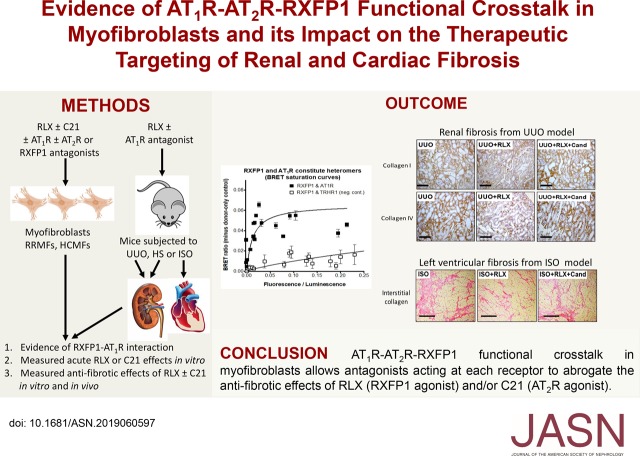
Significance Statement
Studies have shown that the hormone serelaxin, which has organ-protective actions mediated via relaxin family peptide receptor 1 (RXFP1), its cognate G protein–coupled receptor, requires the angiotensin II type 2 receptor (AT2R) to ameliorate renal fibrogenesis in vitro and in vivo. In this study, the authors describe a functional interaction between RXFP1, AT2R, and the angiotensin II type 1 receptor (AT1R), all of which are expressed on extracellular matrix–producing myofibroblasts, the cellular basis of progressive fibrosis. The crosstalk between these G protein–coupled receptors allows antagonists acting at each receptor to directly or allosterically block the antifibrotic actions of agonists acting at AT2R or RXFP1. These findings have significant therapeutic implications for a mechanistic understanding of the concomitant use of drugs acting at each receptor.
Keywords: angiotensin receptors, Relaxin family peptide receptor 1, fibrosis, kidney disease, heart disease
Visual Abstract
Abstract
Background
Recombinant human relaxin-2 (serelaxin), which has organ-protective actions mediated via its cognate G protein–coupled receptor relaxin family peptide receptor 1 (RXFP1), has emerged as a potential agent to treat fibrosis. Studies have shown that serelaxin requires the angiotensin II (AngII) type 2 receptor (AT2R) to ameliorate renal fibrogenesis in vitro and in vivo. Whether its antifibrotic actions are affected by modulation of the AngII type 1 receptor (AT1R), which is expressed on myofibroblasts along with RXFP1 and AT2R, is unknown.
Methods
We examined the signal transduction mechanisms of serelaxin when applied to primary rat renal and human cardiac myofibroblasts in vitro, and in three models of renal- or cardiomyopathy-induced fibrosis in vivo.
Results
The AT1R blockers irbesartan and candesartan abrogated antifibrotic signal transduction of serelaxin via RXFP1 in vitro and in vivo. Candesartan also ameliorated serelaxin’s antifibrotic actions in the left ventricle of mice with cardiomyopathy, indicating that candesartan’s inhibitory effects were not confined to the kidney. We also demonstrated in a transfected cell system that serelaxin did not directly bind to AT1Rs but that constitutive AT1R–RXFP1 interactions could form. To potentially explain these findings, we also demonstrated that renal and cardiac myofibroblasts expressed all three receptors and that antagonists acting at each receptor directly or allosterically blocked the antifibrotic effects of either serelaxin or an AT2R agonist (compound 21).
Conclusions
These findings have significant implications for the concomitant use of RXFP1 or AT2R agonists with AT1R blockers, and suggest that functional interactions between the three receptors on myofibroblasts may represent new targets for controlling fibrosis progression.
Fibrosis results from a failed wound healing response to tissue injury, and is a major contributor to organ dysfunction and failure.1 It is characterized by excessive extracellular matrix (ECM), and in particular, collagen accumulation, and is a pathologic feature of CKD2 and cardiovascular disease,3 irrespective of cause. Numerous studies have shown that fibroblasts and other phenotypically similar mesenchymal cells are the predominant source of ECM, with de novo expression of α-smooth muscle actin (α-SMA) in these cells indicative of their differentiation into activated myofibroblasts.4 Despite fibrosis representing a significant health burden, there are currently no effective strategies that directly ameliorate its progression.
The ovarian and cardiovascular hormone relaxin has several organ-protective actions that are mediated through its cognate G protein–coupled receptor, relaxin family peptide receptor 1 (RXFP1).5 The antifibrotic effects of recombinant human relaxin-2 (serelaxin; RLX) have been consistently demonstrated in experimental models of ageing,6,7 CKD, and cardiovascular disease.5,8–10 RLX directly inhibits angiotensin II (AngII)- and/or TGF-β1–mediated fibroblast differentiation into myofibroblasts, and prevents myofibroblast contraction11 and ECM/collagen synthesis by myofibroblasts.12,13 It also increases collagen-degrading matrix metalloproteinase (MMP) expression and activity.12–15 Additionally, RLX can inhibit inflammatory cell influx postinjury16–18 and influence macrophage polarization toward an M2 prorepair macrophage phenotype.19
Signal transduction studies have shown that activation of RXFP1 on renal and cardiac myofibroblasts by RLX directly increases cGMP20 or signals through an ERK1/2 phosphorylation and neuronal nitric oxide synthase (nNOS)-NO-sGC-cGMP-dependent pathway to inhibit TGF-β1 signal transduction at the level of intracellular Smad2 phosphorylation (phospho-Smad2).13,21–23 This in turn disrupts the profibrotic actions of TGF-β1 on myofibroblast differentiation and aberrant ECM deposition. Likewise, RLX signals through this pathway in renal and dermal myofibroblasts to stimulate expression and activity of various collagen-degrading MMPs.24
Intriguingly, angiotensin II type 2 receptor (AT2R) antagonism or gene deficiency abrogated the effects of RLX in renal myofibroblasts, suggesting that its antifibrotic actions required the AT2R.25 These findings are consistent with the organ protection and inhibition of TGF-β1 signal transduction that is mediated by AT2R activation.26–28 Furthermore, they may explain why RLX only affects injury-induced aberrant ECM/collagen deposition without affecting basal matrix turnover, as the AT2R is poorly expressed in healthy adult tissues and cells, but is dramatically upregulated postinjury or after repeated stimulation and/or inflammation of the kidney and heart.26,29,30
Along with RXFP1 and the AT2R, the angiotensin II type 1 receptor (AT1R) is also expressed on (myo)fibroblasts26,31 and mediates the profibrotic and vasoconstrictor actions of AngII.32 Accordingly, many angiotensin II type 1 receptor blockers (ARBs) are now in clinical use for their antihypertensive and, to a lesser extent, antifibrotic actions.33,34 There is evidence that heteromers constitutively form between AT1R and AT2R, with reports suggesting that this impairs AT1R internalization depending on the cell type studied.35 This may also explain how the AT2R can inhibit the growth effects of the AT1R.36,37 As heteromers can also form between RXFP1 and the AT2R,25 this raises the question as to whether functional interactions can occur between all three receptors on myofibroblasts, and whether this receptor crosstalk can be targeted to regulate fibrosis progression and/or the antifibrotic actions of RLX (acting at RXFP1) or an AT2R agonist (acting at the AT2R). In this study, we have addressed these important questions using primary myofibroblast cultures and murine models of fibrosis, together with selective AT1R and AT2R agonists and antagonists.
Methods
Materials
Recombinant H2 relaxin was generously provided by Corthera Inc. (San Carlos, CA; a subsidiary of Novartis AG, Basel, Switzerland) and is bioactive in rats21,22,24,25,38–40 and mice.15,19,25,39,41,42 Irbesartan was kindly provided by Professor Clive May (Florey Institute of Neuroscience and Mental Health, University of Melbourne, Parkville, Victoria, Australia), candesartan cilexetil was obtained from AstraZeneca (Södertälje, Sweden), CGP42112 was obtained from GL Biochem (Shanghai) Ltd. (Shanghai, China), compound 21 (C21) was obtained from Vicore Pharma AB (Mölndal, Sweden), and PD123319 was obtained from Sigma-Aldrich (St. Louis, MO).
Animals
All animals used in this study were housed in a controlled environment and maintained on a fixed 12 hour light/dark schedule with free access to rodent laboratory chow (Barastock Stockfeeds, Pakenham, Victoria, Australia) and water. All experiments described below were approved by the Florey Institute of Neuroscience and Mental Health’s and Monash University’s Animal Ethics Committees, which adhere to the Australian Code of Practice for the Care and Use of Laboratory Animals for Scientific Purposes.
A rat and mouse model of unilateral ureteric obstruction (UUO), which mimics the pathology of human progressive renal disease,43,44 was used as an experimental model of primary tubulointerstitial fibrosis. In each case, a single ureter was ligated under general anesthesia, and the other kidney remained intact. Tissues were collected from male Sprague–Dawley rats (approximately 250 g in weight; obtained from the Animal Resource Centre, Perth, Western Australia, Australia) for the propagation of renal (myo)fibroblasts. Seven- to eight-week-old male C57BL/6 mice (obtained from Monash University Animal Services, Clayton, Victoria, Australia) were also subjected to UUO, and either left untreated or treated with RLX (0.5 mg/kg per day44), in the absence or presence of candesartan cilexetil (2 mg/kg per day; a dose used to demonstrate its therapeutic efficacy45,46) (n=5–6 mice per treatment group). RLX was delivered to subgroups of mice via 7-day osmotic minipumps (model 1007D; Alzet, Cupertino, CA) from 2 days before injury until 5 days postinjury; mice receiving candesartan cilexetil alone or in combination with RLX were administered the ARB via drinking water over the equivalent 7-day treatment period.
Seven- to eight-week-old male 129sv mice (obtained from the Animal Resource Centre Perth, Western Australia, Australia) were separately subjected to a repeated isoprenaline (ISO)-induced model of cardiomyopathy and fibrosis.42,47 Mice received daily subcutaneous injections of isoprenaline hydrochloride (25 mg/kg; Sigma-Aldrich) over 5 consecutive days, and then left for a further 12 days for left ventricular fibrosis to develop. RLX (0.5 mg/kg per day) was delivered to subgroups of mice via osmotic minipumps (model 2002; Alzet) from days 0 to 17; animals treated with candesartan cilexetil (0.5 mg/kg per day) alone or in combination with RLX received the ARB via drinking water over the 17-day treatment period (n=7–8 mice per treatment group).
Eight- to ten-week-old male FVB/N mice were subjected to a high salt (HS; 5% sodium chloride) diet over 8 weeks. From weeks 5 to 8, subgroups of mice received RLX (0.5 mg/kg per day, via osmotic minipumps, model 2004; Alzet) alone, candesartan cilexetil (2 mg/kg per day; via drinking water) alone, or both combined (n=7–8 mice per group). HS-fed mice that were left untreated over 8 weeks were included as injury controls, whereas mice fed a normal salt (NS; 0.5% sodium chloride) diet were included as no-injury controls.
BP Measurements
Systolic BP was measured in all ISO-injured and HS-fed mice, as well as their respective control groups, using tail cuff plethysmography48 (MC4000 BP Analysis Systems; Hatteras Instruments Inc.). In each case, systolic BP was measured at day 0 and 17 of the ISO model, and weekly over the 8-week HS model. At least 15–20 measurements per time point were pooled to obtain a mean for each animal.
Cell Culture
Primary renal cortical myofibroblasts were propagated from the kidneys of male Sprague–Dawley rats after 3 days of UUO (rat renal myofibroblasts; RRMFs), and maintained as previously described.21,22,24,25 The response of these cells to RLX is very similar to that seen in TGF-β1–stimulated human renal fibroblasts.13 Cultures were maintained in DMEM supplemented with 10% FBS, 2.2% HEPES, 1% L-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml) (DMEM-FBS) at 37°C. All described experiments were independently replicated at three to seven separate times in duplicate or triplicate, with RRMFs used between passages 18–25.
Primary human fetal cardiac fibroblasts (from male fetuses and containing a mixture of atrial and ventricular fibroblasts) were obtained from ScienCell (Carlsbad, CA) and maintained as described before.23 Cells were grown in Medium 199 containing 5% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) plus fibroblast cell growth supplement-2 (FGS-2; ScienCell) (M199-FBS), and maintained at 37°C under 5% CO2. In each case, these cells were stimulated with TGF-β1 (5 ng/ml) to promote their differentiation into myofibroblasts (human primary cardiac myofibroblasts; HCMFs); all experiments on HCMFs were independently carried out at least four separate times in triplicate, with HCMFs used between passages two and four.
Evaluating the Effects of AT1R Blockade on the Antifibrotic Effects of RLX and C21 In Vitro
To determine if a functional interaction existed between RLX and the AT1R, primary RRMFs were seeded into 12-well plates at an equal density of 1–1.25×105 cells per well, and immediately treated with RLX (100 ng/ml; 16.8 nM), in the absence or presence of the AT1R antagonist irbesartan (0.1–10 μM) for 72 hours. The effects of irbesartan alone (0.1–10 μM) were separately evaluated over the same time period, to ensure that it did not affect the various end points measured in the absence of RLX. In all experiments, untreated RRMFs were used as controls.
The effects of AT1R blockade on the antifibrotic effects of RLX (acting at RXFP1), an AT2R agonist (C21), and the combined effects of both RLX and C21 were then evaluated. RRMFs were seeded into 12-well plates at an equal density as above, and immediately treated with RLX (16.8 nM; a dose consistently used to demonstrate its antifibrotic effects in vitro12,14,15,21,22,24,25) or C21 (1 μM; a dose used to demonstrate its therapeutic effects in vitro49,50), in the absence or presence of candesartan (10 μM) or RLX (16.8 nM), C21 (1 μM), and candesartan (10 μM) for 72 hours. Again, untreated RRMFs were used as controls.
After 72 hours, proteins were extracted from cell layers with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions), and media were collected and stored for analysis by gelatin zymography.
To determine the effects of AT1R antagonism on the acute ERK1/2-promoting actions of RLX, RRMFs were seeded into 48-well plates at a density of 1–1.25×105 cells per well and allowed to grow overnight at 37°C in DMEM-FBS. Cells were serum starved for 4–6 hours, then treated with either irbesartan (0.1–10 μM) or candesartan (0.1–10 μM) for 1 hour, and after that with RLX (16.8 nM) for 5 minutes.
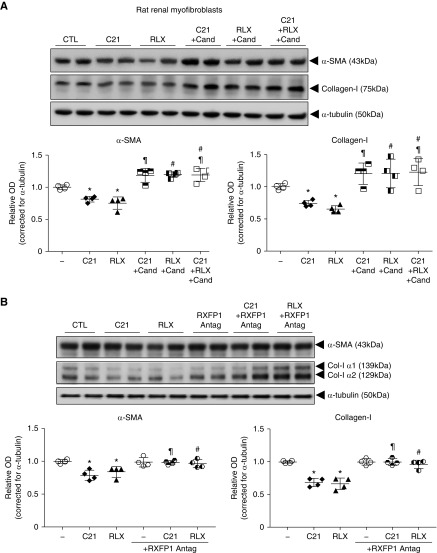
Evaluating the Effects of RXFP1 Blockade on the Antifibrotic Effects of RLX and C21 In Vitro
The effects of RXFP1 blockade on the antifibrotic effects of RLX (acting at RXFP1) or C21 (acting at the AT2R) were also evaluated. RRMFs were seeded into 12-well plates at an equal density as above, and immediately treated with RLX (16.8 nM) or C21 (1 μM), in the absence or presence of an RXFP1 antagonist (1 μM39,51) or the RXFP1 antagonist (1 μM) alone for 72 hours. Again, untreated RRMFs were used as controls. After 72 hours, proteins were extracted from cell layers and media isolated as described above.
Evaluating the Effects of RXFP1 or AT1R±AT2R Blockade on the Acute Effects of RLX and C21 In Vitro
To determine the effects of RXFP1, AT1R±AT2R antagonism on the acute cGMP-promoting actions of RLX and C21, primary human fetal cardiac fibroblasts (containing a mixture of atrial and ventricular fibroblasts) were stimulated with TGF-β1 (5 ng/ml) to stimulate the differentiation of these cells into myofibroblasts (HCMFs). HCMFs were seeded into 48-well plates at a density of 1–1.5×105 cells per well and allowed to grow overnight at 37°C in M199-FBS. The following day, the cells were serum-starved overnight, then treated with the RXFP1 antagonist (1 μM) alone, candesartan (1 μM) alone, the AT2R antagonist PD123319 (1 μM) alone, or candesartan (1 μM) plus PD123319 (1 μM) for 30 minutes, then with either RLX (1 nM) or C21 (1 μM) for 40 minutes.
Western Blotting
Equal amounts of total protein (10–25 μg; from mouse tissue extracts and RRMFs) from each sample were electrophoresed on 10.5% acrylamide gels, as described before.21,25 Blots were probed with primary polyclonal antibodies to either phosphorylated (phospho)-ERK1/2 (#9101 [Thr202/Tyr204], 1:1000 dilution; Cell Signaling Technology, Danvers, MA), nNOS (#610308, 1:500 dilution; BD Biosciences, San Jose, CA), phospho-nNOS (#PA1–032 [Ser1417], 1:1000 dilution; Pierce Biotechnology, Rockford, IL), or TGF-β1 (#sc-146, 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA); or with primary monoclonal antibodies to phospho-Smad2 (#3108 [Ser465/467], 1:1000 dilution; Cell Signaling Technology), α-SMA (#M0851, 1:750 dilution; Dako Corporation, Carpinteria, CA), MMP-13 (#ab75606, 1:500 dilution; Abcam, Cambridge, MA), collagen I (either with an ab88147 mAb [at 1:1000 dilution] that detected a single band corresponding to collagen I [Abcam] or with the ab34710 polyclonal antibody [at 1:1000 dilution] that detected the α1 and α2 chains of collagen I [Abcam]), or the AT1R (#sc-1173 [N-10], 1:1000 dilution; Santa Cruz Biotechnology); and the appropriate secondary antibodies via the use of Dako anti-rabbit or anti-mouse kits (Dako Corporation). Membranes probed with phospho-ERK1/2 and phospho-Smad2 were stripped and reprobed with total ERK1/2 (#4695 [137F5], 1:1000 dilution; Cell Signaling Technology) and Smad2/3 (#3102, 1:1000 dilution; Cell Signaling Technology), respectively. In each experiment, a single replicate membrane was probed for α-tubulin (#3873 [clone DM1A], 1:1000 dilution; Cell Signaling Technology) as a correction for semiquantitative analysis of each other end point measured. All blots were detected with the ECL detection kit and the appropriate bands quantified by densitometry using a GS710 Calibrated Imaging Densitometer and Quantity-One software (Bio-Rad Laboratories, Richmond, CA). The OD of each parameter measured was corrected for α-tubulin protein levels (or total ERK1/2 and Smad2/3 levels for phospho-ERK1/2 and phospho-Smad2, respectively) and expressed relative to the UUO-injured/untreated wild-type mouse group (in vivo studies) or the untreated control group (in vitro) studies, which was expressed as 1 in each case.
Gelatin Zymography
Equal amounts of mouse tissue extracts or cell culture media (5–10 μg per sample) were evaluated by gelatin zymography, using 7.5% acrylamide gels containing 1 mg/ml gelatin (Sigma-Aldrich) as detailed previously.24,25 Gelatinolytic activity was indicated by clear bands and densitometry was used to assess changes in both the combined latent and active forms of either MMP-2 (gelatinase A) or MMP-9 (gelatinase B).
Hydroxyproline Assay
Equivalent tissue portions of kidney tissue (containing cortex and medulla) or left ventricular tissue (apical region) were lyophilized to dry weight, hydrolyzed in 6 M hydrochloric acid, and evaluated for hydroxyproline content as detailed before.21,25,42 Hydroxyproline values were then converted to collagen content and collagen concentration (percentage of collagen content/dry weight tissue52).
Histology, Immunohistochemistry, and Immunofluorescence
Renal collagen IV and collagen I were identified from serial (5 μM) paraffin-embedded kidney sections from each animal subjected to UUO-induced injury, using goat anti-collagen IV (#1340–01, 1:300 dilution; Southern Biotechnology, Birmingham, AL) or rabbit anti-collagen I (#T40777R, 1:1000 dilution; Meridian Life Science, Memphis, TN) primary antibodies, visualized, and morphometrically evaluated by point-counting.25,44 Equivalent areas of the renal cortex were evaluated for each collagen subtype, with glomeruli and large vessels excluded from the analysis. Kidney tissues from NS- and HS-fed mice were subjected to Masson trichrome staining (by Monash Histology Services, Clayton, Victoria, Australia) and analyzed for changes in interstitial collagen deposition and glomerulosclerosis.53
Interstitial left ventricular collagen deposition was identified from paraffin-embedded (5 μm) tissue sections that were stained with 0.1% picrosirius red (Polysciences Inc., Warrington, PA). Images from each section were captured and analyzed as described previously42; and data expressed as percentage of collagen staining per total field area measured.
Immunofluorescence staining of renal phosphorylated-IκB levels (as a surrogate marker of NF-κB activity) was also measured from sections derived from NS- and HS-fed mice, using a rabbit polyclonal primary antibody (#28595, 1:50 dilution; Cell Signaling Technology) and goat anti-rat IgG Alexa Fluor 488 secondary antibody (#A11006, 1:500 dilution; Life Technologies, Camarillo, CA).
All immunohistochemical- and immunofluorescence-stained slides, for targets other than collagen I and IV, were then scanned and captured using the Aperio AT Turbo scanner or Aperio FL scanner, respectively (Leica Biosystems, Nussloch, Germany), and stored as digital high-resolution images on a local server associated with each instrument. Images were viewed and morphometrically analyzed with the Aperio ImageScope v.12.1.0.5029 software (Leica Biosystems). In each case, images from six to eight nonoverlapping fields were captured (at the highest magnification available, ×40), analyzed and data expressed as percentage of staining per total field area.
Competition-Binding Assays
Whole-cell competition bindings assays (n=4 separate times) were conducted in human embryonic kidney (HEK) 293 cells stably transfected with the AT1R54; unlabeled RLX was compared against candesartan (AT1R antagonist) and CGP42112 (AT2R agonist), which were all prepared in DMEM containing 0.1% BSA and evaluated at concentrations ranging from 1 pM to 1 μM. In each separate experiment, each ligand concentration was assessed in triplicate, and the ability of each ligand to inhibit the specific binding of [125I]Sar1Ile8AngII was assessed. Nonlinear regression of the data were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Bioluminescence Resonance Energy Transfer Assays
Bioluminescence resonance energy transfer (BRET) saturation assays were performed in HEK293 cells as described previously25 (n=3 separate times). Cells were transiently cotransfected with a constant amount of Rluc8-tagged AT1R and increasing amounts of Venus-tagged RXFP1. The expression levels of Rluc8- and Venus-tagged constructs for each BRET experiment were detected by luminescence (LUMIstar; BMG Labtech, Mornington, Victoria, Australia) and fluorescence (Envision; Perkin-Elmer, Waltham, MA) measurements, respectively. The actual Receptor-Venus/Receptor-Rluc8 expression ratios were then plotted.
AlphaScreen Phospho-ERK1/2 and cGMP Accumulation Assays
Receptor-mediated phospho-ERK1/2 levels from RRMFs was determined using the ERK1/2 SureFire kit (TGR Biosciences, Hindmarsh, South Australia, Australia), and receptor-mediated cGMP activity was determined from RRMFs and HCMFs using the HTRF cGMP Detection kit (CisBio BioAssays, Bedford, MA), both according to the manufacturer’s instructions. In each case, the nitric oxide (NO) donor 2-(N,N-diethylamino)-diazenolate-2-oxide (10 μM) was used as a positive control for cGMP activity.
Real-Time PCR
A total of 1 μg of RNA from rat renal cortical myofibroblasts (passages 38–40) as well as untreated and AngII (0.1 μM)-treated neonatal rat cardiac ventricular fibroblasts (passages two and three) was extracted (n=2 samples per cell type and treatment) using TRIzol reagent (Invitrogen Pty Ltd., Mt Waverley, Victoria, Australia), and converted to complementary DNA (5× iSCRIPT supermix; Bio-Rad Life Sciences, Gladesville, New South Wales, Australia). RXFP1, AT1aR, and AT2R gene expression were analyzed with TaqMan gene expression assays (Rxfp1: Rn01495351_m1; Agtr1a: Rn02758772_s1; Agt2r: Rn00560677_s1) and were duplexed with the internal housekeeping gene 18s rRNA (Hs99999901_s1; Applied Biosystems, Life Technologies, Mulgrave, Victoria, Australia) using an Applied Biosystems 7900HT Fast RT-PCR system. Calculations of relative expression were carried out using the comparative cycle of threshold fluorescence method.
Statistical Analyses
All data were analyzed by one-way ANOVA followed by the Newman–Keuls post hoc test for multiple comparisons between groups using GraphPad Prism 7 (GraphPad Software). All Western blot, histology/immunohistochemistry, and PCR data are expressed as the mean±SD of the mean or relative mean±SD of the mean, with a value of P<0.05 considered as statistically significant.
Results
The Antifibrotic Actions of RLX In Vitro Are Blocked by the AT1R Antagonist Irbesartan
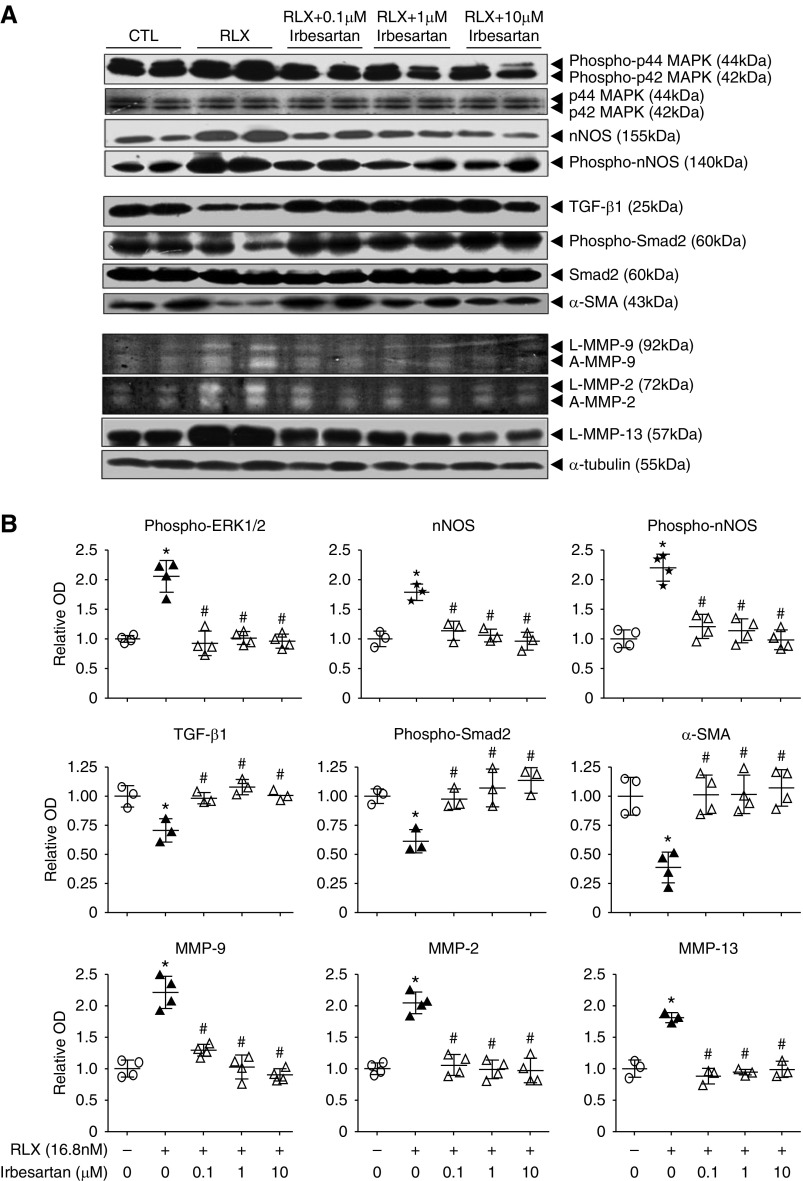
We firstly examined the effect of culturing RRMFs, propagated from animals with UUO, with 100 ng/ml (16.8 nM) RLX for 72 hours. RLX significantly promoted p42/p44 mitogen-activated protein kinase phosphorylation (phospho-ERK1/2), nNOS expression, and nNOS phosphorylation (phospho-nNOS) by approximately 80%–120% (Figure 1A); downregulated TGF-β1, phospho-Smad2 and α-SMA expression (myofibroblast differentiation) levels by approximately 30%–50% (Figure 1B); and upregulated MMP-9 and MMP-2 expression/activity as well as MMP-13 expression levels by approximately 80%–130% (Figure 1C). All of these RLX-induced effects were abrogated by coadministration of irbesartan (at 0.1–10 μM; Figure 1, A–C), whereas irbesartan alone did not affect basal expression of these measures (Supplemental Figure 1).
Figure 1.
The antifibrotic actions of RLX were blocked by the AT1R antagonist irbesartan in vitro. (A) Representative Western blots of renal phosphorylated (phospho)-p44 and p42 MAPK (phospho-ERK1/2), total p44 and p42 MAPK (ERK1/2), nNOS, phospho-nNOS, and α-tubulin; (B) TGF-β1, phospho-Smad2, total Smad2, α-SMA, and α-tubulin; and (C) representative gelatin zymographs of latent (L) and active (A) MMP-9 and MMP-2 levels and representative Western blots of L-MMP-13 and α-tubulin expression from untreated (control) RRMFs and cells treated with RLX (16.8 nM) alone, or in the presence of irbesartan (0.1, 1, and 10 μM) after 72 hours in culture. The total p44 and p42 MAPK (ERK1/2), unphosphorylated Smad2, and α-tubulin blots (A) were included to demonstrate the quality and equivalent loading of protein samples. (B) Also shown are the relative mean±SEM OD levels of phospho-ERK1/2 (corrected for total ERK1/2 levels); nNOS and phospho-nNOS (both corrected for α-tubulin levels); TGF-β1, α-SMA (both corrected for α-tubulin levels), and phospho-Smad2 (corrected for total Smad2 levels); and MMP-9, MMP-2, and MMP-13 (corrected for α-tubulin levels) from each of the groups studied, as determined by densitometry scanning (from n=3–4 separate experiments conducted in duplicate), to that of the untreated group, which was expressed as 1 in each case. *P<0.01 versus untreated cells; #P<0.01 versus RLX alone-treated cells.
The Antifibrotic Actions of RLX In Vivo Are Blocked by the AT1R Antagonist Candesartan Cilexetil
To substantiate these in vitro findings (Figure 1), we examined the effects of 0.5 mg/kg per day of RLX in vivo, a dose that produces circulating levels of approximately 20 ng/ml after 5 days41 and consistent antifibrotic effects.12,15,21,25,38,41 We compared the effects of RLX with the ARB candesartan cilexetil (2 mg/kg per day to produce antihypertensive and antifibrotic effects55), in male wild-type mice subjected to 5 days of UUO-induced tubulointerstitial renal fibrosis. Compared with UUO-injured mice, candesartan cilexetil–treated mice showed a trend toward preventing renal collagen concentration, but was not significantly different from that measured in the untreated UUO group (Supplemental Figure 2), consistent with the slow-acting effects of ARBs.56,57 In comparison, RLX-treated mice had increased renal phospho-ERK1/2 (p42–44 MAPK; by approximately 150%), nNOS expression and phospho-nNOS (both by 150%–160%) (Figure 2A), and latent MMP-13 levels (by approximately 130%) (Figure 2B), but reduced renal TGF-β1 levels, phospho-Smad2, and α-SMA expression (all by 55%–60%) (Figure 2B) as well as total collagen concentration (Figure 2C, Supplemental Figure 2) and collagen I and collagen IV immunostaining (all by 50%–70%) (Figure 2C) by 5 days postinjury. These antifibrotic and signal transduction effects of RLX were abolished by coadministration of candesartan cilexetil over the same time period (Figure 2).
Figure 2.
The antifibrotic actions of RLX in the injured kidney were blocked by the AT1R antagonist candesartan in vivo. (A) Representative Western blots of renal phosphorylated (phospho)-p44 and p42 MAPK (phospho-ERK1/2), total p44 and p42 MAPK (ERK1/2), nNOS, phospho-nNOS, TGF-β1, phospho-Smad2, total Smad2, α-SMA, L-MMP-13, and α-tubulin. (B) Representative images of immunohistochemically stained collagen I and collagen IV as well as total kidney collagen concentration in UUO-injured male C57B6J wild-type mice that were untreated for 5 days or treated with RLX (0.5 mg/kg per day) alone, or RLX (0.5 mg/kg per day) and candesartan cilexetil (2 mg/kg per day) from 2 days before injury until 5 days postinjury. Scale bar, 100 μm. Total p44 and p42 MAPK (ERK1/2), unphosphorylated Smad2, and α-tubulin blots (A) were included to demonstrate the quality and equivalent loading of protein samples. Also shown (B) are the mean±SEM total kidney collagen concentration (expressed as a percentage of the dry weight tissue) or renal collagen I and collagen IV staining (expressed as a percentage of the fractional area stained, respectively) (from n=5–6 animals per treatment group). *P<0.01 versus UUO-injured/untreated group; #P<0.01 versus UUO-injured/RLX-treated group.
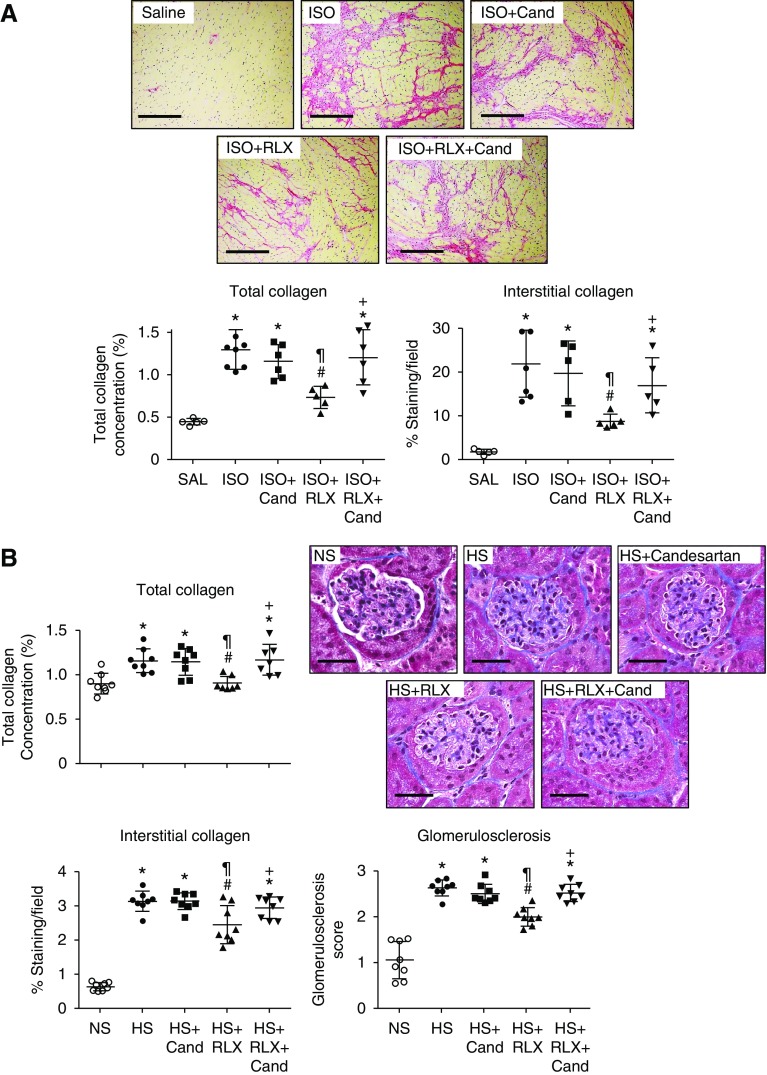
To confirm that these unexpected findings were not confined to the kidney, we also evaluated the cardioprotective effects of RLX (0.5 mg/kg per day) in a murine model of ISO-induced cardiomyopathy, in which RLX reduced cardiac fibrosis.39,42 A lower dose of candesartan cilexetil (0.5 mg/kg per day) was used to avoid any confounding antihypertensive effects. Repeated ISO administration markedly increased left ventricular collagen concentration and picrosirius red–stained interstitial collagen deposition by day 17 (Figure 3A). RLX reduced both the ISO-induced increase in left ventricular collagen concentration and interstitial collagen deposition by approximately 50%–60% (Figure 3A). Candesartan cilexetil alone did not demonstrate any antifibrotic efficacy at this concentration, but inhibited the antifibrotic effects of RLX when coadministered in the ISO model (Figure 3A).
Figure 3.
The antifibrotic actions of RLX in the heart of ISO-injured mice and kidneys of HS-fed mice were blocked by the AT1R antagonist candesartan in vivo. (A) Shown is the mean±SEM total left ventricular collagen concentration, representative picrosirius red–stained images of left ventricular interstitial collagen deposition (scale bar, 100 μm), and the mean±SEM picrosirius red–stained left ventricular interstitial collagen deposition (as a percentage of the fractional area) from uninjured male 129sv mice, ISO (25 mg/kg)-injured mice (after 17 days of injury), and ISO-injured mice treated with candesartan cilexetil (0.5 mg/kg per day) alone, RLX (0.5 mg/kg per day) alone, or RLX (0.5 mg/kg per day) and candesartan (0.5 mg/kg per day) throughout the 17 days of ISO-induced injury (from n=7–8 animals per treatment group). (B) Also shown are the mean±SEM total renal collagen concentration, representative Masson trichrome–stained images of renal interstitial collagen deposition (scale bar, 100 μm), and the mean±SEM interstitial renal collagen staining per field (expressed as percent) as well as the mean±SEM glomerulosclerosis score (as assessed by morphometry of Masson trichrome–stained images), from male FVB/N mice fed an HS (5% sodium chloride) diet for 8 weeks and treated from weeks 5 to 8 with candesartan cilexetil (2 mg/kg per day), RLX (0.5mg/kg per day), or both combined (n=7–8 animals per treatment group). *P<0.01 versus uninjured control groups, respectively; #P<0.01 versus ISO- or HS-injured/untreated group, respectively; ¶P<0.01 versus ISO- or HS-injured plus candesartan-treated group, respectively; +P<0.01 versus ISO- or HS-injured plus RLX-treated group, respectively.
The antifibrotic effects of RLX were additionally evaluated in an HS-induced model of renal fibrosis, a model that displays significantly increased total kidney collagen concentration, interstitial fibrosis, and glomerulosclerosis (by 0.4- to 4-fold) after 8 weeks (Figure 3B) in the absence of any marked hypertension (Supplemental Figure 3). Once again, RLX (0.5 mg/kg per day) normalized HS-induced total kidney collagen concentration and significantly decreased interstitial renal fibrosis and glomerulosclerosis (Figure 3B), when administered subcutaneously from weeks 5 to 8. In comparison, candesartan cilexetil (2 mg/kg per day) alone did not demonstrate any antifibrotic efficacy when administered over the same period, but did lower BP and demonstrate anti-inflammatory effects (Supplemental Figure 3), confirming its therapeutic activity at the dose and time frame investigated. When coadministered with RLX, candesartan again abrogated the antifibrotic effects of RLX in the kidneys of HS-fed mice (Figure 3B); confirming that it could antagonize the antifibrotic effects of RLX in more than one experimental setting/organ.
Constitutive Heteromers Can Form between RXFP1 and the AT1R but RLX Does Not Directly Bind to the AT1R
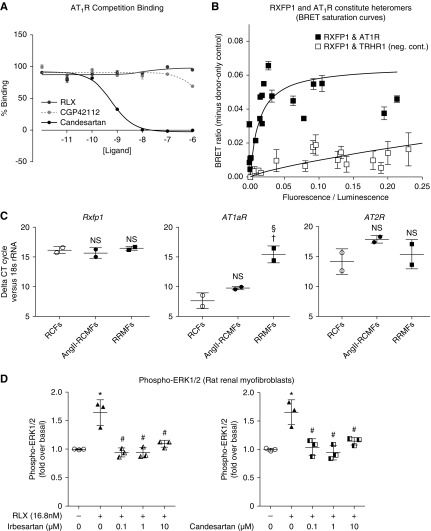
To determine if RLX could directly bind to the AT1R, competition binding assays were performed in HEK293 cells overexpressing the AT1R (Figure 4A). However, only candesartan (positive control) competed for AT1R binding (pIC50: 9.33±0.07), whereas neither RLX nor the AT2R agonist CGP42112 (used as a negative control) demonstrated any binding to the AT1R.
Figure 4.
RLX does not directly interact with the AT1R despite evidence for constitutive AT1R-RXFP1 heteromer formation. (A) Competition-binding assays in HEK293 cells over-expressing the AT1R demonstrated that RLX and an AT2R agonist (CGP42112) did not directly bind to the AT1R, whereas an AT1R antagonist (candesartan) did. (B) BRET saturation curves generated from transiently cotransfected HEK293 cells with a constant amount of Rluc8-tagged AT1R and increasing amounts of Venus-tagged RXFP1 are consistent with heteromers forming between RXFP1 and the AT1R (with saturation demonstrated with a maximum BRET ratio of 0.06), whereas no heteromerization appeared to occur between RXFP1 and the thyrotropin-releasing hormone receptor 1, which was used as a negative control. (C) (Myo)fibroblasts express RXFP1, AT1aR, and AT2R mRNA levels. Shown in the comparative cycle of threshold fluorescence at which RXFP1, AT1aR, and AT2R mRNA expression was detected, relative to the internal housekeeping gene 18s rRNA, by real-time PCR analysis of untreated and AngII (0.1 μM)-treated neonatal rat cardiac ventricular (myo)fibroblasts compared with RRMFs. Data were obtained from n=2 samples per group. NS denotes not significantly different compared with values from the untreated RCF group; †P<0.05 versus values from the RCF group; §P<0.05 versus values from the AngII-RCMF group. (D) The ability of RLX to significantly increase phospho-ERK1/2 levels (after 5 minutes) in RRMFs was abrogated by either candesartan or irbesartan (at 0.1–10 μM) coadministration, indirectly confirming that several RXFP1-expressing cells were also coexpressing AT1R. All data are presented as the mean±SEM relative to the untreated control group (which was expressed as 1), and obtained from n=3 separate experiments. *P<0.01 versus untreated cells; #P<0.01 versus RLX alone-treated cells. RCF, rat cardiac fibroblasts.
BRET saturation assays were next carried out in HEK293 cells to investigate AT1R:RXFP1 heteromerization, and provided evidence that constitutive complex formation between these two receptors could occur, with saturation occurring at a ratio of 0.06 (Figure 4B). On the other hand, no evidence of heteromerization between RXFP1 and the thyrotropin-releasing hormone receptor 1 was observed in a negative control interaction.
As the ability of RLX to stimulate phospho-ERK1/2 in vitro and in vivo was prevented by coadministration of the AT2R antagonist PD123319,25 we reasoned that several RXFP1-expressing cells may coexpress the AT2R. We therefore examined the effects of candesartan or irbesartan on phospho-ERK1/2 responses to acute RLX administration (5 minutes as opposed to versus 72 hours; Figure 1A) in primary RRMFs, which were found to express rxfp1, AT1aR, and AT2R genes (Figure 4C). Phospho-ERK1/2 levels 5 minutes after RLX addition to RRMFs were significantly increased, an effect that was fully blocked by either candesartan or irbesartan (0.1–10 μM) cotreatment (Figure 4C), confirming an acute, functional RXFP1:AT1R interaction in RRMFs.
Evidence for AT1R-AT2R-RXFP1 Crosstalk on Primary RRMFs
Our collective findings strongly suggested that the antifibrotic actions of RLX (through RXFP1) could be abrogated by pharmacologic blockade of either the AT1R or AT2R.25 We therefore determined if functional crosstalk was possible between all three receptors. This was done by evaluating whether the antifibrotic effects of RLX (16.8 nM) or the AT2R agonist C21 (1 μM) were affected by coadministration of the AT1R antagonist candesartan (10 μM) (Figure 5A) or the RXFP1 antagonist B-R13/17K51 (1 μM) (Figure 5B) in RRMFs. C21 and RLX significantly inhibited renal myofibroblast differentiation (α-SMA expression) and collagen I deposition over 72 hours in culture, by approximately 25%–35% of that measured in untreated cultures (all P<0.05 versus untreated group). Strikingly, the antifibrotic effects of either agonist in isolation or combined were fully antagonized by coadministration of candesartan (Figure 5A); however, the antifibrotic effects of either agonist alone were also blocked by coadministration of the RXFP1 antagonist (Figure 5B).
Figure 5.
Evidence for AT1R-AT2R-RXFP1 interactions on RRMFs. (A) Representative Western blots of α-SMA and collagen I from untreated RRMFs and cells treated with C21 (AT2R agonist; 1 μM) or RLX (16.8 nM) alone, C21 (1 μM) plus candesartan (10 μM), RLX (16.8 nM) plus candesartan (10 μM), or C21 (1 μM) plus RLX (16.8 nM) plus candesartan (10 μM) after 72 hours. (B) Representative Western blots of α-SMA and collagen I from untreated RRMFs and cells treated with C21 (1 μM) or RLX (16.8 nM) alone, an RXFP1 antagonist (antag; 1 μM) alone, C21 (1 μM) plus RXFP1 antagonist (1 μM), or RLX (16.8 nM) plus RXFP1 antagonist (1 μM) after 72 hours. (A and B) In each case, α-tubulin staining was included to demonstrate the quality and equivalent loading of protein samples. Also shown are the relative mean±SEM OD levels of α-SMA and collagen I (both corrected for α-tubulin loading) from each of the treatment groups analyzed (from n=4 separate experiments conducted in duplicate). *P<0.05 versus untreated cells; ¶P<0.01 versus C21 alone; #P<0.01 versus RLX-alone.
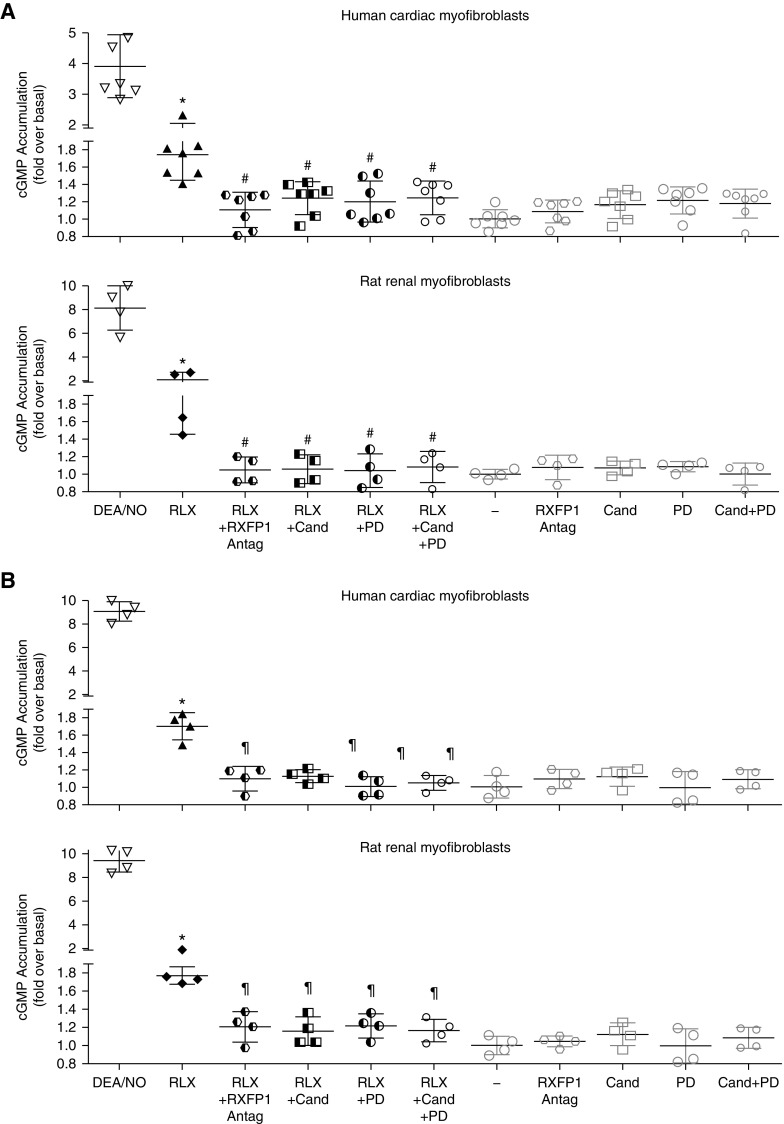
Evidence for AT1R-AT2R-RXFP1 Crosstalk on Acute Second Messenger Signaling in Primary Human Myofibroblasts and RRMFs
To examine if functional interactions between RXFP1, AT1R, and AT2R could also be detected more proximal to the receptors in an acute second messenger assay, we evaluated the effects of RLX (1 nM) and C21 (1 μM) on cGMP signaling, in the absence or presence of antagonists in HCMFs (ScienCell) and primary RRMFs (Figure 6, A and B, respectively). In these studies, the effects of RLX and C21 on cGMP accumulation were investigated, as cGMP has been linked to the effects of both RLX21–25 and C21.58,59 RLX and C21 both significantly increased cGMP levels when administered to HCMFs and RRMFs (Figure 6, A and B, respectively) by 1.6- to 1.8-fold after 40 minutes (all P<0.01 versus no treatment). The cGMP-promoting effects of either RLX (Figure 6A) or C21 (Figure 6B) were significantly inhibited by coadministration of the RXFP1 antagonist (1 μM) alone, candesartan (1 μM) alone, PD123319 (1 μM) alone, or the combined effects of candesartan (1 μM) and PD123319 (1 μM), once again indicating that the antifibrotic effects of an RXFP1 or AT2R agonist could be blocked by antagonists acting at each receptor.
Figure 6.
Evidence for acute AT1R-AT2R-RXFP1 interactions on HCMFs and RRMFs. (A) cGMP accumulation was significantly increased by RLX (1 nM) treatment of HCMFs or RRMFs after 40 minutes (n=4–7 experiments per cell type). (B) Likewise, cGMP accumulation was similarly increased by C21 (1 μM) treatment of HMMFs or RRMFs after 40 minutes (n=4 experiments/cell type). (A and B) In each case, the cGMP-promoting effects of RLX or C21 in HCMFs and RRMFs were significantly inhibited by pretreatment with an RXFP1 antagonist (1 μM) alone, candesartan (1 μM) alone, PD123319 (1 μM) alone, or candesartan (1 μM) plus PD123319 (1 μM). Each antagonist alone, however, did not significantly affect basal cGMP measurements. *P<0.01 versus untreated cells; #P<0.05 versus RLX alone, ¶P<0.05 versus C21 alone.
Discussion
This study investigated, for the first time, whether RLX required the presence of the AT1R to mediate its antifibrotic actions. As evidence has been presented previously for antagonism of AT1R activity being protective against renal60 and cardiac55 fibrosis, it was hypothesized that RLX would disrupt the profibrotic actions of the AngII-AT1R-TGF-β1 axis and that the antifibrotic effects of RLX would be enhanced by AT1R antagonists. However, we found that both acute RLX signaling (Figures 4D and 6) and its ability to downregulate functional end points associated with fibrosis (Figures 1–3 and 5) and upregulate various collagen-degrading MMPs (Figures 1 and 2) were abolished by coadministration of AT1R antagonists (irbesartan, candesartan) in all in vitro and in vivo assays performed. Although candesartan alone did not demonstrate any marked antifibrotic efficacy in the in vivo models studied (Figure 3, Supplemental Figure 2), potentially because of its slow-acting actions,56,57 it consistently blocked the effects of RLX in primary rat and human myofibroblasts (Figures 1, 5, and 6) as well as in the animals models evaluated (Figures 2 and 3). Collectively, this suggested that functional crosstalk could exist between RXFP1 and the AT1R in myofibroblasts when both receptors were expressed at sufficient levels.
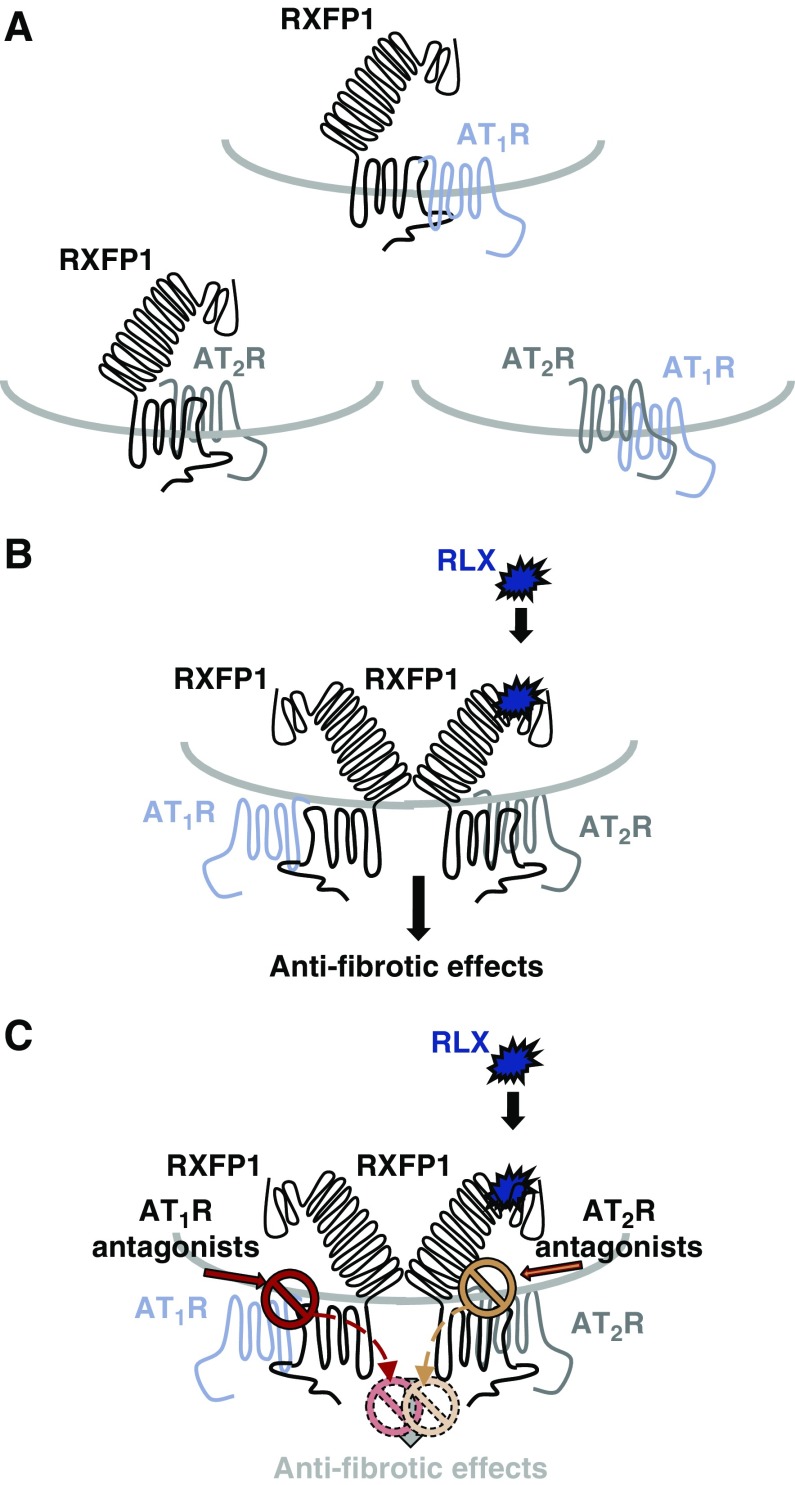
Although HEK293 cells overexpressing AT1Rs were used to confirm that RLX does not directly compete for AT1R binding (Figure 4A), BRET studies demonstrated that RXFP1 could form constitutive heteromers with the AT1R (Figure 4B). Hence, the presence of AT1R-RXFP1 heteromers provides a potential explanation for the finding that AT1R antagonists, acting on AT1Rs on myofibroblasts,26,31 can prevent the antifibrotic actions of RLX that are mediated through RXFP1.21 Further work is required to validate whether these receptors interact via heteromerization in myofibroblasts.
Our collective findings, however, show that the antifibrotic effects of RLX could be blocked by either AT1R or AT2R antagonists,25 and further suggest that interactions beyond RLX-RXFP1-AT1R or RLX-RXFP1-AT2R can occur. It is possible that crosstalk between RXFP1-AT1R and RXFP1-AT2R heteromers allows all three receptors to functionally interact. Such signaling complexes may enable antagonists acting either at the AT1R or AT2R to allosterically block the effects of RLX at RXFP1 (Figure 7). Additionally, it would suggest that RXFP1 and AT1R antagonists may block the actions of an AT2R agonist, and we were able to show that the acute signaling and downstream antifibrotic effects of C21 were blocked by either a RXFP1 or an AT1R antagonist (Figures 5 and 6). These results are strongly supportive of all these events occurring, but only if a functional crosstalk between all three receptors exists on myofibroblasts.
Figure 7.
A schematic illustration of how RLX potentially mediates its antifibrotic effects through AT1R-AT2R-RXFP1 complexes, which in turn, can be regulated by either AT1R or AT2R antagonists. (A) This study provides evidence for AT1R-RXFP1 heteromers forming constitutively, whereas previous studies have indicated that AT2R-RXFP125 and AT1R-AT2R35,37 heteromers can also constitutively form. (B) On the basis of our findings, we propose that crosstalk between RXFP1-AT1R and RXFP1-AT2R heteromers may be taking place on myofibroblasts. (C) This mechanism would allow antagonists acting at either the AT1R or AT2R to allosterically abrogate the antifibrotic effects of RLX that are mediated through these receptor functional interactions, respectively. Likewise, although not shown, this receptor interaction would allow AT1R or RXFP1 antagonists to allosterically abrogate the antifibrotic effects of AT2R agonists.
We are mindful that the expression of these receptors on myofibroblasts does not guarantee that they interact. However, it is becoming increasingly apparent that several class A G protein–coupled receptors,61 including AT1R and AT2R,35 and RXFP125,62 form heteromers or receptor complexes that are required to modulate function. RXFP1 is widely expressed in several organs,5 including the kidney and heart; is moderately decreased after injury in the rodent63 and human64 heart; and is downregulated by β1-adrenoceptor stimulation but upregulated by α1-adrenoceptor stimulation.65 AT1Rs are also widely distributed throughout the heart and kidney in both healthy and diseased states, but are significantly increased in expression in rodents and humans undergoing injury or disease, particularly when the renin-angiotensin system is upregulated.66 In comparison, AT2 receptors are expressed at low levels in tissues and fibroblasts under physiologically quiescent states (at higher levels in females than males29,67), but are dramatically upregulated in pathologic conditions affecting the heart and kidney.26,29,30,68 Therefore, AT1R-AT2R-RXFP1 crosstalk and/or functional interactions are most likely to be observed under pathologic conditions when components of the renin-angiotensin system are upregulated. These conditions that display increased AngII and TGF-β1 are precisely those where RLX8,10,12 and C2126,27 are known to mediate their antifibrotic effects against increased AngII and TGF-β1, whereas these interactions are less likely to occur under physiologic conditions.
Extending beyond the AT1R-AT2R35 or RXFP1-AT2R25 interactions that have previously been reported, our study clearly demonstrates for the first time that the antifibrotic effects of agonists acting at RXFP1 (RLX) or the AT2R (C21) are prevented by AT1R blockade; hence, the crosstalk between more than two receptors can allosterically influence agonist activity at multiple receptors. This implies that the antifibrotic effects of RLX and/or AT2R agonists may be compromised by ARBs (as demonstrated in Figures 1–6). Recent clinical trials evaluating the vasodilatory benefits of RLX in patients with acute heart failure in a phase 3 trial showed that it did improve dyspnea and end-stage mortality at 180 days post-treatment,69 but a more recent, extended phase 3 trial70 failed to demonstrate beneficial effects over patients receiving a placebo (Novartis press release71). The initial phase 3 trial69 was not sufficiently powered to determine if patients receiving ARBs or other medications had compromised benefits to receiving RLX, so it will be of significant interest to determine if the recent trial can shed more light on whether ARBs negatively affected the organ-protective effects of RLX at a clinical level. Furthermore, as one study at least has shown that combining the antifibrotic effects of the ACE inhibitor enalapril with RLX enhanced the antifibrotic efficacy of enalapril alone as well as maintaining the antifibrotic efficacy of RLX,42 it would be of interest to also determine if ACE inhibitors could be used as substitutes to ARBs and used in combination with RLX to offer wider cardioprotection in experimental and clinical settings.
Previous experimental studies combining the effects of ARBs and C21 in vivo49,72,73 have not demonstrated any negation of the organ-protective effects of C21, unlike the data obtained in this study that demonstrated that ARBs consistently negated the antifibrotic effects of RLX in vivo and in vitro. However, combining C21 with losartan or candesartan cilexetil did not demonstrate any additive effects in reducing renal fibrosis73 or atherosclerosis49 in experimental rat or mouse models of diabetes, respectively. Hence, further work is required to explain these discrepant findings in more complex models, particularly to determine whether the lack of any additive effects of the combination therapy on fibrosis regression were due to the inhibitory effects of the ARBs studied on C21 function in myofibroblasts.
In conclusion, we provide evidence that myofibroblasts express RXFP1, AT1R, and AT2R and that heteromers can form between RXFP1 and the AT1R; however, heteromer formation between RXFP1 and the AT2R25 as well as AT1R and AT2R35,37 has previously been proposed. We have also demonstrated in pathophysiologically relevant human and rodent myofibroblast culture models and murine models of fibrosis in vivo that the antifibrotic effects of RLX can be abrogated either by AT1R or AT2R antagonists,25 and that AT1R or RXFP1 blockade also antagonizes the myofibroblast differentiation- and collagen-inhibitory effects of an AT2R ligand acting alone or in combination with RLX (acting at RXFP1) in RRMFs. These findings support the idea that AT1R-AT2R-RXFP1 crosstalk can exist on matrix-producing myofibroblasts, which RLX and C21 can activate to mediate their antifibrotic effects, but in turn, can be allosterically regulated by antagonists acting at the other receptors of this receptor interaction (Figure 7). Moreover, they suggest that this receptor crosstalk may represent a previously unrecognized target for the regulation and treatment of organ fibrosis. Clearly, future work is required to determine if this receptor interaction affects fibrosis progression in other organs where all three receptors are expressed, in nonmyofibroblast cells that may affect the vasodilatory and angiogenic actions of RXFP1 and AT2R agonists, and in conditions such as pregnancy74,75 or preeclampsia,76,77 where an interaction between these receptors may influence relevant outcomes.
Disclosures
Prof. Bathgate reports grants from National Health and Medical Research Council (NHMRC) of Australia, during the conduct of the study; grants from Novartis, outside the submitted work; In addition, Prof. Bathgate has US patent 10,081,662 B2. Associate Prof. Pfleger reports grants from National Health and Medical Research Council, during the conduct of the study; grants, non-financial support and other from Dimerix, grants and non-financial support from BMG Labtech, outside the submitted work. In addition, Associate Prof. Pfleger has a patent WO/2008/055313 licensed to Dimerix has licensed to Excellerate Bioscience, a patent WO/2012/094703 issued, and a patent WO/2019/036753 pending.
Funding
This study was supported by a University of Melbourne Fee Remission Scholarship to Dr. Chow; Australian Postgraduate Scholarships to Mr. Shen, Ms. Y. Wang, and Ms. Barsha; Monash University International Scholarship to Mr. C. Wang; National Health and Medical Research Council of Australia (NHMRC) project grants GNT0436713, GNT0454375, GNT0628634, GNT1045848, and GNT1101552; an NHMRC program grant (GNT1055134) to Prof. Summers; NHMRC Senior Research Fellowships to Prof. Bathgate (GNT1042650), Prof. Denton (GNT1041844), and Associate Prof. Samuel (GNT1041766); and an NHMRC RD Wright Fellowship to Associate Prof. Pfleger (GNT1085842). Research conducted at the Florey Institute of Neuroscience and Mental Health was additionally supported by the Victorian Government’s Operational Infrastructure Support Program.
Supplementary Material
Acknowledgments
We are grateful to Dr. Andreas Loening and Dr. Sanjiv Gambhir (Stanford University, Palo Alto, CA), and Dr. Atsushi Miyawaki (RIKEN Brain Science Institute, Wako City, Japan) for providing complementary DNA constructs (for the bioluminescence resonance energy transfer assays), Prof. Walter Thomas (University of Queensland) for providing the HEK293 cells stably transfected with AT1Rs, and Associate Prof. Julie McMullen (Baker Heart and Diabetes Institute) for providing complementary deoxyribonucleic acid from unstimulated and angiotensin II–stimulated neonatal rat ventricular fibroblasts.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060597/-/DCSupplemental.
Supplemental Figure 1. Irbesartan does not affect the signal transduction end points associated with the antifibrotic actions of RLX, in the absence of RLX.
Supplemental Figure 2. Candesartan alone did not reduce renal fibrosis post-UUO at the time point studied.
Supplemental Figure 3. Candesartan alone reduced systolic BP and renal inflammation post-HS–induced nephropathy.
References
- 1.Wynn TA, Ramalingam TR: Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitson TD: Renal tubulointerstitial fibrosis: Common but never simple. Am J Physiol Renal Physiol 296: F1239–F1244, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Kong P, Christia P, Frangogiannis NG: The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71: 549–574, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby IA, Hewitson TD: Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257: 143–179, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ: Relaxin family peptides and their receptors. Physiol Rev 93: 405–480, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Danielson LA, Welford A, Harris A: Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol 17: 1325–1333, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Martin B, Gabris-Weber BA, Reddy R, Romero G, Chattopadhyay A, Salama G: Relaxin reverses inflammatory and immune signals in aged hearts. PLoS One 13: e0190935, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett RG: Relaxin and its role in the development and treatment of fibrosis. Transl Res 154: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XJ, Hewitson TD, Nguyen MN, Samuel CS: Therapeutic effects of serelaxin in acute heart failure. Circ J 78: 542–552, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Samuel CS, Summers RJ, Hewitson TD: Antifibrotic actions of serelaxin - new Roles for an old player. Trends Pharmacol Sci 37: 485–497, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Gai Y, Yang N, Lu B, Samuel CS, Thannickal VJ, et al.: Relaxin regulates myofibroblast contractility and protects against lung fibrosis. Am J Pathol 179: 2751–2765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, et al.: Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 145: 4125–4133, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Müller GA, et al.: The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int 68: 96–109, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Unemori EN, Amento EP: Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem 265: 10681–10685, 1990 [PubMed] [Google Scholar]
- 15.Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, et al.: Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest 98: 2739–2745, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D: Relaxin inhibits the activation of human neutrophils: Involvement of the nitric oxide pathway. Endocrinology 145: 1106–1112, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Nistri S, Cinci L, Perna AM, Masini E, Mastroianni R, Bani D: Relaxin induces mast cell inhibition and reduces ventricular arrhythmias in a swine model of acute myocardial infarction. Pharmacol Res 57: 43–48, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Brecht A, Bartsch C, Baumann G, Stangl K, Dschietzig T: Relaxin inhibits early steps in vascular inflammation. Regul Pept 166: 76–82, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Sha ML, Li D, Zhu YP, Wang XJ, Jiang CY, et al.: Relaxin abrogates renal interstitial fibrosis by regulating macrophage polarization via inhibition of Toll-like receptor 4 signaling. Oncotarget 8: 21044–21053, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocan M, Sarwar M, Ang SY, Xiao J, Marugan JJ, Hossain MA, et al. ,: ML290 is a biased allosteric agonist at the relaxin receptor RXFP1. Sci Rep 7: 2968, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, et al.: Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J 23: 1219–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Kemp-Harper BK, Kocan M, Ang SY, Hewitson TD, Samuel CS: The anti-fibrotic actions of relaxin are mediated through a NO-sGC-cGMP-dependent pathway in renal myofibroblasts in vitro and enhanced by the NO Donor, diethylamine NONOate. Front Pharmacol 7: 91, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ: Serelaxin-mediated signal transduction in human vascular cells: Bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol 172: 1005–1019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow BS, Chew EG, Zhao C, Bathgate RA, Hewitson TD, Samuel CS: Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: The additional involvement of iNOS. PLoS One 7: e42714, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, et al.: Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86: 75–85, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE: AT2 receptors: Functional relevance in cardiovascular disease. Pharmacol Ther 120: 292–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Del Borgo M, Lee HW, Baraldi D, Hirmiz B, Gaspari TA, et al.: Anti-fibrotic potential of AT2 receptor agonists. Front Pharmacol 8: 564, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peluso AA, Santos RA, Unger T, Steckelings UM: The angiotensin type 2 receptor and the kidney. Curr Opin Nephrol Hypertens 26: 36–42, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, et al.: Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 307: F901–F907, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Widdop RE, Jones ES, Hannan RE, Gaspari TA: Angiotensin AT2 receptors: Cardiovascular hope or hype? Br J Pharmacol 140: 809–824, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Ortega M, Egido J: Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int 52: 1497–1510, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Brewster UC, Setaro JF, Perazella MA: The renin-angiotensin-aldosterone system: Cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 326: 15–24, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schmieder RE, Ruilope LM, Barnett AH: Renal protection with angiotensin receptor blockers: Where do we stand. J Nephrol 24: 569–580, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Düsing R: Pharmacological interventions into the renin-angiotensin system with ACE inhibitors and angiotensin II receptor antagonists: Effects beyond blood pressure lowering. Ther Adv Cardiovasc Dis 10: 151–161, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porrello ER, Pfleger KD, Seeber RM, Qian H, Oro C, Abogadie F, et al.: Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell Signal 23: 1767–1776, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, et al.: The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: Gain-of-function study using gene transfer. Proc Natl Acad Sci U S A 92: 10663–10667, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, et al.: Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens 30: 1176–1184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams EJ, Benyon RC, Trim N, Hadwin R, Grove BH, Arthur MJ, et al.: Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut 49: 577–583, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain MA, Kocan M, Yao ST, Royce SG, Nair VB, Siwek C, et al.: A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1. Chem Sci (Camb) 7: 3805–3819, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasser JM, Cunningham MW Jr., Baylis C: Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 307: F1355–F1362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel CS, Zhao C, Bathgate RA, Bond CP, Burton MD, Parry LJ, et al.: Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis. FASEB J 17: 121–123, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Samuel CS, Bodaragama H, Chew JY, Widdop RE, Royce SG, Hewitson TD: Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension 64: 315–322, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Hewitson TD, Ho WY, Samuel CS: Antifibrotic properties of relaxin: In vivo mechanism of action in experimental renal tubulointerstitial fibrosis. Endocrinology 151: 4938–4948, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Nagai M, Horikoshi K, Izumi T, Seki S, Taniguchi M, Taniguchi I, et al.: Cardioprotective action of perindopril versus candesartan in renovascular hypertensive rats. Cardiovasc Drugs Ther 18: 353–362, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Zhao G, Li H, Liu X, Wang S: Candesartan antagonizes pressure overload-evoked cardiac remodeling through Smad7 gene-dependent MMP-9 suppression. Gene 497: 301–306, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Brooks WW, Conrad CH: Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: Structural and functional correlates. Comp Med 59: 339–343, 2009 [PMC free article] [PubMed] [Google Scholar]
- 48.Widdop RE, Li XC: A simple versatile method for measuring tail cuff systolic blood pressure in conscious rats. Clin Sci (Lond) 93: 191–194, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Chow BS, Koulis C, Krishnaswamy P, Steckelings UM, Unger T, Cooper ME, et al.: The angiotensin II type 2 receptor agonist Compound 21 is protective in experimental diabetes-associated atherosclerosis. Diabetologia 59: 1778–1790, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Fouda AY, Pillai B, Dhandapani KM, Ergul A, Fagan SC: Role of interleukin-10 in the neuroprotective effect of the Angiotensin Type 2 Receptor agonist, compound 21, after ischemia/reperfusion injury. Eur J Pharmacol 799: 128–134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hossain MA, Samuel CS, Binder C, Hewitson TD, Tregear GW, Wade JD, et al.: The chemically synthesized human relaxin-2 analog, B-R13/17K H2, is an RXFP1 antagonist. Amino Acids 39: 409–416, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Gallop PM, Paz MA: Posttranslational protein modifications, with special attention to collagen and elastin. Physiol Rev 55: 418–487, 1975 [DOI] [PubMed] [Google Scholar]
- 53.Hewitson TD, Darby IA, Bisucci T, Jones CL, Becker GJ: Evolution of tubulointerstitial fibrosis in experimental renal infection and scarring. J Am Soc Nephrol 9: 632–642, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE: Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121: 297–303, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Jones ES, Black MJ, Widdop RE: Influence of angiotensin II subtype 2 receptor (AT(2)R) antagonist, PD123319, on cardiovascular remodelling of aged spontaneously hypertensive rats during chronic angiotensin II subtype 1 receptor (AT(1)R) blockade. Int J Hypertens 2012: 543062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noda M, Matsuo T, Fukuda R, Ohta M, Nagano H, Shibouta Y, et al.: Effect of candesartan cilexetil (TCV-116) in rats with chronic renal failure. Kidney Int 56: 898–909, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Higashi K, Oda T, Kushiyama T, Hyodo T, Yamada M, Suzuki S, et al.: Additive antifibrotic effects of pioglitazone and candesartan on experimental renal fibrosis in mice. Nephrology (Carlton) 15: 327–335, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Siragy HM, Carey RM: The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest 97: 1978–1982, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siragy HM, Carey RM: The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klahr S, Morrissey JJ: Comparative study of ACE inhibitors and angiotensin II receptor antagonists in interstitial scarring. Kidney Int Suppl 63: S111–S114, 1997 [PubMed] [Google Scholar]
- 61.Franco R, Martínez-Pinilla E, Lanciego JL, Navarro G: Basic pharmacological and structural evidence for class A G-protein-coupled receptor heteromerization. Front Pharmacol 7: 76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svendsen AM, Vrecl M, Knudsen L, Heding A, Wade JD, Bathgate RA, et al.: Dimerization and negative cooperativity in the relaxin family peptide receptors. Ann N Y Acad Sci 1160: 54–59, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Xu Q, Lekgabe ED, Gao XM, Ming Z, Tregear GW, Dart AM, et al.: Endogenous relaxin does not affect chronic pressure overload-induced cardiac hypertrophy and fibrosis. Endocrinology 149: 476–482, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Dschietzig T, Alexiou K, Kinkel HT, Baumann G, Matschke K, Stangl K: The positive inotropic effect of relaxin-2 in human atrial myocardium is preserved in end-stage heart failure: Role of G(i)-phosphoinositide-3 kinase signaling. J Card Fail 17: 158–166, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Moore XL, Su Y, Fan Y, Zhang YY, Woodcock EA, Dart AM, et al.: Diverse regulation of cardiac expression of relaxin receptor by α1- and β1-adrenoceptors. Cardiovasc Drugs Ther 28: 221–228, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Regitz-Zagrosek V, Auch-Schwelk W, Neuss M, Fleck E: Regulation of the angiotensin receptor subtypes in cell cultures, animal models and human diseases. Eur Heart J 15[Suppl D]: 92–97, 1994 [DOI] [PubMed] [Google Scholar]
- 67.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM: Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Carey RM: Cardiovascular and renal regulation by the angiotensin type 2 receptor: The AT2 receptor comes of age. Hypertension 45: 840–844, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al.: RELAXin in Acute Heart Failure (RELAX-AHF) Investigators : Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 381: 29–39, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Teerlink JR, Voors AA, Ponikowski P, Pang PS, Greenberg BH, Filippatos G, et al.: Serelaxin in addition to standard therapy in acute heart failure: Rationale and design of the RELAX-AHF-2 study. Eur J Heart Fail 19: 800–809, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novartis : Novartis provides update on Phase III study of RLX030 (serelaxin) in patients with acute heart failure. 2017. Available at: https://www.novartis.com/news/media-releases/novartis-provides-update-phase-iii-study-rlx030-serelaxin-patients-acute-heart-failure. Accessed March 22, 2017
- 72.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL: Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 59: 291–299, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Castoldi G, di Gioia CR, Bombardi C, Maestroni S, Carletti R, Steckelings UM, et al.: Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in Zucker diabetic fatty rats. Am J Physiol Renal Physiol 307: F1123–F1131, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Yamasato K, Tsai PS, Davis J, Yamamoto SY, Bryant-Greenwood GD: Human relaxins (RLNH1, RLNH2), their receptor (RXFP1) and fetoplacental growth. Reproduction 154: 67–77, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda-Matsubara Y, Iwai M, Cui TX, Shiuchi T, Liu HW, Okumura M, et al.: Roles of angiotensin type 1 and 2 receptors in pregnancy-associated blood pressure change. Am J Hypertens 17: 684–689, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Conrad KP: G-Protein-coupled receptors as potential drug candidates in preeclampsia: Targeting the relaxin/insulin-like family peptide receptor 1 for treatment and prevention. Hum Reprod Update 22: 647–664, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hladunewich MA, Kingdom J, Odutayo A, Burns K, Lai V, O’Brien T, et al.: Postpartum assessment of the renin angiotensin system in women with previous severe, early-onset preeclampsia. J Clin Endocrinol Metab 96: 3517–3524, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.