Short abstract
This study aimed to explore the role of lncRNA GAS5 in the regulation of the killing effect of NK cells on liver cancer. Compared with a control group, lncRNA GAS5, RUNX3, and NCR1 were down-regulated in NK cells of patients with liver cancer, whereas miR-544 expression was up-regulated in NK cells of patients with liver cancer. Activated NK cells had higher IFN-γ level. Knockdown of GAS5 in activated NK cells decreased IFN-γ secretion, NK cell cytotoxicity, the percentage of CD107a+ NK cells, and the apoptosis rate of HepG2 and Huh7 cells. We also proved the interaction of GAS5 and miR-544, and the negative regulation role of GAS5 on miR-544. GAS5 overexpression in activated NK cells increased RUNX3 expression, IFN-γ secretion, the NK cell cytotoxicity, the percentage of CD107a+ NK cells, and the apoptosis rate of HepG2 cells, while miR-544 mimic abolished the promotion effect of GAS5 overexpression. Finally, in vivo experiments indicated an inhibition effect of GAS5 in tumor growth. LncRNA GAS5 overexpression enhances the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3.
Keywords: lncRNA GAS5, miR-544, RUNX3, natural killer cell, liver cancer
Introduction
Liver cancer is the fifth leading cause of cancer death in the United States, with a 5-yr relative survival rate as low as 18%; it is estimated that 17,414 new deaths occurred in the United States every year.1 Liver transplantation and resection are the main radical cures; however, these treatments always lead to more complications and increase the risk of recurrence.2,3 Therefore, it is important to develop new therapeutic methods for the treatment of liver cancer. Liver has a high proportion of innate immune cells containing NK cells, NKT cells, dendritic cells, and others. Because of the capacity of NK cells to eliminate virus-infected or transformed cells, NK cells are considered as the first line of defense against infection and cancer.4,5 Recently, MRI-guided interventional NK cell delivery permits local delivery to liver cancers to improve clinical outcomes in patients with liver cancer.6 Thus, focus on NK cells might be a new direction to find new therapies for the treatment of liver cancer.
NKp46 is an activating receptor encoded by natural cytotoxicity receptor 1 (NCR1) and can be expressed in resting and activated NK cells, which induces IFN-γ release, enhances the killing effect, and inhibits tumor growth and metastasis.7 Lai et al. found that Runt-related transcription factor 3 (RUNX3) is involved in the transcription regulation of NCR1/NKp46, and enhanced the strength of the NCR1 promoter in NK cells, and RUNX3 overexpression increased NCR1 expression.8 Our previous report has shown that miR-544 promoted immune escape through regulating the RUNX3/NCR1/NKp46 pathway in liver cancer.9 Hence, the RUNX3/NCR1/NKp46 pathway is important in modulating the killing effect of NK cells and cancer progression.
Long non-coding RNAs (lncRNAs) are RNAs longer than 200 nucleotides that modulate apoptosis, invasion, metastasis, epithelial-to-mesenchymal transition regulation, etc.10–12 LncRNA GAS5 (growth arrest-specific transcript 5) is expressed widely in hepatocellular carcinoma, breast cancer, bladder cancer, osteosarcoma, etc.13–15 Sun et al. reported that lncRNA GAS5 was down-regulated in gastric cancer tissues, and GAS5 overexpression decreased gastric cancer cell proliferation and inhibited gastric cancer cells tumorigenesis in vivo.16 Qian et al. found that GAS5 inhibited liver fibrosis, liver tumor migration, and invasion.17 Tao et al. indicated that GAS5 might act as a proto-oncogene in hepatocellular carcinoma.18 Bioinformatics software predicted binding sites between lncRNA GAS5 and miR-544, which indicated the regulation of miR-544 by GAS5. Besides, lncRNA GAS5 is an immune-related lncRNA that can be expressed in CD4+T cells, macrophages, and leukocytes.19–21 Thus, we speculated that lncRNA GAS5 might be involved in the regulation of the killing effect of NK cells on liver cancer through the miR-544/RUNX3//NCR1/NKp46 pathway.
In this study, we found that lncRNA GAS5 was down-regulated in the NK cells of patients with liver cancer, and GAS5 inhibition suppressed the cytotoxicity of NK cells. GAS5 overexpression increased IFN-γ secretion and enhanced NK cell cytotoxicity through miR-544/RUNX3.
Materials and methods
Isolation of primary human NK cells
Venous blood was obtained from healthy controls (n = 12) and patients with liver cancer (n = 20) in The Second Affiliated Hospital and Yuying Children’s Hospital, and PBMCs were isolated from venous blood by Histopaque-1077 (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. All patients signed informed consent. NK cells were harvested using a Human NK cells separation medium kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions, and purified by differential attachment using flow cytometry as previously reported.9
Cell culture and transfection
Human NK cell line NK92 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and cultured in Minimum Essential Medium α (MEM α, Gibco, Waltham, MA, USA) supplemented with 12.5% horse serum (Gibco), 12.5% FBS (Gibco), 2 mM L-glutamine (Sinopharm Chemical Reagent, Shanghai, China) and 1.5 g/l sodium bicarbonate (Sinopharm Chemical Reagent) at 37°C under humidified air with 5% CO2. For the activation of NK92 cells, NK92 cells were stimulated with 100 U/ml of IL-2 (BD Biosciences, Franklin Lakes, NJ, USA) for 24 h. Lentiviral expression vector was constructed by ViraPower™ II Lentiviral Gateway™ Expression System (Invitrogen, Carlsbad, CA, USA). NK92 cells were transfected with lentivirus-mediated GAS5 knockdown (si-GAS5), lentivirus-mediated GAS5 overexpression (lenti-GAS5), lentivirus negative control (lenti-NC), lentivirus-mediated miR-544 mimic, or scramble sequence set as negative control (pre-NC).
Co-culture of NK92 cells and HepG2 cells or Huh7 cells
Human hepatic cancer cell line HepG2 or Huh7 was purchased from ATCC, and 1.5 × 105 cells/ml diluted with DMEM medium (Gibco) supplemented with 10% FBS were seeded in 96-well plates at 100 μl/well. IL-2-activated NK92 cells with different transfection were co-cultured with HepG2 or Huh7 at effector cell/target cell (E:T) ratios of 10:1 for 4 h at 37°C. NK92 cells were collected for testing the cytotoxicity using flow cytometry.
Cytotoxicity assay
NK cell cytotoxicity was detected by CytoTox 96 Non-Radioactive Cytotoxicity Assay according to the manufacturer's instructions. Briefly, 2 × 104 cells/100 μl cells were seeded in 96-well plates, and 10 μl of 10× Lysis Solution/100 μl was added 45 min before adding CytoTox 96® Reagent. Then, 50 μl of the CytoTox 96® Reagent was added to each well. The plate was incubated at dark for 30 min at room temperature before adding 50 μl of Stop Solution to each well; absorbance at 490 nm was recorded within 1 h. Percent cytotoxicity = 100 × Experimental LDH Release (OD490)/Maximum LDH Release (OD490).
ELISA
NK92 cells or primary NK cells were seeded in 96-well plates at a density of 5 × 103 cells/well for 4 h, and centrifuged at 200 g for 4 min. The supernatants were collected to detect the levels of IFN-γ using IFN-γ Human ELISA Kit (Invitrogen). Optical density (OD) was measured at 450 nm with an iMark reader (Bio-Rad, Hercules, CA, USA).
Analysis of CD107a+ NK cells
NK92 cells were incubated with PE Mouse anti-human CD107a (BD Biosciences) for 30 min at 4°C. After washing twice with PBS, cells were incubated with FITC-labeled goat anti-mouse IgG (BD Biosciences) in the dark for 30 min at 4°C, and analyzed by FACSCalibur flow cytometer (BD Biosciences).
Annexin V-PI double staining assay
HepG2, Huh7, or primary liver cancer cells were collected and washed in cold PBS. Annexin-binding buffer (1X) was prepared, then 5 μl of the 1 mg/ml PI solution was diluted in 45 μl 1X annexin-binding buffer. Cells were re-suspended in 1X annexin-binding buffer to 1 × 106 cells/ml. FITC annexin V (5 μl) and PI solution (1 μl) were added to 100 μl cell suspension for 15 min at room temperature (25°C). Then, 400 μl 1X annexin-binding buffer was added, gently mixed, and the mixtures were kept on ice. The stained cells were analyzed by flow cytometry.
Quantitative real-time RCR (qRT-PCR)
Total RNAs from primary human NK cells or NK92 cells were extracted by Trizol (Invitrogen), and inversely transcribed into cDNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). qRT-PCR was conducted to measure GAS5, miR-544, and NCR1 expression using PowerUp™ SYBR™ Green Master Mix (Invitrogen). The relative expressions of GAS5, miR-544 and NCR1 were calculated using the 2-ΔΔCt method. Specific primers for GAS5, miR-544, and NCR1 were as follows: GAS5, F: 5′- AGCTGGAAGTTGAAATGG-3′ and R: 5′-CAAGCCGACTCTCCATACC-3′; miR-544, F: 5′-GGAACTCGAGCCGCTGCCTTAAGTCACTCTCTAT-3′ and R: 5′-GGAAGGATCCCACAACTGACCACAGTTT-3′; NCR1, F: 5′-TCTAGACGGCAGTAGAAGGTC-3′ and R: 5′-CTTGCTGGATCTGGTGGTAA-3′.
Western blot
NK cells, NK92 cells, and tumor tissues were lysed in Radio Immunoprecipitation Assay (RIPA) buffer (Beijing Solarbio Science and Technology, China). Protein samples was separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated with primary Abs against RUNX3 (1:1000; Cell Signaling Technology, Danvers, MA, USA), NCR1 (1:500; Abcam, Cambridge, MA, USA) at 4°C overnight, and then incubated with HRP-conjugated secondary Ab (Abcam, USA) at room temperature for 1 h. Bands were visualized by an enhanced chemiluminescence reagent (Bio-Rad), and band intensities were quantified using image software Image Lab (Bio-Rad). β-Actin (Abcam) was used as an internal control.
RNA immunoprecipitation (RIP) assay
RIP was performed using Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore). NK cell lysate was prepared from 2 × 107 cells using 100 μl RIP lysis buffer added with 0.25 μl RNase inhibitor and 0.5 μl protease inhibitor on ice. After centrifugation of cell lysate, the supernatant was incubated with RIP buffer containing protein-A/G-Sepharose beads conjugated with anti-AGO2 Ab or negative control IgG. After obtained the RNA-binding protein complex, GAS5, and miR-544 in the precipitates were detected by qRT-PCR.
RNA pull-down
The biotin labeled lncRNA GAS5 was transcribed with the Biotin RNA Labeling Mix (Roche, Basel, Switzerland) and T7 RNA polymerase (Roche). NK cell extract was prepared by 2 × 107 cells in RIP buffer, mixed with biotin-labeled GAS5 for 1 h at 4°C, and then adding beads and incubating for 1 h at room temperature. Western blotting was used to detect AGO2 in GAS5 pull-down complex, and qRT-PCR was used to detect miR-544 in the precipitates.
Xenograft in nude mice
Male BALB/c nude mice (7 wk old, 18–20 g) were purchased from Laboratory animal center of Wenzhou Medical University. All animal experiments were approved by the Ethics Committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. HepG2 cells (6 × 106 cells) were injected subcutaneously into the armpit of the right forelimb of BALB/c nude mice. IL-2 stimulated LNK cells (3 × 106 cells) transfected with lenti-GAS5 or lenti-NC were injected intravenously twice at 2 h after HepG2 implantation and at d 7, so nude mice were divided into lenti-GAS5 group (n = 6) or lenti-NC group (n = 6). Tumor volume was measured every 5 d, and the nude mice were sacrificed at d 20. Tumor tissues were collected and GAS5, miR-544, and RUNX3 expressions were detected by qRT-PCR and Western blot.
Statistical analysis
Data were presented as mean ± SD, and analyzed by SPSS software (version 18.0). The differences between groups were assessed by two-sided Student’s t-test or ANOVA with a P value < 0.05 considered statistically significant.
Results
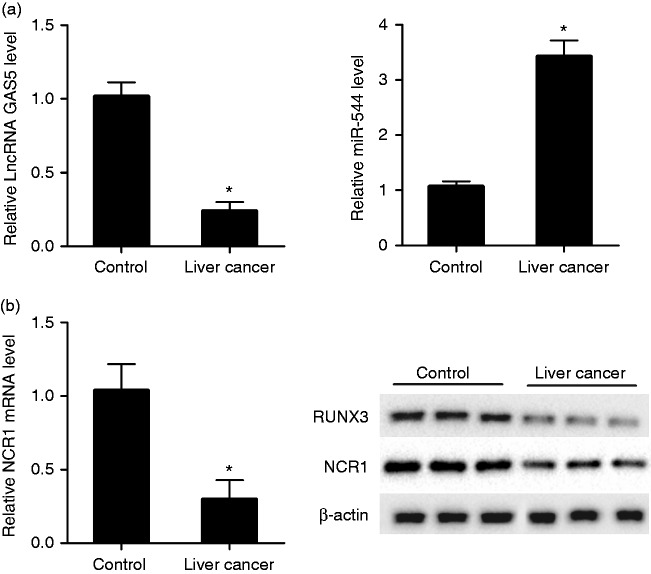
LncRNA GAS5 was down-regulated in NK cells of patients with liver cancer
To investigate the abnormal expression of lncRNA GAS5, miR-544, RUNX3, and NCR1 in patients with liver cancer, qRT-PCR and Western blot were performed. We observed that lncRNA GAS5 was down-regulated in NK cells of patients in the liver cancer group compared with the control group, whereas miR-544 was up-regulated in NK cells of the liver cancer group compared with the control group (Figure 1a). mRNA level of activating NK cell receptor NCR1 and protein level of RUNX3 and NCR1 were down-regulated in NK cells of the liver cancer group compared with the control group (Figure 1b).
Figure 1.
lncRNA GAS5 was down-regulated in NK cells of patients with liver cancer. NK cells were isolated from peripheral blood of healthy controls (n = 12) and patients with liver cancer (n = 20). (a) qRT-PCR was used to detect the relative expression of lncRNA GAS5 and miR-544 in control and liver cancer groups. Compared with the control group, lncRNA GAS5 was down-regulated in NK cells of the liver cancer group, whereas miR-544 was up-regulated in NK cells of liver cancer group. (b) qRT-PCR was used to measure the relative mRNA level of NCR1 in control and liver cancer groups, and Western blot was used to measure the protein level of RUNX3 and NCR1 in control and liver cancer groups. Compared with control group, mRNA level of NCR1 and protein level of RUNX3 and NCR1 were down-regulated in NK cells of the liver cancer group. *P < 0.05, compared with control.
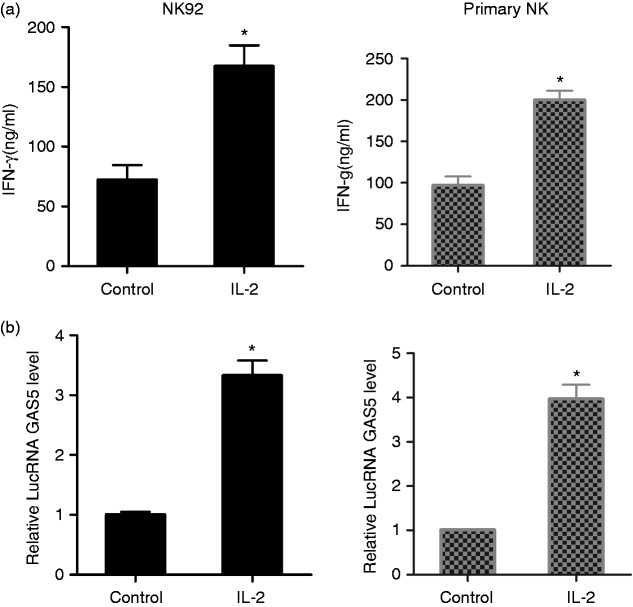
LncRNA GAS5 was up-regulated in activated NK cells
To observe the effect of lncRNA GAS5 on activated NK cells, NK92 cells were stimulated with 100 U/ml IL-2. As shown in Figure 2a, IFN-γ secretion in the supernatant of NK92 cells was increased in the IL-2 group compared with the control group, which indicated that the killing effect of NK cells was enhanced. And lncRNA GAS5 was up-regulated in NK92 cells of the IL-2 group compared with the control group (Figure 2b). In addition, primary NK cells were isolated from PBMC of healthy controls and stimulated with 100 U/ml IL-2. IFN-γ secretion in the supernatant of primary NK cells was increased in the IL-2 group compared with the control group (Figure 2a), and lncRNA GAS5 was up-regulated in primary NK cells of IL-2 group compared with the control group (Figure 2b).
Figure 2.
lncRNA GAS5 was up-regulated in activated NK cells. Human NK cell line NK92 was divided into two groups: control group and IL-2 group (stimulated by 100 U/ml IL-2). Primary NK cells were isolated from PBMC of healthy controls and were divided into two groups: control group and IL-2 group (stimulated by 100 U/ml IL-2). (a) IFN-γ secreted by NK92 cells was increased in IL-2 group compared with the control group. IFN-γ secreted by primary NK cells was increased in the IL-2 group compared with the control group. (b) lncRNA GAS5 was up-regulated in NK cells of IL-2 group compared with the control group. lncRNA GAS5 was up-regulated in primary NK cells of the IL-2 group compared with the control group. *P < 0.05, compared with control.
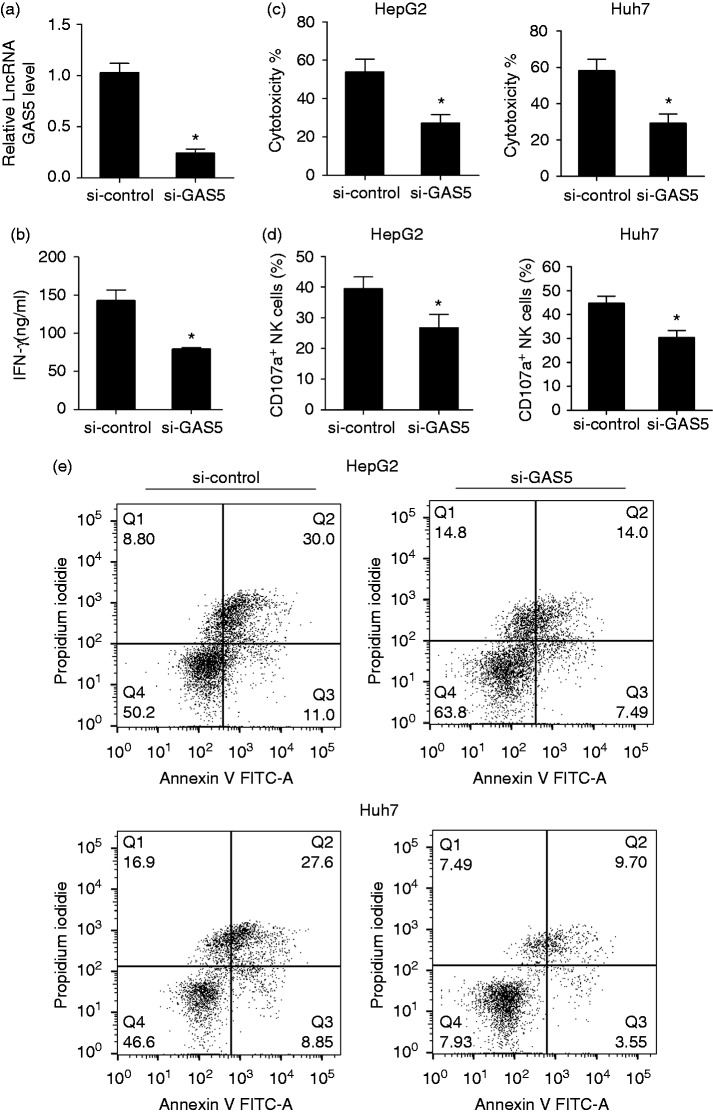
LncRNA GAS5 knockdown inhibited the killing effect of NK cells
Based on the up-regulation of lncRNA GAS5 on activated NK cells, we detected a role for lncRNA GAS5 in the killing effect of NK cells. We isolated peripheral NK cells from patients with liver cancer. Peripheral NK cells were transfected with si-GAS5 or si-control, then co-cultured with primary liver cancer cells. Cytotoxicity in the si-GAS5 group was decreased compared with the si-control group, and the apoptosis rate in si-GAS5 group was reduced compared with the si-control group (53.1% vs 25.4%, Supplementary Figure 1). After activation, NK92 cells were transfected with si-control or si-GAS5. We found that lncRNA GAS5 was down-regulated in NK92 cells of si-GAS5 group (Figure 3a). IFN-γ secretion in the supernatant of NK92 cells was decreased in the si-GAS5 group, which indicated that the killing effect of NK cells was decreased (Figure 3b). After co-cultured NK92 cells and HepG2 cells, the cytotoxicity was decreased in si-GAS5 group than si-control group (Figure 3c). After co-culturing NK92 cells and Huh7 cells, cytotoxicity was decreased in the si-GAS5 group compared with the si-control group (Figure 3c). In addition, the percentage of CD107a+ of NK cells was decreased in the si-GAS5 group compared with the si-control group (Figure 3d). The annexin V-PI double staining assay was used to detect apoptosis of HepG2 and Huh7 cells (Figure 3e). HepG2 cells were divided into si-control and si-GAS5 groups, and the apoptosis rate in the si-GAS5 group was reduced compared with that of the si-control group (41% vs 21.49%). Huh7 cells were divided into si-control and si-GAS5 groups, and the apoptosis rate in si-GAS5 group was reduced compared with that of the si-control group (36.45% vs 13.25%). These findings indicated that inhibition of si-GAS5 suppressed the killing effect of NK cells.
Figure 3.
lncRNA GAS5 knockdown inhibited the killing effect of NK cells. NK92 cells were activated by IL-2 (100 U/ml) and transfected with si-control or si-GAS5. (a) lncRNA GAS5 was down-regulated in the si-GAS5 group compared with the si-control group. (b) IFN-γ secretion in the supernatant was decreased in the si-GAS5 group compared with the si-control group. (c) After transfection with si-control or si-GAS5, NK cells were co-cultured with HepG2 cells, and cytotoxicity was detected by the CytoTox 96 non-radioactive cytotoxicity assay. Cytotoxicity was decreased in the si-GAS5 group compared with the si-control group. After co-culturing NK92 cells and Huh7 cells, cytotoxicity was decreased in the si-GAS5 group compared with the si-control group. (d) Lysosome marker CD107a of NK cells was detected by flow cytometry. The percentage of CD107a+ of NK cells was decreased in the si-GAS5 group compared with the si-control group. (e) Annexin V-PI double staining assay was used to detect the apoptosis of HepG2 and Huh7 cells. HepG2 cells were divided into si-control and si-GAS5 groups, and the apoptosis rate in si-GAS5 group was reduced compared with si-control group (41% vs 21.49%). Huh7 cells were divided into si-control and si-GAS5 groups, and the apoptosis rate in si-GAS5 group was reduced compared with si-control group (36.45% vs 13.25%). *P < 0.05, compared with si-control.
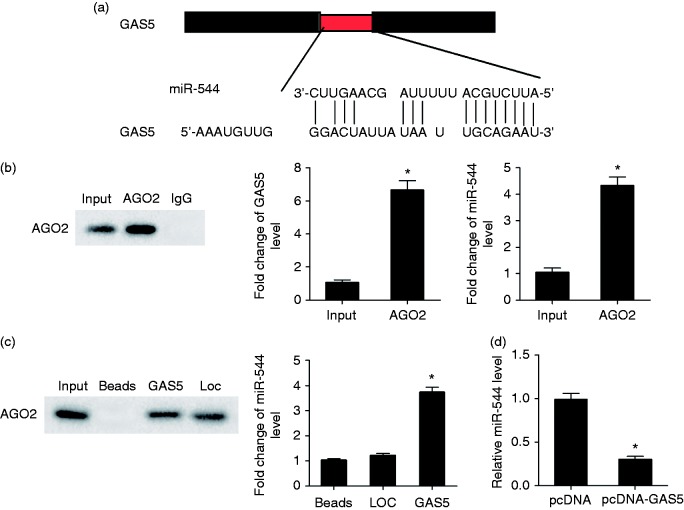
Regulation role of lncRNA GAS5 in miR-544
According to the bioinformatics software DIANA tools (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/index), there were binding sites between GAS5 and miR-544 (Figure 4a). AGO2 Ab was used for RNA immunoprecipitation. AGO2 protein was detected in Input and AGO2 groups by IP-Western (Figure 4b). GAS5 and miR-544 levels in the AGO2 group were higher than that of the Input group (Figure 4b). AGO2 was observed in the GAS5 pull-down complex, and miR-544 was enriched in the GAS5 pull-down complex, whereas miR-544 was slightly increased in the LOC pull-down complex compared with beads alone (Figure 4c). After transfection of lenti-GAS5, the miR-544 level was significantly decreased (Figure 4d), which indicated that miR-544 was negatively regulated by GAS5.
Figure 4.
Regulation role of lncRNA GAS5 in miR-544. (a) Bioinformatics software predicted binding sites between GAS5 and miR-544. (b) AGO2 Ab was used for RNA immunoprecipitation. AGO2 protein level was detected by IP-Western, and GAS5 and miR-544 were detected by qRT-PCR. GAS5 and miR-544 were enriched in AGO2. (c) AGO2 in GAS5 pull-down complex was detected by Western blot, and miR-544 enrichment in GAS5 pull-down complex was detected by qRT-PCR. Loc, negative control of GAS5 pull-down complex. (d) NK92 cells were transfected with lenti-NC or lenti-GAS5. Compared with the lenti-NC group, miR-544 level was significantly decreased in lenti-GAS5 group. *P < 0.05, compared with lenti-NC.
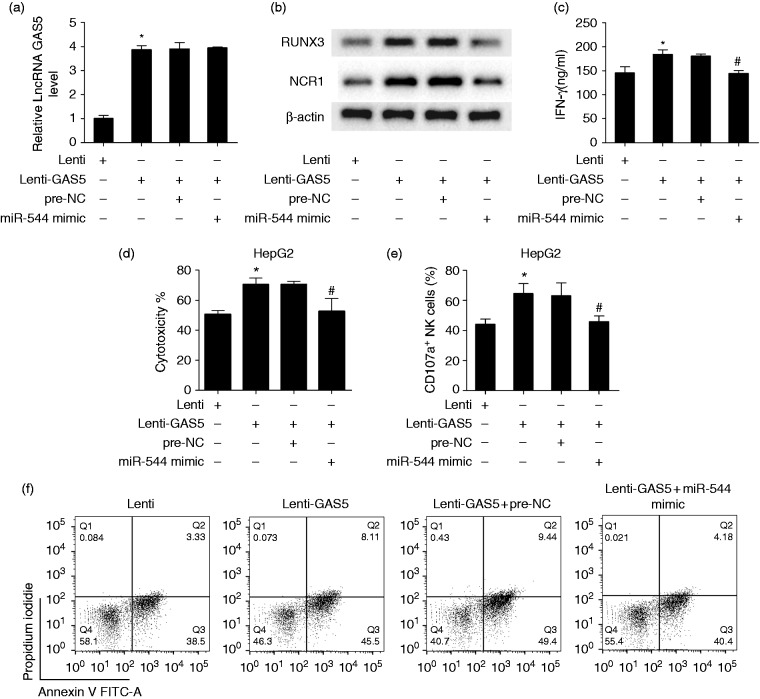
LncRNA GAS5 enhanced the killing effect of NK cells through miR-544/RUNX3
Based on the regulation of RUNX3 by miR-544 in liver cancer, the lncRNA GAS5/miR-544/RUNX3 pathway might involve regulation of the killing effect of NK cells in liver cancer. To demonstrate whether lncRNA GAS5 enhanced the killing effect of NK cells through miR-544/RUNX3, we transfected activated NK92 cells with lenti-NC, lenti-GAS5, lenti-GAS5+pre-NC, or lenti-GAS5+miR-544 mimic. As shown in Figure 5a, GAS5 expression was up-regulated in the lenti-GAS5 group, and miR-544 mimic did not change the GAS5 level. Protein level of RUNX3 and NCR1 was up-regulated in the lenti-GAS5 group, and miR-544 mimic reversed the promotion effect of lenti-GAS5 (Figure 5b). IFN-γ secretion in the supernatant was increased in the lenti-GAS5 group, and miR-544 mimic reversed the promotion effect of lenti-GAS5 (Figure 5c). Then, NK cells were co-cultured with HepG2 cells. The cytotoxicity of NK cells was enhanced in the lenti-GAS5 group, and miR-544 mimic reversed the promotion effect of lenti-GAS5 (Figure 5d). The percentage of CD107a+ of NK cells was increased in the lenti-GAS5 group, and miR-544 mimic reversed the promotion effect of lenti-GAS5 (Figure 5e). Annexin V-PI double staining assay showed that the apoptosis rates of HepG2 cells in lenti-NC, lenti-GAS5, lenti-GAS5 + pre-NC, or lenti-GAS5 + miR-544 mimic were 41.83%, 53.61%, 58.84%, and 44.58%, respectively (Figure 5f).
Figure 5.
lncRNA GAS5 enhanced the killing effect of NK cells through miR-544/RUNX3. NK92 cells were activated by IL-2 (100 U/ml), transfected with lenti-GAS5 or miR-544 mimic, and divided into lenti-NC, lenti-GAS5, lenti-GAS5 + pre-NC, lenti-GAS5 + miR-544 mimic groups. (a) GAS5 expression was up-regulated in the lenti-GAS5 group compared with the lenti-NC group, and an miR-544 mimic did not change the GAS5 level. (b) Protein level of RUNX3 was up-regulated in the lenti-GAS5 group compared with the lenti-NC group, and an miR-544 mimic reversed the promotion effect of lenti-GAS5. (c) IFN-γ secretion in the supernatant was increased in the lenti-GAS5 group, and miR-544 mimic reversed the promotion effect of lenti-GAS5. (d) After transfection, NK cells were co-cultured with HepG2 cells. Cytotoxicity was enhanced in the lenti-GAS5 group, and an miR-544 mimic reversed the promotion effect of lenti-GAS5. (e) The percentage of CD107a+ of NK cells was increased in the lenti-GAS5 group, and an miR-544 mimic reversed the promotion effect of lenti-GAS5. (f) Annexin V-PI double staining assay showed that apoptosis rate of HepG2 cells in lenti-NC, lenti-GAS5, lenti-GAS5 + pre-NC, or lenti-GAS5 + miR-544 mimic was 41.83%, 53.61%, 58.84%, and 44.58%, respectively. *P < 0.05, compared with lenti-NC; #P < 0.05, compared with lenti-GAS5 + pre-NC.
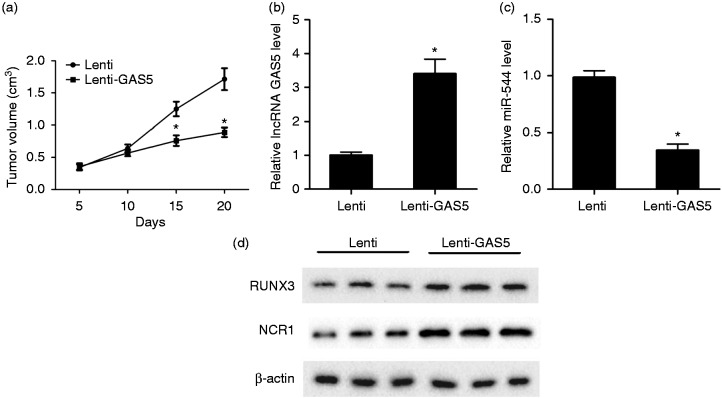
GAS5 over-expression inhibited tumor growth in HepG2 xenograft in nude mice
To further confirm the role of GAS5 in tumor growth in vivo, HepG2 cells were injected subcutaneously into BALB/c nude mice, and activated NK cells transfected with lenti-GAS5 or lenti-NC were injected intravenously twice at 2 h after HepG2 implantation and at d 7. Tumor volume in lenti-GAS5 was decreased than lenti-NC group (Figure 6a). Besides, lncRNA GAS5 was up-regulated in the lenti-GAS5 group (Figure 6b), miR-544 was down-regulated in the lenti-GAS5 group (Figure 6c), and RUNX3 and NCR1 were up-regulated in lenti-GAS5 group (Figure 6d). These data indicated that GAS5 overexpression suppressed tumor growth, and that GAS5 negatively regulates miR-544 and positively regulates RUNX3 in vivo.
Figure 6.
Xenograft in nude mice. HepG2 cells (6 × 106 cells) were injected subcutaneously into BALB/c nude mice. NK cells transfected with lenti-GAS5 or lenti-NC were injected intravenously twice at 2 h after HepG2 implantation and at d 7, and nude mice were divided into lenti-GAS5 or lenti-NC groups, with six mice in each group. (a) Tumor volume was measured every 5 d, and the nude mice were sacrificed at d 20. (b) lncRNA GAS5 was up-regulated in the lenti-GAS5 group. C. miR-544 was down-regulated in the lenti-GAS5 group. (d) RUNX3 was up-regulated in the lenti-GAS5 group. *P < 0.05, compared with lenti-NC.
Discussion
This study investigated the role of lncRNA GAS5 in the killing effect of NK cells in liver cancer, and its interaction with the miR-544/RUNX3 pathway. Our data showed that, compared with healthy controls, expression levels of lncRNA GAS5, NCR1, and RUNX3 were down-regulated in NK cells of patients with liver cancer, whereas miR-544 expression was up-regulated NK cells of patients with liver cancer. Activated NK cells had higher IFN-γ levels, which indicated the enhanced killing effect of NK cells. After knockdown of GAS5 in activated NK cells, IFN-γ secretion and NK cell cytotoxicity decreased, and the percentage of CD107a+ NK cells was reduced. We proved the interaction of GAS5 and miR-544, and the negative regulation of GAS5 on miR-544. After GAS5 overexpression in activated NK cells, IFN-γ secretion, NK cell cytotoxicity, and the percentage of CD107a+ NK cells increased, while an miR-544 mimic abolished the promotion effect of GAS5 over-expression. In vivo experiments strongly indicated an inhibition effect of GAS5 in tumor growth.
Numerous studies have suggested that enhancing immune function and suppressing immune escape can suppress the growth and metastasis of liver cancer.22–24 NK cells have natural cytotoxicity ability, and the percentage of NK cells in liver is more than five times as high as the percentage in peripheral blood, which indicates that NK cells may play a vital role in the immune function of the liver.25 Recently, more and more researchers have focused on NK cell-based immunotherapy in the treatment of liver cancer. Kamiya et al. reported that IL-2 activated NK cells exerted higher cytotoxicity against liver cancer cell lines than NK cells without stimulation.26 Inhibition of the activity of NK cells and decrease of the number of NK cells facilitated tumor growth and metastases in a murine liver metastasis model.27 In this study, IL-2 activated NK cells had higher IFN-γ secretion, indicating the enhanced killing effect of NK cells in liver cancer, which was consistent with previous reports.26
A number of studies have reported that lncRNAs can be expressed abnormally in liver cancer, and play vital roles in regulating the proliferation and migration of liver cancer cells, self-renewal of liver tumor-initiating cells and acquired immune system, etc.28–30 Several studies have shown that lncRNA GAS5 was down-regulated in liver cancer tissue or cell lines, and indicated a poor prognosis in liver cancer.31,32 However, the expression of lncRNA GAS5 in NK cells and the role of lncRNA GAS5 in the killing effect of NK cells in liver cancer are still not known. In this study, we report for the first time that lncRNA GAS5 is down-regulated in NK cells of patients with liver cancer compared with healthy controls, and lncRNA GAS5 is up-regulated in IL-2 activated NK cells compared with NK cells without stimulation. Our previous report found that the miR-544/RUNX3/NCR1/NKp46 pathway was involved in promotion of immune escape in liver cancer.9 Based on this result, we further confirmed the interaction between lncRNA GAS5 and miR-544, and found that GAS5 negatively regulated miR-544 and positively regulated RUNX3. GAS5 overexpression decreased tumor volume markedly in HepG2 xenograft nude mice, which indicated that GAS5 could be a biological target for the treatment of liver cancer.
In conclusion, our study found that lncRNA GAS5 was down-regulated in NK cells of patients with liver cancer. GAS5 overexpression in activated NK cells increased IFN-γ secretion, NK cell cytotoxicity, and the percentage of CD107a+ NK cells through miR-544/RUNX3, which indicated that the killing effect of NK cell was enhanced, thus providing potential targets for the treatment of liver cancer.
Supplemental Material
Supplemental Material for LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3 by Peipei Fang, Luxia Xiang, Weilai Chen, Shaoxun Li, Shanshan Huang, Jie Li, Lu Zhuge, Lingxiang Jin, Wenke Feng, Yiping Chen and Chenwei Pan in Innate Immunity
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by Zhejiang Provincial Natural Science Foundation of China (LY17H160054).
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Zaydfudim V, Vachharajani N, Klintmalm G, et al. Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann Surg 2016; 264: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee W, Lee C, Cheng C, et al. Outcomes of liver resection for hepatocellular carcinoma in liver transplantation era. Eur J Surg Oncol 2015: 41; 1144–1152. [DOI] [PubMed] [Google Scholar]
- 4.Eckert C, Klein N, Kornek M, et al. The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation. Front Immunol 2015; 6: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenne C, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- 6.Su Z, Wang X, Zheng L, et al. MRI-guided interventional natural killer cell delivery for liver tumor treatment. Cancer Med 2018; 7: 1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gras Navarro A, Björklund A, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol 2015; 6: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai C, Mager D. Role of runt-related transcription factor 3 (RUNX3) in transcription regulation of natural cytotoxicity receptor 1 (NCR1/NKp46), an activating natural killer (NK) cell receptor. J Biol Chem 2012: 287; 7324–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan C, Xiang L, Pan Z, et al. MiR-544 promotes immune escape through down-regulation of NCR1/NKp46 via targeting RUNX3 in liver cancer. Cancer Cell Int 2018; 18: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arase M, Horiguchi K, Ehata S, et al. Transforming growth factor-β-induced lncRNA-Smad7 inhibits apoptosis of mouse breast cancer JygMC(A) cells. Cancer Sci 2014; 105: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Zhao X, Wang C, et al. Systematic analyses reveal long non-coding RNA (PTAF)-mediated promotion of EMT and invasion-metastasis in serous ovarian cancer. Mol Cancer 2018; 17: 96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chen M, Xia Z, Chen C, et al. LncRNA MALAT1 promotes epithelial-to-mesenchymal transition of esophageal cancer through Ezh2-Notch1 signaling pathway. Anticancer Drugs 2018; 29(8): 767–773. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Guo C, Wang L, et al. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis 2018; 9: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Zhou J, Wang Z, et al. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed Pharmacother 2018; 104: 451–457. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Kong D. LncRNA GAS5 represses osteosarcoma cells growth and metastasis via sponging MiR-203a. Cell Physiol Biochem 2018; 45: 844–855. [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Jin F, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 2014; 14: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian X., Xu C., Zhao P., et al. Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein, Virology 2016; 492: 155–165. [DOI] [PubMed] [Google Scholar]
- 18.Tao R, Hu S, Wang S, et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis 2015; 36: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Pan Y, Xu X, et al. Sialidase deficiency in Porphyromonas gingivalis increases IL-12 secretion in stimulated macrophages through regulation of CR3, IncRNA GAS5 and miR-21. Front Cell Infect Microbiol 2018; 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayama T, Marr A, Kino T. Differential expression of glucocorticoid receptor noncoding RNA repressor Gas5 in autoimmune and inflammatory diseases. Horm Metab Res 2016; 48: 550–557. [DOI] [PubMed] [Google Scholar]
- 21.Suo Q, Sheng J, Qiang F, et al. Association of long non-coding RNA GAS5 and miR-21 levels in CD4 T cells with clinical features of systemic lupus erythematosus. Exp Ther Med 2018; 15: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Meng X, Jia Q, et al. Herbal compound Songyou Yin and moderate swimming suppress growth and metastasis of liver cancer by enhancing immune function. Integr Cancer Ther 2016; 15: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng M, Zhi K, Gao X, et al. Activation of cellular immunity and marked inhibition of liver cancer in a mouse model following gene therapy and tumor expression of GM-SCF, IL-21, and Rae-1. Mol Cancer 2013; 12: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedroza-Gonzalez A, Verhoef C, Ijzermans J, et al. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology 2013; 57: 183–194. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Sun C, Tian Z, et al. NK cells in immunotolerant organs. Cell Mol Immunol 2013; 10: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamiya T, Chang Y, Campana D. Expanded and Activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res 2016; 4: 574–581. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Tsujimoto H, Ono S, et al. Abdominal infection suppresses the number and activity of intrahepatic natural killer cells and promotes tumor growth in a murine liver metastasis model. Ann Surg Oncol 2016; 23 Suppl 2: S257–S265. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Liang Y, Song R, et al. Long non-coding RNA NEAT1 -modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol Cancer 2018; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji J, Yin Y, Ju H, et al. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis 2018; 9: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Liu Y, Yao L, et al. The long noncoding RNA lncZic2 drives the self-renewal of liver tumor-initiating cells via the protein kinase C substrates MARCKS and MARCKSL1. J Biol Chem 2018; 293: 7982–7992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Chang L, Li C, Lan T, et al. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol Med Rep 2016; 13: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L, Ye H, Huang G, et al. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol 2016; 37: 2691–2702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3 by Peipei Fang, Luxia Xiang, Weilai Chen, Shaoxun Li, Shanshan Huang, Jie Li, Lu Zhuge, Lingxiang Jin, Wenke Feng, Yiping Chen and Chenwei Pan in Innate Immunity