Abstract
The high frequency of cognitive impairment in individuals on hemodialysis is well characterized. In-center hemodialysis patients are disproportionately affected by cognitive impairment compared with other dialysis populations, identifying hemodialysis itself as a possible factor. The pathophysiology of cognitive impairment has multiple components, but vascular-mediated cerebral injury appears to contribute based on studies demonstrating increased cerebral ischemic lesions and atrophy in brain imaging of patients on hemodialysis. Patients on hemodialysis may be at increased risk for cerebral ischemic injury disease due to vasculopathy associated with ESKD and from their comorbid diseases, such as hypertension and diabetes. This review focuses on the intradialytic cerebral hypoperfusion that can occur during routine hemodialysis due to the circulatory stress of hemodialysis. This includes a review of current methods used to monitor intradialytic cerebral perfusion and the structural and functional cognitive outcomes that have been associated with changes in intradialytic cerebral perfusion. Monitoring of intradialytic cerebral perfusion may become clinically relevant as nephrologists try to avoid the cognitive complications seen with hemodialysis. Identifying the appropriate methods to assess risk for cerebral ischemic injury and the relationship of intradialytic cerebral hypoperfusion to cognitive outcomes will help inform the decision to use intradialytic cerebral perfusion monitoring in the clinical setting as part of a strategy to prevent cognitive decline.
Keywords: hemodialysis, cognitive impairment, cerebral perfusion
Cognitive Impairment in Dialysis and Implication for Patients
In the United States, the ESKD population is over 700,000 and growing.1 Cohort studies demonstrate that two thirds of patients on hemodialysis (HD) suffer from cognitive impairment (CI) and half of those have severe impairment.2–5 CI is associated with higher mortality and hospitalization rates, and lower functional status and quality of life.6,7 CI reduces patients’ ability to adhere to the medications and dietary restrictions that are central to care and compromises their decision-making capacity. This is particularly important as we try to incorporate shared decision making regarding dialysis initiation and withdrawal, and in determining overall goals of care. The pathophysiology of cognitive decline in this population is unclear. However, one leading hypothesis suggests that cerebral ischemia during HD sessions may contribute to or accelerate cognitive decline. This review will summarize the current literature on HD-related cognitive decline, focusing on the importance of HD-related cerebral ischemic disease, reviewing the methods used to monitor intradialytic cerebral perfusion, and providing the results of studies evaluating intradialytic cerebral perfusion and cognitive outcomes.
The Role of HD in CI
Multiple factors may contribute to CI in the dialysis population, including the complications of renal disease such as anemia and uremia. However, it appears that the HD population suffers more CI when compared with patients with ESKD receiving peritoneal dialysis (PD), another form of dialysis. In a retrospective study of 121,623 Medicare subjects, those who initiated dialysis on HD had twice the cumulative incidence of dementia over the subsequent 3-year period compared with those who initiated dialysis on PD.8 This risk persisted after adjustment for differences in baseline demographics and comorbidities. However, these studies could not account for potential physician bias of directing patients with more cognitive ability toward PD.
Prospective studies evaluating cognitive differences between persons receiving PD and HD show similar results. A study of 25 PD and 41 HD participants with a median follow-up of 12 months demonstrated cognition declined faster in those on HD compared with PD, despite similar baseline Montreal Cognitive Assessment (MoCA) scores and adjustment for education and demographics.9 Participants with lower MoCA scores had reduced decision-making capacity. In another prospective study with participants matched on age, comorbidity, education, and employment status, patients receiving PD had better cognitive scores.10 However, testing environments were not comparable, with cognitive testing being done during HD in the unit compared with a quiet room for patients on PD. It is also important to note that none of the studies can fully account for differences between the two dialysis populations. However, the sole randomized controlled trial of PD versus HD needed to address inherent differences was stopped prematurely, due to poor enrollment.11 In contrast to the aforementioned studies, Kalirao et al.12 use nine cognitive tests and found similar levels of moderate to severe CI among patients receiving PD and HD (66.6% and 73.4%, respectively). A different study of 114 patients on PD had a lower rate of CI at 29%, but this study only used the MoCA score to classify patients.13 In the Kalirao study it is important to note that more participants on PD than HD had normal cognition (26% versus 13%). Participants receiving PD were also more likely to be of nonwhite race (45% versus 17%), which was independently associated with CI. It also demonstrated that participants on PD had greater impairments in the domain of memory (58%) compared with executive function (28%). In HD cohorts, executive function is more commonly impaired and is thought to reflect more vascular-mediated damage. A meta-analysis evaluating cognitive function and risk of dementia in patients on PD and HD, using many of the studies mentioned above, found that patients treated with PD had better cognitive outcomes and lower risk of dementia than those treated with HD.14 Despite some variability in the literature, there is greater support for more CI in patients with ESKD on HD, specifically vascular disease–mediated CI, compared with PD. This warrants further investigation to evaluate for HD process–related cognitive decline.
Mechanism for CI in HD
The mechanism for CI in HD is likely multifactorial but preliminary work shows that cerebral ischemic disease may contribute. In a large sample of Medicare recipients, there was a four- to eightfold increase in stroke rate in the months after HD initiation that then decreased but still remained double the cohort’s pre-HD baseline rate at 1 year.15 This demonstrates an increased risk of ischemic cerebrovascular disease after HD initiation. There is also extensive literature demonstrating severe white matter disease, lacunae and infarcts, and atrophy on brain imaging in patients on HD.2,16–18 In the general population, there is clinicopathological evidence that cerebrovascular disease is linked to CI.19 Types of parenchymal changes that are often seen in vascular-mediated CI include small or micro- and subcortical infarcts, cerebral structural atrophy, and demyelination in white matter. Consistent with the general population, the degree of white matter damage in patients on HD correlates with worse cognitive test scores,20 suggesting cerebrovascular disease contributes to cognitive dysfunction in the HD population.
The high degree of cerebral ischemic damage in patients on HD led investigators to evaluate systemic and cerebral perfusion during the HD treatment as a risk factor. Most patients on HD experience a decline in BP during HD; approximately 30% become hypotensive.21 HD-induced BP fluctuations, myocardial dysfunction, and vascular dysfunction from inflammation and endotoxin release may lead to hemodynamic instability precipitating perfusion abnormalities and end-organ damage.22–26 The brain has high blood flow, receiving 15%–20% of cardiac output, and is thus susceptible to ischemic damage. In normal physiology, blood flow to the brain remains relatively constant over a wide range of cerebral perfusion pressures (60–160 mm Hg).27 This is mainly due to the ability of large and small cerebral vasculature to dilate and constrict in response to pressure changes. This vascular tone can be impaired in some disease states. Chronic renal disease is associated with decreased cerebral blood flow (CBF), thought to be mediated by vascular endothelial damage.28 Patients with ESKD on HD may have an increased severity of risk factors that can impair the physiologic response and increase susceptibility to cerebral ischemic during the hemodynamic stress of HD. Vascular stiffness can be from ESKD-specific factors such as inflammation and vascular calcification from impaired calcium and phosphate regulation, along with common comorbidities including diabetes, hypertension, atherosclerosis, and older age.29–31 This vasculopathy decreases the ability to dilate and constrict effectively. Furthermore, local vasoactive substances, such as hydrogen ions and nitric oxide which are released during hypoxia to induce vasodilation, are already at abnormal levels in patients on HD and may not be effective.27,32 Once cerebral ischemia occurs, local lactic acidosis can further impair postischemic recovery due to direct neuron toxicity.33 The model of HD-induced circulatory stress in the setting of impaired cerebral autoregulation leading to cerebral hypoperfusion and ischemic injury is a common hypothesis illustrated in Figure 1.34–36 A recent study provides preliminary support by demonstrating a correlation between increased variation in systemic intradialytic BP and worse white matter disease.20 To better determine if white matter injury is from cerebral hypoperfusion resulting from HD-induced hemodynamic variation requires the ability to monitor cerebral perfusion during HD. This may be done using newer and innovative technology outlined below.
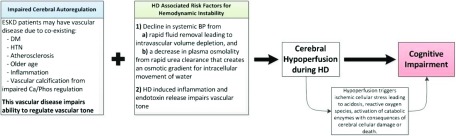
Figure 1.
This schematic illustrates the how ESKD patients on hemodialysis may be at risk for cerebral hypoperfusion and subsequent cognitive impairment. ESKD patients have risk factors that contribute to vascular disease and lead to impaired cerebral autoregulation. Pairing this vascular disease with the hemodynamic stress of HD may result in cerebral hypoperfusion during an HD session. Cerebral hypoperfusion may cause cerebral cellular damage and subsequent cognitive impairment. Ca/Phos, calcium/phosphate; DM, diabetes mellitus; HTN, hypertension.
Results of Studies Evaluating Cerebral Perfusion during HD
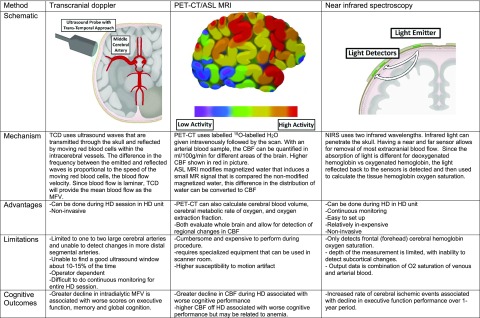
Three main methods are used to evaluate intradialytic cerebral perfusion: blood flow velocity by transcranial Doppler (TCD), CBF using computed tomography (CT) scanning and magnetic resonance imaging (MRI), and cerebral hemoglobin oxygen saturation using near-infrared spectroscopy (NIRS). Each method has its unique advantages and drawbacks with some variation in findings (see Figure 2).
Figure 2.
Overview of technology used to measure cerebral perfusion in patients on HD including information on mechanism, the advantages and limitations of each technology and cognitive outcomes noted when using the various measurements. ASL, arterial spin labeling; H2O, water; 15O, oxygen-15; O2, oxygen.
CBF Velocity Using TCD
TCD can be performed at the bedside and detects Doppler signals through the cranium, allowing for real-time measurement of blood flow velocity of intracranial arteries. The mechanism along with advantages and limitations is further described in Figure 2. The main limitations noted with this technique are that blood flow velocity measurements are restricted to larger proximal arteries and the method is technically difficult, relying on finding a good ultrasound window for the probe, which can be absent in 10%–15% of participants.37 TCD has been used to measure cerebral blood velocity in HD cohorts since the 1980s. In the initial small studies measuring cerebral blood velocity in main cranial arteries, the mean flow velocity (MFV) overall decreased after HD, by an order of 11%–21%, although the magnitude of decline varied.38–41 The decrease in MFV is associated with increased ultrafiltration (UF) volume.41 However, it is difficult to know if decreases in MVF lead to cerebral ischemia, because a dilated vessel may decrease blood flow velocity but still maintain blood flow to tissues. This is illustrated by increased hematocrit (which correlates with decreased MFV) being associated with vessel dilation and preserved oxygen delivery. To understand if reductions in MFV during HD indicate cerebral ischemia, a larger study used TCD to evaluate MFV during HD and its relationship to outcomes of cerebral structure and function.42 In this study of 97 participants on HD, the MVF decreased by about 10% during HD and the decrease correlated with higher UF. The association between MFV decrease and cognitive outcomes is described in the cognitive outcomes section below. Overall the literature on TCD indicates a reduction in MFV during HD. However, most studies were small (<30 participants), requiring replication in larger cohorts to confirm findings. Furthermore, more investigation of determinants of and outcomes associated with decrease in MFV in cerebral vessels is needed, as this may not always indicate cerebral ischemia.
CBF Using CT Scans and MRI
Directly measuring CBF can be done using positron emission tomography–CT (PET-CT) scan or arterial spin labeling MRI. Figure 2 provides a brief overview of this technique along with advantages and limitations. The main advantage is that whole brain scans can be done, thus allowing for the ability to detect regional changes in CBF (in ml/100 g per minute). However, the technique is burdensome and most studies using this method are not conducted while patients are on HD. In one of the first studies using PET to measure CBF in patients on HD, the authors evaluated the effect of anemia and found that seven patients treated with HD (not during HD) had higher CBF compared with six nondialysis controls.43 The CBF then decreased to near control levels (from 9.8±1.3 to 14.2±0.6 g/dl) once anemia in the patients on HD was corrected. This demonstrates the brain’s ability to increase blood flow to compensate for reduced oxygen carrying capacity and that, although in a small sample size, CBF was preserved in patients treated with HD when not receiving HD. Using arterial spin labeling MRI to measure CBF, Jiang et al.44 similarly found that CBF was higher in patients with ESKD compared with healthy controls, and that CBF had a strong negative correlation with hemoglobin level. Thus, baseline CBF is preserved or higher in patients treated with HD (off HD) compared with patients not on dialysis, but this may be due to anemia and is unlikely to indicate improved cerebral oxygen delivery.
To evaluate the effect of HD on CBF, Polinder-Bos et al.45 recently measured CBF in 12 elderly participants during an HD session by conducting HD in the scanner room. Global CBF had a mean decrease of 10%±15% from the pre-HD reading to the end HD reading, with loss of consciousness in one patient who had a 20% decrease in CBF during HD. The authors did not find a relationship between baseline hematocrit and change in CBF, but they did not evaluate the relationship between change in hematocrit and change in CBF during HD. These studies measuring CBF demonstrate a clear association with anemia levels when off HD, but also indicate there are changes in CBF during HD that indicate lack of adequate perfusion.
Cerebral Oxygenation Using NIRS Technology
NIRS is a noninvasive method for measuring cerebral hemoglobin oxygen saturation in the clinical setting. The sensors use wavelengths that penetrate the skull to measure cerebral hemoglobin oxygen saturation (hereafter referred to as cerebral oxygen saturation). Figure 2 provides an overview of this technique along with the main advantages and limitations. Cerebral oxygen saturation is largely determined by cerebral metabolic rate of oxygen and oxygen delivery to the brain. Cerebral oximetry is monitored in clinical settings when risk of cerebral ischemia is high, such as carotid endarterectomy. Advantages include ease of use and continuous monitoring. The main disadvantages are limitations in coverage (usually restricted to the frontal lobe) and inability to detect subcortical flow, which would be important for detecting potential ischemia in white matter tracts. In the HD cohort studies, pre-HD baseline cerebral oxygen saturation measured by NIRS is between 40% and 55% compared with 70% in healthy controls.46–48 This baseline reduction seems to be partly explained by anemia common to patients on HD, as improvement of anemia with blood transfusion significantly increased cerebral oxygen saturation levels in a cohort of patients on HD.49 Anemia can lead to increased oxygen extraction from hemoglobin. In addition, lower serum pH, having diabetes, and more years on HD are associated with lower cerebral oxygen saturation values at baseline, likely due to shifts in the hemoglobin-oxygen disassociation curve and endothelial dysfunction.50
Although pre and post-HD cerebral oxygen saturation measurements appear stable, when evaluating the entire HD session there are periods of decline.46–48 This intradialytic decline is associated with a faster UF rate.48 In the largest study to date with 58 participants and 635 HD sessions, authors found cerebral ischemia, defined as a >15% relative drop in cerebral oxygen saturation for at least 2 minutes, occurred in 24% of HD sessions.51 The rate of cerebral ischemia increased with greater decline in mean arterial BP (MAP), particularly when MAP fell below 60 mm Hg. However, change in MAP was not able to predict cerebral ischemia during HD. This may be due to the heterogeneity in cerebral autoregulation impairment in the study population, with only 32% of participants having impaired cerebral autoregulation (determined by a liner change in cerebral oxygen saturation for each MAP level). This rate may be due to the relatively low prevalence (38%) of ESKD due to diabetes or hypertension in the cohort. Prevalence of impaired cerebral autoregulation in the United States HD population may be higher as both diabetes and hypertension are more common and can cause vasculopathy.
Overall the current literature shows that patients on HD have decreased levels of cerebral oxygen saturation compared with healthy controls and that declines in cerebral oxygen saturation can occur during an HD session in some patients. The effects of intradialytic declines in cerebral oxygen saturation on cognitive function and brain structure have not been well studied but the limited results are noted in the following section.
Association between Cerebral Perfusion and Cognitive Outcomes
Although changes in CBF and oxygenation occur during the HD treatment, it is not clear if this affects the patients’ cognitive ability or leads to cerebral structural changes. The association of CI and cerebral structural disease with intradialytic changes in CBF or cerebral oxygenation is still undetermined. Several of the studies in this review evaluated for associations between cerebral perfusion and cognitive test scores with conflicting results. In one study, lower CBF off HD was associated with a higher cognitive score.44 The authors hypothesized this could be due to greater uremic toxin delivery. Another explanation is the confounding role of anemia in CI, as adjustment for hemoglobin reduced the association. This study did not evaluate for an association with CBF and cerebral structural lesions. Conversely, when looking at changes in CBF during HD, Polinder-Bos et al.45 found that patients with a decrease in CBF during the HD had lower scores on a measure of attention and executive function. This indicates the importance of timing of the CBF measurement: comparing a baseline measure off HD versus detecting changes during HD. In the larger study using TCD to measure MFV during HD, there was a decline in performance on global cognition, executive function tests, and memory tests from interdialytic assessment to intradialytic assessment, and the decline correlated with the percentage decline in MFV.42 In addition, although cognitive performance for the overall cohort was similar from baseline to 12 month follow-up, those with a higher percentage decline of MFV during HD had greater decline in scores on global cognition and executive function. They did not find any correlation between baseline MFV and change in cerebral structures on MRI but were underpowered with only 24 participants having repeat imaging. The cognitive performance results are consistent with results from NIRS data, where greater decline in cognitive score was associated with increased time with cerebral ischemia (a decrease of 15% in cerebral oxygen saturation from baseline) during HD.51
The data on measurements of intradialytic cerebral perfusion in relationship to cognitive outcomes remain limited with some variability in results. The variability in the relationship with CI may be due to differences in the tests used to measure cognition. Commonly used measures of cognitive performance include the Mini-Mental Status Exam and MoCA (which are less sensitive screening tests of global cognition) to more domain-specific tests measuring memory, executive function, and attention. Although executive function is noted to be commonly impaired, even the affected domains vary between studies and cognitive tests have overlap in the domains they measure that cannot be separated out. Due to inherent variability in the heterogeneous HD population, a comprehensive battery of tests is likely needed to detect changes in cognition. However, despite some variability in results, the overall preliminary evidence indicates that changes in intradialytic cerebral hemodynamics and oxygenation have been associated with harmful effects on cognitive ability.
Summary
Cognitive decline occurs in patients on HD and there is evidence that cerebral ischemic injury contributes to this. This ischemic injury may be occurring due to HD-induced cerebral hypoperfusion. There are various methods to evaluate for intradialytic cerebral perfusion changes and each has their own advantages and limitations. Although some methods allow for greater evaluation of regional hypoperfusion, such as PET-CT, they are also cumbersome and cannot be done in a usual clinical setting. More mobile methods such as TCD and NIRS allow for measurement in the dialysis units but they only measure specific regions. NIRS is easily operated and capable of continuous monitoring and thus avoids missing any changes that could occur during HD. However, the sensitivity of NIRS to detect small changes may be limited. In a comparison of change in CBF during HD as measured by PET-CT and change in cerebral oxygen saturation measured by NIRS, the parameters were variably correlated in a small study of 12 participants.52 This is not surprising given the difference in parameters that are measured by each technique. Promisingly, across the various techniques measuring cerebral perfusion parameters, results consistently indicate that a decrease in cerebral perfusion likely occurs during HD. To better understand the pathophysiology of CI in HD, we must fully examine the relationship between intradialytic changes in cerebral perfusion and patient cognition and cerebral lesions. It is important to refine methods to accurately capture changes in cerebral perfusion during HD and then determine the level of change in cerebral perfusion associated with cognitive or structural changes. Using that information, we may be able to use intradialytic monitoring of cerebral perfusion as a point-of-care clinical tool to prevent unintended cognitive consequences of HD. Preserving cognition is important for our patients’ ability to manage their disease and make important decisions on their healthcare. Preventing cognitive decline will also improve the health-related quality of life for patients on HD.
Disclosures
None.
Funding
Dr. Wolfgram is supported by a National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK113119.
Acknowledgments
Dr. Theresa Maatman created all illustrations used in the figures for this review.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. : US renal data system 2018 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 73[3S1]: A7–A8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ: Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci 134: 83–88, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Pereira AA, Weiner DE, Scott T, Sarnak MJ: Cognitive function in dialysis patients. Am J Kidney Dis 45: 448–462, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Wolfgram DF, Sunio L, Vogt E, Smith HM, Visotcky A, Laud P, et al. : Haemodynamics during dialysis and cognitive performance. Nephrology (Carlton) 19: 771–776, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al. : Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kimmel PL, Thamer M, Richard CM, Ray NF: Psychiatric illness in patients with end-stage renal disease. Am J Med 105: 214–221, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Wolfgram DF, Szabo A, Murray AM, Whittle J: Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 35: 189–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyasere O, Okai D, Brown E: Cognitive function and advanced kidney disease: Longitudinal trends and impact on decision-making. Clin Kidney J 10: 89–94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann D, Mau W, Wienke A, Girndt M: Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int 93: 430–438, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, et al. ; NECOSAD Study Group: Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: A randomized controlled trial. Kidney Int 64: 2222–2228, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, et al. : Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 57: 612–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea YF, Lam MF, Lee MS, Mok MY, Lui SL, Yip TP, et al. : Prevalence of cognitive impairment among peritoneal dialysis patients, impact on peritonitis and role of assisted dialysis. Perit Dial Int 36: 284–290, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian X, Guo X, Xia X, Yu H, Li X, Jiang A: The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 98: e14390, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray AM, Seliger S, Lakshminarayan K, Herzog CA, Solid CA: Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 24: 1166–1173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimitsu T, Hirakata H, Fujii K, Kanai H, Hirakata E, Higashi H, et al. : Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin Nephrol 53: 445–451, 2000 [PubMed] [Google Scholar]
- 17.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, et al. : Anatomic brain disease in hemodialysis patients: A cross-sectional study. Am J Kidney Dis 61: 271–278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seliger SL, Weiner DE: Cognitive impairment in dialysis patients: Focus on the blood vessels? Am J Kidney Dis 61: 187–190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalaria RN: Cerebrovascular disease and mechanisms of cognitive impairment: Evidence from clinicopathological studies in humans. Stroke 43: 2526–2534, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Eldehni MT, Odudu A, Mcintyre CW: Brain white matter microstructure in end-stage kidney disease, cognitive impairment, and circulatory stress. Hemodial Int 23: 356–365, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, et al. : Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 9: 2124–2132, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. : Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre CW: Recurrent circulatory stress: The dark side of dialysis. Semin Dial 23: 449–451, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Marants R, Qirjazi E, Grant CJ, Lee TY, McIntyre CW: Renal perfusion during hemodialysis: Intradialytic blood flow decline and effects of dialysate cooling. J Am Soc Nephrol 30: 1086–1095, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim M, Behairy M, El-Ashry M, Mostafa AE: Cardiovascular risk of circulating endotoxin level in prevalent hemodialysis patients. Egypt Heart J 70: 27–33, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cipolla MJ: Control of cerebral blood flow. In: The Cerebral Circulation, edited by Cipolla MJ, San Rafael, CA, Morgan & Claypool Life Sciences, 2009 [PubMed] [Google Scholar]
- 28.Sedaghat S, Vernooij MW, Loehrer E, Mattace-Raso FU, Hofman A, van der Lugt A, et al. : Kidney function and cerebral blood flow: The rotterdam study. J Am Soc Nephrol 27: 715–721, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YS, Seifert T, Brassard P, Rasmussen P, Vaag A, Nielsen HB, et al. : Impaired cerebral blood flow and oxygenation during exercise in type 2 diabetic patients. Physiol Rep 3: e12430, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, et al. : Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Pires PW, Dams Ramos CM, Matin N, Dorrance AM: The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar SR, Kaitwatcharachai C, Levin NW: Nitric oxide and hemodialysis. Semin Dial 17: 224–228, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kalimo H, Rehncrona S, Söderfeldt B, Olsson Y, Siesjö BK: Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab 1: 313–327, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA: Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol 24: 353–363, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIntyre CW, Goldsmith DJ: Ischemic brain injury in hemodialysis patients: Which is more dangerous, hypertension or intradialytic hypotension? Kidney Int 87: 1109–1115, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Markus HS: Transcranial Doppler ultrasound. Br Med Bull 56: 378–388, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Postiglione A, Faccenda F, Gallotta G, Rubba P, Federico S: Changes in middle cerebral artery blood velocity in uremic patients after hemodialysis. Stroke 22: 1508–1511, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Hata R, Matsumoto M, Handa N, Terakawa H, Sugitani Y, Kamada T: Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke 25: 408–412, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Metry G, Spittle M, Rahmati S, Giller C, Giller A, Kaufman A, et al. : Online monitoring of cerebral hemodynamics during hemodialysis. Am J Kidney Dis 40: 996–1004, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Stefanidis I, Bach R, Mertens PR, Liakopoulos V, Liapi G, Mann H, et al. : Influence of hemodialysis on the mean blood flow velocity in the middle cerebral artery. Clin Nephrol 64: 129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Findlay MD, Dawson J, Dickie DA, Forbes KP, McGlynn D, Quinn T, et al. : Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol 30: 147–158, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metry G, Wikström B, Valind S, Sandhagen B, Linde T, Beshara S, et al. : Effect of normalization of hematocrit on brain circulation and metabolism in hemodialysis patients. J Am Soc Nephrol 10: 854–863, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Jiang XL, Wen JQ, Zhang LJ, Zheng G, Li X, Zhang Z, et al. : Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: An arterial-spin labeling MR imaging. Metab Brain Dis 31: 929–936, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Polinder-Bos HA, García DV, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP, et al. : Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol 29: 1317–1325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, et al. : Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract 126: 57–61, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E: Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861–1869, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Malik J, Kudlicka J, Lachmanova J, Valerianova A, Rocinova K, Bartkova M,et al. : Tissue ischemia worsens during hemodialysis in end-stage renal disease patients. J Vasc Access 18: 47–51, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Ookawara S, Ueda Y, Miyazawa H, Kofuji M, Hayasaka H, et al. : Changes in cerebral oxygenation associated with intradialytic blood transfusion in patients with severe anemia undergoing hemodialysis. Nephron Extra 7: 42–51, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito K, Ookawara S, Ueda Y, Goto S, Miyazawa H, Yamada H, et al. : Factors affecting cerebral oxygenation in hemodialysis patients: Cerebral oxygenation associates with pH, hemodialysis duration, serum albumin concentration, and diabetes mellitus. PLoS One 10: e0117474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L: Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 28: 2511–2520, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polinder-Bos HA, Elting JWJ, Aries MJ, García DV, Willemsen AT, van Laar PJ,et al. : Changes in cerebral oxygenation and cerebral blood flow during hemodialysis - A simultaneous near-infrared spectroscopy and positron emission tomography study [published online ahead of print December 12, 2018]. J Cereb Blood Flow Metab doi:10.1177/0271678X18818652 [DOI] [PMC free article] [PubMed] [Google Scholar]