Significance Statement
Necroptosis has emerged as an important cell death pathway that contributes to inflammation and injury of many organs, including the kidney. The mechanisms underlying necroptosis are not well understood. The authors have identified a previously unrecognized important role of integrin-linked kinase (ILK) in mediating necroptosis in collecting duct epithelial cell using genetically engineered mice to lack Ilk in the collecting duct principal cells of the kidney. These Ilk-knockout mice develop acute tubular injury, interstitial fibrosis and inflammation in the kidneys. Treating both the ILK inhibited cultured cells and ILK-deficient mice with a necroptosis inhibitor, necrostatin-1, reduced the harmful effects associated with the loss of ILK. The study shows that ILK plays an important role in regulating necroptosis in kidney tubular cells.
Keywords: necroptosis, integrin-linked kinase (ILK), collecting duct principal cells
Visual Abstract
Abstract
Background
Necroptosis is a newly discovered cell death pathway that plays a critical role in AKI. The involvement of integrin-linked kinase (ILK) in necroptosis has not been studied.
Methods
We performed experiments in mice with an Ilk deletion in collecting duct (CD) principal cells (PCs), and cultured tubular epithelial cells treated with an ILK inhibitor or ILK siRNA knockdown.
Results
Ilk deletion in CD PCs resulted in acute tubular injury and early mortality in mice. Progressive interstitial fibrosis and inflammation associated with the activation of the canonical TGF-β signaling cascade were detected in the kidneys of the mice lacking ILK in the CD PCs. In contrast to the minimal apoptosis detected in the animals’ injured CDs, widespread necroptosis was present in ILK-deficient PCs, characterized by cell swelling, deformed mitochondria, and rupture of plasma membrane. In addition, ILK deficiency resulted in increased expression and activation of necroptotic proteins MLKL and RIPK3, and membrane translocation of MLKL in CD PCs. ILK inhibition and siRNA knockdown reduced cell survival in cultured tubular cells, concomitant with increased membrane accumulation of MLKL and/or phospho-MLKL. Administration of a necroptosis inhibitor, necrostatin-1, blocked cell death in vitro and significantly attenuated inflammation, interstitial fibrosis, and renal failure in ILK-deficient mice.
Conclusions
The study demonstrates the critical involvement of ILK in necroptosis through modulation of the RIPK3 and MLKL pathway and highlights the contribution of CD PC injury to the development of inflammation and interstitial fibrosis of the kidney.
CKD affects approximately 10% of the world’s population and places a significant burden on economy and health care worldwide.1,2 Effective therapies to prevent or halt CKD progression are lacking, largely due to limited understanding of the pathologic and molecular basis of the disease. It is increasingly recognized that AKI is a major contributor to the development of CKD.3 Therefore, it is critical to understand the injury and recovery mechanisms of renal tubular cells and their roles in mediating inflammatory response and fibrosis in the kidney.
There are two main types of cell death that occur during kidney tubular injury: apoptosis and necrosis. Apoptotic cells are frequently detected in the renal proximal tubule, distal tubule, and loop of Henle in various AKI models.3 Tubular cell apoptosis was once considered to be a key form of cell death leading to CKD.4 However, this point of view was recently challenged because inhibition of apoptosis, e.g., by caspase inhibitors, failed to alleviate renal injury.5 Therefore, an alternative pathology has to be sought. In recent years, breakthrough studies show that necrosis can occur in a highly regulated manner through complex molecular interactions, and it plays a critical role in mediating tissue injury and interstitial fibrosis.6,7
Necroptosis is the most-characterized pathway of regulated necrosis in higher eukaryotic cells.6 Necroptosis is activated via the formation of the necrosome, which includes receptor interacting protein kinase 1 (RIPK1), RIPK3, and mixed lineage kinase domain-like pseudokinase (MLKL). The homotypic interaction of RIPK3 and RIPK1 drives RIPK3 phosphorylation, which in turn recruits and phosphorylates MLKL.8 Phosphorylated MLKL oligomerizes and translocates to the plasma membrane, eventually resulting in membrane rupture.9 When cells undergo necroptosis, they present with organelle and cell swelling, permeabilization of the plasma membrane, and spilling of intracellular contents.10 Recent studies indicate necroptosis is an important player in some high-effect diseases, such as myocardial ischemia and reperfusion injury (IRI), sepsis, and intestinal inflammation.11–13 Furthermore, it has been shown to contribute to the pathogenesis of renal IRI and AKIs induced by nephrotoxic agents such as cisplatin and iodinated contrast.14–16 The involvement of many signaling pathways that are critically associated with cell adhesion and survival, such as integrin and its downstream integrin-linked kinase (ILK), has not yet been studied in necroptosis.
ILK is a critical scaffold protein located in focal adhesions. Through interacting with the cytoplasmic domain of β-integrins, ILK transduces integrin signaling to the interior of the cell and mediates diverse cellular processes, including cell survival, proliferation, adhesion, differentiation, and migration.17 In vivo studies have suggested that ILK plays a critical role in developing and maintaining the structure and function of many organs, including the kidney.18–20 For example, inactivation of ILK in mouse kidney podocytes leads to podocyte damage, progressive proteinuria, glomerulosclerosis, and severe tubulointerstitial fibrosis.21–23 Aberrant expression of ILK is associated with renal tubulointerstitial fibrosis, renal cell carcinoma, diabetic glomerulopathy, and congenital nephrotic syndrome.24–28 To further investigate the potential function of ILK in collecting duct (CD) principal cells (PCs), we generated knockout (KO) mice with Ilk deletion in PCs. We found that deletion of ILK in PCs led to profound tubular injury and renal failure, unexpectedly without significant apoptosis of tubular cells. Further investigation demonstrated that deleting ILK in PCs induces significant necroptosis of CD PCs instead. ILK deficiency in PCs activates necroptotic signaling and promotes inflammation and interstitial fibrosis in the kidney. More importantly, blocking necroptosis using necrostatin-1 (Nec-1), a chemical inhibitor of RIPK1, attenuates CD injury and fibrosis in Ilk KO mice. Therefore, our data support a previously unrecognized, important function of ILK in mediating necroptosis in CD epithelial cells.
Methods
See Supplemental Methods for a detailed description.
Experimental Animals
All animal experiments were approved by the Massachusetts General Hospital (MGH) Subcommittee on Research Animal Care, in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All mice were on the C57BL/6J background. B6N;129-Ilktm1Star/J mice carrying loxP sites have been previously described and are available from the Jackson laboratory (stock number 023310).29 Transgenic mice harboring aquaporin 2 (Aqp2) Cre recombinase (Aqp2-Cre+) were generated by Dr. Günther Schütz’s group (Heidelberg, Germany)30 and obtained from Dr. Wenzheng Zhang (Albany, NY).31 Homozygous, floxed Ilk mice (Ilk fl/fl) were crossed with Aqp2-Cre+ mice to generate PC Ilk KO mice (Ilk fl/fl; Aqp2-Cre+). The littermates without Aqp2-Cre (Ilk fl/fl; Aqp2-Cre−) were used as control.
For the Nec-1 (Sigma-Aldrich, St. Louis, MO) treatment experiment, 2-week-old wild-type and Ilk fl/fl; Aqp2-Cre+ mice were given 1.65 mg/kg Nec-1 or PBS containing 1% DMSO through intraperitoneal injection every 2 days for 2 weeks. Mice were euthanized at the age of 4 weeks.
Kidney Tissue Preparation
Kidney tissues were collected as previously described.32 Briefly, mice were anesthetized using isoflurane (3% inhalant). The right kidney was snap frozen in liquid nitrogen and stored at −80°C for protein and RNA analysis. The left kidney was fixed by perfusion through the left ventricle with paraformaldehyde-lysine-periodate fixative. After postfixation and extensive washing with PBS, kidneys were embedded in optimal cutting temperature compound or paraffin, and cut into 4-μm (cryostat) or 3-μm (paraffin) sections.
Measurement of Serum Creatinine and BUN
Serum creatinine and BUN content were measured using the QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA) and Stanbio Urea Nitrogen Kit (Stanbio Laboratory, Boerne, TX), respectively, following the manufacturers’ instructions. Approximately 60 μl of serum was used for each measurement.
Immunofluorescence Staining and Immunoblotting
Paraffin-embedded kidney sections were deparaffinized. Cryostat kidney sections were rehydrated in PBS. Routine immunostaining was performed as previously described.33 Briefly, sections were blocked with 1% BSA and incubated with primary antibodies of various dilutions from 1:100 to 1:3000 (Supplemental Table 1) or corresponding normal IgG of 1:100 dilution, followed by the fluorophore-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Slides were viewed under a Nikon Eclipse 90i epifluorescence (Nikon Instruments; Melville, NY) or a Zeiss LSM800 confocal microscope (Carl Zeiss Microscopy; Thornwood, NY). Analysis of fluorescence intensity was performed using ImageJ software. Immunofluorescence staining of kidney with MLKL, CD68, and lymphocyte antigen 6 complex locus G (Ly6G) was performed on cryosectioned slides. The rest of the immunostaining was performed on paraffin sections. Quantification of the number of F4/80- or Ly6G-positive cells was performed manually in ten randomly selected fields (magnification, 400×) for each specimen. Data are presented as the number of F4/80- or Ly6G-positive cells per high power field with a magnification of 400×.
Immunofluorescence staining of cultured cells was performed as previously described.34 Briefly, cells grown on glass coverslips were fixed with 4% paraformaldehyde for 20 minutes, and then permeabilized with 0.01% Triton X-100 for 4 minutes. After blocking with 1% BSA, they were subjected to routine immunostaining as mentioned above.
Immunoblotting was performed as previously described.34 Briefly, homogenized kidney tissues or cell lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (MilliporeSigma, Burlington, MA). Membranes were incubated with primary antibodies (Supplemental Table 1) followed by horseradish peroxidase–conjugated secondary antibodies (Santa Cruz, Dallas, TX), and then developed using the ECL Pro Kit (PerkinElmer, Waltham, MA). Relative intensity of protein expression was quantified by OD using the Quantity One software (Bio-Rad).
Hematoxylin and Eosin Staining
Hematoxylin and eosin (H&E) staining was conducted using kidney paraffin sections as previously described.35 The tubular injury score was calculated based on the percentage of tubules in kidney sections that displayed tubular damage, including tubular dilation or atrophy, tubular necrosis, loss of the brush border, or cast formation.36 The degree of injury was graded semiquantitatively and blindly by two independent researchers from ten randomly chosen fields of each kidney section, according to the extent of injury involved in each field as follows: 0, normal; 1, <10%; 2, 11%–25%; 3, 26%–75%; 4, >75% of the observed tubules.
Picrosirius Red Staining and Masson Trichrome Staining
Paraffin-embedded kidney sections were stained using the Picrosirius Red Stain Kit (Polysciences, Warrington, PA) or Trichrome Stain Kit (Masson; Sigma-Aldrich) following the manufacturers’ instructions, and examined by a Zeiss LSM800 confocal microscope. Using picrosirius red staining, type 1 collagen fibers stain yellow to orange and type 3 collagen fibers stain green under polarized light. Using Masson trichrome staining, collagen fibers usually stain blue, cytoplasm stains pink, and nuclei stain dark brown.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) was performed as previously described without modification.37 Briefly, paraformaldehyde-lysine-periodate–fixed kidney slices were postfixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C for 24 hours. The slices were incubated with 1% osmium tetroxide in cacodylate buffer at room temperature for 1 hour, followed by several rinses in 0.1 M sodium cacodylate buffer. After dehydration in a graded ethanol series from 50% to 100%, followed by brief dehydration in propylene oxide, kidney slices were infiltrated with Eponate resin (Ted Pella, Redding, CA) and embedded in fresh Eponate at 60°C. Ultra-thin sections were cut, stained with uranyl acetate and lead citrate, and examined under a JEOL JEM 1011 transmission electron microscope (JEOL, Peabody, MA). Images were taken using an AMT digital imaging system (Advanced Microscopy Techniques, Danvers, MA).
RNA Isolation and Quantitative Real-Time PCR
Mouse kidney RNA was extracted using QIAshredder and RNeasy purification kits (Qiagen, Valencia, CA). The synthesis of cDNA from mRNA was performed following the protocol of the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was carried out using a QuantStudio 3 Real-Time PCR system and the PowerUp SYBR Green PCR Master Mix (Life Technology, Carlsbad, CA). The level of specific mRNA was normalized to β-actin gene expression. The relative mRNA expression was determined by the 2−∆∆Ct method. The sequences of primers are summarized in Supplemental Table 2.
Cell Culture and Cell Viability Assays
LLC-PK1 cells and mCCDC11 cells were cultured in DMEM containing 10% FBS in a 5% carbon dioxide/95% air-humidified atmosphere at 37°C. Cells were trypsinized in 0.25% trypsin-EDTA, and plated on dishes. Cells were allowed to grow for 24 hours, 0.5, 1, and 2 μM of the ILK inhibitor cpd22 (407331; Calbiochem, San Diego, CA) was added, and then incubated with cells for 24 hours. Control cells were treated with 0.1% DMSO. After treatment for 24 hours, cells were harvested, lysed in lysis buffer, and prepared for electrophoresis (SDS-PAGE) and immunoblotting. All experiments were repeated at least three times.
The LLC-PK1 were seeded on 96-well plates and grown to approximately 80% confluence. Cells were then treated with 0.2% DMSO or ILK inhibitor cpd22 in the presence or absence of the necroptotic inhibitor Nec-1 (50 μM) for 24 hours. Both the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Affymetrix, Santa Clara, CA) assay and DNA quantification assay (Invitrogen) were applied to assess cell viability by measuring OD values or fluorescence intensity, respectively. Relative cell viability as the percentage of treated group over control group was calculated as OD treat/OD control×100% for the MTT assay, and fluorescencetreat/fluorescencecontrol×100% for the DNA quantification assay.
Small interfering RNA Transfection
ILK small interfering RNA (siRNA) (sense sequence, AAGGACACAUUCUGGAAGGGG; antisense sequence, CCUUCCAGAAUGUGUCCUUGG) was purchased from GE Dharmacon (Lafayette, CO). mCCDC11 cells were seeded in six-well plates and transfected with ILK siRNA (50 nM) or scrambled siRNA mixed with 5 μl Lipofectamine 2000 (Invitrogen) in 1 ml DMEM for 6 hours. At 24 hours after transfection, some control cells (transfected with or without scrambled siRNA) were incubated with 2 μM cpd22, and 1 ng/ml recombinant TNF-α (300-01A; PeproTech), respectively. The cell viability assay and immunofluorescence staining were performed 48 hours after ILK siRNA transfection.
Statistical Analyses
Data are shown as mean±SEM of independent replicates (n≥3). Experimental data were analyzed using the t test for two groups or with one-way ANOVA for multiple groups, using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA). A P value <0.05 was considered statistically significant.
Results
Renal Failure and Kidney Tubular Injury Occur in Mice with Ilk Deletion in PCs
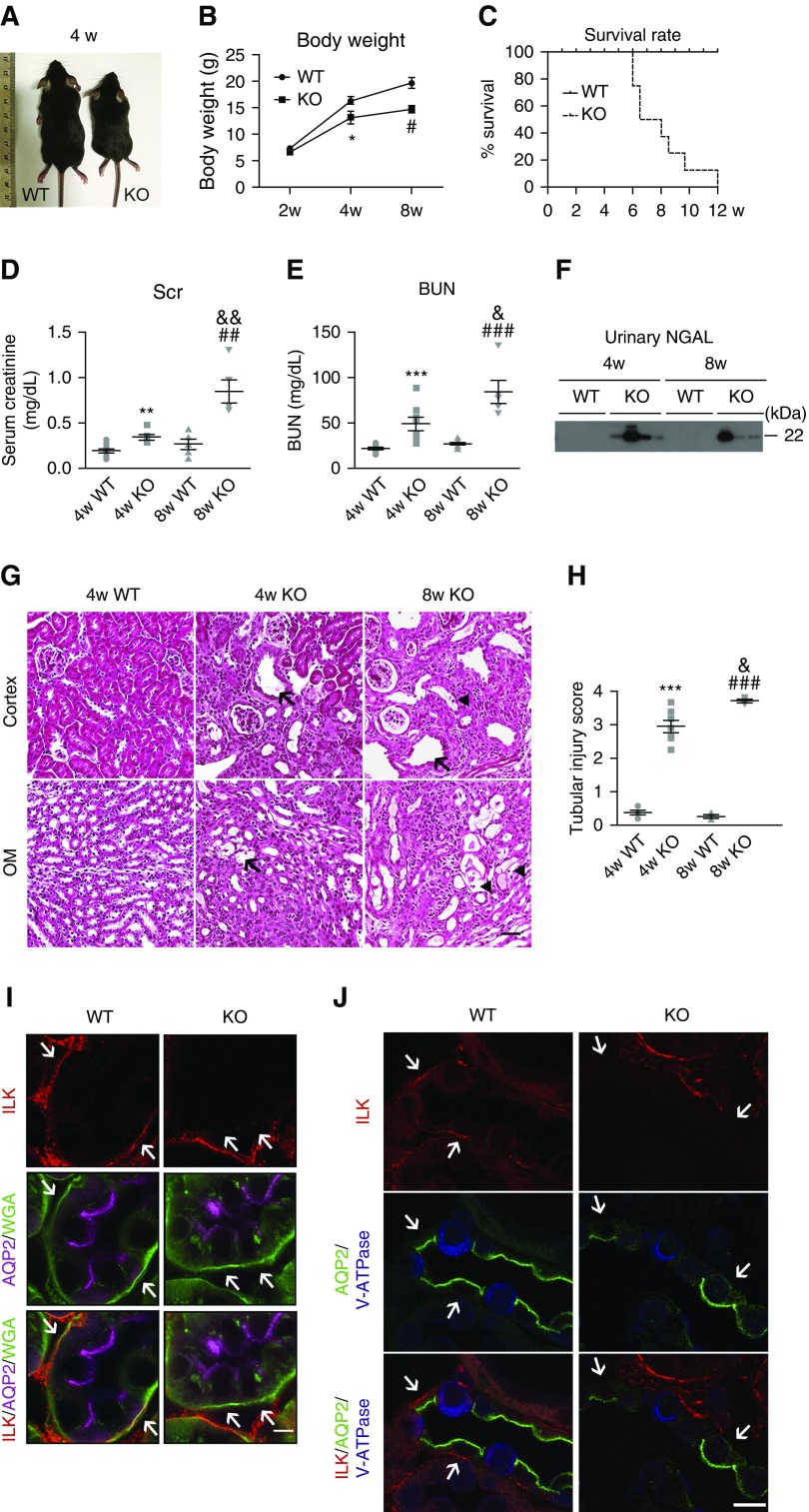
To delete ILK in CD PCs, we crossed Aqp2-Cre and floxed Ilk transgenic mice. The expression of Cre recombinase is controlled by the Aqp2 promoter, which is expressed specifically in CD PCs after embryonic day 18.5. The Ilk fl/fl; Aqp2-Cre (Ilk KO) mice appeared similar to wild-type littermates at birth and within 2–3 weeks after birth. However, at around 4 weeks, KO mice exhibited significantly lower body weight and were less active compared with their littermates (Figure 1, A and B). At 8 weeks old, more than half of the KO mice became severely distressed, exhibiting hunched posture, ruffled fur, and emaciation. The Kaplan–Meier curve revealed a mortality of approximately 60% at 8 weeks of age (Figure 1C).
Figure 1.
Renal failure and kidney tubular injury are present in PC Ilk KO mice. (A) Representative images of wild-type (WT) and PC Ilk KO mice at 4 weeks old. (B) Body weight. *P<0.05 versus 4-week-old wild type; #P<0.05 versus 8-week-old wild-type mice. (C) Survival rate was shown by a Kaplan–Meier curve. n=8–10 mice per group. (D and E) Elevated serum creatinine (Scr) and BUN in PC Ilk KO mice. n=6–10. **P<0.01, ***P<0.001 versus 4-week-old wild-type mice; ##P<0.01, ###P<0.001 versus 8-week-old wild-type mice; &P<0.05, &&P<0.01 versus 4-week-old KO mice. (F) Increased urinary excretion of NGAL in PC Ilk KO mice as shown by immunoblotting. (G) Representative images of H&E staining of kidney cortex and outer medulla (OM) of wild-type and KO mice. Tubular dilation and microcysts were present in Ilk KO kidney (arrows). Detached and dead tubular cells were observed within tubular lumen (arrowheads). Scale bar, 50 μm. (H) Tubular injury score was calculated based on the percentage of damaged tubules. The degree of injury was graded blindly in ten randomly chosen fields as follows: 0, normal; 1, <10%; 2, 11%–25%; 3, 26%–75%; 4, >75%. ***P<0.001 versus 4-week-old wild-type mice; ###P<0.001 versus 8-week-old wild-type mice; &P<0.05 versus 4-week-old KO mice. n=4–7. Original magnification, 200×. (I) Immunofluorescence staining using an Airyscan confocal microscope confirmed deletion of ILK in PCs in KO kidney. ILK (red) was expressed on the basal membrane that was costained with wheat germ agglutinin (WGA)–FITC (green) in both PCs (stained purple with anti-AQP2 antibody) and ICs (negative for AQP2 staining) in wild-type kidney, whereas membrane expression of ILK (red) was significantly reduced or even absent in Ilk KO PCs. Arrows indicate ILK expression in WGA-positive basal membranes in wild-type and KO CDs, respectively. Scale bar, 5 μm. (J) Immunofluorescence staining revealed reduced ILK expression (red) in PCs in the KO kidney. PC-specific marker AQP2 stained green and IC-specific marker V-ATPase stained blue. ICs had low level of expression of ILK in general and there was no detectable change in ILK expression in KO kidney compared with the wild type. Arrows indicate ILK expression in basal membranes in wild-type and KO PCs, respectively. Scale bar, 10 μm. Data are presented as mean±SEM. Statistics were performed using the t test.
Serum creatinine and BUN were significantly increased in 4-week-old KO mice and continued to rise at 8 weeks, compared with wild-type mice, indicating progressive renal failure in PC Ilk KO mice (Figure 1, D and E). Increased urine secretion of kidney injury marker neutrophil gelatinase-associated lipocalin (NGAL) was also detected in KO mice (Figure 1F). The concentration of urinary NGAL in 8-week-old KO mice was estimated to be 0.542 mg/ml (Supplemental Figure 1). H&E staining revealed increased tubular dilation and tubular cell atrophy in 4-week-old KO kidney (Figure 1G). Kidney architecture was severely disrupted with tubular damage and expansion of extracellular matrix (ECM) in 8-week-old KO kidneys. Detached tubular cells were clustered within the dilated tubules. Tubular injury score was significantly higher in KO mice than wild-type mice (Figure 1H).
Downregulation of ILK expression in PC Ilk KO kidney was examined by immunofluorescence staining. In wild-type kidney, ILK expression was clearly detected in the basal membrane in AQP2-expressing PCs, and was costained with wheat germ agglutinin–FITC (Figure 1, I and J, Supplemental Figure 2). Conversely, in PC Ilk KO mice, ILK expression was significantly diminished or even absent in the basal membrane in PCs (Figure 1, I and J, Supplemental Figure 2). There was no detectable alteration of ILK expression in intercalated cells (ICs) in PC Ilk KO and wild-type mice (Figure 1J).
PC Ilk KO Induces Interstitial Fibrosis and Activation of TGF-β/Smad Signaling Pathway in Kidney
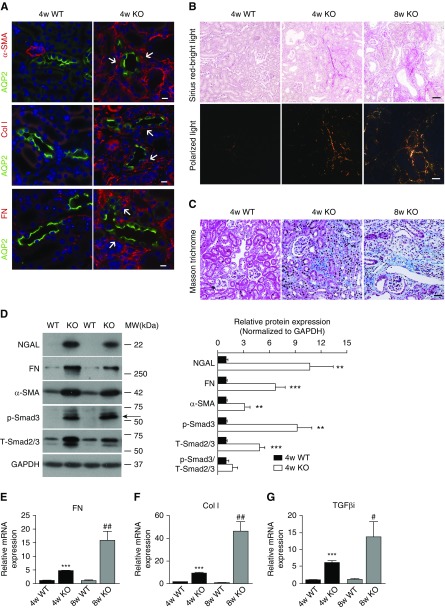
Immunofluorescence staining for markers of activated fibroblasts, α-smooth muscle actin (α-SMA), and ECM including collagen type 1 and fibronectin was performed in 4-week-old mouse kidney. α-SMA, collagen I, and fibronectin staining was barely detectable in wild-type kidney, but markedly increased in the interstitium of Ilk KO kidney, in proximity to or surrounding AQP2-positive CDs (Figure 2A, Supplemental Figure 3). Picrosirius red staining and Masson trichrome staining further revealed excessive deposition of collagen fibrils in 4- and 8-week-old KO kidney compared with the wild type (Figure 2, B and C). A significant elevation of NGAL, fibronectin, and α-SMA in 4-week-old KO kidney was revealed by immunoblotting (Figure 2D). In addition, the expression of several ECM genes, including fibronectin and collagen I, was significantly increased in 4-week-old KO kidney and even more dramatically at 8 weeks old by quantitative real-time PCR, indicating an escalating fibrogenic gene expression over time in KO kidney (Figure 2, E and F).
Figure 2.
Ilk KO in PCs causes kidney fibrosis and activates the TGF-β/Smad signaling pathway. (A) Immunofluorescence staining revealed the increased expression of α-SMA (red and arrows, top panels), ECM collagen type 1 (Col I; red and arrows, middle panels), and fibronectin (FN; red and arrows, bottom panels) in the cortex of 4-week-old KO mice. CD PCs were highlighted by immunostaining with anti-AQP2 antibody (green). Nuclei were stained blue with DAPI. Scale bar, 10 μm. (B) Deposition of collagen fibrils in 4-week-old wild-type (WT) and 4- and 8-week-old KO kidney was examined by picrosirius red staining. Collagen type 1 (red) and type 3 fibrils (green) were observed under polarized light (bottom three panels). Top three panels are images obtained under the bright light. Scale bar, 50 μm. (C) Masson trichrome staining revealed the excessive deposition of collagen fibers in 4- and 8-week-old KO kidney compared with the control. Scale bar, 50 μm. (D) Representative immunoblotting revealed increased expression of NGAL, FN, α-SMA, phosphorylated Smad3 (Ser423/425, p-Smad3, arrows), and total Smad2/3 (T-Smad2/3) in 4-week-old Ilk KO kidney (left). Individual protein signal intensity was quantified by densitometry and plotted over the intensity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The ratio of p-Smad3 over T-Smad2/3 is also shown in the graph (right). **P<0.01, ***P<0.001 versus wild-type mice. (E–G) Real-time PCR confirmed the augmented mRNA levels of (E) FN, (F) Col I, and (G) TGF-βi in Ilk KO kidney. Expression of individual genes was normalized to β-actin. ***P<0.001 versus 4-week-old wild-type mice; #P<0.05, ##P<0.01 versus 8-week-old wild-type mice. Data presented as mean±SEM, n=6–7 for (D–G). Statistics were performed using the t test. MW, molecular weight.
The canonical signaling pathway of TGF-β and its downstream effector molecules, Smads, were then investigated in PC Ilk KO kidney. Total Smad2/3 was mildly increased whereas phosphorylated Smad3 was dramatically increased in KO kidney (Figure 2D). TGF-β–induced protein (TGF-βi) is a target protein induced by active TGF-β and is excreted to ECM. TGF-βi mRNA was also increased six times and 13.5 times more in 4- and 8-week-old KO kidney, respectively, compared with wild type (Figure 2G), indicating a robust activation of the canonic TGF-β signaling pathway.
PC Ilk Deletion Induces Extensive Inflammation in Kidney
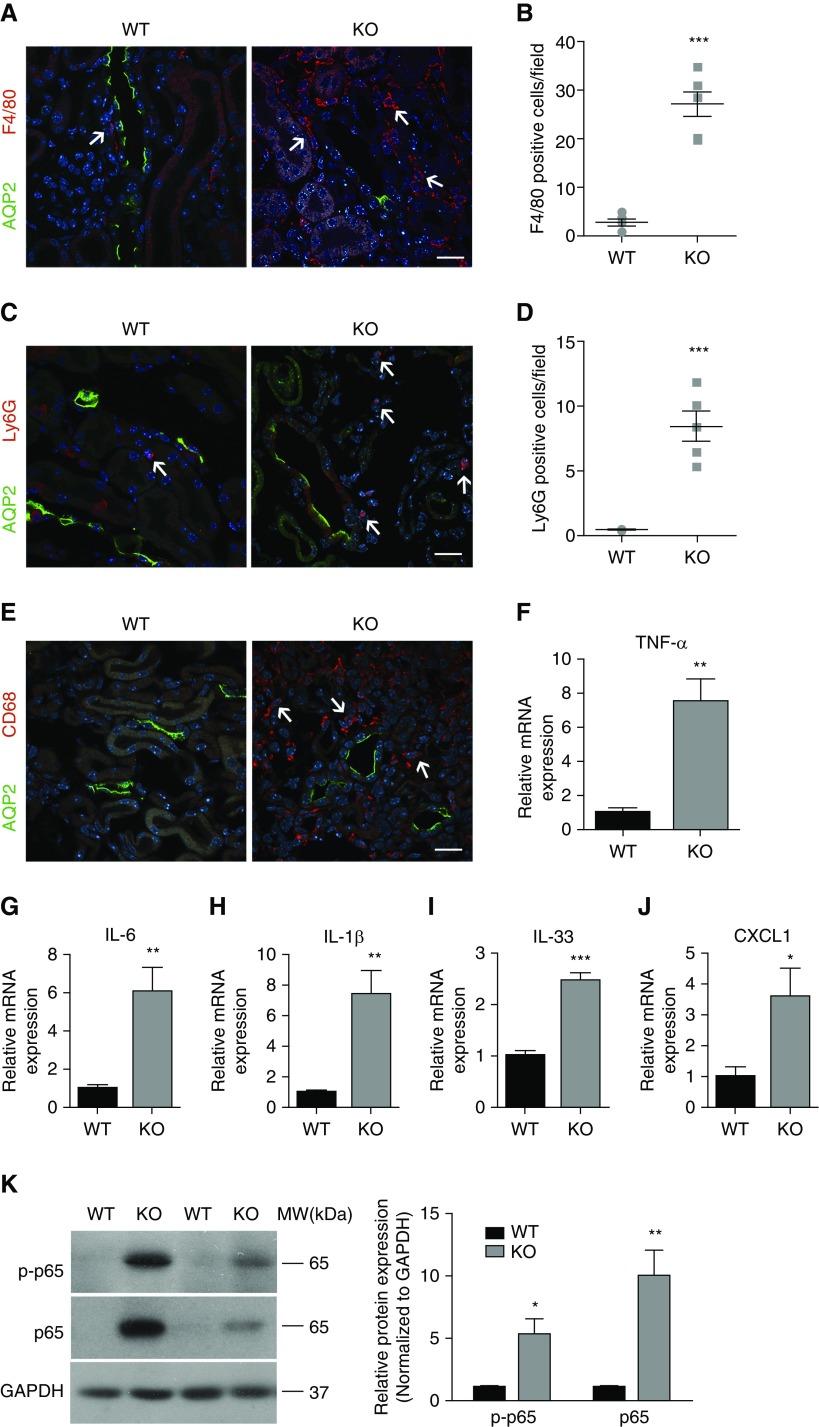
We further examined the presence of inflammatory signals in PC Ilk KO kidney because inflammation contributes to the exacerbation of AKI and renal fibrosis.38,39 Immunofluorescence staining with the macrophage marker F4/80, CD68, and neutrophil marker Ly6G revealed a significantly increased number of macrophages and neutrophils in KO kidney (Figure 3, A–E). Furthermore, KO kidney showed significantly augmented gene expression of proinflammatory cytokines, including TNF-α, IL-6, IL-1β, IL-33, and chemokine CXCL1 by quantitative real-time PCR (Figure 3, F–J). In addition, both the phosphorylated and total NF-κB RelA/p65 subunit were elevated in KO kidney by immunoblotting (Figure 3K), indicating the activation of the NF-κB pathway, an important regulator of inflammation.40 We also examined inflammatory cytokine expression in other tissues. Despite a significantly increased expression of IL-33 and CXCL1 in KO kidney, mRNA levels of IL-33 and CXCL1 in liver and spleen were not significantly altered in 4-week-old KO mice, suggesting that PC Ilk KO caused inflammation predominantly in the kidney.
Figure 3.
PC-specific ablation of Ilk induces renal inflammation. (A, C, and E) Representative immunofluorescence staining revealed increased F4/80-positive macrophages (red and arrows, A), Ly6G-positive neutrophils (red and arrows, C) and CD68-positive macrophages (red and arrows, E) in 4-week-old KO kidney. AQP2 stained green and nuclei stained blue. Scale bar, 20 μm. (B) F4/80-positive cells and (D) Ly6G-positive cells in 4-week-old kidney was quantified in ten randomly chosen fields for each specimen. Data are presented as the number of positive cells per high power field (mean±SEM/field). n=4–6. Original magnification, 400×. (F–J) Increased gene expression of (F) TNF-α, (G) IL-6, (H) IL-1β, (I) IL-33, and (J) CXCL1 in 4-week-old Ilk KO kidney was revealed by real-time PCR. Expression of individual genes was normalized to the expression of β-actin. (K) Representative immunoblotting images showed increased expression of phosphorylated (Ser536) and total NF-κB p65 subunit in 4-week-old KO kidney (left). The ratio of phosphorylated p65 and total NF-κB p65 over glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is plotted in the graph (right). *P<0.05, **P<0.01, ***P<0.001 versus wild-type (WT) mice. Data presented as mean±SEM, n=5–8 for (F–K). Statistics were performed using the t test. MW, molecular weight.
CD Epithelium Injury in PC Ilk KO Mice Is Revealed by Electron Microscopy
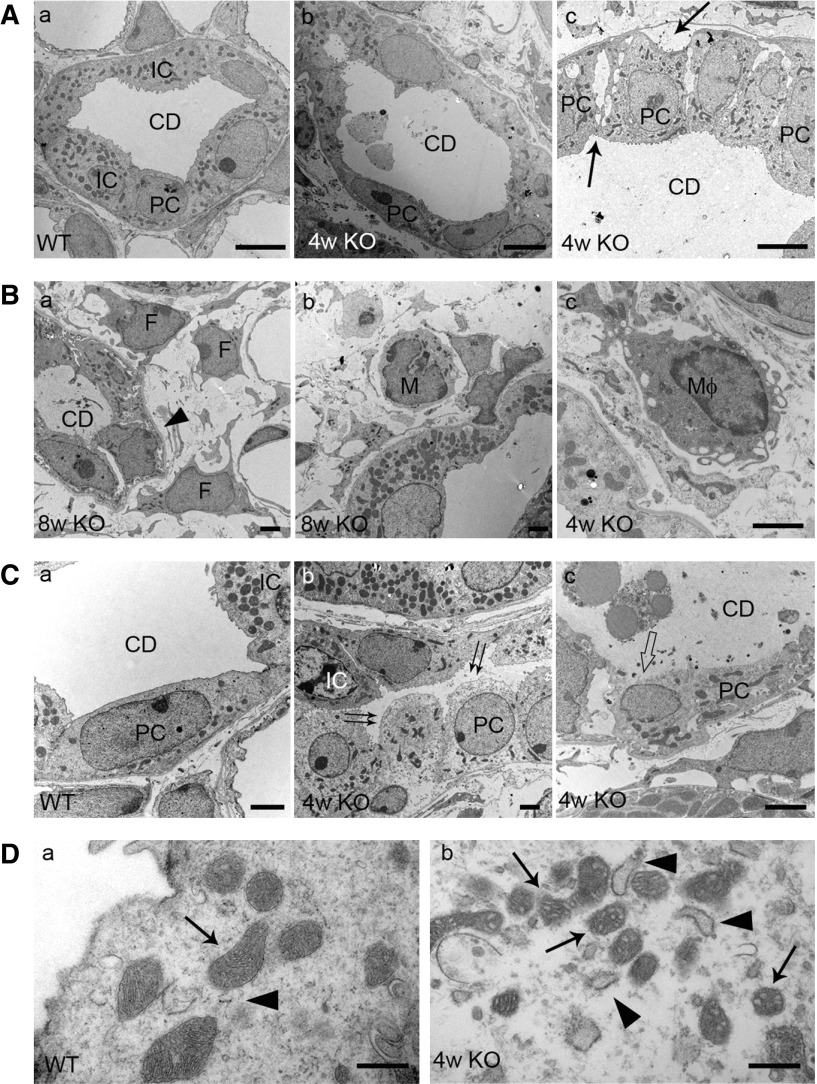
We next examined the CD epithelial cells using TEM in Ilk KO kidney. The ultrastructure of the glomerulus, segments of proximal tubules, and thin and thick ascending and descending loops appeared intact in 4-week-old KO and wild-type kidney (data not shown). CDs appeared intact in wild-type mice (Figure 4A, a). However, in KO kidney, CDs were severely distorted, with disrupted intercellular junctions between adjacent CD tubular cells and increased spacing between PCs and the basement membrane (Figure 4A, b and c). Detached epithelial cells and cellular debris were frequently observed in the tubular lumen (Figure 4A, b).
Figure 4.
CD epithelial cell injury and interstitial fibrosis are revealed by TEM in 4- and 8-week-old Ilk KO kidney. (A, a) CD appeared intact in 4-week-old wild-type (WT) kidney. (b and c, arrows) Increased intercellular junction and gaps between PCs and basement membrane were observed in CDs of 4-week-old Ilk KO kidney. (b) Detached tubular cells and cell debris were seen within the tubular lumen. Scale bar, 5 μm. (B) Increased interstitial fibrosis and inflammatory cell infiltration in Ilk KO kidney. (a) Extracellular matrix and fibrils were accumulated around CDs in KO kidney. The CD basement membrane was thickened (arrowhead). (a) Activated fibroblasts (F), (b) monocytes (M), and (c) macrophages (Mφ) were detected in the interstitium next to CDs in KO kidney. Scale bar, 2 μm. (C) Ilk KO PCs were undergoing necroptosis as indicated by cell swelling with (b, double arrows) lucent cytoplasm, (c, hollow arrow) apical membrane rupture, and (c) release of abundant amorphous cellular contents that were filled in the lumen of CDs. (a) Wild-type PCs remained intact. Scale bar, 2 μm. (D) Deformed mitochondria (arrows) and swollen endoplasmic reticulum (arrowheads) were present in (b) Ilk KO PCs, compared with (a) wild-type PCs. Scale bar, 500 nm.
The CD basement membranes were often thickened, and there was a large accumulation of ECM and fibrils surrounding the CDs in 4-week-old KO kidney, which was more prominent in 8-week-old KO kidney (Figure 4B, a). Activated fibroblasts with enlarged nuclei and expanded long processes were frequently observed together with increased infiltration of monocytes and macrophages in interstitium surrounding the CDs (Figure 4B).
In 4-week-old KO kidney, PCs were swollen with lucent cytoplasm, in contrast to the dense cytoplasm in adjacent ICs and PCs in wild-type kidney (Figure 4C, a and b). Many PCs had lost plasma membrane continuity and were ruptured in KO kidney (Figure 4C, c). Amorphous cellular contents, associated with or without vesicular membrane structures, were released and filled the lumen of CDs in KO kidney (Figure 4C, c). Furthermore, mitochondria in Ilk KO PCs appeared deformed and the endoplasmic reticulum was swollen (Figure 4D, b). Despite dramatic morphologic alteration, the nuclear membrane and nucleus of ILK-deficient PCs appeared intact. Approximately 75% of observed PCs had undergone necroptosis in PC Ilk KO kidney, based on TEM examination. In contrast, ICs appeared grossly intact with seemingly normal plasma membrane, dense cytosol content, and seemingly normal mitochondria in PC Ilk KO kidney.
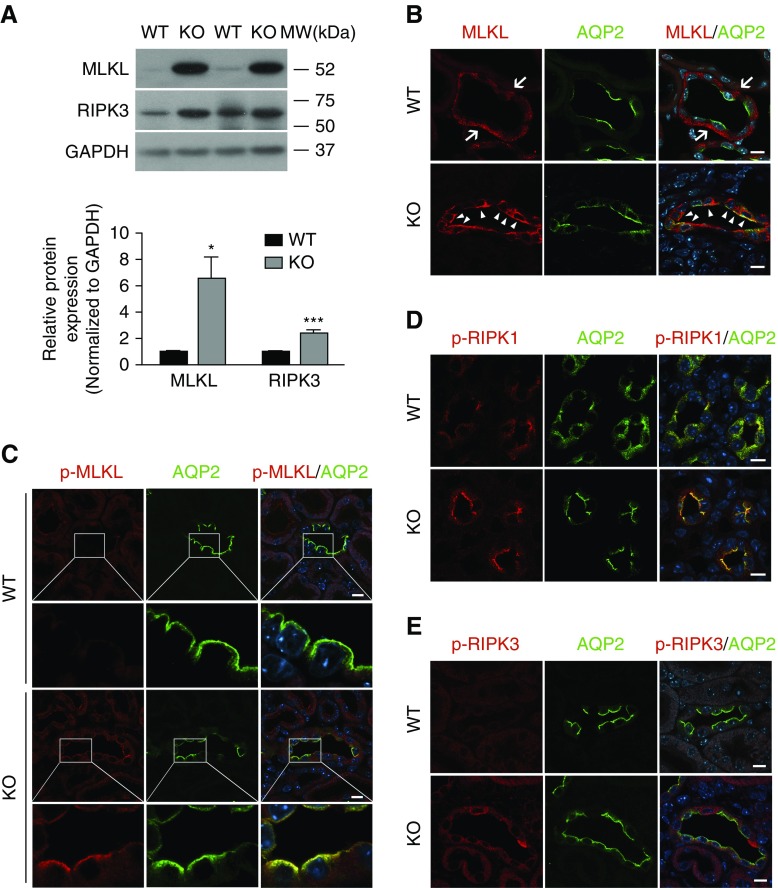
Ilk Deletion in PC Causes Upregulation and Activation of Necroptosis Signal in Kidney
Necroptosis in Ilk KO kidney was first studied by immunoblotting. Immunoblotting showed that expression of MLKL and its upstream kinase RIPK3 was significantly elevated in KO kidney (Figure 5A). Next, necroptosis signaling was examined by immunofluorescence staining using antibodies against total and phospho-MLKL, RIPK1, and RIPK3. The total MLKL signal was located diffusely inside PCs and in the PC basal membrane in wild-type kidney (Figure 5B). However, in KO kidney, immunostaining of MLKL appeared more intense and, interestingly, it clearly accumulated in the apical membrane in Ilk KO PCs (Figure 5B, Supplemental Figure 4). Subsequent immunofluorescence staining using antibody against phosphorylated MLKL (Ser345) revealed a dramatic apical membrane accumulation of phospho-MLKL in Ilk KO PCs (Figure 5C). Furthermore, immunofluorescence staining with anti–phospho-RIPK1 (Ser166) and anti–phospho-RIPK3 (Thr231/Ser232) antibodies revealed increased phospho-RIPK1 and RIPK3 signals in Ilk KO PCs compared with the wild type (Figure 5, D and E).
Figure 5.
Necroptosis signal is induced in CDs of Ilk KO mice. (A) Representative immunoblotting showed the upregulation of expression of MLKL and RIPK3 in 4-week-old KO mouse kidney (top panel). Expression of MLKL and RIPK3 was quantified by densitometry and normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (bottom panel). *P<0.05, ***P<0.001 versus wild-type (WT) mice. Data presented as mean±SEM, n=6–8. Statistics were performed using the t test. (B) Immunofluorescence staining revealed increased apical membrane expression and accumulation of MLKL (red, arrowheads) in AQP2-positive PCs (green) in Ilk KO mice, whereas the staining of MLKL (red, arrows) was more diffuse inside cells and in the basal region in wild-type PCs (green). Scale bar, 10 μm. (C) Increased apical membrane accumulation of phosphorylated MLKL (Ser345, p-MLKL, red) signal was clearly detected in Ilk KO PCs (AQP2 stained green) by Airyscan fluorescence confocal microscopy. Scale bar, 10 μm. (D and E) Increased expression of phosphorylated RIPK1 (Ser166, p-RIPK1, red) and phosphorylated RIPK3 (Thr231/Ser232, p-RIPK3, red) was also detected in Ilk KO PCs (green) by immunofluorescence staining. Scale bar, 10 μm. MW, molecular weight.
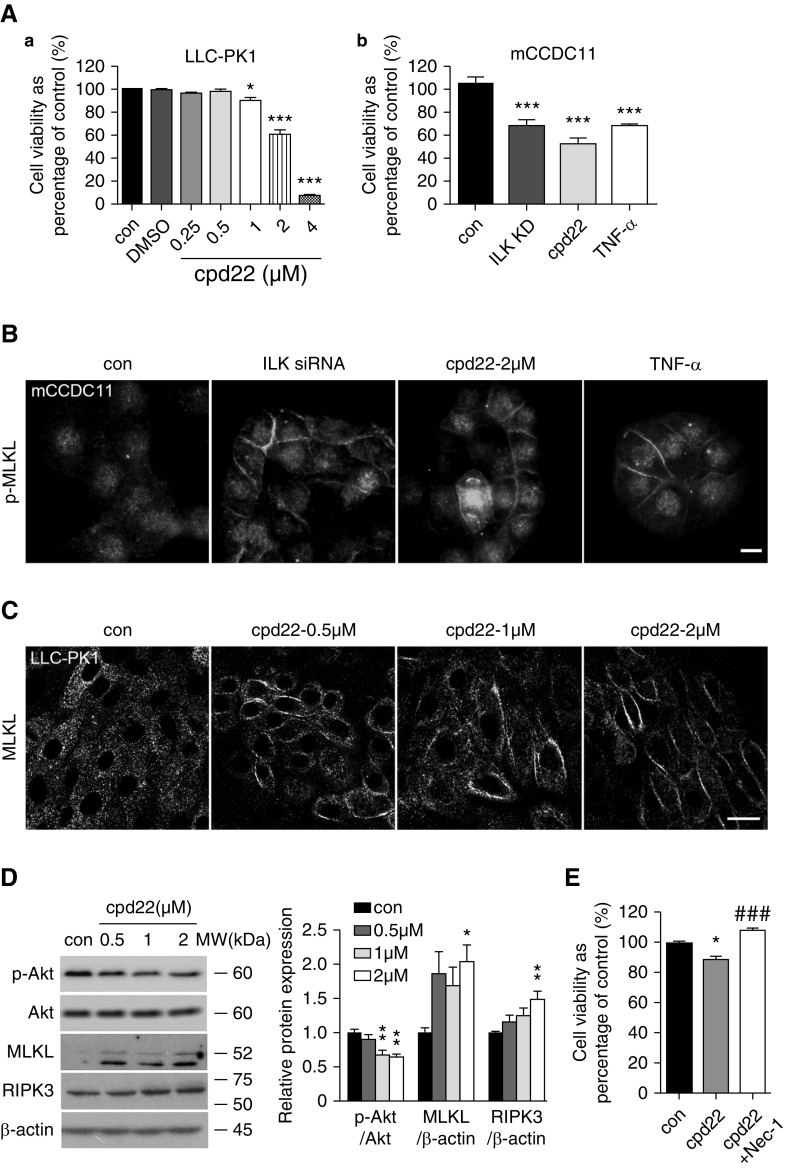
ILK Inhibition and Knockdown Increases Cell Death and Upregulates Necroptotic Signal in Cultured Renal Tubular Epithelial Cells
We next explored the role of ILK inhibition and knockdown in cultured kidney tubular cells, LLC-PK1, and a mouse CD cell line, mCCDC11, respectively. A well known ILK inhibitor, cpd22, was used to block ILK activity, as indicated by inhibition of the phosphorylation of Akt, a key downstream target of ILK (Figure 6D). Treatment of LLC-PK1 cells with 0.25–4 μM cpd22 for 24 hours reduced cell viability in a dose-dependent manner as measured by MTT (half maximal inhibitory concentration of 2.855 μM; Figure 6A, a). ILK siRNA knockdown in mCCDC11 increased cell loss, as measured by the DNA quantification assay, to the same degree as cells treated with TNF-α or cpd22 (Figure 6A, b). Immunofluorescence staining detected increased plasma membrane accumulation of phospho-MLKL in mCCDC11 cells with ILK siRNA knockdown and in cells treated with cpd22 or TNF-α (Figure 6B). Immunostaining of cpd22-treated LLC-PK1 revealed a similar translocation of MLKL signal from cytosol to the plasma membrane (Figure 6C), concomitant with the increased expression of MLKL and RIPK3 detected by immunoblotting (Figure 6D). We then applied a necroptosis inhibitor Nec-1, which is an allosteric inhibitor of RIPK1.41 When LLC-PK1 cells were incubated with Nec-1 in the presence of cpd22, Nec-1 was able to reverse the reduction of cell survival caused by ILK inhibition (Figure 6E).
Figure 6.
ILK inhibition and knockdown upregulates necroptotic signal in cultured renal tubular epithelial cells. (A, a) LLC-PK1 relative cell viability was reduced with the treatment of cpd22 in a dose-dependent manner as measured by MTT assay. Cpd22 (0.25–4 μM) was incubated with cells for 24 hours. *P<0.05, ***P<0.001 versus control (con). (b) ILK siRNA knockdown (ILK KD) or treatment with TNF-α or cpd22 (2 μM) caused cell loss as measured by DNA quantification assay in mCCDC11. ***P<0.001 versus control. (B) Increased plasma membrane accumulation of phospho-MLKL (p-MLKL, red) was clearly detected in mCCDC11 cells transfected with ILK siRNA. Similar membrane accumulation of phospho-MLKL was also present in cells treated with cpd22 or TNF-α as detected by immunofluorescence staining. Scale bar, 10 μm. (C) Immunofluorescence staining revealed that cpd22 treatment caused translocation of MLKL signals (red) from cytosol to the plasma membrane in LLC-PK1, compared with the control. Nuclei were stained blue with DAPI. Scale bar, 20 μm. (D) Representative immunoblotting showed the reduction of phosphorylated Akt (Ser 473, p-Akt) and upregulation of MLKL and RIPK3 in LLC-PK1 with cpd22 treatment for 24 hours (left panel). Bar graph represents the ratio of p-Akt over total Akt, and the ratio of MLKL and RIPK3 over β-actin expression as quantified by densitometry (right panel). *P<0.05, **P<0.01 versus control. (E) Nec-1 treatment of LLC-PK1 cells reversed the reduction of cell viability induced by cpd22. LLC-PK1 cells were treated with cpd22 (2 μM) in the presence or absence of Nec-1 (50 μM) for 24 hours. Relative cell viability was assessed by measuring the cellular DNA content. *P<0.05 versus control; ###P<0.001 versus 2 μM cpd22 treatment group. Data presented as mean±SEM. Experiments were repeated at least three times. Statistics were performed using one-way ANOVA.
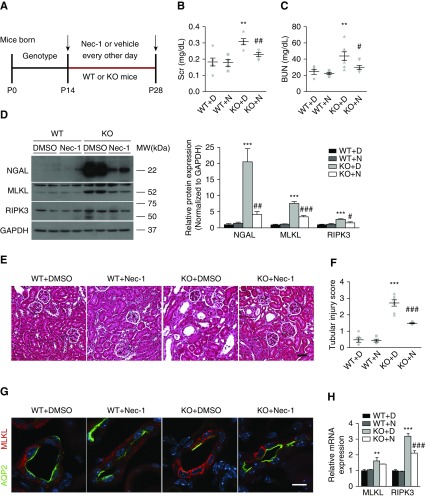
Nec-1 Inhibits Necroptosis and Attenuates AKI in PC Ilk KO Mice
We next examined an essential role of necroptosis in mediating kidney injury in PC Ilk KO mice using the necroptosis inhibitor Nec-1. We defined our treatment window of Nec-1 to be between 2 and 4 weeks after birth (Figure 7A). Compared with vehicle-treated KO mice, Nec-1–treated KO mice showed significant reduction in serum creatinine and BUN (Figure 7, B and C), as well as renal NGAL expression (Figure 7D). In wild-type mice treated with Nec-1 or vehicle, there was no detectable difference in serum creatinine or BUN (Figure 7, B and C). H&E staining of the kidney revealed that Nec-1 treatment significantly improved tubular atrophy and dilation, and overall tubular injury score in KO mice (Figure 7, E and F).
Figure 7.
Inhibition of necroptosis attenuates tubular injury and renal function decline in PC Ilk KO mice. (A) Diagram of Nec-1 administration schedule for wild-type (WT) and Ilk KO mice. After genotyping, 2-week-old wild-type and KO mice were treated with 1.65 mg/kg Nec-1 or vehicle (1% DMSO in PBS) by intraperitoneal injection every 2 days for 2 weeks. (B) Nec-1 reduced the elevation of serum creatinine (Scr) and (C) BUN in Ilk KO mice. **P<0.01 versus wild-type with DMSO mice; #P<0.05, ##P<0.01 versus KO with DMSO mice. (D) Representative immunoblotting showed that Nec-1 reduced expression of NGAL, MLKL, and RIPK3 in Ilk KO kidney (left). The expression of NGAL, MLKL, and RIPK3 was quantified by densitometry, and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (right). ***P<0.001 versus wild-type with DMSO mice; #P<0.05, ##P<0.01, ###P<0.001 versus KO with DMSO mice. (E) Representative images of H&E staining in kidney cortex. Nec-1–treated Ilk KO mice showed less tubular dilation and microcyst formation, compared with wild-type kidney treated with or without Nec-1. Scale bar, 50 μm. (F) Nec-1 treatment improved the tubular injury score in Ilk KO kidney. ***P<0.001 versus wild type with DMSO mice; ###P<0.001 versus KO with DMSO mice. (G) Immunofluorescence staining showed that Nec-1 blocked translocation of MLKL signal (red) from cytosol to plasma membrane in Ilk KO PCs (AQP2 stained green). Scale bar, 10 μm. (H) Gene expression of MLKL and RIPK3 was evaluated by real-time PCR. Expression of individual genes was normalized to β-actin. **P<0.01, ***P<0.001 versus wild-type with DMSO mice; ###P<0.001 versus KO with DMSO mice. Data presented as mean±SEM, n=5–7. Statistics were performed using one-way ANOVA. KO+D, KO mice treated with 1% DMSO in PBS; KO+N, KO mice treated with Nec-1; P, postnatal day; WT+D, wild-type mice treated with 1% DMSO in PBS; WT+N, wild-type mice treated with Nec-1.
We further examined the necroptosis pathway in Nec-1–treated KO mice. Immunoblotting showed that Nec-1 treatment significantly reversed the upregulation of MLKL and RIPK3 in KO kidney compared with vehicle (Figure 7D). Quantitative real-time PCR showed that RIPK3 mRNA was also significantly reduced whereas MLKL mRNA was slightly reduced in Nec-1–treated KO mice (Figure 7H). In addition, Nec-1 decreased the membrane translocation of MLKL in ILK-deficient PCs compared with wild-type PCs, indicating that Nec-1 blocked the MLKL activation induced by ILK deficiency (Figure 7G, Supplemental Figure 4).
Nec-1 Ameliorates Inflammation and Kidney Fibrosis in PC Ilk KO Mice
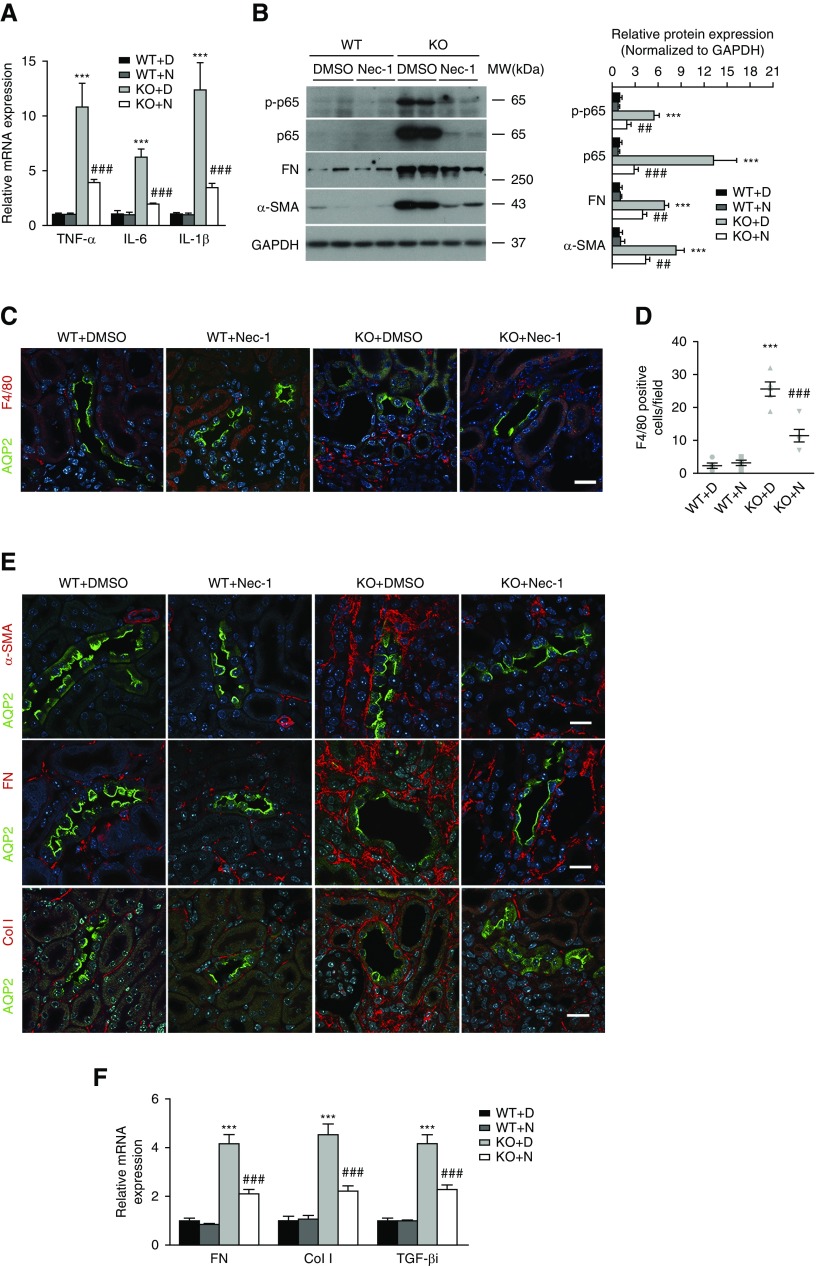
We investigated further inflammatory responses and kidney fibrosis in Ilk KO mice after Nec-1 treatment. Intriguingly, Nec-1 caused a marked reduction in the gene expression of TNF-α, IL-6, and IL-1β in KO kidney (Figure 8A). Nec-1 also significantly blocked the upregulation of the phosphorylated NF-κB p65 and total p65 subunit, as well as the F4/80-positive macrophage infiltration in KO mice (Figure 8, B–D). In addition, immunofluorescence staining revealed a dramatic reduction of α-SMA, fibronectin, and collagen type 1 in the interstitium in Nec-1–treated KO kidney compared with vehicle-treated KO mice (Figure 8E). Consistently, Nec-1 also blocked the increase of fibronectin and α-SMA protein, as well as the mRNA of fibronectin, collagen type 1, and TGF-βi in KO kidney (Figure 8, B and F). Therefore, inhibiting necroptosis by Nec-1 effectively antagonizes inflammation and interstitial fibrosis in the kidney induced by ILK deficiency in PCs.
Figure 8.
Nec-1 blocks inflammatory response and fibrosis in PC Ilk KO kidney. (A) Nec-1 suppressed gene expression of TNF-α, IL-6, and IL-1β in Ilk KO kidney as revealed by real-time PCR. Expression of individual genes was normalized to β-actin. ***P<0.001 versus wild-type (WT) with DMSO mice; ###P<0.001 versus KO with DMSO mice. (B) Representative immunoblotting showed that Nec-1 inhibited the upregulation of phosphorylated NF-κB p65 (p-p65), total p65, fibronectin (FN), and α-SMA in Ilk KO kidney (left). Protein expression of p-p65, total p65, FN, and α-SMA was quantified by densitometry and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (right). ***P<0.001 versus wild-type with DMSO mice; ##P<0.01, ###P<0.001 versus KO with DMSO mice. (C) Representative immunofluorescence staining showed reduced infiltration of F4/80-positive macrophages (red) in Nec-1–treated KO kidney. CD PCs were indicated by staining of AQP2 (green). Scale bar, 20 μm. (D) The number of F4/80-positive cells was quantified per field (mean±SEM/field). ***P<0.001 versus wild-type with DMSO mice; ###P<0.001 versus KO with DMSO mice. Original magnification, 400×. (E) Representative immunofluorescence images revealed decreased expression of α-SMA (red), FN (red), and collagen type 1 (Col I, red) in Nec-1–treated Ilk KO kidney. Green fluorescence signal indicates the AQP2-positive CDs. Nuclei were stained blue with DAPI. Scale bar, 20 μm. (F) Nec-1 decreased gene expression of FN, Col I, and TGF-βi in Ilk KO kidney by real-time PCR. Expression of individual genes was normalized to β-actin. ***P<0.001 versus wild-type with DMSO mice; ###P<0.001 versus KO with DMSO mice. Data presented as mean±SEM, n=5–7. Statistics were performed using one-way ANOVA. KO+D, KO mice treated with 1% DMSO in PBS; KO+N, KO mice treated with Nec-1; WT+D, wild-type mice treated with 1% DMSO in PBS; WT+N, wild-type mice treated with Nec-1.
Discussion
The goal of this study was to address the specific role of ILK in maintaining tubular structure and function. By deleting ILK in PCs in mouse kidney, we unexpectedly uncovered the critical involvement of ILK in mediating necroptosis of CD PCs, which led to profound inflammation and interstitial fibrosis in the kidney. This study highlights the essential function of ILK signaling in promoting PC survival through modulating necroptosis, and provides a novel understanding of the crucial contribution of the ECM-integrin-ILK signaling in PCs to tubular injury, inflammation, and interstitial fibrosis of the kidney.
Apoptosis and necrosis are considered two major mechanisms of cell death in acute tubular injury. The role of ILK in cell apoptosis has been studied in multiple organs.42,43 Hepatocyte- or cardiomyocyte-specific ILK deficiency induced apoptosis in mice.19,44 More recently, deletion of ILK in distal tubules of kidney was reported to cause kidney failure and tubular injury, which was also attributed to apoptosis of tubular cells.45 However, in our PC Ilk KO mice, we have not observed prevailing apoptosis of CD cells (data not shown). Further investigation led to the discovery of necroptosis in CDs induced by ILK deficiency. We have revealed ultrastructural characteristics of necroptosis in ILK-deficient PCs by TEM, and demonstrated upregulation and activation of key necroptotic complex proteins, MLKL, RIPK1, and RIPK3, in KO kidney. These results suggest the involvement of ILK signaling in tubular cell necroptosis.
How does ILK affect the necroptotic process? We have shown that MLKL translocates from the cytosol to the plasma membrane in Ilk KO PCs and cpd22-treated cultured cells. Significant membrane accumulation of phospho-MLKL is present in Ilk KO PC in mice and in ILK knockdown mCCDC11 cells. It is known that MLKL activation (phosphorylation) and subsequent homo-oligomerization is required for necroptosis to occur.9 However, it remains unclear by which mechanism MLKL translocates to the plasma membrane. A recent study revealed that heat shock protein 90 is capable of mediating necroptosis directly through regulating membrane translocation of activated MLKL.46 We hypothesize that, through regulating cytoskeletal dynamics, ILK may directly or indirectly modulate intracellular trafficking of MLKL and/or other necroptotic complex factors. ILK is shown to interact with scaffold protein IQGAP1 and effector mDia to promote the trafficking of caveolin-1 to the plasma membrane by stabilizing microtubules.47 We have recently reported that ILK regulates a water channel AQP2 to re-entry in the exocytotic pathway through modulating F-actin polymerization.48 Therefore, it is likely that ILK may affect necroptosis through regulating the trafficking of necroptotic complex proteins, such as MLKL. ILK was once considered a serine/threonine kinase,49,50 but more recent studies suggest that ILK is likely a “pseudokinase.”51–55 Our data suggest that ILK signaling is involved in modifying the phosphorylation of RIPK1, RIPK3, and MLKL. However, how the deletion of ILK phosphorylates necroptotic complex proteins, directly or indirectly, warrants further investigation.
ILK may also be involved in mediating the balance between apoptosis and necroptosis. Multiple previous studies have revealed that ILK deficiency induces cell apoptosis in many tissues.19,44 Necroptosis and apoptosis are known to share complex signaling pathways and are closely intertwined.56 What determines apoptosis versus necroptosis is highly dependent on the specific context. For example, various segments of kidney tubules may undergo different forms of cell death, even in response to similar insults. Unilateral ureteral obstruction predominantly elicits necrosis in the proximal tubules but apoptosis in CDs, whereas IRI causes apoptosis and necroptosis in proximal tubules.57–59 Isolated CD necroptosis has not been reported in known AKI models. In our study, absence of the ILK signal in PCs may alter the balance toward necroptosis as opposed to apoptosis.
Moreover, cell detachment caused by ILK deficiency may also trigger necroptosis. Anchorage dependence has long been recognized as an essential requirement for cell survival.60 The disruption or loss of integrin binding to ECM initiates a form of caspase-dependent apoptosis that is termed anoikis. As a focal adhesion adaptor molecule engaged by integrins, ILK functions as a pivotal effector in the transduction of survival signals from ECM and growth factors.61 We did observe cell detachment from the basement membrane in CDs of Ilk KO kidney, therefore cannot rule out the possibility that ILK deficiency leads to cell detachment and detached PCs subsequently undergo necroptosis. Whether cell detachment induces necroptosis directly has not been described. Our in vitro study in attached cultured cells has shown that inhibiting ILK or ILK knockdown causes upregulation and activation of necroptotic proteins such as MLKL, indicating that ILK deficiency alone is capable of activating necroptosis signaling.
Emerging studies suggest that the CD of the kidney may function as an essential regulator of inflammation in response to renal injury in addition to transporting water and salt.37,62,63 Why is CD injury “inflammatory?” It could be due to PC necroptosis, which is known to be highly inflammatory in general. During necroptosis, cell membrane disruption releases a number of cellular contents serving as damage-associated molecular patterns. These damage-associated molecular patterns trigger the production and release of proinflammatory cytokines and chemokines to amplify the inflammatory response.64,65 Therefore, PC necroptosis is capable of provoking massive kidney inflammation and fibrosis.
The process of cell death occurred during kidney injury is highly complex. Besides apoptosis and necroptosis, ferroptosis is recently reported to be involved in certain AKIs.66,67 In addition, multiple pathways may crosstalk and work synergistically to drive AKI.68 Despite being one of the major inhibitors of necroptosis, Nec-1 is also reported to inhibit ferroptosis.67 Attenuating PC necroptosis by Nec-1 in Ilk KO mice, or protecting cells from death induced by ILK inhibition in vitro cannot rule out the involvement of ferroptosis in our study. Therefore, whether ILK also modulates ferroptosis in addition to necroptosis in kidney CD PCs remains to be elucidated.
In summary, we have demonstrated a critical novel function of ILK in promoting CD PC survival by modulating the RIPK3-MLKL–dependent necroptosis pathway. In addition, our study highlighted the critical contribution of CD injury to interstitial inflammation and fibrosis of the kidney.
Disclosures
None.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01-DK-096015 and R21-DK-092619, NephCure Kidney International, a Gottschalk research grant from the American Society of Nephrology, the S&R Foundation Ryuji Ueno award, and MGH Executive Committee on Research support to Dr. H. A. J. Lu. The Microscopy Core facility of the MGH Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (NIDDK grant DK-57521) and from the Center for the Study of Inflammatory Bowel Disease (NIDDK grant DK43351). Dr. W. Lu is supported by NIH grants R01-DK078226. Dr. Păunescu is supported by the MGH Executive Committee on Research. Dr. Yang is supported by the National Natural Science Foundation of China grant 81620108029.
Supplementary Material
Acknowledgments
We thank Dr. Dennis Brown (MGH and Harvard Medical School, Boston, MA) for valuable advice on image acquisition and analysis.
Ms. M. Huang, Dr. H. Huang, and Dr. H. A. J. Lu conceived and designed research; Ms. M. Huang, Ms. Zhu, Ms. He, Dr. Tsuji, Mr. Jin, Dr. Xie, Dr. Ham, and Ms. Capen performed experiments; Ms. M. Huang and Ms. Zhu analyzed data; Ms. M. Huang, Ms. Zhu, Dr. H. Huang, Dr. Tsuji, and Ms. Capen interpreted results of experiments; Ms. M. Huang prepared figures; Ms. M. Huang, Dr. Yang, and Dr. H. A. J. Lu drafted manuscript; Ms. M. Huang, Dr. W. Lu, Dr. Păunescu, Dr. Yang, and Dr. H. A. J. Lu edited and revised manuscript; Ms. M. Huang, Dr. Yang, and Dr. H. A. J. Lu approved final version of manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111162/-/DCSupplemental.
Supplemental Table 1. List of primary antibodies used for immunoblotting (IB) and immunofluorescence staining (IF).
Supplemental Table 2. List of primers used for quantitative real-time PCR.
Supplemental Figure 1. Increased urinary NGAL secretion in 8 weeks old Ilk KO mice was revealed by immunoblotting, normalized to NGAL standard.
Supplemental Figure 2. ILK expression in wild type and PC Ilk KO kidney was revealed by immunofluorescence staining under low magnification.
Supplemental Figure 3. Immunofluorescence staining using isotype-matched immunoglobulin G (IgG) revealed minimal, nonspecific background staining in the wild type and fibrotic Ilk KO kidney.
Supplemental Figure 4. Necrostatin-1 (Nec-1) treatment blocked membrane accumulation of MLKL in Ilk KO PCs by immunofluorescence staining viewed under low magnification.
References
- 1.Humphreys BD: Mechanisms of renal fibrosis. Annu Rev Physiol 80: 309–326, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Wang F, Saran R, He Z, Zhao MH, Li Y, et al.: Mortality risk of chronic kidney disease: A comparison between the adult populations in urban China and the United States. PLoS One 13: e0193734, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sancho-Martínez SM, López-Novoa JM, López-Hernández FJ: Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin Kidney J 8: 548–559, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, et al.: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Yuan J: Necroptosis in health and diseases. Semin Cell Dev Biol 35: 14–23, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Fang Y, Wu J, Chen H, Zou Z, Zhang X, et al.: RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis 9: 878, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al.: Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Li W, Ren J, Huang D, He WT, Song Y, et al.: Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24: 105–121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasparakis M, Vandenabeele P: Necroptosis and its role in inflammation. Nature 517: 311–320, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Gonzalez G, Vandenabeele P, Krysko DV: Necroptosis: A novel cell death modality and its potential relevance for critical care medicine. Am J Respir Crit Care Med 194: 415–428, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Zhe-Wei S, Li-Sha G, Yue-Chun L: The role of necroptosis in cardiovascular disease. Front Pharmacol 9: 721, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierdomenico M, Negroni A, Stronati L, Vitali R, Prete E, Bertin J, et al.: Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol 109: 279–287, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al.: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al.: A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkermann A, Heller JO, Prókai A, Weinberg JM, De Zen F, Himmerkus N, et al.: The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice [published correction appears in J Am Soc Nephrol 25: 2942, 2014]. J Am Soc Nephrol 24: 1545–1557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yen CF, Wang HS, Lee CL, Liao SK: Roles of integrin-linked kinase in cell signaling and its perspectives as a therapeutic target. Gynecol Minim Invasive Ther 3: 67–72, 2014 [Google Scholar]
- 18.Cano-Peñalver JL, Griera M, Serrano I, Rodríguez-Puyol D, Dedhar S, de Frutos S, et al.: Integrin-linked kinase regulates tubular aquaporin-2 content and intracellular location: A link between the extracellular matrix and water reabsorption. FASEB J 28: 3645–3659, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, Guo L, et al.: Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology 45: 1025–1034, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Smeeton J, Zhang X, Bulus N, Mernaugh G, Lange A, Karner CM, et al.: Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development 137: 3233–3243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, et al.: Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17: 1334–1344, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, et al.: Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, et al.: Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Blattner SM, Kretzler M: Integrin-linked kinase in renal disease: Connecting cell-matrix interaction to the cytoskeleton. Curr Opin Nephrol Hypertens 14: 404–410, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y: Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretzler M, Teixeira VP, Unschuld PG, Cohen CD, Wanke R, Edenhofer I, et al.: Integrin-linked kinase as a candidate downstream effector in proteinuria. FASEB J 15: 1843–1845, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Guo L, Sanders PW, Woods A, Wu C: The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol 159: 1735–1742, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman Mde F, Grande RM, Naves MA, de Franco MF, de Paulo Castro Teixeira V: Integrin-linked kinase (ILK) expression correlates with tumor severity in clear cell renal carcinoma. Pathol Oncol Res 19: 27–33, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Terpstra L, Prud’homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, et al.: Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol 162: 139–148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, et al.: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, et al.: Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol 24: 243–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji K, Păunescu TG, Suleiman H, Xie D, Mamuya FA, Miner JH, et al.: Re-characterization of the glomerulopathy in CD2AP deficient mice by high-resolution helium ion scanning microscopy. Sci Rep 7: 8321, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vedovelli L, Rothermel JT, Finberg KE, Wagner CA, Azroyan A, Hill E, et al.: Altered V-ATPase expression in renal intercalated cells isolated from B1 subunit-deficient mice by fluorescence-activated cell sorting. Am J Physiol Renal Physiol 304: F522–F532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D: Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Tanwar PS, Zhang L, Kaneko-Tarui T, Curley MD, Taketo MM, Rani P, et al.: Mammalian target of rapamycin is a therapeutic target for murine ovarian endometrioid adenocarcinomas with dysregulated Wnt/β-catenin and PTEN. PLoS One 6: e20715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al.: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamuya FA, Xie D, Lei L, Huang M, Tsuji K, Capen DE, et al.: Deletion of β1-integrin in collecting duct principal cells leads to tubular injury and renal medullary fibrosis. Am J Physiol Renal Physiol 313: F1026–F1037, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng XM, Nikolic-Paterson DJ, Lan HY: Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10: 493–503, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Christian F, Smith EL, Carmody RJ: The regulation of NF-κB subunits by phosphorylation. Cells 5: 12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N: Necrostatin-1 blocks both RIPK1 and IDO: Consequences for the study of cell death in experimental disease models. Cell Death Differ 20: 185–187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards LA, Thiessen B, Dragowska WH, Daynard T, Bally MB, Dedhar S: Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene 24: 3596–3605, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Friedrich EB, Liu E, Sinha S, Cook S, Milstone DS, MacRae CA, et al.: Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol 24: 8134–8144, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Matsui T, Abel ED, Dedhar S, Gerszten RE, Seidman CE, et al.: Deep sequence analysis of gene expression identifies osteopontin as a downstream effector of integrin-linked kinase (ILK) in cardiac-specific ILK knockout mice. Circ Heart Fail 7: 184–193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raman A, Reif GA, Dai Y, Khanna A, Li X, Astleford L, et al.: Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J Am Soc Nephrol 28: 2708–2719, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, et al.: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7: e2051, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, et al.: Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 19: 574–588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mamuya FA, Cano-Peñalver JL, Li W, Rodriguez Puyol D, Rodriguez Puyol M, Brown D, et al.: ILK and cytoskeletal architecture: An important determinant of AQP2 recycling and subsequent entry into the exocytotic pathway. Am J Physiol Renal Physiol 311: F1346–F1357, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Legate KR, Montañez E, Kudlacek O, Fässler R: ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31, 2006 [DOI] [PubMed] [Google Scholar]
- 50.McDonald PC, Fielding AB, Dedhar S: Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci 121: 3121–3132, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Ghatak S, Morgner J, Wickström SA: ILK: A pseudokinase with a unique function in the integrin-actin linkage. Biochem Soc Trans 41: 995–1001, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Wickström SA, Lange A, Montanez E, Fässler R: The ILK/PINCH/parvin complex: The kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaynberg J, Fukuda K, Lu F, Bialkowska K, Chen Y, Plow EF, et al.: Non-catalytic signaling by pseudokinase ILK for regulating cell adhesion. Nat Commun 9: 4465, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuda K, Gupta S, Chen K, Wu C, Qin J: The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell 36: 819–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda K, Knight JD, Piszczek G, Kothary R, Qin J: Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ILK): Cross-validation of the pseudokinase. J Biol Chem 286: 21886–21895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG: The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol 39: 63–69, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, et al.: Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63: 564–575, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Chen B, Wang Y, Meng C, Huang H, Huang XR, et al.: RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc Natl Acad Sci U S A 115: E1475–E1484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havasi A, Borkan SC: Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stupack DG, Cheresh DA: Get a ligand, get a life: Integrins, signaling and cell survival. J Cell Sci 115: 3729–3738, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Dedhar S: Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol 12: 250–256, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Fujiu K, Manabe I, Nagai R: Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L, Moriguchi T, Kaneko H, Hayashi M, Hasegawa A, Nezu M, et al.: Reducing inflammatory cytokine production from renal collecting duct cells by inhibiting gata2 ameliorates acute kidney injury. Mol Cell Biol 37: e00211–e00217, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulay SR, Linkermann A, Anders HJ: Necroinflammation in kidney disease. J Am Soc Nephrol 27: 27–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaczmarek A, Vandenabeele P, Krysko DV: Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, et al.: Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol 28: 218–229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al.: Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180–1191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, et al.: Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci 74: 3631–3645, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.