Significance Statement
Effective advance care planning is a national priority for nephrologists. Yet, multiple barriers stymie patient–provider communication about treatment goals and patient preferences, including limited provider time and reluctance to initiate these conversations. This study examined a single-item measure eliciting patients’ treatment preference when confronted with a serious illness. This simple measure associated with two validated tools measuring health outcome priorities as well as acceptability of hypothetical end-of-life scenarios, including chronic dialysis. These results suggest that the serious illness treatment preference question is an efficient measure that can serve as an important point of entry for initiating goals-of-care communication and discussing the trade-offs of aggressive treatments (e.g., dialysis) with respect to patients’ overarching goals.
Keywords: advance care planning, serious illness, end of life, goals of care, health outcome priority
Visual Abstract
Abstract
Background
Patient-centered care for older adults with CKD requires communication about patient’s values, goals of care, and treatment preferences. Eliciting this information requires tools that patients understand and that enable effective communication about their care preferences.
Methods
Nephrology clinic patients age ≥60 years with stage 4 or 5 nondialysis-dependent CKD selected one of four responses to the question, “If you had a serious illness, what would be important to you?” Condensed versions of the options were, “Live as long as possible;” “Try treatments, but do not suffer;” “Focus on comfort;” or “Unsure.” Patients also completed a validated health outcome prioritization tool and an instrument determining the acceptability of end-of-life scenarios. Patient responses to the three tools were compared.
Results
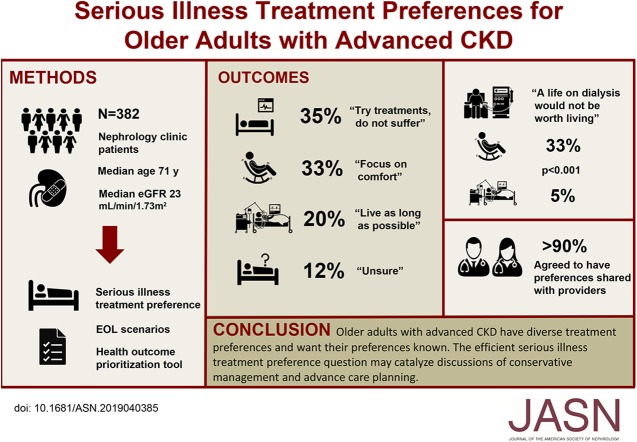
Of the 382 participants, 35% (n=134) selected “Try treatments, but do not suffer;” 33% (n=126) chose “Focus on comfort;” 20% (n=75) opted for “Live as long as possible;” and 12% (n=47) selected “Unsure.” Answers were associated with patients’ first health outcome priority and acceptability of end-of-life scenarios. One third of patients with a preference to “Focus on comfort” reported that a life on dialysis would not be worth living compared with 5% of those who chose “Live as long as possible” (P<0.001). About 90% of patients agreed to share their preferences with their providers.
Conclusions
Older adults with advanced CKD have diverse treatment preferences and want to share them. A single treatment preference question correlated well with longer, validated health preference tools and may provide a point of entry for discussions about patient’s treatment goals.
Older patients with advanced CKD often experience disturbing symptoms, functional decline, long-term institutionalization, or burdensome treatments such as dialysis.1–3 In this setting, nephrologists’ limited understanding of their patients’ values4,5 and missed opportunities to engage their patients in shared decision making may negatively affect patient quality of life, quality of dying, and patient-centered care.6–11 This may also contribute to treatment regret regarding dialysis initiation,6 poorer end-of-life care compared with patients with other serious illnesses,9 and high health care utilization at the end of life.12,13
Leading nephrology organizations have advocated for advance care planning as a clinical priority.14 Unfortunately, nephrologists face multiple barriers with respect to advance care planning, namely, that patients with advanced CKD are medically complex, receive fragmented care, and have uncertain trajectories. Nephrologists also report an unclear locus of responsibility, limited clinical time, and limited communication skills to initiate and lead these discussions.10,15–18 In addition, data on patient treatment preferences and possible advance care planning interventions in patients with CKD are limited.19 Although patients have a variety of life experiences that affect their perspectives toward advance care planning,20 a majority prefer for providers to discuss prognosis and present multiple treatment options when faced with disease progression.6,20,21 However, many providers report reluctance to initiate these discussions, with fewer than 5% of nephrologists reporting a discussion of prognosis with their patients in one study.10,22,23
Efficient measures that can be easily integrated into routine clinical activities may help nephrologists elicit patient values, assess patient readiness to engage in advance care planning conversations, and facilitate more informed, shared decision making discussions regarding intensive treatments, such as dialysis.24 We hypothesized that in older patients with advanced CKD, a single-item instrument that assessed patient preferences when confronted with a hypothetical serious illness would associate with health outcome priorities as well as responses on the acceptability of common, hypothetical end-of-life scenarios. We also hypothesized that most patients would be willing to have this information documented in their medical charts and shared with their providers.
Methods
Study Setting and Participants
Patients followed by a nephrology provider at an academic clinic in the southern United States were enrolled between November 2016 and October 2018. Patient eligibility criteria, as previously described in related studies,5,25,26 were age ≥60 years and nondialysis-dependent CKD stages 4–5, defined by the Modification of Diet in Renal Disease Study equation.27 We excluded patients with clinically apparent moderate to severe dementia, dialysis dependence, history of kidney transplantation, or recent AKI (baseline eGFR ≥60 ml/min per 1.73 m2 within the prior 12 months or baseline eGFR ≥40 ml/min per 1.73 m2 within the prior 4 months).
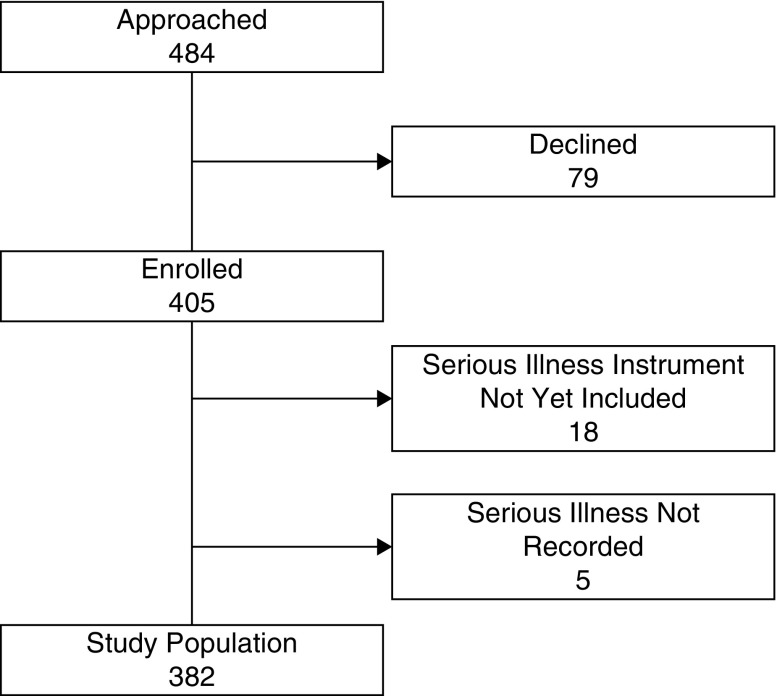
We approached 484 patients and 405 (84%) consented (Figure 1). For this analysis, 23 patients were excluded (18 who were enrolled before the serious illness question was included in the protocol and five for unrecorded responses to the serious illness question after its inclusion) and the remaining 382 patients are described here. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (approval number 161523) and adhered to the principles of the Declaration of Helsinki.
Figure 1.
Study flow diagram.
Serious Illness Treatment Preference Question
As previously described,5 trained research staff collected information regarding health values, including several measures evaluating health outcome priorities. Research staff then asked patients the single-item serious illness treatment preference question adapted from the landmark Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT):28 “If you had a serious illness, what would be important to you?” There were four response options: “I want treatments to try to live as long as possible. I would not want to stop treatment even if I was in pain, could not feed or care for myself, or needed machines to live” (i.e., treatments to live as long as possible); “I want to try treatments for a period of time, but I would not want to suffer. If after a period of time the treatments do not help or I am suffering, I would want to stop.” (i.e., try treatments, but do not suffer); “I want to focus on my quality of life and being comfortable, even if it means having a shorter life.” (i.e., focus on comfort); and “I am not sure.” The interpretation of what constituted a serious illness was left to the patient to define.
Health Outcome Priorities
As previously described, patients also completed a validated health outcome prioritization tool.5,24 Patients were presented with a script and were asked to rank health outcome priorities (“maintaining independence;” “keeping you alive;” “reducing or eliminating pain;” and “reducing or eliminating symptoms” such as dizziness, fatigue, shortness of breath) on an enlarged visual analog scale with a range of 0 (lowest priority) to 100 (highest priority). Patients could not rank two priorities equally. Because the health outcome priority rankings demonstrated excellent reliability in previous studies whereas the numerical visual analog scale scores demonstrated substantial variation, we chose a priori to capture rank orderings only.24
End-of-Life Scenarios
We used a publicly available advance care planning workbook29 to capture patient preferences in common end-of-life scenarios (e.g., cannot recognize family or friends, kept alive by kidney dialysis, and live in a nursing home permanently). Research staff introduced the material, spoke about the importance of understanding the patients’ responses should their illness worsen, and asked patients to carefully consider their values and preferences when answering the items. Each item prompted the patients to judge whether they would find life in a described condition “difficult but acceptable;” “worth living, but just barely;” or “not worth living.” The patients were prompted to consider each of the described scenarios as permanent and were permitted to respond “Can’t answer now” if unable to provide a response or if they found the question distressing. Patients were permitted to opt out of each question and were provided with emotional support if necessary.
Willingness to Share with Providers and Document in Electronic Health Record
Six months after study initiation, we added an additional question immediately after the serious illness item, to assess whether patients would be willing to have their response to the serious illness item shared with their doctors and recorded in their electronic health record (EHR). Response options were yes or no.
Patient Characteristics and Other Measures
Upon enrollment, patients completed a brief questionnaire to collect information on sociodemographics and past medical history. We also performed manual chart reviews and structured data abstraction to supplement this information with documented comorbidities and common clinical measurements (e.g., vital signs, laboratory test values, etc.) from the prior 12 months. With information on comorbidities, we calculated a Charlson comorbidity index (CCI) for each patient.30 We measured independence in activities of daily living (ADL) and instrumental activities of daily living (iADL) with the Katz31 and Lawton32 indices, respectively. ADL and iADL scores ranged from 0 to 5 and 0 to 8, respectively, with higher scores indicating greater independence in activities. We used the Palliative Care Outcome Scale symptom list for end-stage renal disease measure2,33 to assess patient symptoms over the prior week generating a total score, ranging from 0 to 68, with higher scores indicating a higher symptom burden.
Statistical Analyses
Patient characteristics are presented as medians and interquartile ranges (IQRs) for continuous variables and counts with percentages for categorical variables. We tested the association between serious illness treatment preferences and (1) first health outcome priority and (2) acceptability of common end-of-life scenarios with chi-squared tests.
We used binary logistic regression to compare patients who answered “Unsure” to the serious illness treatment preference to the remainder of the cohort. Patients who responded “Unsure” were similar to respondents with a delineated preference. We therefore excluded patients who responded “Unsure” from our multivariable regression model to focus on patients with known preferences. We then modeled serious illness treatment preference responses using multivariable ordinal logistic regression with a rank order starting with aggressive care and progressing to less aggressive care (i.e., “Live as long as possible” ordered as 0; “Try treatments, but do not suffer” ordered as 1; and “Focus on comfort” ordered as 2). Independent variables in the model were selected a priori on the basis of plausibility of an association: age, sex, race, marital status, insurance, income (as a continuous variable in $10,000 U), education, eGFR, Palliative Care Outcome Scale symptom list for end-stage renal disease score, and comorbidities (coronary artery disease, cerebrovascular disease, peripheral vascular disease, heart failure, and diabetes). Variables were checked for collinearity. We repeated the regression analysis using the CCI instead of individual comorbidities. Continuous predictors were included in regression models as restricted cubic splines to allow for possible nonlinear association. For continuous variables, odds ratios (ORs) were scaled to express the odds per an increase equal to the variable’s IQR. For categorical variables, ORs were expressed against specified references.
To better characterize the association between patients’ response to the serious illness treatment preference question and their first health outcome priority, the relationship was assessed using an unadjusted logistic regression model, with the dependent variable being the dichotomized first health outcome priority (staying alive versus any other priority i.e., pain, symptoms, or independence). After a linear association was graphically demonstrated with the serious illness treatment preference response, the serious illness treatment preference response was treated numerically (i.e., “Live as long as possible” ordered as 0; “Try treatments, but do not suffer” ordered as 1; and “Focus on comfort” ordered as 2). In addition, we assessed rank-order correlation of the dichotomized first health outcome priority with the serious illness treatment preference response using the Goodman and Kruskal γ to account for ties.34–36 P values of <0.05 were considered statistically significant. We performed all analyses using R (version 3.4.4).37
Results
The 382 participants included 46% women, 16% black Americans, 62% with at least some college education, and 69% with Medicare insurance (Table 1). Participants had a median age of 71 years (IQR, 66–77), a median CCI score of 6 (IQR, 4–7), a median eGFR at enrollment of 23 ml/min per 1.73 m2 (IQR, 17–28), and most patients were independent in their ADL and iADL (Table 1).
Table 1.
Baseline characteristics by serious illness treatment preference response
| Characteristic | Combined (n=382) | Live as Long as Possible (n=75) | Try Treatments, but Do Not Suffer (n=134) | Focus on Comfort (n=126) | Unsure (n=47) |
|---|---|---|---|---|---|
| Age, yr | 71 [66–77] | 70 [65–75] | 71 [66–78] | 73 [68–78] | 68 [65–74] |
| Women | 46 (174) | 41 (31) | 44 (59) | 46 (58) | 55 (26) |
| Race | |||||
| Black | 16 (61) | 24 (18) | 12 (16) | 11 (14) | 28 (13) |
| White | 84 (317) | 73 (55) | 87 (117) | 88 (111) | 72 (34) |
| Other | 1 (4) | 3 (2) | 1 (1) | 1 (1) | 0 (0) |
| Marital status | |||||
| Married | 65 (249) | 60 (45) | 70 (94) | 61 (77) | 70 (33) |
| Divorced | 11 (42) | 19 (14) | 8 (11) | 10 (13) | 9 (4) |
| Widowed | 18 (70) | 11 (8) | 18 (24) | 24 (30) | 17 (8) |
| Single/other | 5 (21) | 11 (8) | 4 (5) | 5 (6) | 4 (2) |
| Insurance | |||||
| Private | 27 (104) | 28 (21) | 30 (40) | 22 (28) | 32 (15) |
| Medicaid/medical assistance | 3 (13) | 11 (8) | 1 (2) | 2 (2) | 2 (1) |
| Medicare | 69 (262) | 60 (45) | 69 (92) | 75 (94) | 66 (31) |
| Education | |||||
| Some high school | 10 (38) | 16 (12) | 10 (14) | 7 (9) | 6 (3) |
| High school diploma or equivalent | 28 (106) | 24 (18) | 22 (30) | 30 (38) | 43 (20) |
| Some college | 23 (89) | 24 (18) | 28 (37) | 17 (22) | 26 (12) |
| College degree or higher | 39 (149) | 36 (27) | 40 (53) | 45 (57) | 26 (12) |
| Household income (annual, in thousands) | |||||
| <20 | 15 (57) | 23 (17) | 10 (14) | 13 (16) | 21 (10) |
| 20–40 | 25 (94) | 25 (19) | 25 (34) | 21 (26) | 32 (15) |
| 40–60 | 19 (71) | 19 (14) | 23 (31) | 17 (22) | 9 (4) |
| 60–80 | 14 (55) | 8 (6) | 15 (20) | 16 (20) | 19 (9) |
| ≥80 | 23 (88) | 21 (16) | 24 (32) | 27 (34) | 13 (6) |
| Decline to answer | 4 (17) | 4 (3) | 2 (3) | 6 (8) | 6 (3) |
| Comorbidities | |||||
| Hypertension | 96 (368) | 99 (74) | 99 (132) | 94 (118) | 94 (44) |
| Diabetes mellitus | 49 (189) | 37 (28) | 54 (73) | 48 (61) | 57 (27) |
| CAD | 28 (108) | 27 (20) | 31 (42) | 25 (31) | 32 (15) |
| CVD | 18 (67) | 11 (8) | 17 (23) | 21 (27) | 19 (9) |
| PVD | 11 (41) | 16 (12) | 7 (10) | 10 (13) | 13 (6) |
| Cardiovascular disease | 40 (151) | 35 (26) | 42 (56) | 39 (49) | 43 (20) |
| Heart failure | 20 (78) | 21 (16) | 20 (27) | 20 (25) | 21 (10) |
| Cancer | 27 (104) | 28 (21) | 28 (38) | 26 (33) | 26 (12) |
| Chronic lung disease | 14 (53) | 13 (10) | 18 (24) | 11 (14) | 11 (5) |
| CCI | 6 [4–7] | 5 [4–7] | 6 [5–7] | 5 [4–7] | 6 [4–7] |
| BMI, kg/m2 | 30.3 [26.0–35.1] | 29.8 [25.5–33.8] | 30.3 [25.8–34.9] | 30.2 [26.3–35.8] | 33.2 [28.2–37.1] |
| eGFR (MDRD)a | 23 [17–28] | 24 [16–29] | 23 [18–28] | 23 [18–27] | 23[17–28] |
| ADL score | 5 [5–5] | 5 [5–5] | 5 [5–5] | 5 [5–5] | 5 [5–5] |
| iADL score | 8 [7–8] | 8 [7–8] | 8 [7–8] | 8 [6–8] | 8 [7–8] |
| POS-S Renal | 8 [4–15] | 7.5 [3–12] | 10 [5–15] | 8 [4–15] | 7 [4–15] |
Continuous variables are presented as median [IQR], categorical variables are presented as percent (n). CAD, coronary artery disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; BMI, body mass index; MDRD, Modification of Diet in Renal Disease; POS-S Renal, Palliative Care Outcome Scale symptom list for end-stage renal disease.
In ml/min per 1.73 m2.
Serious Illness Preference
Approximately 33% of patients (n=126) chose “Focus on comfort;” 35% (n=134) chose “Try treatments, but do not suffer;” 20% (n=75) chose “Live as long as possible;” and 12% (n=47) chose “Unsure” as their serious illness treatment preference (P<0.001). Patients who responded “Unsure” were modestly younger and more likely to be black than their cohort counterparts (median age, 68 [IQR, 65–74] versus 71 [IQR, 66–77] years; black, 28% [n=13] versus 14% [n=48]) but were otherwise like respondents with a delineated preference.
Association with Health Outcome Priority and Advance Care Planning Responses
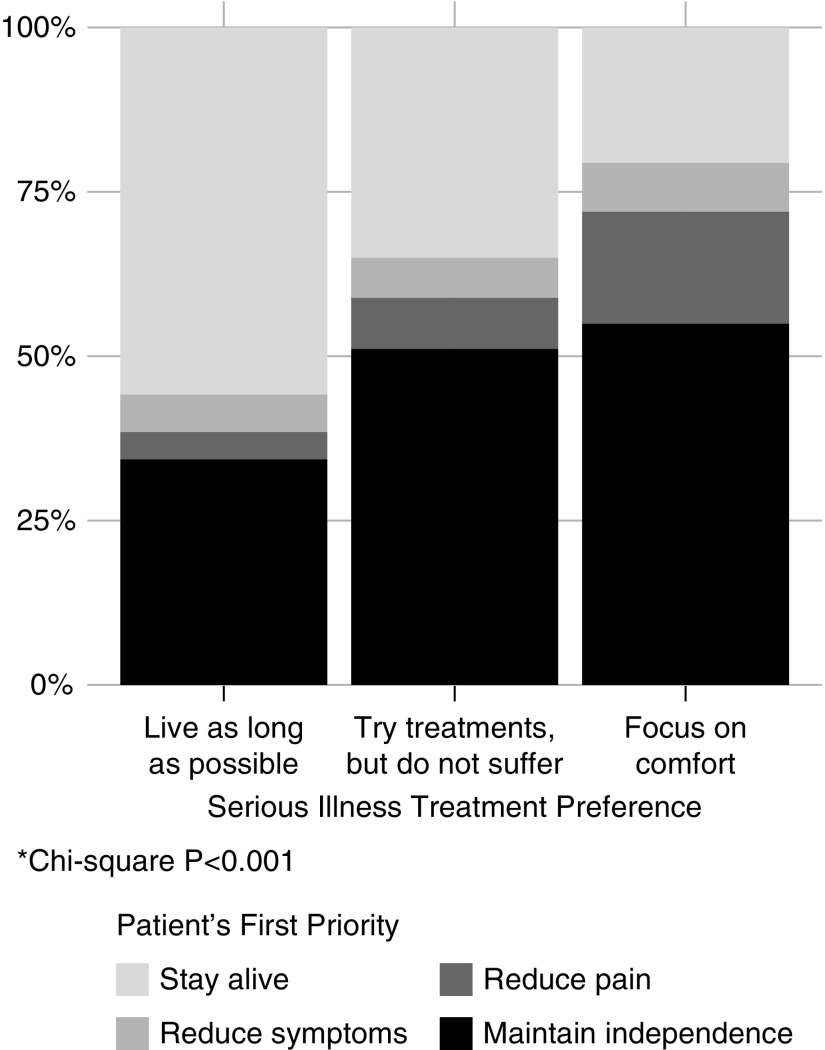
Patients’ serious illness treatment preferences were associated with patients’ first health outcome priority (Figure 2, Supplemental Table 1; P<0.001). Maintaining independence was the first health outcome priority for 55% and 51% of patients who selected “Focus on comfort” or “Try treatments, but do not suffer,” respectively, compared with 34% of patients who selected “Live as long as possible” as their treatment preference (“Live as long as possible” versus “Try treatments, but do not suffer” P=0.02; “Live as long as possible” versus “Focus on comfort” P=0.006; “Try treatments, but do not suffer” versus “Focus on comfort” P=0.55]. In addition, staying alive was the first health outcome priority for 56%, 35%, and 20% of patients who selected “Live as long as possible;” “Try treatments, but do not suffer;” and “Focus on comfort” as their serious illness preference, respectively (“Live as long as possible” versus “Try treatments, but do not suffer” P=0.005; “Live as long as possible” versus “Focus on comfort” P<0.001; “Try treatments, but do not suffer” versus “Focus on comfort” P=0.01).
Figure 2.
Patients’ first health outcome priority by serious illness treatment preference. *chi-squared P<0.001.
In the binary logistic regression model, each one-point increase toward less aggressive treatment in the serious illness treatment preference question (e.g., “Live as long as possible” to “Try treatments, but do not suffer”) was associated with an increased odds of a first health outcome priority of pain, symptoms, or maintaining independence (OR, 2.21; 95% confidence interval [95% CI], 1.60 to 3.05) versus staying alive. The rank-order correlation between the serious illness treatment preference and the dichotomized first health outcome priority was moderate (0.46; 95% CI, 0.30 to 0.62). The degree of positive and negative correlation between patients’ serious illness treatment preferences and the first health outcome priority is shown in Supplemental Figure 1.
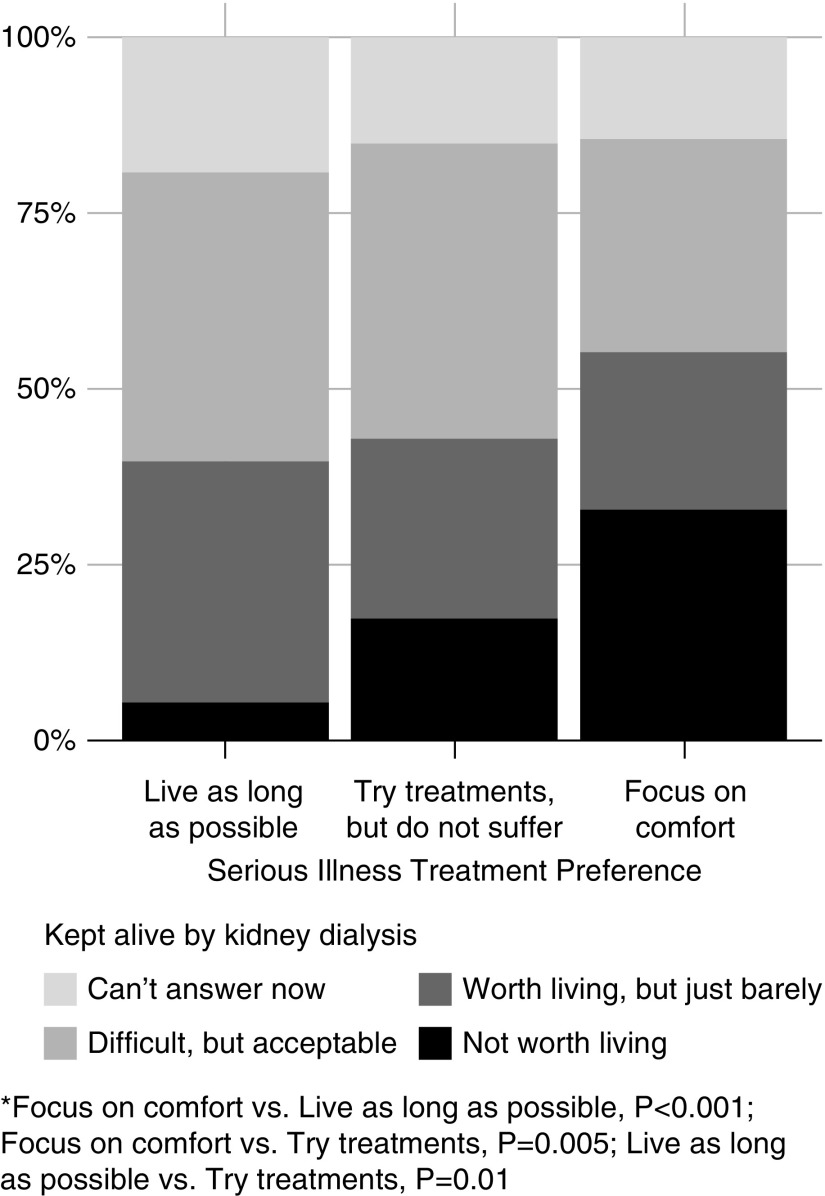
Patients’ serious illness treatment preference selection was associated with acceptability of the hypothetical end-of-life scenarios (Table 2). Patients who chose “Try treatments, but do not suffer” or “Focus on comfort” as their serious illness preference were more likely to say life was not worth living for all the scenarios except “Cannot walk but can use a wheelchair” (Table 2). Approximately 33% of patients (n=41) who chose “Focus on comfort” reported that a life on dialysis would not be worth living compared with 5% (n=4) of patients who chose “Live as long as possible” (P<0.001; Figure 3).
Table 2.
Serious illness treatment preference and acceptance of common end-of-life scenarios
| Common End-of-Life Scenarioa | Live as Long as Possible (n=74) | Try Treatments, but Do Not Suffer (n=133) | Focus on Comfort (n=126) |
|---|---|---|---|
| Cannot recognize family/friends | |||
| Difficult, but acceptable | 51 (38) | 23 (31) | 19 (24) |
| Worth living, but just barely | 18 (13) | 19 (25) | 14 (18) |
| Not worth living | 14 (10) | 47 (62) | 51 (64) |
| Can’t answer now | 18 (13) | 11 (15) | 16 (20) |
| Cannot think or talk clearly | |||
| Difficult, but acceptable | 51 (38) | 20 (26) | 18 (23) |
| Worth living, but just barely | 28 (21) | 27 (36) | 21 (26) |
| Not worth living | 10 (7) | 46 (61) | 47 (59) |
| Can’t answer now | 11 (8) | 8 (10) | 14 (18) |
| Cannot respond to commands | |||
| Difficult, but acceptable | 43 (32) | 16 (21) | 12 (15) |
| Worth living, but just barely | 20 (15) | 17 (22) | 24 (30) |
| Not worth living | 19 (14) | 57 (76) | 50 (63) |
| Can’t answer now | 18 (13) | 11 (14) | 14 (18) |
| Cannot walk but can use a wheelchair | |||
| Difficult, but acceptable | 69 (51) | 66 (87) | 52 (65) |
| Worth living, but just barely | 22 (16) | 21 (28) | 28 (35) |
| Not worth living | 3 (2) | 7 (9) | 10 (12) |
| Can’t answer now | 7 (5) | 5 (7) | 11 (14) |
| Cannot go outside, must stay home | |||
| Difficult, but acceptable | 66 (49) | 63 (84) | 44 (56) |
| Worth living, but just barely | 22 (16) | 26 (34) | 34 (43) |
| Not worth living | 4 (3) | 5 (7) | 9 (11) |
| Can’t answer now | 8 (6) | 6 (8) | 13 (16) |
| In severe untreatable pain most of the time | |||
| Difficult, but acceptable | 32 (24) | 14 (18) | 10 (13) |
| Worth living, but just barely | 39 (29) | 29 (39) | 18 (23) |
| Not worth living | 16 (12) | 47 (63) | 52 (66) |
| Can’t answer now | 12 (9) | 10 (13) | 19 (24) |
| In severe discomfort most of the time | |||
| Difficult, but acceptable | 40 (29) | 24 (31) | 19 (24) |
| Worth living, but just barely | 32 (23) | 39 (52) | 34 (43) |
| Not worth living | 16 (12) | 28 (37) | 32 (40) |
| Can’t answer now | 12 (9) | 9 (12) | 15 (19) |
| Kept alive by kidney dialysis | |||
| Difficult, but acceptable | 41 (30) | 42 (56) | 30 (38) |
| Worth living, but just barely | 34 (25) | 26 (34) | 22 (28) |
| Not worth living | 5 (4) | 17 (23) | 33 (41) |
| Can’t answer now | 19 (14) | 15 (20) | 14 (18) |
| Kept alive by breathing machines | |||
| Difficult, but acceptable | 29 (21) | 14 (18) | 5 (6) |
| Worth living, but just barely | 22 (16) | 18 (24) | 13 (17) |
| Not worth living | 27 (20) | 58 (76) | 69 (87) |
| Can’t answer now | 22 (16) | 11 (14) | 13 (16) |
| Full-time care required | |||
| Difficult, but acceptable | 36 (26) | 26 (35) | 14 (17) |
| Worth living, but just barely | 24 (17) | 27 (36) | 26 (32) |
| Not worth living | 21 (15) | 39 (52) | 49 (61) |
| Can’t answer now | 19 (14) | 7 (10) | 12 (15) |
| Cannot control bladder or bowels | |||
| Difficult, but acceptable | 36 (26) | 20 (27) | 15 (19) |
| Worth living, but just barely | 31 (22) | 40 (53) | 27 (34) |
| Not worth living | 17 (12) | 32 (42) | 44 (55) |
| Can’t answer now | 17 (12) | 8 (10) | 14 (18) |
| Live in nursing home permanently | |||
| Difficult, but acceptable | 37 (27) | 27 (35) | 24 (30) |
| Worth living, but just barely | 29 (21) | 28 (37) | 19 (24) |
| Not worth living | 15 (11) | 34 (45) | 42 (53) |
| Can’t answer now | 19 (14) | 11 (15) | 15 (19) |
| Emotional or financial burden on family | |||
| Difficult, but acceptable | 30 (22) | 20 (27) | 9 (12) |
| Worth living, but just barely | 25 (18) | 21 (28) | 23 (29) |
| Not worth living | 25 (18) | 47 (63) | 47 (59) |
| Can’t answer now | 20 (15) | 11 (15) | 21 (26) |
Numbers expressed as percent (N).
n=333 because of exclusion of 47 participants who were unsure of their serious illness treatment preference and two participants with missing end-of-life scenario responses.
Figure 3.
Serious illness treatment preference and the acceptability of being kept alive by chronic dialysis. *“Focus on comfort” versus “Live as long as possible,” P<0.001; “Focus on comfort” versus “Try treatments, but do not suffer,” P=0.005; “Live as long as possible” versus “Try treatments, but do not suffer,” P=0.01.
Patients’ Willingness to Share Preferences
After an item assessing patients’ willingness to share their care preferences was added to the study protocol, 251 patients (66% of analytic cohort) were asked about their willingness to share treatment preferences with their doctors and have it documented in their EHR. About 88% (n=221 out of 251) of queried patients reported a desire to share their preferences and have them documented. After excluding patients who responded “Unsure” to the serious illness treatment preference item, the results were similar with 91% (n=200 out of 220) of patients reporting a desire to share and document their serious illness care preferences (Supplemental Figure 2, Supplemental Table 2). There was no association between serious illness treatment preference response and willingness to have preferences documented (Supplemental Table 2).
Predictors of Serious Illness Preference Response
In the multivariable model, being a widow/widower (reference: married) (OR, 1.94; 95% CI, 1.02 to 3.68), higher income (OR, 2.32; 95% CI, 1.32 to 4.09), and the presence of cerebrovascular disease (OR, 2.48; 95% CI, 1.30 to 4.73) were associated with a preference toward less aggressive care (i.e., treatment goals that prioritize comfort) (Supplemental Table 3). Not having graduated high school (reference: high school diploma or equivalent) (OR, 0.44; 95% CI, 0.20 to 0.99) and private insurance (reference: Medicare) (OR, 0.54; 95% CI, 0.31 to 0.94) were associated with a preference toward more aggressive care (Supplemental Table 3).
Discussion
We found that older adults with advanced CKD had diverse preferences when confronted with a hypothetical serious illness, and only one fifth reported a preference for treatments to live as long as possible. Further, the single-item serious illness measure captured important information about patient priorities and goals of care. The serious illness response associated with patients’ willingness to accept nearly all common end-of-life scenarios and about one third of patients who preferred treatments that focused on comfort reported that a life on dialysis would be unacceptable. In addition, patients’ serious illness responses associated with their stated primary health outcome priority, assessed using a validated health outcome priority tool.5,24 Importantly, the vast majority of patients were willing to share their serious illness preference with their providers and to have them documented in the EHR.
Across specialties, goals-of-care communication between patients and providers has been shown to improve important patient and health care system outcomes.38–42 In the setting of multiple barriers to advance care planning,10,15–18 a shortage of hospice and palliative care physicians,43 and some patients’ preferences to not involve a palliative care team,6 nephrologists need tools that help to initiate conversations to elicit care preferences. The single-item serious illness treatment preference question is simple, comprehensible to patients, and takes little time to collect. Further, as our results suggest, the measure provides useful insight on patient health outcome priorities and preferences regarding end-of-life treatment choices. These characteristics make it well suited for collection during routine nephrology care and future research targeting improvements in end-of-life care in advanced CKD. In addition, the structured response categories facilitate EHR integration (like the near ubiquitous status of “full code” and “do-not-resuscitate” orders).
Generalizable strategies that improve patient–provider communication about treatment goals are needed in nephrology. Although lengthy educational material and preappointments with social workers have been shown to have a moderate effect on advance care planning discussions and documentation in the general population and cancer patients,44–47 many of the tools currently used lack evidence48 or are not practical for routine use during a nephrology office visit because of their length or resource burden. Recently, a preconversation, communication-priming questionnaire during routine outpatient visits in general patients with a serious illness was shown to increase occurrence, documentation, and quality of goals-of-care communication.49 Our findings indicate that the serious illness treatment preference question provides general insight on patient care preferences and should be studied further as an easy-to-use tool to (1) assess patient readiness for advance care planning, (2) prime patients for more in-depth advance care planning conversations, and (3) give providers an entryway to discuss these issues and improve patient–provider communication about goals of care.50 Notably, nearly 90% of patients were agreeable to documenting their serious illness preference response, indicating the measure is likely to be acceptable and pragmatic to integrate into routine medical care. Future studies should examine the stability of serious illness treatment preferences in patients with advanced CKD over time and the potential effect of available responses on surrogate decision makers during severe illnesses.
Nephrologists report difficulty in identifying patients most appropriate for conservative management or a time-limited dialysis trial.51 Patients with advanced CKD who choose to “Focus on comfort” or to “Try treatments, but do not suffer” are likely to benefit from a more thorough discussion of these options. Such patients were more likely to select “Maintaining independence” as their first health outcome priority and a majority reported that life was not worth living if they required full-time care or lived in a nursing home; outcomes that are common in morbid or frail older adults who initiate chronic dialysis.3,52 Nearly one third of patients who preferred treatments that “Focus on comfort” reported that a life on dialysis would be unacceptable, possibly helping nephrologists identify patients who are more likely to perceive conservative care as consistent with their values and preferences.6 In addition, this approach may mitigate the continual pressure to reconsider dialysis in patients with advanced CKD with a clearly stated preference for conservative care.8 For example, EHR documentation of a stable patient preference for comfort-focused care may give providers adequate reassurance on the consistency of patients’ choices concerning dialysis.
Strengths and Weaknesses
Our study has several strengths. First, older adults with advanced CKD frequently receive high-intensity care near the end of life, yet providers have a limited understanding of their preferences.5 Our results give voice to the diversity of these preferences. Second, the number of participants in our study was relatively high compared with relatable studies in advanced CKD. Third, our participation rate was also high reducing selection biases. Fourth, the serious illness treatment preference measure we used was simple, thereby enhancing generalizability and facilitating future adoption.
Our study also has several limitations. First, we measured patient responses to a hypothetical serious illness, which may not reflect patient preferences when faced with the actual situation. Patient treatment preferences are affected by perceptions of treatment burden, possible outcomes, and the likelihoods of these outcomes53; factors we cannot account for in the absence of a firm definition of “serious illness.” However, the serious illness question is meant to catalyze shared decision making and advance care planning, not replace it, and may have wider clinical adoption than lengthier questionnaires.54 Second, we only measured patient responses once. Future studies should examine whether serious illness treatment responses map with actual end-of-life decisions. Third, our patient population was largely white and had high educational attainment, possibly limiting generalizability of our results. Fourth, although patients with clinically diagnosed moderate to severe dementia were excluded, patients with other levels of cognitive impairment were included. Hence, some patients’ subclinical cognitive impairment may have influenced their ability to communicate their true preferences. Fifth, the correlation between the serious illness treatment preference and the first health outcome priority was only moderate, indicating that this tool should not act as a substitute for more meaningful, in-depth advance care planning discussions. Future research should examine whether completion of the serious illness treatment preference question affects initiation of advance care planning conversations or health outcome priority concordance with providers. Finally, we did not capture the treatment preferences of patients’ health care proxies. These individuals are often tasked with medical decision making near the end of life.
In conclusion, our findings show that more than two thirds of older adults with advanced CKD would prioritize treatments that focus on comfort or do not cause suffering when faced with a potential serious illness. Almost all patients were willing to share these preferences with their providers and to document them in their record. Patients’ serious illness treatment preferences also captured important differences in the acceptability of aggressive therapies, including dialysis. The simple, single-item serious illness treatment measure may serve as an important point of entry for initiating more frequent and effective goals-of-care communication.
Disclosures
Dr. Abdel-Kader reports grants from Satellite Health Care Dr. Siew reports personal fees from Akebia Therapeutics Inc., other from DaVita, outside the submitted work; and received honorarium as a contributing author for UpToDate, as well as serving as Associate Editor for CJASN.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK090304 (to Abdel-Kader) and National Center for Advancing Translational Sciences Clinical and Translational Science Awards UL1TR000445 and UL1TR002243, all from the National Institutes of Health, the Satellite Health Norman Coplon Extramural Grant Program (Abdel-Kader), and the Vanderbilt Center for Kidney Disease.
Supplementary Material
Acknowledgments
Abdel-Kader designed the study. Baddour, Salat, and Abdel-Kader collected the data. Mason and Stewart analyzed the data. All authors participated in data interpretation. Baddour and Abdel-Kader drafted the manuscript. All authors revised the manuscript and approved the final version of the manuscript. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
We thank Dr. Beatrice Concepcion for guidance and assistance in creating the visual abstract. We also thank the patients and providers at the Vanderbilt University Medical Center nephrology clinic for their participation during this study.
The funders of this study had no role in study design, data collection, analysis, interpretation, manuscript preparation, or the decision to report the findings.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019040385/-/DCSupplemental.
Supplemental Table 1. Serious illness treatment preference and first choice health outcome priority.
Supplemental Table 2. Patient willingness to share serious illness preference with provider and medical chart.
Supplemental Table 3. Odds of a serious illness treatment preference choice favoring less aggressive care.
Supplemental Figure 1. Visual assessment of association between patients’ serious illness treatment preference and first health outcome priority.
Supplemental Figure 2. Patients’ willingness to share serious illness treatment preference.
References
- 1.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, et al.: Chronic kidney disease and functional limitation in older people: Health, aging and body composition study. J Am Geriatr Soc 54: 750–756, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Murphy EL, Murtagh FE, Carey I, Sheerin NS: Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: Use of a short patient-completed assessment tool. Nephron Clin Pract 111: c74–c80, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, James MT, Klarenbach SW, Manns BJ, Ravani P, et al.: Alberta Kidney Disease Network : A population-based cohort study defines prognoses in severe chronic kidney disease. Kidney Int 93: 1217–1226, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Eneanya ND, Paasche-Orlow MK, Volandes A: Palliative and end-of-life care in nephrology: Moving from observations to interventions. Curr Opin Nephrol Hypertens 26: 327–334, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Ramer SJ, McCall NN, Robinson-Cohen C, Siew ED, Salat H, Bian A, et al.: Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers’ perceptions. J Am Soc Nephrol 29: 2870–2878, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong SP, Hebert PL, Laundry RJ, Hammond KW, Liu CF, Burrows NR, et al.: Decisions about renal replacement therapy in patients with advanced kidney disease in the US department of veterans affairs, 2000–2011. Clin J Am Soc Nephrol 11: 1825–1833, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SPY, McFarland LV, Liu CF, Laundry RJ, Hebert PL, O’Hare AM: Care practices for patients with advanced kidney disease who forgo maintenance dialysis. JAMA Intern Med 179: 305–313, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL: Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med 176: 1095–1102, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell JO, Patel UD, Steinhauser KE, Ammarell N, Tulsky JA: Discussions of the kidney disease trajectory by elderly patients and nephrologists: A qualitative study. Am J Kidney Dis 59: 495–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong SP, Kreuter W, O’Hare AM: Healthcare intensity at initiation of chronic dialysis among older adults. J Am Soc Nephrol 25: 143–149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong SP, Kreuter W, O’Hare AM: Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med 172: 661–663, discussion 663–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, et al.: American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, Ravani P, et al.: Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open 1: e184852, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hare AM, Szarka J, McFarland LV, Taylor JS, Sudore RL, Trivedi R, et al.: Provider perspectives on advance care planning for patients with kidney disease: Whose job is it anyway? Clin J Am Soc Nephrol 11: 855–866, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holley JL, Carmody SS, Moss AH, Sullivan AM, Cohen LM, Block SD, et al.: The need for end-of-life care training in nephrology: National survey results of nephrology fellows. Am J Kidney Dis 42: 813–820, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Combs SA, Culp S, Matlock DD, Kutner JS, Holley JL, Moss AH: Update on end-of-life care training during nephrology fellowship: A cross-sectional national survey of fellows. Am J Kidney Dis 65: 233–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luckett T, Sellars M, Tieman J, Pollock CA, Silvester W, Butow PN, et al.: Advance care planning for adults with CKD: A systematic integrative review. Am J Kidney Dis 63: 761–770, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Goff SL, Eneanya ND, Feinberg R, Germain MJ, Marr L, Berzoff J, et al.: Advance care planning: A qualitative study of dialysis patients and families. Clin J Am Soc Nephrol 10: 390–400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine A, Fontaine B, Kraushar MM, Rich BR: Nephrologists should voluntarily divulge survival data to potential dialysis patients: A questionnaire study. Perit Dial Int 25: 269–273, 2005 [PubMed] [Google Scholar]
- 22.Davison SN, Simpson C: Hope and advance care planning in patients with end stage renal disease: Qualitative interview study. BMJ 333: 886–889, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wachterman MW, Marcantonio ER, Davis RB, Cohen RA, Waikar SS, Phillips RS, et al.: Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med 173: 1206–1214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH: Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med 171: 1854–1856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salat H, Javier A, Siew ED, Figueroa R, Lipworth L, Kabagambe E, et al.: Nephrology provider prognostic perceptions and care delivered to older adults with advanced kidney disease. Clin J Am Soc Nephrol 12: 1762–1770, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javier AD, Figueroa R, Siew ED, Salat H, Morse J, Stewart TG, et al.: Reliability and utility of the surprise question in CKD stages 4 to 5. Am J Kidney Dis 70: 93–101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 28.The SUPPORT Principal Investigators : A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 274: 1591–1598, 1995 [PubMed] [Google Scholar]
- 29.Pearlman R, Starks H, Cain K, Cole W, Rosengren D, Patrick D: Your Life, Your Choices: Planning for Future Medical Decisions, Collingdale, PA, Diane Publishing, 2010 [Google Scholar]
- 30.Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, et al.: Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc 87: 817–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz S: Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31: 721–727, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM: Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179–186, 1969 [PubMed] [Google Scholar]
- 33.Hearn J, Higginson IJ: Development and validation of a core outcome measure for palliative care: The palliative care outcome scale. Palliative care core audit project advisory group. Qual Health Care 8: 219–227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agresti A: Categorical Data Analysis, Hoboken, NJ, John Wiley & Sons, Inc, 2002 [Google Scholar]
- 35.Goodman LA, Kruskal WH: Measures of association for cross classifications. J Am Stat Assoc 49: 737–764, 1954 [Google Scholar]
- 36.Goodman LA, Kruskal WH: Measures of association for cross classifications III: Approximate sampling theory. J Am Stat Assoc 58: 310–364, 1963 [Google Scholar]
- 37.R Foundation for Statistical Computing : R: A Language and Environment for Statistical Computing, 2013. Available at: http://www.r-project.org/. Accessed June 4, 2019
- 38.Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al.: Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300: 1665–1673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, et al.: Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med 169: 480–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Detering KM, Hancock AD, Reade MC, Silvester W: The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. BMJ 340: c1345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright AA, Keating NL, Ayanian JZ, Chrischilles EA, Kahn KL, Ritchie CS, et al.: Family perspectives on aggressive cancer care near the end of life. JAMA 315: 284–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV: Medical care inconsistent with patients’ treatment goals: Association with 1-year Medicare resource use and survival. J Am Geriatr Soc 50: 496–500, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force : Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage 40: 899–911, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC: Tools to promote shared decision making in serious illness: A systematic review. JAMA Intern Med 175: 1213–1221, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayton JM, Butow PN, Tattersall MH, Devine RJ, Simpson JM, Aggarwal G, et al.: Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 25: 715–723, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Pearlman RA, Starks H, Cain KC, Cole WG: Improvements in advance care planning in the Veterans Affairs System: Results of a multifaceted intervention. Arch Intern Med 165: 667–674, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Sudore RL, Landefeld CS, Barnes DE, Lindquist K, Williams BA, Brody R, et al.: An advance directive redesigned to meet the literacy level of most adults: A randomized trial. Patient Educ Couns 69: 165–195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler M, Ratner E, McCreedy E, Shippee N, Kane RL: Decision aids for advance care planning: An overview of the state of the science. Ann Intern Med 161: 408–418, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, et al.: Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: A randomized clinical trial. JAMA Intern Med 178: 930–940, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tulsky JA, Beach MC, Butow PN, Hickman SE, Mack JW, Morrison RS, et al.: A research agenda for communication between health care professionals and patients living with serious illness. JAMA Intern Med 177: 1361–1366, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Parvez S, Abdel-Kader K, Pankratz VS, Song MK, Unruh M: Provider knowledge, attitudes, and practices surrounding conservative management for patients with advanced CKD. Clin J Am Soc Nephrol 11: 812–820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al.: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fried TR, Bradley EH, Towle VR, Allore H: Understanding the treatment preferences of seriously ill patients. N Engl J Med 346: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Fried TR, Bradley EH, Towle VR: Assessment of patient preferences: Integrating treatments and outcomes. J Gerontol B Psychol Sci Soc Sci 57: S348–S354, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.