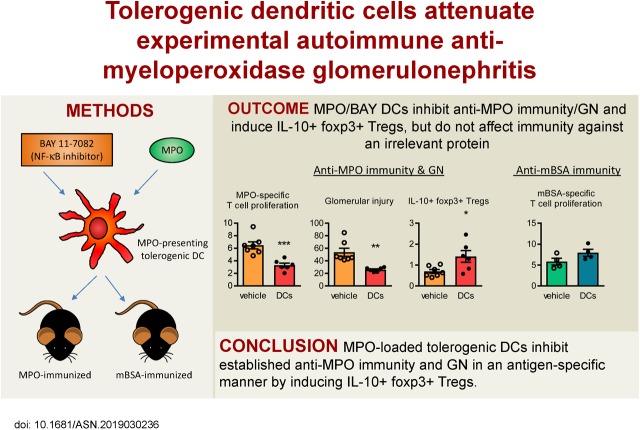
Significance Statement
Current treatments for autoimmune anti-myeloperoxidase (anti-MPO) GN are only partially effective and have many adverse effects, including broad immunosuppression. Thus, safer, more targeted therapies are needed, ideally ones that induce MPO-specific immunosuppression. Tolerogenic dendritic cells can deliver antigen-specific immunosuppression and have shown efficacy in various disease models. To investigate the therapeutic efficacy of this approach in a mouse model of anti-MPO GN, the authors generated MPO-loaded tolerogenic dendritic cells, induced by ex vivo treatment with an NFκB inhibitor. They demonstrated that these tolerogenic dendritic cells attenuated established anti-MPO autoimmunity and GN in mice in an antigen-specific manner, generating IL-10–expressing regulatory CD4+Foxp3+ T cells via inducible costimulator. These findings suggest that antigen-exposed tolerogenic dendritic cells may offer a novel antigen-specific therapeutic option for anti-MPO GN.
Keywords: ANCA, glomerulonephritis, immunosuppression, immunology and pathology
Visual Abstract
Abstract
Background Because of their capacity to induce antigen-specific immunosuppression, tolerogenic dendritic cells are a promising tool for treatment of autoimmune conditions, such as GN caused by autoimmunity against myeloperoxidase (MPO).
Methods
We sought to generate tolerogenic dendritic cells to suppress anti-MPO GN by culturing bone marrow cells with an NFκB inhibitor (BAY 11-7082) and exposing them to a pulse of MPO. After administering these MPO/BAY dendritic cells or saline to mice with established anti-MPO or anti–methylated BSA (mBSA) immunity, we assessed immune responses and GN. We also examined mechanisms of action of MPO/BAY dendritic cells.
Results
MPO/BAY dendritic cells decreased anti-MPO immunity and GN without inhibiting immune responses against mBSA; they also induced IL-10–producing regulatory T cells in MPO-immunized mice without affecting IL-10+ CD4+Foxp3− type 1 regulatory T cells or regulatory B cells. MPO/BAY dendritic cells did not inhibit anti-MPO immunity when CD4+Foxp3+ cells were depleted in vivo, showing that regulatory T cells are required for their effects. Coculture experiments with dendritic cells and CD4+Foxp3− or CD4+Foxp3+ cells showed that MPO/BAY dendritic cells generate Foxp3+ regulatory T cells from CD4+Foxp3− cells through several pathways, and induce IL-10+ regulatory T cells via inducible costimulator (ICOS), which was confirmed in vivo. Transfer of MPO/BAY dendritic cell–induced regulatory T cells in vivo, with or without anti–IL-10 receptor antibody, demonstrated that they suppress anti-MPO immunity and GN via IL-10.
Conclusions
MPO/BAY dendritic cells attenuate established anti-MPO autoimmunity and GN in an antigen-specific manner through ICOS-dependent induction of IL-10–expressing regulatory T cells. This suggests that autoantigen-loaded tolerogenic dendritic cells may represent a novel antigen-specific therapeutic option for anti-MPO GN.
In rapidly progressing crescentic-necrotizing GN, most patients present with inflammation and damage of glomeruli (i.e., vasculitis) caused by autoimmunity against neutrophil proteins, often myeloperoxidase (MPO).1 Evidence from patients with anti-MPO–associated vasculitis (MPO-AAV) and animal models indicates this disease is mediated by T cells and MPO-specific autoantibodies, known as anti-neutrophil cytoplasmic antibodies (ANCA) or MPO-ANCA.2–6
Much of our knowledge about the pathogenesis of anti-MPO GN has come from animal models. One model involves immunizing mice with MPO to generate MPO-specific autoimmunity, then injecting MPO-ANCA3 or low-dose anti–glomerular basement membrane (anti-GBM) globulin (Ig)7 to activate and lodge neutrophils in glomeruli, allowing deposition of MPO for subsequent recognition by infiltrating autoreactive T cells. Supported by evidence from patients with vasculitis, it has been shown that CD44,8 and CD8 T cells,8,9 and cytokines IL-17A10,11 and IFNγ12,13 are pathogenic in this model, whereas CD4+Foxp3+ regulatory T cells (Tregs) are protective.14–16
Current treatments for MPO-AAV are only partially effective, involve nonspecific immunosuppression, and have significant adverse effects. Induction therapy, consisting of high-dose corticosteroids and cyclophosphamide or a B cell–depleting antibody (Ab) (rituximab), induces remission in 70%–90% of patients.1,17,18 However, the incidence of dialysis or death at 5 years is approximately 30%, with the majority of deaths caused by side effects, mainly infections, often due to treatment-induced broad immunosuppression.1,17,19 Therefore, new, more specific therapies are desperately needed. Such therapies would ideally provide antigen-specific immunosuppression in that they would inhibit only MPO-specific autoimmunity, thus avoiding the complications of nonantigen-specific treatments such as cyclophosphamide and rituximab.
Dendritic cells (DCs) are critical antigen-presenting cells (APCs) in the development of adaptive immunity. Following activation, they upregulate MHC-II and various immune mediators including costimulatory molecules and cytokines to induce antigen-specific T cell responses to promote protective immunity against pathogens and cancers. However, through autoantigen presentation, activated DCs also play a central role in autoimmune diseases. In contrast, immature or semi-mature DCs are critical to the maintenance of peripheral tolerance by inducing T cell hyporesponsiveness.20,21
Several factors promote the tolerogenic phenotype of DCs including IL-10, TGFβ, vitamin D; downregulation of costimulatory molecules including CD80, CD86, and CD40; or inhibition of nuclear factor κB (NFκB), a major proinflammatory pathway within DCs.22–26 Antigen-exposed tolerogenic DCs, including those deficient in NFκB or treated with NFκB inhibitors such as BAY-11-7082 (BAY), have induced antigen-specific immunosuppression and attenuated organ damage in transplantation27 and experimental autoimmune diseases, including rheumatoid arthritis (RA), multiple sclerosis (MS), and type 1 diabetes.28–30 Tolerogenic DCs inhibit T cells by promoting anergy and apoptosis, and by inducing Tregs and regulatory B cells (Bregs),22,31,32 but the mechanism of immunosuppression is context dependent and varies between differently modified DCs which exhibit different phenotypes.
Tolerogenic DCs have entered the clinic, being tested in phase 1 trials in patients with autoimmune diseases—such as RA, type 1 diabetes, and Crohn disease—with promising results indicating they are biologically active and well tolerated, producing no side effects.33–35 Administration of BAY-modified DCs to RA patients showed that they were safe and induced immunoregulatory and anti-inflammatory effects.33
Successful antigen-specific immunosuppression leading to disease reversal may not be applicable to all autoimmune diseases, but is likely to be achievable in MPO-AAV for several reasons. Firstly, antigen-specific immunomodulation requires clarity regarding the nature of the disease-causing autoantigen and its immunodominant peptides. Some autoimmune diseases such as RA and type 1 diabetes feature reactivity to many different autoantigens with little knowledge of the dominant disease-causing antigen. In MPO-AAV, not only is autoreactivity usually restricted to MPO, but the immunodominant nephritogenic MPO peptide recognized by ANCA in patients presenting with acute MPO-AAV is known.36 In our murine model of this disease, we have defined the immunodominant MPO CD4+ T cell37 and CD8+ T cell9 epitopes, both of which are relevant to the human MHC (i.e., HLA). These epitopes have striking homology and overlap with the human peptide.36 This MPO epitope hot spot thus makes studies of antigen-specific immunomodulation in this model relevant to human disease. Secondly, in autoimmune diseases such as MS, intermolecular epitope spreading has been clearly documented and makes antigen-specific immunosuppression difficult, whereas there is no evidence of this occurring in MPO-AAV. Finally, in many autoimmune diseases, including type 1 diabetes and Hashimoto disease, patients typically present with advanced, irreversible end organ damage. In contrast, in most patients presenting with acute MPO-AAV, with effective treatment, renal function can be preserved or improved to levels sufficient to avoid transplantation or dialysis.
Through their capacity to induce antigen-specific immunosuppression, tolerogenic DCs represent a potentially more specific therapy for MPO-AAV. However, their therapeutic efficacy in anti-MPO GN has never been tested. In these preclinical studies, we sought to determine whether BAY-treated, MPO-loaded DCs can suppress established MPO-specific autoimmunity and consequently renal injury in an antigen-specific manner and define their mechanisms of action.
Methods
Mice
All mice used for experiments were 8- to 12-week-old males on a C57BL/6 background. Wild-type (WT) C57BL/6 mice were obtained from Monash University Animal Services (Melbourne, Australia). DREG mice expressing diphtheria toxin (DT) receptor/green fluorescent protein (GFP) under the Foxp3 promoter were originally provided by Dr. Tim Sparwasser (Centre for Experimental and Clinical Infection Research, TWINCORE, Hanover, Germany) and Dr. Katharina Lahl (Technical University of Munich, Munich, Germany).38 Foxp3-GFP mice were provided by Prof. Alexander Rudensky, University of Washington, Seattle, WA. All mice were bred, kept, and experimented on under specific-pathogen-free conditions at the Monash Medical Centre Animal Facility (Clayton, Australia). All experiments were approved by the Monash University Animal Ethics Committee and are summarized in Table 1.
Table 1.
Summary of in vivo experiments
| Experiment | Rationale | Groups (n) | Results |
|---|---|---|---|
| MPO/BAY DCs in mice with established anti-MPO immunity | To assess the effect of MPO/BAY DCs on anti-MPO immunity and GN | Saline (7) | Figures 2, 3, and 5, Supplemental Figure 2 |
| MPO/BAY DCs (6) | |||
| MPO/BAY DCs in mBSA-immunized mice | To test the effect of MPO/BAY DCs on immunity against an irrelevant antigen | Saline (4) | Figure 4 |
| MPO/BAY DCs (4) | |||
| MPO/DMSO DCs in MPO-immunized mice | To assess if control (MPO/DMSO) DCs are suppressive | Saline (6) | Supplemental Table 1 |
| MPO/DMSO DCs (6) | |||
| Treg depletion in DREG mice | To test if Tregs are required for MPO/BAY DC effects on anti-MPO immunity | Saline (4) | Figure 6, Supplemental Figure 3 |
| DT+saline (6) | |||
| DT+MPO/BAY DCs (6) | |||
| ICOS blockade | To determine if MPO/BAY DCs promote Tregs via ICOS | Rat IgG+saline (6) | Figure 7, C and D |
| Rat IgG+MPO/BAY DCs (6) | |||
| Anti-ICOS+saline (6) | |||
| Anti-ICOS+MPO/BAY DCs (6) | |||
| Treg transfer±anti–IL-10R Ab | To determine if MPO/BAY DC-induced Tregs suppress anti-MPO immunity/GN via IL-10 | Saline (6) | Figure 8, Supplemental Figure 5 |
| Tregs (6) | |||
| Saline+anti–IL-10R Ab (6) | |||
| Tregs+anti–IL-10R Ab (6) |
Generation and Phenotype of Tolerogenic DCs
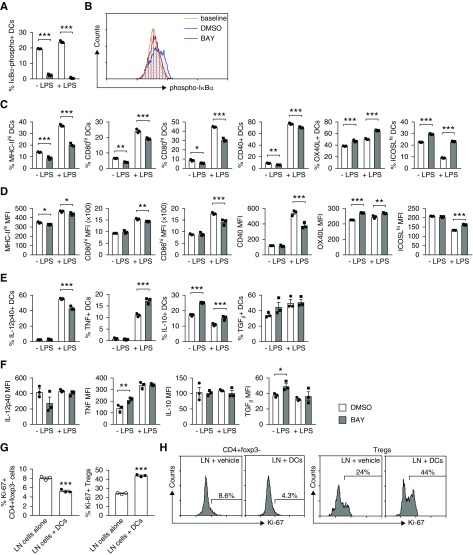
DCs were generated by culturing mouse bone marrow cells for 8 days in the presence of GM-CSF.39 BAY (5 μM; Sigma) or DMSO (Sigma) were present for the last 4 days of culture. DCs were enriched by phycoerythrin (PE) anti-CD11c Ab (HL3; BD Biosciences) followed by anti-PE microbeads (Miltenyi) to obtain >95% purity (CD11c+ cells), and then pulsed with heat-inactivated recombinant mouse MPO40 (50 μg/ml) for 1.5 hours. The phenotype of DMSO or BAY-treated DCs was assessed by flow cytometry after 24-hour culture in the presence or absence of LPS (1 μg/ml; Sigma) by measuring their surface expression of MHC-II, CD80, CD86, CD40, programmed death-ligand 1 (PD-L1), inducible costimulator (ICOS) ligand (ICOSL), and OX40L, as well as intracellular levels of IL-10, TGFβ, TNF, and IL-12p40, using these Abs: APC/Cy7 anti–MHC-II (M5/114), APC/Cy7 anti-CD86 (GL1), PE anti-OX40L (RM134L), Alexa488 anti–IL-10 (JES5-16E3), and PE anti-TGFβ/LAP (TW7-20B9) (all from Biolegend); FITC anti-CD40 (3/23), FITC anti-TNF (MP6-XT22), and PE anti–IL-12p40 (C15.6) (all from BD Biosciences); FITC anti-CD80 (16-10A1) (eBioscience); and protein G–purified and fluorochrome-conjugated (Molecular Probes, grown in-house) Alexa488 anti-PD-L1 (MIH5) and Alexa647 anti-ICOSL (HK5.3). For cytokine detection, brefeldin A (BFA, 5 μg/ml; Sigma) was present in the culture. Phosphorylated inhibitor of nuclear factor κBα (IκBα) was detected by intracellular staining using rabbit anti-mouse IκBα−phospho S36 Ab (EPR6235[2]; Abcam) followed by Alexa488 chicken anti-rabbit IgG (Thermo Fisher). To confirm the effect of BAY on IκBα-phospho levels, DMSO or BAY-treated DCs were unstimulated or stimulated with LPS (24 hours), washed, and lysed. Protein concentration in DC lysates was determined by the Bradford assay. Phosphorylated IκBα was measured by ELISA as per the manufacturer’s instructions (catalog number 85-86062-11; Invitrogen).
Induction of Anti-MPO GN
Mice were immunized subcutaneously (s.c.) with mouse MPO40 (20 μg) in CFA (inguinal regions; Sigma) on day 0 and boosted s.c. with MPO (10 μg) in Incomplete Freund Adjuvant (axillary areas; Sigma) on day 7. Control mice were injected with equal doses of methylated BSA (mBSA; Sigma). GN was triggered by an intravenous injection of low-dose sheep anti-mouse GBM Ig7 on day 21 and disease was assessed on day 26.
Administration of DCs, Anti-ICOS Ab, and DT
DCs (1×106 per mouse) or vehicle (saline) were injected s.c. (inguinal regions) 14 days after the initial MPO immunization. In some experiments, mice receiving saline or BAY DCs also received control rat IgG (Sigma) or blocking anti-ICOS Ab (7E.17G9; BioXcell) intraperitoneally (i.p.) every second day, starting 1 day before DC injection (i.e., from day 13 to 25; 0.2 mg/injection). DT (1.25 μg/mouse; Sigma) was injected i.p. for 3 consecutive days.
Treg Transfer and IL-10 Receptor–Neutralizing Experiments
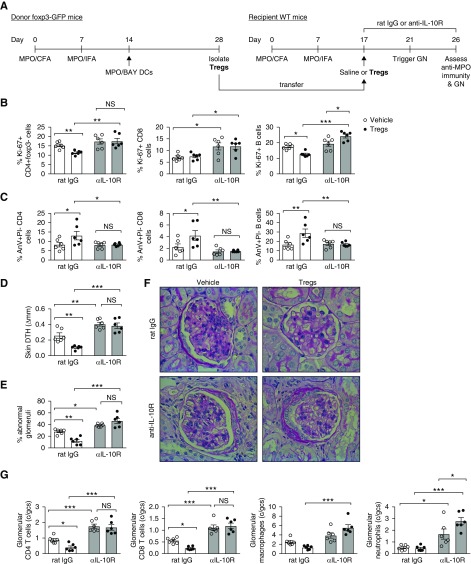
BAY-treated, MPO-pulsed (MPO/BAY) DCs were administered to Foxp3-GFP mice 14 days after MPO immunization. On day 28, Tregs were isolated from draining lymph nodes (LNs) and spleen by anti-CD4 microbeads (Miltenyi) followed by sorting of GFP+ cells to obtain 90% Treg purity. Saline or Tregs (5×105 per mouse) were then injected i.p. into WT mice which had been immunized with MPO 17 days earlier. The mice also received 1 mg of control rat IgG (Sigma) or blocking anti–IL-10 receptor (anti–IL-10R) Ab (1B1.3A; BioXCell) i.p. on day 17 (4 hours before Treg transfer) and day 24. GN was triggered on day 21, and disease/immunity was assessed on day 26.
T Cell and B Cell Responses
T cell and B cell responses were assessed in draining (inguinal, axillary) LNs ex vivo by flow cytometry. LN cells were restimulated with recombinant MPO (5 μg/ml) for 48 hours. For cytokines, BFA (5 μg/ml; Sigma) was present for the last 18 hours of culture. Proliferation of effector T cells (CD4+Foxp3−), Tregs (CD4+Foxp3+), CD8 T cells (CD8+), and B cells (CD19+) was determined by Ki-67 staining.41 Cytokines were detected by intracellular staining for IFNγ, IL-17A, IL-4, TGFβ, and IL-10.41 Activation and apoptosis were measured by CD44 and AnnexinV (AnV)/propidium iodide (PI) staining, respectively.42 T follicular helper (Tfh) cells were identified as CD4+CXCR5+PD-1+ cells.43 For Treg activation markers, cells were stained for surface ICOS, OX40, TNF receptor 2 (TNFR2), and PD-1, and intracellularly for Foxp3 and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), using these Abs: PE anti-OX40 (OX-86), PE or APC anti-Foxp3 (FJK-16s), and PE anti-ICOS (7E.17G9) (all from eBioscience); PE anti-TNFR2 (TR75-89), APC/Cy7 anti–PD-1 (29F.1A12), and APC anti-CTLA-4 (UC10-4B9) (all from Biolegend).
Circulating Ab Levels and Skin Delayed-Type Hypersensitivity
Titers of anti-MPO and anti-mBSA IgG and IgG subclasses (IgG1, IgG2b, IgG2c, and IgG3) were measured by ELISA44,45 in serum collected at the end of experiments. Total circulating IgE levels were determined by ELISA according to the manufacturer’s instructions (catalog number 432401; Biolegend). Sera from naive mice were used as a baseline. Skin delayed-type hypersensitivity (DTH) was measured as the difference in swelling between footpads injected 24 hours earlier with saline or immunizing antigens (MPO or mBSA; 10 μg).7
Renal Injury and Leukocyte Accumulation
The proportion of abnormal glomeruli was determined on formalin-fixed, paraffin-embedded kidney sections stained with periodic acid–Schiff, assessing at least 50 glomeruli per animal. Glomerular abnormalities included segmental necrosis, crescent formation (at least two cell layers in Bowman’s space), mesangial proliferation, and capillary wall thickening.43 Proteinuria was measured by the Bradford method42 on urine collected during the last 24 hours of the experiments. CD4 and CD8 T cells, macrophages, and neutrophils were assessed in at least 20 glomeruli per animal on periodate-lysine-paraformaldehyde–fixed frozen kidney sections by immunohistochemistry,9,42 using rat anti-mouse CD4 (GK1.5; BioXCell), rat anti-mouse CD8 (53-6.7; BioXCell), rat anti-mouse CD68 (FA/11; Invitrogen), and rat anti-mouse Gr-1 (RB6.8C5; BioXCell), respectively. Results are expressed as cells per glomerular cross section.
Phenotype of Renal Macrophages
Single cell suspensions from collagenase/DNase-digested kidneys were surface stained for CD45 and F4/80, followed by fixation/permeabilization (BD Biosciences) and intracellular staining for inducible nitric oxide synthase (iNOS) and mannose receptor (MR).41 To measure cytokine production, digested kidney cells were incubated for 18 hours with BFA (5 μg/ml), surface stained for CD45 and F4/80, fixed/permeabilized, and intracellularly stained for IL-10 and IL-12p40 using APC anti–IL-10 (JES5-16A3; eBiosciences) and PE anti–IL-12p40.
In Vitro DC/T Cell Coculture Experiments
LN cells (5×105) from WT MPO-immunized mice (day 14) were cultured in the presence of MPO (5 μg/ml) for 48 hours, with or without MPO/BAY DCs (2×104). CD4+Foxp3+ (Tregs) and CD4+Foxp3− cells were purified from LNs/spleen of MPO-immunized Foxp3-GFP mice (day 14) by anti-CD4 microbeads, followed by GFP+ and GFP− sorting.46 Tregs or CD4+Foxp3− cells (2×104) were cultured for 48 hours with or without MPO/BAY DCs (2×104) in the presence of control rat/mouse/hamster IgG (rat/mouse IgG, Sigma; hamster IgG, BioXCell) or blocking Abs against ICOS, CTLA4, TNFR2, CD80, CD86, OX40L, TGFβ, IL-10R, or CD40L (10 μg/ml each). The Abs used were: anti-TGFβ (1D11.16.8), anti-TNFR2 (TR75-54.7), anti–CTLA-4 (UC10-4710-11), anti–IL-10R (1B1.3A), and anti-ICOS (7E.17G9) (all from BioXCell); anti-CD40L (MR1), anti-OX40L (RM134L), anti-CD86 (GL1), and anti-CD80 (16-10A1) (all grown in-house and protein-G purified). LPS was added at 1 μg/ml to all cultures.
Statistical Analyses
The two-tailed unpaired t test was used to compare means between two groups. When comparing more than two groups, ANOVA followed by the Sidak or Dunnett multiple comparison test was used. Results are expressed as the mean±SEM. All statistical analyses were performed using GraphPad Prism (GraphPad software, San Diego, CA). Results were considered to be statistically significant if P<0.05.
Results
NFκB Inhibition Induces a Tolerogenic Phenotype in DCs
Tolerogenic DCs were generated by exposure to BAY which irreversibly inhibits NFκB by preventing phosphorylation of IκBα.47 The phenotype of BAY DCs was assessed after 24-hour culture with or without LPS, and compared with DMSO-treated cells. We confirmed that BAY blocked IκBα phosphorylation in DCs (Figure 1, A and B, Supplemental Figure 1A). By assessing the proportion of cells expressing various molecules and/or the level of expression of those molecules per cell (mean fluorescence intensity [MFI]), we observed that, basally, BAY DCs had less MHC-II, CD80, CD86, and CD40, but more OX40L, ICOSL, IL-10, TNF, and TGFβ (Figure 1, C–F). After LPS stimulation, BAY DCs had less MHC-II, CD80, CD86, CD40, and IL-12p40, but more OX40L, ICOSL, TNF, and IL-10 (Figure 1, C–F). BAY marginally decreased PD-L1 (Supplemental Figure 1, B and C).
Figure 1.
NFκB inhibition induces a tolerogenic phenotype in DCs. (A–F). DMSO or BAY-treated DCs were stimulated with or without LPS for 24 hours. DC phenotype and IκBα phosphorylation were assessed by flow cytometry. (A) The percentage of DCs containing phosphorylated IκBα. Results are presented as minus baseline. (B) Representative flow cytometry histograms showing phosphorylated IκBα in DMSO or BAY-treated DCs. Baseline, no anti–phospho-IκBα Ab. (C) The proportion of DCs expressing MHC-II, CD80, CD86, CD40, OX40L, and ICOSL. (D) The level of DC expression of MHC-II, CD80, CD86, CD40, OX40L, and ICOSL (MFI). (E) The proportion of DCs expressing IL-12p40, TNF, IL-10, and TGFβ. (F) The intensity of DC expression of IL-12p40, TNF, IL-10, and TGFβ (MFI). (G and H) LN cells from MPO-immunized mice were cultured with MPO and LPS, and with or without MPO/BAY DCs. (G and H) Proliferation of CD4+Foxp3− T effectors and Foxp3+ Tregs was determined by Ki-67 staining (flow cytometry). (H) Representative flow cytometry histograms showing CD4+Foxp3− and CD4+Foxp3+ (Treg) proliferation. Data are presented as scatter plots with the mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
To confirm that BAY DCs are overall inhibitory, we cocultured LN cells from MPO-immunized mice with MPO and with or without MPO/BAY DCs, in the presence of LPS. MPO/BAY DCs decreased proliferation of MPO-specific CD4+Foxp3− effector T cells, while increasing proliferation of Foxp3+ Tregs (Figure 1, G and H).
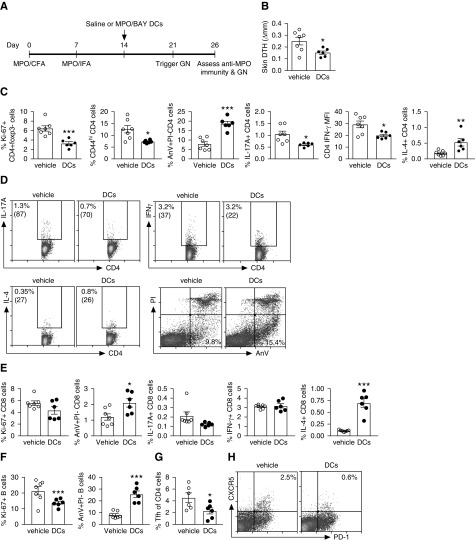
MPO/BAY DCs Decrease Established MPO-Specific Immunity
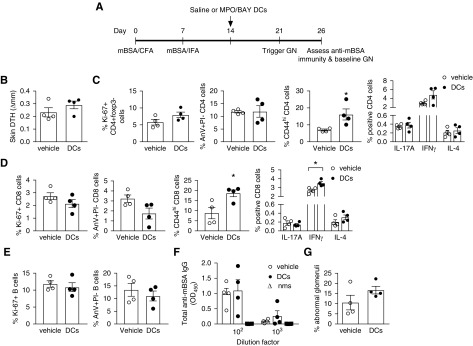
To test whether tolerogenic DCs can reduce established anti-MPO immunity and consequently GN, we administered MPO/BAY DCs or vehicle (saline) to mice 14 days after the first MPO immunization. GN was triggered with low-dose anti-GBM Ig on day 21 and experiments ended on day 26 (Figure 2A). MPO/BAY DCs decreased MPO-specific, T cell–driven skin DTH (Figure 2B). In the LNs, they inhibited MPO-specific proliferation of effector CD4 T cells, decreased CD4 activation (CD44), and enhanced CD4 apoptosis (Figure 2, C and D). The DCs also lowered the proportion of CD4 cells producing IL-17A and suppressed their expression of IFNγ (MFI), while increasing the percentage of IL-4+ CD4 cells (Figure 2, C and D). CD4 expression of IL-17A and IL-4 (MFI), and the proportion of IFNγ+ CD4 cells, was similar between saline and DC-treated mice (data not shown). They also increased CD8 T cell apoptosis and augmented the proportion of IL-4+ CD8 cells (Figure 2E). The DCs caused a trend toward reduced CD8 proliferation (Figure 2L; P=0.1) and the percentage of IL-17A+ CD8 cells (Figure 2E; P=0.07), whereas IFNγ (Figure 2E) and CD44 expression by CD8 cells was not affected (data not shown). They also decreased proliferation and increased apoptosis (Figure 2F) of B cells, in line with reduced Tfh cells (Figure 2, G and H) in the LNs. The DCs did not affect titers of anti-MPO IgG or IgG subclasses in serum (Supplemental Figure 2, A and B), but they increased total circulating levels of IgE (Supplemental Figure 2C).
Figure 2.
MPO/BAY DCs attenuate established anti-MPO immunity. (A) Experimental design. MPO/BAY DCs (n=6) or saline (n=7) were administered 14 days after the first MPO immunization, 7 days before triggering GN, and experiments ended on day 26. (B) Dermal DTH (change in skin swelling between MPO- and saline-injected footpads). (C–H) T cell and B cell responses, as assessed by flow cytometry using MPO-restimulated LN cells. (C) CD4 T cell proliferation, activation (CD44), and apoptosis (AnV+PI−); proportion of CD4 T cells producing IL-17A and IL-4, and the level of expression of IFNγ by CD4 T cells (MFI). (D) Representative flow cytometry plots showing IL-17A, IFNγ, and IL-4 production by CD4 T cells, and the proportion of apoptotic (AnV+PI−) CD4 T cells. Numbers in brackets indicate MFI. (E) CD8 T cell proliferation and apoptosis, and the proportion of CD8 cells producing IL-17A, IFNγ, or IL-4. (F) B cell proliferation and apoptosis. (G) The percentage of Tfh cells. (H) Representative flow cytometry plots showing the proportion of Tfh cells (CXCR5+PD-1+) of CD4 cells. Data are presented as scatter plots with the mean±SEM. *P<0.05, **P<0.01, ***P<0.001. CFA, Complete Freund Adjuvant; IFA, Incomplete Freund Adjuvant.
BAY-Treated, MPO-Exposed DCs Attenuate Anti-MPO GN
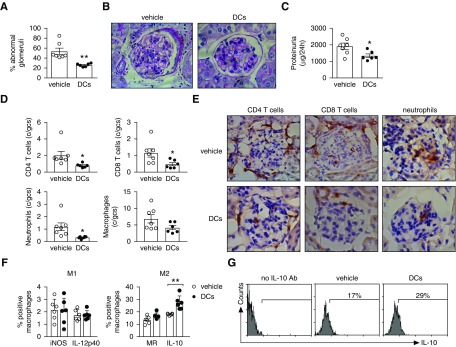
In line with reduced anti-MPO immunity, MPO/BAY DCs reduced glomerular injury (Figure 3, A and B) and proteinuria (Figure 3C). This was associated with fewer glomerular CD4 and CD8 T cells, and neutrophils (Figure 3, D and E). The DC-induced decrease in glomerular macrophages was not statistically significant (Figure 3D; P=0.1). However, the DCs caused a shift in the phenotype of renal macrophages toward an anti-inflammatory M2, indicated by an increased percentage of cells producing IL-10 (Figure 3, F and G) and a trend toward enhanced proportion of MR+ macrophages (P=0.06), whereas M1 markers (iNOS and IL-12p40) were not altered (Figure 3F).
Figure 3.
MPO/BAY DCs attenuate anti-MPO GN. MPO/BAY DCs (n=6) or saline (n=7) were administered to mice with established anti-MPO immunity (day 14) and GN assessed on day 26 (experimental design shown in Figure 2A). (A) The proportion of abnormal glomeruli. (B) Representative photomicrographs showing glomerular injury in mice treated with vehicle or BAY DCs. Formalin-fixed kidney sections were stained with periodic acid–Schiff (hematoxylin counterstain). Original magnification, ×400. (C) Proteinuria (in the last 24 hours). (D) Glomerular CD4 T cells, CD8 T cells, neutrophils, and macrophages. (E) Representative photomicrographs showing glomerular CD4 cells, CD8 cells, and neutrophils. Leukocytes (brown) were assessed by immunohistochemistry (hematoxylin counterstain). Original magnification, ×400. (F and G) Collagenase/DNase-digested kidneys were stained for macrophages (CD45+F4–80+) and M1 (iNOS, IL-12p40) and M2 markers (MR, IL-10), and analyzed by flow cytometry. (F) The proportion of renal macrophages expressing iNOS, IL-12p40, MR, or IL-10. (G) Representative flow cytometry plots showing the proportion of IL-10+ renal macrophages. Data are presented as scatter plots with the mean±SEM. *P<0.05, **P<0.01. c/gcs, cells per glomerular cross section.
BAY Treatment Is Required for the Tolerogenic Effects of DCs in Anti-MPO GN
To determine if DC exposure to BAY is required for their suppressive effects on anti-MPO GN, we administered control (DMSO)-treated, MPO-pulsed DCs or saline to MPO-immunized mice on day 14. Other than reducing CD4 and CD8 apoptosis and increasing the proportion of IL-4+ CD8 cells, these DCs did not alter renal injury or anti-MPO immunity in the LNs (Supplemental Table 1).
BAY-Treated, MPO-Loaded DCs Do Not Inhibit Immunity against an Irrelevant Antigen
To determine if MPO/BAY DCs suppress anti-MPO immunity in an antigen-specific manner, we administered saline or MPO/BAY DCs to mBSA-immunized mice (Figure 4A). MPO/BAY DCs did not suppress anti-mBSA CD4 (Figure 4, B and C), CD8 (Figure 4D), or B cell responses (Figure 4, E and F), but they increased CD4 (Figure 4C) and CD8 (Figure 4D) CD44 expression, and augmented the proportion of IFNγ+ CD8 cells (Figure 4D). The mild baseline histologic injury due to anti-GBM Ig injection was also unaffected (Figure 4G), indicating the therapeutic efficacy of MPO/BAY DCs in anti-MPO GN is not due to suppressing baseline injury caused by anti-GBM Ig.
Figure 4.
MPO/BAY DCs do not inhibit immunity against an irrelevant antigen. (A) Experimental design. MPO/BAY DCs (n=4) or saline (n=4) were administered to mice with established anti-mBSA immunity (day 14), 7 days before triggering GN, and experiments ended on day 26. (B) Dermal DTH (change in skin swelling between mBSA and saline-injected footpads). (C–E) T cell and B cell responses, assessed by flow cytometry using mBSA-restimulated LN cells. (C) CD4 T cell proliferation, apoptosis (AnV+PI−), and activation (CD44), and the proportion of CD4 T cells expressing IL-17A, IFNγ, or IL-4. (D) CD8 T cell proliferation, apoptosis, and CD44 expression, and the proportion of CD8 T cells producing IL-17A, IFNγ, or IL-4. (E) B cell proliferation and apoptosis. (F) Total serum anti-mBSA IgG levels (ELISA). (G) The proportion of abnormal glomeruli. Data are presented as scatter plots with the mean±SEM. *P<0.05. nms, normal mouse serum. CFA, Complete Freund Adjuvant; IFA, Incomplete Freund Adjuvant.
BAY-Modified, MPO-Exposed DCs Promote IL-10+ Tregs in Anti-MPO Vasculitis
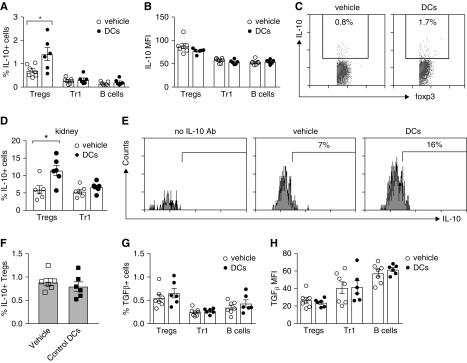
To explore the mechanisms by which MPO/BAY DCs suppress anti-MPO immunity, we analyzed how they affected regulatory cells in mice with anti-MPO GN, including Foxp3+ Tregs, type 1 Tregs (Tr1; IL-10/TGFβ+ CD4+Foxp3−) and Bregs. MPO/BAY DCs increased the proportion of IL-10+ Tregs, but not IL-10+ Tr1s or Bregs in the LNs (Figure 5, A and C). The level of IL-10 expression (MFI) was not altered by any of the regulatory cells (Figure 5B). The proportion of IL-10+ Tregs, but not Tr1s, was also elevated in the kidneys of mice receiving MPO/BAY DCs (Figure 5, D and E). Induction of IL-10+ Tregs required BAY treatment of DCs because DMSO-treated, MPO-loaded DCs did not increase IL-10+ Tregs in LNs (Figure 5F). MPO/BAY DCs did not alter TGFβ expression in any regulatory cell subset (Figure 5, G and H).
Figure 5.
MPO/BAY DCs enhance IL-10–producing Foxp3+ Tregs in anti-MPO GN. (A–E, G, and H) Saline (n=7) or MPO/BAY DCs (n=6) were administered to mice with established anti-MPO immunity (day 14), 7 days before GN trigger (experimental design shown in Figure 2A). IL-10 and TGFβ expression by CD4+Foxp3+ Tregs, Tr1s (CD4+Foxp3−), and B cells (CD19+) were assessed on day 26 by flow cytometry, using MPO-restimulated LN cells or digested kidneys. (A) The proportion of LN Tregs, Tr1s, and B cells producing IL-10 in mice receiving saline or MPO/BAY DCs. (B) The level of expression of IL-10 (MFI) by LN Tregs, Tr1s, and B cells in saline or MPO/BAY DC-treated mice. (C) Representative flow cytometry plots showing IL-10+ Tregs in LNs from mice receiving saline or MPO/BAY DCs. (D) The proportion of renal Tregs and Tr1 cells expressing IL-10 in saline or MPO/BAY DC-treated mice. (E) Representative flow cytometry plots showing IL-10+ Tregs in the kidneys of mice receiving saline or MPO/BAY DCs. (F) Saline (n=6) or control (DMSO-treated) MPO-pulsed DCs (n=6) were administered to MPO-immunized mice on day 14 instead of MPO/BAY DCs as above and the proportion of IL-10+ Tregs analyzed in LNs on day 26. (G) The proportion of TGFβ+ and (H) the level of expression of TGFβ (MFI) by Tregs, Tr1s, and B cells in mice receiving saline or MPO/BAY DCs. Data are presented as scatter plots with the mean±SEM. *P<0.05.
Tregs Are Required for MPO/BAY DC-Mediated Effects on Anti-MPO Immunity
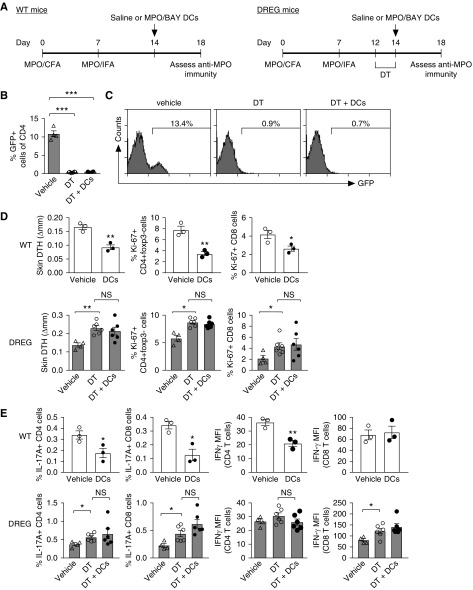
To test whether MPO/BAY DCs inhibit anti-MPO immunity via Tregs, we injected saline or MPO/BAY DCs into MPO-immunized WT or Treg-depleted DREG mice on day 14, and assessed MPO-specific responses on day 18 (Figure 6A). We confirmed Treg depletion in LNs of DT-injected DREG mice (Figure 6, B and C). The DCs decreased CD4 and CD8 immunity in WT mice (Figure 6, D and E). As before (Figure 2), they inhibited DTH, CD4, and CD8 proliferation (Figure 6D), the proportion of IL-17A+ CD4 and CD8 cells, and the level of CD4 IFNγ expression (MFI) (Figure 6E). In DREG mice, Treg depletion increased CD4 and CD8 responses (Figure 6, D and E, Supplemental Figure 3) and, in the absence of Tregs, the DCs did not inhibit anti-MPO immunity (Figure 6, D and E).
Figure 6.
Foxp3+ Tregs are required for MPO/BAY DC-mediated effects on anti-MPO immunity. (A) Experimental design. Saline or MPO/BAY DCs were administered to WT (saline, n=3; DCs, n=3) or DREG mice (expressing DT receptor and GFP on Foxp3+ cells) with established anti-MPO immunity (day 14). DREG mice also received DT (n=6 per group) on days 12–14 to deplete Tregs. Another group of MPO-immunized DREG mice were untreated (no DT or DCs; n=4). Anti-MPO immunity was assessed on day 18 using MPO-restimulated LN cells. (B) Efficacy of Treg depletion in DREG mice, shown by the proportion of GFP+ CD4 cells in LNs on day 18. (C) Representative flow cytometry plots showing GFP+ cells in DREG mice receiving saline, DT, or DT and DCs. (D) Dermal DTH (change in skin swelling between MPO- and saline-injected footpads) and proliferation of LN CD4 and CD8 cells. (E) The proportion of CD4 and CD8 cells producing IL-17A, and the level of IFNγ production (MFI) by CD4 and CD8 cells. Data are presented as scatter plots with the mean±SEM. *P<0.05, **P<0.01, ***P<0.001. CFA, Complete Freund Adjuvant; IFA, Incomplete Freund Adjuvant.
MPO/BAY DCs Induce IL-10+ Tregs via ICOS
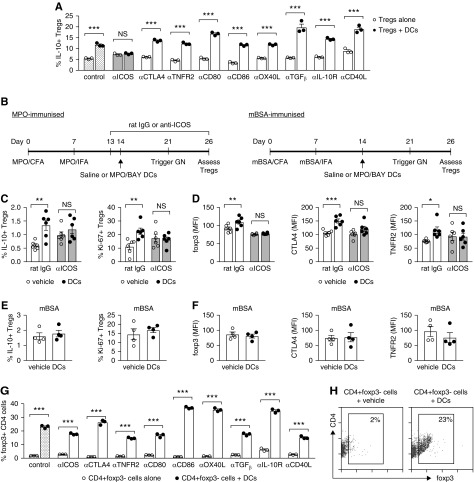
To define the pathways through which MPO/BAY DCs induce IL-10+ Tregs, we cocultured purified Tregs from MPO-immunized mice, with or without MPO/BAY DCs, in the presence of control or blocking Abs against various DC-derived mediators or their receptors. The proportion of IL-10+ Tregs was increased by the DCs in the presence of control IgG, and this effect was abrogated when ICOS was neutralized (Figure 7A). Induction of IL-10+ Tregs by MPO/BAY DCs was not prevented by blocking any of the other molecules (Figure 7A). When the same data are expressed as a fold increase in the proportion of IL-10+ Tregs by the DCs, it shows more clearly that other molecules including CD80, CD86, and TGFβ downregulate this process, because MPO/BAY DCs promoted IL-10+ Tregs to a greater extent when those proteins were blocked (Supplemental Figure 4A).
Figure 7.
MPO/BAY DCs promote IL-10+ Tregs and Treg activation via ICOS and induce Foxp3+ from CD4+Foxp3− cells. (A) Tregs (CD4+Foxp3+ cells), isolated from MPO-immunized Foxp3-GFP mice, were cocultured with or without MPO/BAY DCs, in the presence of LPS and control IgG or blocking Abs against various molecules. The proportion of IL-10+ Tregs was assessed by flow cytometry. (B) Experimental design for data presented in (C–F). MPO-immunized mice received MPO/BAY DCs (n=6) or saline (n=6) on day 14, and either control rat IgG (n=6) or a blocking anti-ICOS Ab (n=6) from day 13. Alternatively, saline (n=4) or MPO/BAY DCs (n=4) were given to mBSA-immunized mice. GN was triggered (day 21) and Tregs assessed on day 26 by flow cytometry, using MPO or mBSA-restimulated LN cells. (C) The proportion of IL-10+ Tregs and Treg proliferation, and (D) Treg expression of Foxp3, CTLA-4, and TNFR2, in MPO-immunized mice. (E) The proportion of IL-10+ Tregs and Treg proliferation, and (F) Treg expression of Foxp3, CTLA-4, and TNFR2 in mBSA-immunized mice. (G and H) CD4+Foxp3− cells, isolated from MPO-immunized Foxp3-GFP mice, were cocultured with or without MPO/BAY DCs, in the presence of LPS and control IgG or blocking Abs as in the DC/Treg cultures above, and induction of Foxp3+ cells measured by flow cytometry. (G) The proportion of CD4+Foxp3+ cells of all CD4. (H) Representative flow cytometry plots showing MPO/BAY DC-mediated induction of Foxp3+ from CD4+Foxp3− cells. Data are presented as scatter plots with the mean±SEM. *P<0.05, **P<0.01, ***P<0.001. CFA, Complete Freund Adjuvant; IFA, Incomplete Freund Adjuvant.
To confirm that MPO/BAY DCs induce IL-10+ Tregs via ICOS in vivo, we administered saline or MPO/BAY DCs to MPO-immunized mice on day 14, but the mice also received control IgG or a blocking anti-ICOS Ab from day 13 (Figure 7B). Confirming in vitro data, the DCs increased the proportion of IL-10+ Tregs in the LNs in the presence of rat IgG, but not when ICOS was neutralized (Figure 7C). MPO/BAY DCs also increased MPO-specific Treg proliferation, as well as Treg expression of Foxp3, CTLA4, and TNFR2 (markers associated with increased Treg-mediated suppression) in mice receiving rat IgG, but not anti-ICOS Ab (Figure 7D), without affecting Treg ICOS, OX40, or PD-1 (data not shown). These effects on Tregs were antigen specific, because MPO/BAY DCs did not affect Treg responses in mBSA-immunized mice (Figure 7, B, E, and F).
MPO/BAY DCs Promote the Generation of CD4+Foxp3+ Tregs from CD4+Foxp3− Cells
To test if MPO/BAY DCs can induce CD4+Foxp3+ Tregs from CD4+Foxp3− cells and examine how this occurs, we cocultured purified CD4+Foxp3− cells from MPO-immunized mice, with or without MPO/BAY DCs, in the presence of control or blocking Abs as in the DC/Treg cultures. MPO/BAY DCs generated Foxp3+ from Foxp3− cells in the presence of control IgG (Figure 7, G and H), which could not be abolished by blocking any of the molecules tested (Figure 7G). Expressing the same data as a DC-mediated fold increase in the proportion of Tregs shows more evidently that several molecules (ICOS, CD80, TGFβ, IL-10R, and CD40L) promote, whereas CD86/CTLA4 and OX40L inhibit, the induction of Tregs by MPO/BAY DCs (Supplemental Figure 4B). This is because DC-induced Treg generation was lower when ICOS, CD80, TGFβ, IL-10R, or CD40L were blocked; but higher when CD86, CTLA4, or OX40L were inhibited (Supplemental Figure 4B).
MPO/BAY DC-Induced Tregs Suppress Anti-MPO Immunity and Vasculitis via IL-10
To determine if MPO/BAY DC-induced Tregs inhibit anti-MPO GN via IL-10, we administered Tregs (isolated from MPO-immunized, BAY DC–treated Foxp3-GFP mice) or saline, and either control or blocking IL-10R Ab, to mice with established anti-MPO immunity (Figure 8A). Tregs decreased proliferation (Figure 8B) and increased apoptosis (Figure 8C) of LN CD4 cells and B cells in the absence but not in the presence of anti–IL-10R Ab. They did not affect IL-17A+ or IFNγ+ CD4 cells, but did increase IL-4+ CD4 T cells only in rat IgG–treated mice (Supplemental Figure 5). Tregs also reduced CD8 survival (Figure 8C), but not proliferation (Figure 8B), only in the absence of anti–IL-10R Ab. In line with these effects on anti-MPO immunity, Tregs attenuated MPO-specific skin DTH (Figure 8D), as well as glomerular injury (Figure 8, E and F) and leukocyte accumulation in glomeruli (Figure 8G), only when IL-10R signaling was intact.
Figure 8.
MPO/BAY DC-induced Foxp3+ Tregs suppress anti-MPO immunity and GN via IL-10. (A) Experimental design. Tregs were isolated from MPO-immunized BAY DC-treated Foxp3-GFP mice. Then, vehicle (saline; n=6 per group) or Tregs (n=6 per group), and either control rat IgG or a blocking IL-10R Ab, were given to mice with established anti-MPO immunity (day 17), 4 days before triggering GN. Disease and MPO-specific immunity (using MPO-restimulated LN cells; flow cytometry) were assessed on day 26. (B) Proliferation of CD4 T cells, CD8 T cells, and B cells. (C) CD4, CD8, and B cell apoptosis. (D) Dermal DTH (change in skin swelling between MPO- and saline-injected footpads). (E) The proportion of abnormal glomeruli. (F) Representative photomicrographs showing glomerular injury. Formalin-fixed kidney sections were stained with periodic acid–Schiff (hematoxylin counterstain). Original magnification, ×400. (G) Glomerular leukocytes. *P<0.05, **P<0.01, ***P<0.001. CFA, Complete Freund Adjuvant; c/gcs, cells per glomerular cross section; IFA, Incomplete Freund Adjuvant.
Discussion
Tolerogenic DCs are an appealing cellular tool for the treatment of autoimmune diseases due to their capacity to deliver antigen-specific immunosuppression. As such, they have shown therapeutic efficacy in various disease models and are being clinically tested in different autoimmune conditions. The current studies demonstrate for the first time that autoantigen-loaded tolerogenic DCs can suppress established autoimmunity and thus renal damage in experimental MPO-AAV.
In line with other studies using mouse and human DCs30,33 and NFκB promoting DC activation,48 BAY-mediated inhibition of NFκB induced an anti-inflammatory DC phenotype. This was evident from reduced expression of MHC-II and costimulatory molecules, including CD86 and CD40, and increased production of anti-inflammatory cytokines IL-10 and TGFβ. The net immunosuppressive effect of MPO/BAY DCs was verified by in vitro coculture with LN cells from MPO-immunized mice showing DC-mediated inhibition of MPO-specific effector T cell proliferation.
In vivo, MPO/BAY DCs suppressed established MPO-specific autoimmunity and consequently GN. They blunted responses of CD4 and CD8 T cells, both of which are pathogenic in anti-MPO GN.4,9 They had the greatest effect on CD4 cells, reducing their activation/proliferation/survival and causing a shift from Th1/Th17 to Th2 immunity. The DCs also decreased proliferation and survival of B cells and reduced Tfh cells which are known to promote B cell responses.49 Importantly, these effects of MPO/BAY DCs were antigen specific because they did not inhibit T or B cell immunity against an irrelevant antigen. Hence, autoantigen-loaded tolerogenic DCs may be a valuable novel therapy for MPO-AAV capable of inhibiting pathogenic autoimmunity, without altering protective immunity against pathogens, unlike the current immunosuppressants.
Increased Th2 immunity in mice receiving MPO/BAY DCs likely contributes to their immunosuppressive effects by turning off pathogenic Th1 and Th17 cells. However, Th2 responses are also well known to be allergy promoting and, indeed, MPO/BAY DCs elevated total serum IgE levels, suggesting that increased risk of allergic responses may be a side effect of this cellular therapy.
Decreased injury in BAY DC–treated mice was not due to reduction in MPO-ANCA because anti-MPO Ab titers were not affected and this model is ANCA independent.4 Thus, the translation of the results from this study to patients with AAV should be done cautiously because this disease is known to be mediated by both MPO-specific T cells and ANCA. However, reduced B cell expansion may have contributed to decreased anti-MPO immunity and GN in mice receiving MPO/BAY DCs because B cells also have Ab-independent roles in AAV (potentially as APCs) because patients receiving a B cell–depleting Ab reach remission before their ANCA levels drop.18
Tolerogenic DCs can inhibit immunity by promoting T cell anergy/apoptosis, and inducing regulatory cells including Foxp3+ Tregs, IL-10/TGFβ-producing CD4+Foxp3− Tr1s and Bregs.22,24,31,32 However, the mechanism of immunosuppression is context dependent and varies between different types of tolerogenic DCs. Here, MPO/BAY DCs specifically promoted Tregs, which are protective in anti-MPO GN,14–16 without affecting Tr1s or Bregs. The DCs enhanced MPO-specific Treg proliferation, and expression of Foxp3, CTLA4, and TNFR2, indicating increased Treg suppressive capacity,50–52 with the most marked effect on Tregs being the induction of IL-10+ cells.
Transfer of tolerogenic DCs to DREG mice in which Tregs were specifically depleted with DT confirmed that MPO/BAY DC–mediated inhibition of anti-MPO immunity is Treg dependent. MPO/BAY DCs failed to suppress both CD4 and CD8 T cell responses when Tregs were depleted, indicating they inhibit both T cell types via Tregs. This is consistent with other studies showing CD4+Foxp3+ Tregs can suppress CD4 and CD8 T cells.53 MPO/BAY DCs are not likely to directly turn off CD8 T cells via crosspresentation of MPO because they are CD8− and CD8− DCs, in contrast to CD8+ DCs, do not efficiently crosspresent antigens.54
Further mechanistic studies in which MPO/BAY DCs were cocultured with Tregs or CD4+Foxp3− cells from MPO-immunized mice showed that these DCs promote Treg responses by directly interacting with Tregs to induce IL-10+ cells and by generating Foxp3+ from Foxp3− cells. Blocking various DC-expressed mediators or their receptors on T cells showed that the induction of IL-10+ Tregs was completely dependent on DC ICOSL and Treg ICOS. This was confirmed in vivo and is supported by findings showing ICOS promotes IL-10+ Tregs.55 DC-mediated induction of Foxp3+ from Foxp3− cells also occurs in other systems.56 Moreover, our data show that although MPO/BAY DCs induce IL-10+ Tregs via ICOSL and generate Foxp3+ from Foxp3− cells through several pathways (including ICOS, IL-10R, CD40L, CD80, and TGFβ), both processes, interestingly, occur in a regulated fashion because the induction of IL-10+ Tregs was counter-regulated by CD80, CD86, and TGFβ, whereas the latter was suppressed by OX40L and CD86 which are known to block the generation of Tregs.57,58 These results indicate that MPO/BAY DCs interact with Tregs and CD4+Foxp3− cells via multiple molecules to augment Treg responses overall, but this occurs in a regulated manner, which may prevent overexuberant Treg-mediated immunosuppression potentially leading to increased risk of infections. They also suggest blocking Treg-inhibitory molecules on BAY DCs such as CD86, which suppressed both IL-10+ Tregs and the generation of CD4+Foxp3+ from CD4+Foxp3− cells, may further increase the immunosuppressive capacity of these DCs in vivo.
Consistent with the DCs having the greatest effect on the expansion of IL-10+ Tregs, Treg transfer/IL-10R blockade experiments showed BAY DC–induced Tregs suppress established MPO-specific CD4/CD8 and B cell immunity and thus GN via IL-10. Similarly, Tregs can inhibit other forms of GN via IL-10.59 MPO/BAY DCs enhanced the proportion of IL-10+ Tregs not only in the LNs, but also in the kidneys, suggesting these Tregs also migrate to the target organ where they likely inhibit the local anti-MPO autoimmune effector response.
Our IL-10R blockade and Treg depletion studies also provide new insights into the pathogenesis of anti-MPO GN. We show that endogenous IL-10R signaling is inhibitory in this disease and, although endogenous Tregs prevent the generation of anti-MPO responses,15 these studies demonstrate they also suppress established pathogenic MPO-specific autoimmunity, inhibiting both CD4 and CD8 T cells, as well as Th1 and Th17 immunity. This is important and particularly relevant to therapies aiming to augment endogenous immunosuppressive mediators such as Tregs to inhibit MPO-specific immunity which is established in patients presenting with MPO-AAV.
Our results are supported by reports in models of other autoimmune diseases (e.g., RA, MS) and transplantation, showing that NFκB-deficient or BAY-treated DCs reduced injurious immunity in an antigen-specific manner.27–30 Similar to our data, immunosuppression in those studies was associated with Treg expansion27,28 or was IL-10 dependent.24,30 BAY DCs also expanded Tregs in RA patients,33 suggesting similar pathways occur in humans. Our studies add novel insights into the mechanisms by which NFκB-inhibited DCs suppress pathogenic autoimmunity by showing that they generate Foxp3+ from Foxp3− cells through several pathways including ICOS, IL-10R, CD40L, CD80, and TGFβ, and induce IL-10+ Tregs specifically via ICOS/ICOSL.
MPO/BAY DCs are likely to persist in recipient mice for a few weeks, but their immunosuppressive effects are expected to be much longer lasting, as seen with other types of tolerogenic DCs in models of type 1 diabetes and allergic asthma.60–62 Administration of multiple doses of MPO/BAY DCs should induce stronger, longer-lasting effects on MPO-specific immunity and thus GN, and in case pathogenic autoimmunity returns, reapplication of these cells should turn it off again, as reported for other inhibitory DCs in models of allergy and RA.30,62
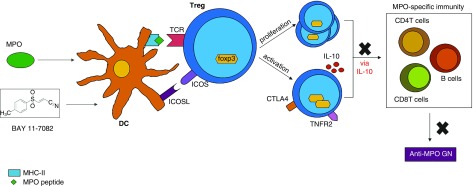
In summary, MPO-exposed, BAY-modified DCs suppress established MPO-specific immunity and thus GN in an antigen-specific manner by ICOS-dependent induction of IL-10–producing Tregs (Figure 9). Phase 1 trials in autoimmune conditions have shown tolerogenic DCs are safe and well tolerated, producing no major adverse effects.33–35 Therefore, MPO-loaded tolerogenic DCs are a promising new and likely more-targeted therapy for MPO-AAV which warrant further research to be developed into a clinically testable therapeutic.
Figure 9.
Summary of the pathway through which MPO/BAY DCs attenuate anti-MPO GN. DCs are (1) treated with BAY which inhibits NFκB and causes upregulation of ICOSL, and (2) exposed to MPO so they can take it up and present it to T cells. These DCs interact with MPO-specific CD4+Foxp3+ Tregs, and through the ICOSL/ICOS pathway, cause Treg proliferation and activation (upregulation of Foxp3, CTLA4, TNFR2, and IL-10). MPO/BAY DC-induced Tregs then suppress MPO-specific immunity, including CD4 and CD8 T cells, as well as B cells, via IL-10, to attenuate anti-MPO GN. TCR, T cell receptor.
Disclosures
Prof. Kitching reports grants from National Health and Medical Research Council of Australia, during the conduct of the study; grants from National Health and Medical Research Council of Australia, grants from Roche Australia, personal fees from CSL Limited, and personal fees from Ablynx, outside the submitted work. All of the remaining authors have nothing to disclose.
Funding
The funds for these studies were used from grants provided by the National Health and Medical Research Council of Australia.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Anh Cao (Monash University, Clayton, Victoria, Australia) for technical assistance, Dr. Tim Sparwasser (Centre for Experimental and Clinical Infection Research, TWINCORE, Hanover, Germany) and Dr. Katharina Lahl (Technical University of Munich, Munich, Germany) for providing DREG mice, and Prof. Alexander Rudensky (University of Washington, Seattle, WA) for Foxp3-GFP mice.
Dr. Odobasic designed the studies, performed experiments, collected and analyzed data, and wrote the manuscript; Ms. Oudin collected data and reviewed the paper; Dr. Gan performed experiments and reviewed the paper; Dr. Ito collected data and reviewed the manuscript; Prof. Kitching analyzed data and reviewed the manuscript; Prof. Holdsworth designed the studies, analyzed data, and reviewed the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019030236/-/DCSupplemental.
Supplemental Table 1. The effect of DMSO-treated MPO-pulsed DCs on anti-MPO immunity and GN.
Supplemental Figure 1. The effect of BAY on DC phospho-IκBα levels and expression of PD-L1.
Supplemental Figure 2. The effect of tolerogenic DCs on antibody levels.
Supplemental Figure 3. T cell cytokine production in wildtype or Treg-depleted DREG mice receiving vehicle or MPO/BAY DCs.
Supplemental Figure 4. The mechanisms by which MPO/BAY DCs regulate Treg responses.
Supplemental Figure 5. The effect of MPO/BAY DC-induced Tregs in the presence or absence of an IL-10R antibody on cytokine production by CD4 T cells.
References
- 1.Kallenberg CG: Key advances in the clinical approach to ANCA-associated vasculitis. Nat Rev Rheumatol 10: 484–493, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR: Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol 10: 499–506, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Gan PY, Holdsworth SR, Kitching AR, Ooi JD: Myeloperoxidase (MPO)-specific CD4+ T cells contribute to MPO-anti-neutrophil cytoplasmic antibody (ANCA) associated glomerulonephritis. Cell Immunol 282: 21–27, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, et al.: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al.: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper L, Radford D, Plant T, Drayson M, Adu D, Savage CO: IgG from myeloperoxidase-antineutrophil cytoplasmic antibody-positive patients stimulates greater activation of primed neutrophils than IgG from proteinase 3-antineutrophil cytosplasmic antibody-positive patients. Arthritis Rheum 44: 921–930, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Gan PY, Summers SA, Ooi JD, O’Sullivan KM, Tan DS, Muljadi RC, et al.: Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol 23: 1955–1966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Sullivan KM, Lo CY, Summers SA, Elgass KD, McMillan PJ, Longano A, et al.: Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 88: 1030–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Eggenhuizen P, O’Sullivan KM, Alikhan MA, Holdsworth SR, Ooi JD, et al.: CD8+ T cells effect glomerular injury in experimental anti-myeloperoxidase GN. J Am Soc Nephrol 28: 47–55, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, et al.: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira E, Hamour S, Sawant D, Henderson S, Mansfield N, Chavele KM, et al.: Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 25: 2209–2217, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Summers SA, Steinmetz OM, Gan PY, Ooi JD, Odobasic D, Kitching AR, et al.: Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum 63: 1124–1135, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M, Iwahori T, Nakabayashi I, Akashi M, Watanabe T, Yoshikawa N: In vitro production of myeloperoxidase anti-neutrophil cytoplasmic antibody and establishment of Th1-type T cell lines from peripheral blood lymphocytes of patients. Clin Exp Rheumatol 23: 227–230, 2005 [PubMed] [Google Scholar]
- 14.Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL, et al.: Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum 65: 1922–1933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan DS, Gan PY, O’Sullivan KM, Hammett MV, Summers SA, Ooi JD, et al.: Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J Am Soc Nephrol 24: 573–585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Borstel A, Sanders JS, Rutgers A, Stegeman CA, Heeringa P, Abdulahad WH: Cellular immune regulation in the pathogenesis of ANCA-associated vasculitides. Autoimmun Rev 17: 413–421, 2018 [DOI] [PubMed] [Google Scholar]
- 17.King C, Harper L: Avoidance of harm from treatment for ANCA-associated vasculitis. Curr Treatm Opt Rheumatol 3: 230–243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al.: European Vasculitis Society (EUVAS) : Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-Year results of a randomised trial. Ann Rheum Dis 74: 1178–1182, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al.: European Vasculitis Study Group : Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 70: 488–494, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa H, Matsumoto T: Mechanisms of tolerance induction by dendritic cells in vivo. Front Immunol 9: 350, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al.: Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med 206: 549–559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engman C, Garciafigueroa Y, Phillips BE, Trucco M, Giannoukakis N: Co-stimulation-impaired bone marrow-derived dendritic cells prevent dextran sodium sulfate-induced colitis in mice. Front Immunol 9: 894, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, et al.: 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol 192: 4210–4220, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Martin E, O’Sullivan B, Low P, Thomas R: Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity 18: 155–167, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Tai N, Yasuda H, Xiang Y, Zhang L, Rodriguez-Pinto D, Yokono K, et al.: IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin Immunol 139: 336–349, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Thomas DC, Wong FS, Zaccone P, Green EA, Wållberg M: Protection of islet grafts through transforming growth factor-β-induced tolerogenic dendritic cells. Diabetes 62: 3132–3142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Ge W, et al.: Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol 178: 5480–5487, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Iruretagoyena MI, Sepúlveda SE, Lezana JP, Hermoso M, Bronfman M, Gutiérrez MA, et al.: Inhibition of nuclear factor-kappa B enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther 318: 59–67, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Qian S, Liang X, Wang L, Woodward JE, Giannoukakis N, et al.: Prevention of diabetes in NOD mice by administration of dendritic cells deficient in nuclear transcription factor-kappaB activity. Diabetes 52: 1976–1985, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Martin E, Capini C, Duggan E, Lutzky VP, Stumbles P, Pettit AR, et al.: Antigen-specific suppression of established arthritis in mice by dendritic cells deficient in NF-kappaB. Arthritis Rheum 56: 2255–2266, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Qian C, Qian L, Yu Y, An H, Guo Z, Han Y, et al.: Fas signal promotes the immunosuppressive function of regulatory dendritic cells via the ERK/β-catenin pathway. J Biol Chem 288: 27825–27835, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH: Induction of tolerance by IL-10-treated dendritic cells. J Immunol 159: 4772–4780, 1997 [PubMed] [Google Scholar]
- 33.Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, et al.: Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med 7: 290ra87, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M: Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 34: 2026–2032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jauregui-Amezaga A, Cabezón R, Ramírez-Morros A, España C, Rimola J, Bru C, et al.: Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: A phase I study. J Crohn’s Colitis 9: 1071–1078, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, et al.: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, et al.: The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 109: E2615–E2624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al.: Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 204: 57–63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odobasic D, Kitching AR, Yang Y, O’Sullivan KM, Muljadi RC, Edgtton KL, et al.: Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood 121: 4195–4204, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR: The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods 308: 167–178, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Odobasic D, Ruth AJ, Oudin V, Kitching AR, Holdsworth SR: OX40 ligand is inhibitory during the effector phase of crescentic glomerulonephritis. Nephrol Dial Transplant 34: 429–441, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR: Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 18: 760–770, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Dick J, Gan PY, Kitching AR, Holdsworth SR: The C3aR promotes macrophage infiltration and regulates ANCA production but does not affect glomerular injury in experimental anti-myeloperoxidase glomerulonephritis. PLoS One 13: e0190655, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ooi JD, Gan PY, Chen T, Eggenhuizen PJ, Chang J, Alikhan MA, et al.: FcγRIIB regulates T-cell autoreactivity, ANCA production, and neutrophil activation to suppress anti-myeloperoxidase glomerulonephritis. Kidney Int 86: 1140–1149, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Odobasic D, Jia Y, Kao W, Fan H, Wei X, Gu R, et al.: Formyl peptide receptor activation inhibits the expansion of effector T cells and synovial fibroblasts and attenuates joint injury in models of rheumatoid arthritis. Int Immunopharmacol 61: 140–149, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Gan PY, Tan DS, Ooi JD, Alikhan MA, Kitching AR, Holdsworth SR: Myeloperoxidase peptide-based nasal tolerance in experimental ANCA-associated GN. J Am Soc Nephrol 27: 385–391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al.: Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272: 21096–21103, 1997 [DOI] [PubMed] [Google Scholar]
- 48.O’Sullivan BJ, Thomas R: CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J Immunol 168: 5491–5498, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Stebegg M, Kumar SD, Silva-Cayetano A, Fonseca VR, Linterman MA, Graca L: Regulation of the germinal center response. Front Immunol 9: 2469, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Bäumel M, Männel DN, Howard OM, Oppenheim JJ: Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 179: 154–161, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Read S, Malmström V, Powrie F: Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192: 295–302, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan YY, Flavell RA: Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445: 766–770, 2007 [DOI] [PubMed] [Google Scholar]
- 53.von Boehmer H: Mechanisms of suppression by suppressor T cells. Nat Immunol 6: 338–344, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, et al.: The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A 103: 10729–10734, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al.: Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 204: 105–115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Dawicki W, Zhang X, Town J, Gordon JR: Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol 185: 5003–5010, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C: Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) -adaptive regulatory T cell generation. J Immunol 180: 6566–6576, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, et al.: OX40 costimulation turns off Foxp3+ Tregs. Blood 110: 2501–2510, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ostmann A, Paust HJ, Panzer U, Wegscheid C, Kapffer S, Huber S, et al.: Regulatory T cell-derived IL-10 ameliorates crescentic GN. J Am Soc Nephrol 24: 930–942, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo J, Xia CQ, Peng R, Clare-Salzler MJ: Immature dendritic cell therapy confers durable immune modulation in an antigen-dependent and antigen-independent manner in nonobese diabetic mice. J Immunol Res 2018: 5463879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N: Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol 173: 4331–4341, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR: Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: Differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J Immunol 189: 72–79, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.