pleomorphic rhabdomyosarcoma of the adult. Am J Clin Oncol 2006; 29: 102–103. [DOI] [PubMed] [Google Scholar]
- 33.Chen K. Fatal thrombocytopenia associated with intracardiac mass. J Cardiovasc Dis Res 2012; 3: 65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan D, Chalouhi N, Jabbour P, et al. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflammation 2012; 9: 222–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14: 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]