Short abstract
Chronic enteropathy (CE) in dogs is characterized retrospectively per treatment response as food-responsive enteropathy (FRE), antibiotic-responsive enteropathy (ARE), and immunosuppressant-responsive enteropathy (IRE) – the latter most resembling inflammatory bowel disease in people. The aim of this study was to characterize duodenal macrophages (Mϕ) in CE using immunohistochemistry; with calprotectin (CAL) as a marker of early differentiated Mϕ and CD163 expression as a marker for resident Mϕ in the duodenum before and after treatment. Prior to treatment, dogs with FRE and IRE had a lower CD163+/CAL+ ratio than control dogs (CTRL) in crypts; this increased significantly and normalized compared with CTRL after treatment. Conversely, the CD163+/CAL+ ratio in dogs with ARE was comparable to that in healthy dogs before and after treatment. In summary, these results suggest that Mϕ play a role in the pathogenesis of CE in FRE and IRE, with a decrease in resident Mϕ and an increase in early differentiated Mϕ, but not in ARE dogs. Mϕ normalize after successful treatment.
Keywords: Canine, CD163, chronic enteropathy, inflammatory bowel disease, macrophage
Introduction
Macrophages (Mϕ) are part of the innate immune system and play several important roles in the intestinal mucosa, including maintenance of immunological and epithelial homeostasis as well as interaction with the intestinal microbiota.1 Intestinal Mϕ are continuously replenished via migration and maturation of monocytes from the peripheral blood, differing from resident Mϕ of liver and lungs that undergo local proliferation.2
The term “macrophage waterfall” has been used to describe the maturation stages from monocytes to mature resident Mϕ in the intestinal lamina propria.3 In mice, early stages of Mϕ maturation are characterized by high expression of Ly6C, which is down-regulated during later stages of maturation. These later stages of maturation are characterized by expression of CD163, a scavenger-receptor for the hemoglobin-haptoglobin complex (scavenger receptor cysteine-rich superfamily group B), and increasing expression of CX3CR1, a chemokine receptor.3,4 In man, the same maturation process of Mϕ has been described with decreased expression of CD14, a co-receptor of TLR 4 for bacterial LPS, during maturation, and increased expression of CD163 and CD206 (mannose receptor) in later stages.4
Mϕ can undergo a spectrum of differentiation in tissues, with the two extremes characterized as M1- and M2- Mϕ. The M1-Mϕ initiate and sustain inflammation, whereas M2-Mϕ are associated with resolution of chronic inflammation and promotion of tissue repair.5 In health, resident intestinal Mϕ are in an immunologically tolerant state.6,7 Mechanisms to promote immune tolerance include down-regulation of CD14, and increased IL-10 production following contact with commensal bacteria.7,8 In both mouse and humans models, the intestinal Mϕ equilibrium is altered during inflammation with an increase in early stage Mϕ, which promotes inflammation, and a decrease in tolerant mature stages.3,4
Chronic enteropathy (CE) in dogs is classified retrospectively depending on treatment response in food-responsive (FRE), antibiotic-responsive (ARE), and immunosuppressant-responsive enteropathy (IRE, which includes steroid-responsive enteropathy).9 IRE is considered to be similar to inflammatory bowel disease (IBD) in man.
Information on intestinal Mϕ is sparse in dogs, but considering the similarities between human IBD and canine CE, it seems plausible that they play an underappreciated role in perpetuating intestinal inflammation. To date, Mϕ distribution has been described mostly in healthy dogs and dogs with CE using a single Ab, MAC387, directed against the intracellular myeloid-related protein also named calprotectin (CAL).10–14 One disadvantage of calprotectin to assess Mϕ is its lack of specificity, as CAL is also expressed by granulocytes.15 Furthermore, there is some evidence that CAL may be present only in the early stages of monocyte differentiation in dogs, and other markers might be needed to detect more mature or resident Mϕ.15,16
Recently, two Abs against scavenger receptors (CD204 and CD163) have been described in dogs; these Abs have been used to differentiate canine histiocytic sarcoma from other sarcomas or round cell tumors.17 Both CD204 and CD163 were shown to be useful in detecting Mϕ in canine formalin-fixed samples, with no indication of cross reactivity with dendritic cells; CD163 has also been used to identify Mϕ in formalin-fixed canine brain tissue.18 Although CD163 is associated with M2-phenotype (anti-inflammatory), recent studies suggest that this receptor can be expressed by both M1- and M2- Mϕ.19,20 To date, there is only one published article assessing CD163 in dogs with CE at diagnosis, and none assessing changes following treatment.21
The aims of this study were to (1) assess CAL and CD163 expression in the duodenum of healthy dogs and dogs with CE, and (2) compare CAL and CD163 expression in dogs with CE before treatment and after resolution of clinical signs.
Material and methods
The study had full Animal Ethics Committee (AEC) institutional approval for all dogs used in the respective institutions where they were enrolled. Fully informed and signed owner consent was obtained for each dog. Enrollment of dogs for the clinical trial took place at Murdoch University (MU, R2262/09), the University of Melbourne (UoM, UMVH 2014-06), and Iowa State University (ISU, 1-11-7061-K).
Clinical trial
Dogs with signs consistent with chronic enteropathy (gastrointestinal clinical signs (e.g., mass loss, diarrhea, vomiting) for longer than 3-wk duration) were prospectively enrolled after work-up to rule out extra-intestinal disease as previously described.22 Clinical investigation included upper and lower gastro-intestinal endoscopy at the time of enrollment and again after clinical response. Multiple endoscopic mucosal pinch biopsies were taken from the stomach, duodenum, ileum, and colon. For the purposes of this study, only duodenal samples were analyzed. Treatment trials of a minimum of 2 wk each included first dietary trials (hydrolyzed or selected protein diets), followed by antibiotic in non-responders, and then steroid in non-responders. Oxytetracycline was used for the antibiotic trial at a dose of 10 mg/kg PO q12h (Slade Pharmacy, Docklands 3008, Australia). Prednisolone was administered at a dosage of 1 mg/kg PO q24h (Pred-X, Apex Laboratories Pty Limited, Somersby 2250, Australia) for the corticosteroid trial. Two dogs received chlorambucil in addition at a dosage of 4 mg/m2 (Leukeran, Aspen Australia, St Elonards 2065, Australia). Response to treatment was used to classify dogs retrospectively with FRE, ARE, or IRE (Supplemental Figure S1). The term IRE was used rather than steroid-responsive enteropathy, as two dogs received a combination of prednisolone and chlorambucil. Treatment was deemed successful if the canine IBD activity index, CIBDAI, was reduced by at least 75% after starting treatment for a minimum of 5 wk, at which time endoscopy was repeated.23
Healthy dogs
Endoscopic biopsies were taken from seven healthy adult mixed-breed dogs (CTRL, four males and three females) for a project unrelated to this study (AEA1112209 and 1112075). The exact age of these dogs is unknown, but they were all young adults (1–3 yr old); CTRL dogs were current with vaccination and parasite prophylaxis and were deemed to be healthy based on physical examination and laboratory results (routine hematology and serum biochemistry). All CTRL dogs were fed the same commercial pet food.
Histopathology
Histopathology grading was performed by a single pathologist (AS) blinded to the study following published criteria of the World Small Animal Veterinary Association for intestinal histopathology assessment.24 Briefly, 10 parameters are assessed and graded from normal to marked changes (score 0–3) and individual parameter scores summed (total score 0–30). Descriptive results are given as median and range.
Immunohistochemistry and cell counting
Four serial formalin-fixed paraffin-embedded sections of the duodenum for each dog at each time point were used for immunohistochemistry (IHC) with the following primary Abs: AM-3K, mouse IgG1 monoclonal anti-CD163 (AM-3K, #KAL-KT013; TransGenic Inc, Kobe, Japan) and MAC387, mouse IgG1 monoclonal anti-calprotectin (MAC387, #M0747; Dako Australia Pty. Ltd., North Sydney, Australia). For CD163, heat-induced epitope retrieval (97°C for 20 min) was performed using DAKO Target Retrieval Solution Ready to Use S1700 (#S170084-2; Dako Australia Pty. Ltd., North Sydney, Australia). Slides for calprotectin IHC were incubated with proteinase K (#S300402; Dako Australia Pty. Ltd., North Sydney, Australia) diluted 1:400 for 20 min at room temperature.
Endogenous peroxidase activity was quenched with 3% H2O2 for 5 min. Blocking was performed with FBS (#10099-133, Gibco by Life technologies, Mulgrave, Australia). Slides were incubated with the primary Ab (AM-3K 1:200 for 1 h or MAC387 1:400 for 30 min). Slides were incubated with biotinylated link (LSAB+ System-HRP, # K0690, Dako Australia Pty. Ltd., North Sydney, Australia) for 20 min and then incubated with streptavidin-HRP for 20 min. Slides were finally incubated with VECTOR NovaRED Peroxidase (HRP) Substrate kit (#SK-4800; VECTOR Novared, Abacus ALS Pty. Ltd., Waterford, QLD, Australia) as per the manufacturer’s instruction. Slides were lightly counterstained with hematoxylin and lithium carbonate prior mounting in dibutylphthalate polystyrene xylene (Merck DPX new mounting Medium, #1.00579.0500; Trajan Scientific and Medical, Ringwood, VIC, Australia). The primary Ab was replaced by a non-relevant isotype matched Ab (#14-4714-85, Thermo Fischer Scientific, Scoresby, Australia) for negative controls at the same concentration as the primary Ab.
Normal lymph nodes from a dog were used as positive control. Five areas per slide in the villous region and crypt region were chosen randomly. Slides and areas were coded using a random number generator (www.random.org) to ensure blinding of the author about the provenance of each slide and area. Care was taken to select properly orientated villi and corresponding crypt region if available or otherwise from an adjacent region. Analysis of the collected images was carried out via a custom macro in the FIJI distribution of Image J as follows.25 The area of interest for analysis was selected manually using a region of interest (ROI) after exclusion of epithelium, lacteals, and large vessels. The separate color channels (AEC and hematoxylin) were separated using color deconvolution. Nuclei were segmented by applying a Gaussian blur (sigma = 2) and using watershed segmentation to isolate individual nuclei. A Gaussian blur was applied to the isolate AEC channel and positive cells were selected via a threshold and binarised. Binary reconstruction was used to select the positive DAB cells that were associated with a positive nuclear signal (i.e., cells).
Results were expressed as the labelling index (the ratio number of positively stained cells/total number of cells × 100) for calprotectin-positive cells (CAL+) or CD163-positive cells (CD163+) per 10,000 square microns.26
Statistical analyses
Labelling index
Data were assessed for distribution and skew and reasonably well controlled by applying a logit transformation. Mixed-effects generalized linear regression models of the logit transformed proportions of cells labelled (i.e., equivalent to a logistic regression, accounting for repeated measurement) were used to compare the labelling index between groups (CTRL, FRE, ARE, and IRE) with healthy dogs (CTRL) treated as the reference group, and to compare within groups pre- and post-treatment (PRE, POST). The models accounted for repeated measurement in individual dogs and individual dog variability, and were fit using the lme4 library in the R statistical package version 3.4.1.27,28 Post-estimation, residuals were checked for major departures from normality using the epiR library, contrasts, least-mean squares (predicted marginal means) and their 95% confidence intervals (CI) were estimated with Bonferroni correction to adjust for multiple comparisons using the library “lsmeans”.29,30
CD163+/CAL+ ratio Data were assessed for distribution, and severe skew was well controlled by logarithmic transformation (to the base 2, to aid interpretation). A mixed-effects linear regression model was used to compare the log-transformed ratio between groups (CTRL, FRE, ARE, and IRE) with normal dogs treated as the reference group, and to compare within groups pre- and post-treatment (PRE, POST), accounting for repeated measurement and individual dog variability. Post-estimation, residuals were checked for major departures from normality, contrasts, least-mean squares (predicted marginal means) and their 95% CIs were estimated with Bonferroni correction to adjust for multiple comparisons. Model outputs were visualised using the ggplot2 library.31
Results
Clinical trial
A total of 28 dogs were enrolled in the study (MU n = 6, UoM n = 15, and ISU n = 7) with endoscopy at inclusion. One MU dog did not complete the treatment trial due to sudden death (disease-related) and one UoM dog also did not complete the trial because of owner non-compliance. The 26 remaining dogs responded to one of the three treatment trials; details of signalment, origin, treatment response, and clinical scores are given in Supplemental Table S1. The FRE group included ten dogs (MU n = 4 and UoM n = 6), ARE group seven dogs (MU n = 1 and UoM n = 6), and IRE group nine dogs (ISU n = 7 and UoM n = 2).
The food used in the FRE group was based on individual cases, and included Hill’s z/d™ (Hill’s Pet Nutrition, Sydney 2001, Australia), Purina® Pro Plan Veterinary Diets® HA Hydrolyzed™ (Purina®, Rhodes 2138, Australia), Royal Canin™ Sensitivity Control, and Royal Canin™ Hypoallergenic (Royal Canin™, Melbourne 3030, Australia). All dogs that completed the study had a second endoscopy a minimum of 5 wk after resolution of clinical signs. The CIBDAI (median and range) prior to treatment was 6 (3–11) for FRE group, 7 (4–9) for ARE, and 6 (5–16) for IRE group and post treatment 0 (0–1), 0 (0–1), and 0 (0–0), respectively.
Histology
Healthy dogs had very mild histologic changes such as mild lacteal dilation and mild lympho-plasmacytic infiltrate. The median histology score (across all 10 parameters) of healthy dogs was 2 out of a maximum of 30 (range 0–3). Inflammation in 19 out of 26 dogs with CE was characterized by lympho-plasmacytic infiltrate. The median FRE group histology score was 3 before (range 1–5) and 2.5 after therapy (range 0–4). The median ARE group histology score was 2 before (range 1–6) and 1 after therapy (range 0–4). The median IRE group histology score was 4 before (range 2–8) and 3 after therapy (range 1–9).
CD163- and calprotectin-positive cells
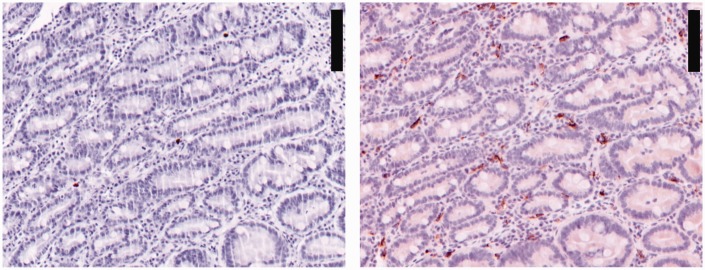
There was not enough crypt tissue for cell counting in one dog with IRE post treatment and in two dogs with FRE both pre- and post-treatment sampling; accordingly, these dogs were removed from relevant subsequent analyses. The number of dogs included for each location is reported in Supplemental Table S2. An example of crypt staining can be seen in Figure 1. Labelling index for CD163 and CAL are summarized in Figure 2 and Supplemental Table S2. Overall for all groups before or after treatment and CTRL, the number of CD163+ was higher than CAL+ in villi and crypts.
Figure 1.
Duodenal crypt region labelled for calprotectin on the left and CD163 on the right. These are serial sections at the same localization. Scale bar: 100 µm. Note the higher number of CD163 stained cells than calprotectin on serial sections.
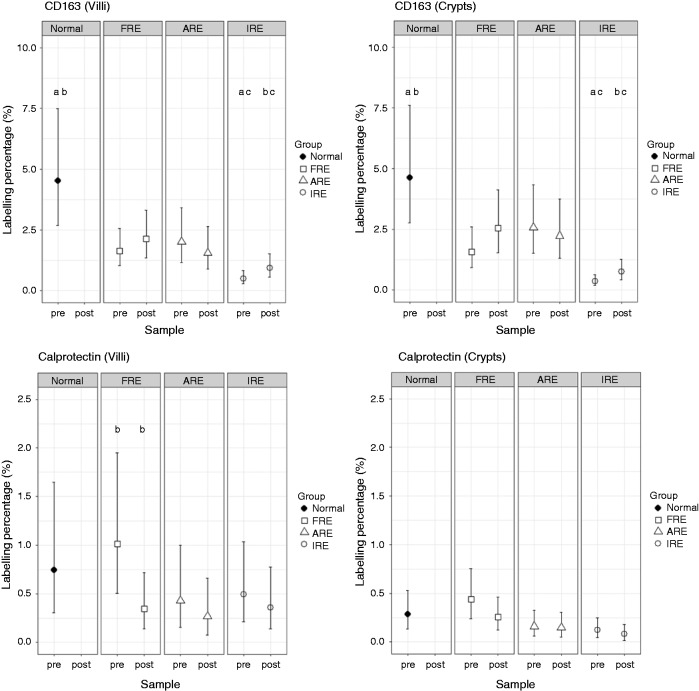
Figure 2.
Least-squares means and Bonferroni-corrected 95% CI of calprotectin and CD163 labelling index in duodenal villi and crypts. ARE: Antibiotic responsive enteropathy; FRE: Food responsive enteropathy; IRE: Immune-suppressive responsive enteropathy; Normal: healthy dogs; pre: pre-treatment; post: post-treatment. Dot plots available in Supplemental Figure S2.
Both in the villi and the crypts, there was an increase in the labelling index of CD163-positive cells (CD163+) after treatment for FRE and IRE dogs (Figure 2 and Supplemental Table S2). The percentage increase was statistically significant for IRE dogs both in villi and crypts (increase of 0.43% (CI: 0.01%, 1.14%) and 0.39% (CI: 0.04%, 0.98%) respectively, Table 1). Despite the increase post treatment in CD163+ in IRE dogs, CD163+ remained markedly lower than CTRL both before and after treatment in the villi and in the crypts (both with P < 0.01). The increase in FRE dogs did not reach statistical significance. Conversely to dogs with FRE and IRE, dogs with ARE had a slight decrease in both locations of CD163+ cells following treatment that was not statistically significant.
Table 1.
Mixed-effects generalized linear regression model outputs for the absolute differences (%) in labelling index of CD163+ or calprotectin+ cells in the villi and crypts.
|
CD163 |
Calprotectin |
|||||||
|---|---|---|---|---|---|---|---|---|
| Difference | 95% CI | P-Value | Difference | 95% CI | P-Value | |||
| Villi | ||||||||
| FREpre | vs | CTRL | −2.90 | −4.04, 0.32 | 0.066 | 0.27 | −0.60, 4.08 | 0.712 |
| FREpost | vs | CTRL | −2.42 | −3.87, 1.64 | 0.162 | −0.40 | −0.75, 1.15 | 0.390 |
| FREpost | vs | FREpre | 0.48 | −0.39, 1.91 | 0.337 | −0.67 | −0.88, −0.27 | 0.006 |
| AREpre | Vs | CTRL | −2.52 | −3.97, 1.90 | 0.171 | −0.32 | −0.74, 1.85 | 0.564 |
| AREpost | vs | CTRL | −2.99 | −4.12, 0.47 | 0.073 | −0.48 | −0.77, 1.03 | 0.306 |
| AREpost | vs | AREpre | −0.48 | −1.22, 0.86 | 0.402 | −0.16 | −0.36, 0.27 | 0.352 |
| IREpre | vs | CTRL | −4.04 | −4.43, − 2.87 | <0.001 | −0.25 | −0.72, 1.90 | 0.640 |
| IREpost | vs | CTRL | −3.61 | −4.29, − 1.62 | 0.007 | −0.39 | −0.75, 1.30 | 0.429 |
| IREpost | vs | IREpre | 0.43 | 0.01, 1.14 | 0.045 | −0.13 | −0.36, 0.32 | 0.473 |
| Crypts | ||||||||
| FREpre | vs | CTRL | −3.04 | −4.18, 0.35 | 0.066 | 0.15 | −0.20, 1.16 | 0.541 |
| FREpost | vs | CTRL | −2.09 | −3.87, 3.08 | 0.289 | −0.03 | −0.26, 0.64 | 0.870 |
| FREpost | vs | FREpre | 0.96 | −0.12, 2.73 | 0.090 | −0.18 | −0.33, 0.09 | 0.149 |
| AREpre | Vs | CTRL | −2.05 | −3.89, 3.46 | 0.315 | −0.13 | −0.30, 0.40 | 0.474 |
| AREpost | vs | CTRL | −2.27 | −3.95, 2.88 | 0.255 | −0.14 | −0.30, 0.36 | 0.422 |
| AREpost | vs | AREpre | −0.20 | −1.26, 1.61 | 0.783 | −0.01 | −0.12, 0.19 | 0.875 |
| IREpre | vs | CTRL | −4.26 | −4.57, − 3.32 | <0.001 | −0.16 | −0.31, 0.25 | 0.296 |
| IREpost | vs | CTRL | −3.87 | −4.45, − 2.13 | 0.003 | −0.21 | −0.32, 0.13 | 0.149 |
| IREpost | vs | IREpre | 0.39 | 0.04, 0.98 | 0.023 | −0.04 | −0.12, 0.09 | 0.435 |
P-Value obtained with a mixed-effect generalized model incorporating dog as random effect to account for repeated measurement and Bonferroni correction for multiple comparisons. Bold values are significant P values. ARE: Antibiotic-responsive enteropathy. CI: confidence interval (Bonferroni-corrected). n: number of dogs; CTRL: healthy dogs; FRE: food responsive enteropathy; PRE: pre-treatment; POST: post-treatment; IRE: immune-suppressive responsive enteropathy.
For CAL, the only statistically significant change after treatment was a decrease in the labelling index of calprotectin-positive cells (CAL+) in the villi of the FRE (–0.67% (CI: –0.88%, –0.27%); Table 1). No statistically significant change was seen in CAL+ in the crypts after treatment. There was no statistically significant difference in CAL+ compared with the CTRL dogs for any groups before or after treatment.
CD163+/CAL+ratio
To further assess the relative changes in CD163+ in relation to CAL+ rather than absolute changes in labelling index, the CD163+/CAL+ ratio was determined (Figure 3 and Supplemental Table S3). P-Values of the mixed-effects logistic regression model are reported in Table 2.
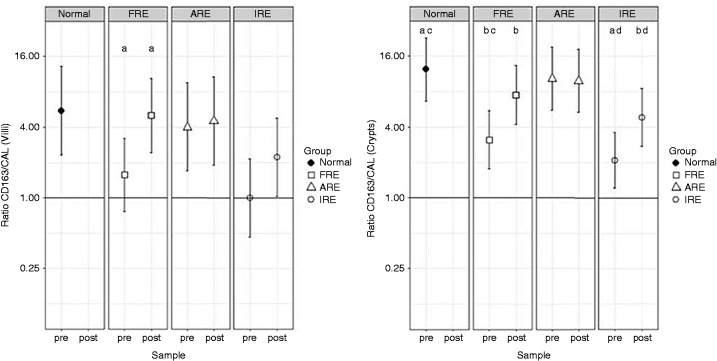
Figure 3.
Least-squares means and Bonferroni-corrected 95% CI of the ratio of CD163 and calprotectin labelling index in duodenal villi and crypts. The horizontal line represents a ratio of 1. Ratios are presented on logged scales. CAL: calprotectin; ARE: Antibiotic responsive enteropathy; FRE: Food responsive enteropathy; IRE: Immune-suppressive responsive enteropathy; Normal: healthy dogs; pre: pre-treatment; post: post-treatment. Columns with the same letter are statistically significantly different: aP < 0.01; b, c, dP < 0.05. Dot plots available in Supplemental Figure S3.
Table 2.
Differences in ratios (expressed as fold changes) from a mixed-effects logistic regression model comparing labelling ratio of CD163 and calprotectin in villi and crypts.
|
Villi |
Crypts |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Fold change in ratios | (95% CI) | P-Value | Fold change in ratios | (95% CI) | P-Value | |
| FREpre | vs | CTRL | −3.5 | (−21.1, + 1.7) | 0.16 | −4.0 | (−15.1, − 1.0) | 0.048 |
| FREpost | vs | CTRL | −1.1 | (−6.6, + 5.4) | 0.92 | −1.7 | (−6.3, + 2.3) | 0.45 |
| FREpost | vs | FREpre | +3.2 | (+1.5, + 7.0) | 0.004 | +2.4 | (+1.1, + 5.1) | 0.023 |
| AREpre | vs | CTRL | −1.4 | (−9.6, + 5.0) | 0.74 | −1.2 | (−4.8, + 3.3) | 0.79 |
| AREpost | vs | CTRL | −1.2 | (−8.5, + 5.7) | 0.83 | −1.2 | (−5.0, + 3.2) | 0.75 |
| AREpost | vs | AREpre | +0.8 | (−2.3, + 2.9) | 0.81 | −1.0 | (−2.3, + 2.2) | 0.92 |
| IREpre | vs | CTRL | −5.5 | (−34.4, + 1.1) | 0.065 | −5.9 | (−21.6, − 1.6) | 0.009 |
| IREpost | vs | CTRL | −2.5 | (−15.5, + 2.5) | 0.32 | −2.6 | (−9.5, + 1.5) | 0.16 |
| IREpost | vs | IREpre | +2.5 | (1.0, + 5.1) | 0.058 | +2.3 | (+1.4, + 4.9) | 0.028 |
CI and P-values obtained with a mixed-effect generalized model incorporating dog as random effect to account for repeated measurement and additionally Bonferroni corrected for multiple comparisons. Bold values are significant P values. ARE: Antibiotic-responsive enteropathy. CI: confidence interval (Bonferroni-corrected). n: number of dogs; CTRL: healthy dogs; FRE: food responsive enteropathy; PRE: pre-treatment; POST: post-treatment; IRE: immune-suppressive responsive enteropathy. CD163+/CAL+ ratio for Group 1 and Group 2 is listed in Supplemental Table S3.
A statistically significant increase in CD163+/CAL+ ratio was seen in the FRE group after treatment compared with before both in villi and in the crypts (increases of 3.2- and 2.4-fold, respectively). A statistically significant increase was also seen in IRE group in the crypts after treatment (5.9-fold increases). The increase in IRE group in the villi after treatment (5.6-fold) did not reaching statistical significance.
The CD163+/CAL+ ratio was statistically significantly decreased in the FRE and IRE groups before treatment compared with CTRL dogs in crypts (4.0- and 5.9-fold lower, respectively). In comparison, the CD163+/CAL+ ratios of the FRE or IRE groups after treatment were highly comparable to the CD163+/CAL+ ratio of the CTRL dogs.
In contrast, dogs with ARE had a similar CD163+/CAL+ ratio to the CTRL group at the start of the study and after treatment.
There was only a weak-to-low level of correlation between the total histological score and other metrics (Supplemental Table S4).
Discussion
It is increasingly apparent that Mϕ play an important role in IBD in mouse models and people, with an alteration in Mϕ maturation from migrating monocytes to intestinal resident Mϕ.4 In comparison, the role of Mϕ has been poorly defined in canine CE, and this is the first study to assess CAL and CD163 expression in the duodenum of dogs before and after treatment for CE. Most studies in dogs to date have assessed intestinal Mϕ using CAL expression only, except for two recent studies assessing CAL with other putative Mϕ markers in dogs with histiocytic ulcerative colitis or chronic enteropathy.11,12,14,21,32
Calprotectin (CAL) is a 24-kDa heterodimer of two calcium binding proteins, S100A8 (MRP8) and S100A9 (MRP14); it is present in neutrophil cytoplasm, monocytes and M1- Mϕ, and can be released in the extracellular space after cell activation.33 There is some evidence that CAL expression is lost during maturation of canine Mϕ; for example, in dogs with leishmaniasis, Mϕ with amastigotes are CAL negative and in granulomatous colitis, PAS positive Mϕ are also CAL negative.16,32 This suggests that CAL expression might be more suited to assess migrating monocytes and early stages of Mϕ maturation, rather than resident Mϕ -population.
Several studies have assessed CAL in intestinal biopsies from dogs with CE. Similar to our study, no difference was found between dogs treated with immunosuppressant (treatment response not reported) after ruling out FRE or ARE in one recent study.21 Another study showed a higher number of CAL+ cells in the intestine of dogs with IRE compared with normal dogs, but did not detect a difference between FRE or ARE dogs and CTRL dogs (as in our study).11 Conversely, another study found a significant overall increase in CAL+ (using a semi-quantitative scale on full-thickness biopsies) in dogs with CE, although only a small number of dogs with lymphoplasmacytic enteritis were included (five dogs) and no information was available about CE subtype (i.e., FRE vs ARE vs IRE).12
Only one study has previously assessed intestinal Mϕ in dogs before and after treatment; this study compared double positive cells for NFκB and CAL (NFκB+CAL+) in dogs with FRE and other causes of CE (including dogs that responded to steroids or cyclosporine and dogs that did not respond).14 A higher count of NFκB +CAL+ cells was noted in all dogs with CE (no breakdown was available for the subtypes of CE) prior to treatment compared with healthy dogs, and there was a significant decrease in NFκB +CAL+ cells in FRE after treatment. A significant decrease in dogs treated with steroid was also noted regardless of treatment response (responsive vs non-responsive).
In our study, overall CAL+ were low both in CTRL and dogs with CE and there was no significant difference between CTRL dogs and FRE, ARE, or IRE dogs prior or after treatment. The only statistically significant change after treatment was a reduction in CAL+ in the villi of FRE dogs. Our results are consistent with the study of Luckschander et al. in suggesting a reduction in CAL+ after successful treatment in FRE dogs.14 Another study assessing mononuclear cells infiltrate (without IHC) also reported a reduction in mononuclear cells after treatment in FRE dogs although the exact cell line was not determined.34 These findings support a reduction in populations of early differentiated Mϕ in the duodenum with resolution of clinical signs in FRE dogs.
As outlined previously, there are conflicting results on differences in CAL+ between CE dogs and CTRL dogs, and no difference was observed in our study. Furthermore, no significant reduction in CAL+ in IRE dogs was noted as reported in some other studies.11,14 One limitation of the present study is the number of dogs in each group, which might be insufficient to reach statistical significance. Consistent with previous reports in multiple countries, most dogs enrolled in the Australian clinical trial had FRE, followed by ARE and IRE.35–37 For this reason, dogs diagnosed and treated in the USA with IRE were included. Although this was essential to increase the number of dogs with IRE from two to nine, we cannot exclude that geographical, environmental, or genetic differences between both countries could influence the underlying cause for CE, as reported in humans.38,39
Comparisons between studies can be problematic for several reasons including differences in control populations, staining technique, definition of CE subtypes, and cell counting method (positive cells per area vs labelling index vs semi-quantitative scale). We expressed our results as a percentage of positive cells per total of nucleated cells (labelling index). This enabled us to count the proportion of positive cells as a percentage of the total number of cells rather than positive cells per area to account for the inflammatory infiltrates in dogs with CE. Strategies to reduce bias included blinding of the investigators analyzing the slides and ROIs as well as using a customized macro developed in FIJI to calculate the labelling index rather than manual count. Finally, in statistical analysis we accounted for repeated measurement and individual dog effects and corrected for multiple comparisons. These stringent criteria might account for some results being close to but not achieving statistical significance at a P < 0.05 threshold (for example the difference in CD163+ of FRE before treatment compared with CTRL).
The other marker assessed in our study is CD163, which is expressed mainly by Mϕ and has been linked to a M2-phenotype in man and mouse.40 Little information is currently available in dogs, but a recent study has characterized the phenotypic features of canine M1- and M2- Mϕ obtained in vitro, with transcriptome analysis, suggesting CD163 to be transcribed mainly in M2- Mϕ.41 In vivo data in dogs with Leishmania also suggest that CD163 is expressed by M2- Mϕ.42
We found that healthy dogs had a higher number of CD163+ than CAL+ cells both in villi and crypts, which is consistent with previous observations in dogs with histiocytic ulcerative colitis and CE.21,32 Furthermore, CD163+ was decreased in dogs with IRE prior to treatment compared with CTRL dogs as reported recently in dogs with CE where information about treatment response is unknown.21 Response to treatment in both FRE and IRE dogs was characterized by an increase in CD163+ (significant in IRE dogs). Despite this increase, CD163+ remained lower in IRE dogs after treatment compared with CTRL dogs.
Interestingly, ARE dogs, in contrast to FRE and IRE dogs, showed no clear change in CD163+ following treatment, with no significant difference compared with healthy dogs before or after treatment despite clinical improvement. This finding suggests that a reduced number of CD163+ cells is part of the pathogenesis in FRE and IRE dogs with an increase in CD163+ cells after treatment, whereas other cells are the key players in the pathogenesis of ARE.
Several studies in humans have reported an increase in CD163 cells both in ulcerative colitis (UC) and Crohn’s disease (CD), which is very different from our findings.43–45 This difference is likely due to differences in inflammatory infiltrates between dogs and humans. Canine CE is usually characterized by a lympho-plasmacytic infiltrate, whereas Crohn’s disease is characterized by a high percentage of granulomas.46,47
Although CD163 Mϕ are considered to have a M2-phenotype, this also depends on other co-expressed molecules. For example, Barman et al. have shown that CD163+ cells could be further separated in pro-inflammatory CD160low and anti-inflammatory CD160 high Mϕ.48 Interestingly, glucocorticoid administration can induce in vitro and in vivo (peripheral blood) CD163 expression on Mϕ.49 If monocytes replenish intestinal Mϕ in canine intestine as described in human and mouse, this could explain our observation in IRE dogs, but would not account for the same finding in FRE dogs.3 Our observation that CD163+ are higher in CTRL dogs than IRE dogs, with the same trend in FRE dogs, supports the assertion that CD163+ cells in dogs correspond to resident Mϕ as described in mouse and man. However, further work is needed to confirm if these cells have an anti-inflammatory phenotype.
To further assess the changes in CAL+ in relation to CD163+ rather than the total number of cells, the ratio CD163+/CAL+ was determined in dogs with CE before and after treatment. Findings in FRE and IRE groups were similar with significantly reduced CD163+/CAL+ ratios in the crypts prior to treatment in comparison with CTRL dogs and approaching statistical significance in the villi for the IRE dogs. Successful treatment was characterized by marked increase in this ratio for both groups in crypts region and for FRE dogs in the villi. After treatment, there was no significant difference in CD163+/CAL+ ratio between dogs with FRE or IRE and CTRL dogs both in villi and crypts. Again, the picture was very different in ARE group with similar CD163+/CAL+ ratio to CTRL group prior treatment and no significant change in ratio after successful treatment.
Our hypothesis is that CAL+ are likely to represent migrating monocytes and early maturation stages of Mϕ whereas CD163+ represent resident Mϕ. However, further studies are needed to confirm this hypothesis. A limitation of this study is that we have only looked at two putative Mϕ markers, namely CAL and CD163. Further canine markers are required to better assess changes in the Mϕ population. Although CD64, a high affinity receptor for IgG, has often been used as a pan-marker for humans Mϕ, there is indication that it is not expressed by intestinal Mϕ in human and only rarely in dogs.7,32
Another important limitation of this study is that the phenotype of CAL+ and CD163+ was not further assessed. Cytokine and chemokine profiling is needed to clarify their lineage (M1 vs M2). There is some evidence that up-regulation of CD163 in monocytes also occurs in humans with IBD, and it would be of interest to assess if this is also the case in dogs.44
Our study included dogs enrolled in different institutions. Although this was useful to obtain a meaningful number of dogs in each disease category with samples before and after treatment, we cannot exclude the possibility that sample preparation in the different laboratories could have influenced the results. However, there was no obvious difference in the immunohistochemistry results between the different institutions to suggest that this was a problem. While ideal, we were also unable to obtain age- and breed-matched healthy controls due to ethical considerations. Most dogs were mixed-breed, which limits bias due to a breed-associated phenotype.
Finally, it would be of interest to assess dogs that do not respond to treatment, but all dogs that were enrolled went into remission. The dog that was excluded from the study initially responded, but then relapsed when the owner changed the treatment against recommendations. Because of owner compliance, this dog had to be excluded from the study and the owner was not ready to come back for the second endoscopic examination.
Our findings indicate that FRE and IRE dogs have a shift in their Mϕ populations compared with healthy dogs, similar to findings reported in mice and humans.3,50 This is characterized by a decreased CD163+/CAL+ ratio. These results are also consistent with observations in dogs with granulomatous colitis or CE with an increase in CAL+, and decrease in CD163+ compared with healthy dogs.21,32 Furthermore, we have shown in this study that, following remission, there was a significant increase in CD163+ in both groups and decrease in CAL+ in the villi of FRE dogs. The overall result is a normalization of the CD163+/CAL+ ratio after treatment in both FRE and IRE groups. The CD163+ labelling index in post-treatment FRE dogs also reverted to normal, but population of CD163+ cells remained reduced in IRE dogs compare to CTRL dogs. In contrast, population of CD163+ cells do not seem to be altered with antibiotic treatment in ARE dogs.
In summary, our study indicates that Mϕ play an important role in CE for dogs with FRE and IRE. CD163+/CAL+ ratio is lower than healthy dogs in these two groups at diagnosis and normalized after treatment in FRE dogs. This is very different from ARE dogs for which only negligible changes in the ratio were noted before or after treatment, which suggest that these cells do not play such an important role in this condition. Further studies are needed to better characterize canine intestinal Mϕ and their different phenotypes in health and disease.
Supplemental Material
Supplemental material for Changes in duodenal CD163-positive cells in dogs with chronic enteropathy after successful treatment by Julien RS Dandrieux, Lina Maria Martinez Lopez, Andrew Stent, Albert Jergens, Karin Allenspach, Cameron J Nowell, Simon M Firestone, Wayne Kimpton and Caroline S Mansfield in Innate Immunity
Acknowledgements
The authors would like to thank Dr Nathalee Prakash for help with tissue sampling, Barbara Bacci for her help with optimization of CD163, and Paul Benham for technical help with the IHC.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Joeris T, Muller-Luda K, Agace WW, et al. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol 2017; 10: 845–864. [DOI] [PubMed] [Google Scholar]
- 2.Mowat AM, Scott CL, Bain CC. Barrier-tissue macrophages: functional adaptation to environmental challenges. Nat Med 2017; 23(11): 1258–1270. [DOI] [PubMed] [Google Scholar]
- 3.Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 2012; 42: 3150–3166. [DOI] [PubMed] [Google Scholar]
- 4.Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013; 6: 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229: 176–185. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Dominguez E, Samaniego R, Flores-Sevilla JL, et al. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukocyte Biol 2015; 98: 453–456. [DOI] [PubMed] [Google Scholar]
- 7.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005; 115: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayama H, Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity. Int Immunol 2012; 24: 673–680. [DOI] [PubMed] [Google Scholar]
- 9.Dandrieux JRS. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract 2016; 57: 589–599. [DOI] [PubMed] [Google Scholar]
- 10.German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol 1999; 120: 129–145. [DOI] [PubMed] [Google Scholar]
- 11.German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med 2001; 15: 14–25. [DOI] [PubMed] [Google Scholar]
- 12.Kleinschmidt S, Meneses F, Nolte I, et al. Characterization of mast cell numbers and subtypes in biopsies from the gastrointestinal tract of dogs with lymphocytic-plasmacytic or eosinophilic gastroenterocolitis. Vet Immunol Immunopathol 2007; 120: 80–92. [DOI] [PubMed] [Google Scholar]
- 13.Kleinschmidt S, Meneses F, Nolte I, et al. Distribution of mast cell subtypes and immune cell populations in canine intestines: evidence for age-related decline in T cells and macrophages and increase of IgA-positive plasma cells. Res Vet Sci 2008; 84: 41–48. [DOI] [PubMed] [Google Scholar]
- 14.Luckschander N, Hall JA, Gaschen F, et al. Activation of nuclear factor-kappaB in dogs with chronic enteropathies. Vet Immunol Immunopathol 2010; 133: 228–236. [DOI] [PubMed] [Google Scholar]
- 15.German AJ, Hall EJ, Kelly DF, et al. An immunohistochemical study of histiocytic ulcerative colitis in boxer dogs. J Comp Pathol 2000; 122: 163–75. [DOI] [PubMed] [Google Scholar]
- 16.Mozos E, Perez J, Day MJ, et al. Leishmaniosis and generalized demodicosis in three dogs: a clinicopathological and immunohistochemical study. J Comp Pathol 1999; 120: 257–268. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Murakami M, Hoshino Y, et al. The Class A macrophage scavenger receptor CD204 is a useful immunohistochemical marker of canine histiocytic sarcoma. J Comp Pathol 2013; 148: 188–196. [DOI] [PubMed] [Google Scholar]
- 18.Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet Pathol 2012; 49: 682–692. [DOI] [PubMed] [Google Scholar]
- 19.Buechler C, Eisinger K, Krautbauer S. Diagnostic and prognostic potential of the macrophage specific receptor CD163 in inflammatory diseases. Inflamm Allergy Drug Targets 2013; 12: 391–402. [DOI] [PubMed] [Google Scholar]
- 20.Barros MH, Hauck F, Dreyer JH, et al. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PloS one 2013; 8: e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner A, Junginger J, Lemensieck F, et al. Immunohistochemical characterization of gastrointestinal macrophages/phagocytes in dogs with inflammatory bowel disease (IBD) and non-IBD dogs. Vet Immunol Immunopathol 2018; 197: 49–57. [DOI] [PubMed] [Google Scholar]
- 22.Allenspach K, Bergman PJ, Sauter S, et al. P-glycoprotein expression in lamina propria lymphocytes of duodenal biopsy samples in dogs with chronic idiopathic enteropathies. J Comp Pathol 2006; 134: 1–7. [DOI] [PubMed] [Google Scholar]
- 23.Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003; 17: 291–297. [DOI] [PubMed] [Google Scholar]
- 24.Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008; 138 Suppl 1: S1–43. [DOI] [PubMed] [Google Scholar]
- 25.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol 2014; 9: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. 2017.
- 29.Stevenson M, Nunes T, Heuer C, et al. epiR: Tools for the Analysis of Epidemiological Data https://CRAN.R-project.org/package=epiR (2017, accessed 5 April 2018).
- 30.Lenth RV. Least-Squares Means: The R Package lsmeans. J Stat Softw 2016; 1: https://www.jstatsoft.org/v069/i01
- 31.Wickham H. ggplot2: Elegant Graphics for Data New York: Springer-Verlag, http://ggplot2.org (2009).
- 32.Nolte A, Junginger J, Baum B, et al. Heterogeneity of macrophages in canine histiocytic ulcerative colitis. Innate immunity 2017; 23: 228–239. [DOI] [PubMed] [Google Scholar]
- 33.Striz I, Trebichavsky I. Calprotectin – a pleiotropic molecule in acute and chronic inflammation. Physiol Res 2004; 53: 245–253. [PubMed] [Google Scholar]
- 34.Walker D, Knuchel-Takano A, McCutchan A, et al. A comprehensive pathological survey of duodenal biopsies from dogs with diet-responsive chronic enteropathy. J Vet Intern Med 2013; 27: 862–874. [DOI] [PubMed] [Google Scholar]
- 35.Marks SL, Laflamme DP, McAloose D. Dietary trial using a commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease. Vet Ther 2002; 3: 109–118. [PubMed] [Google Scholar]
- 36.Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 2007; 21: 700–708. [DOI] [PubMed] [Google Scholar]
- 37.Mandigers PJ, Biourge V, van den Ingh TS, et al. A randomized, open-label, positively-controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. J Vet Intern Med 2010; 24: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 38.Dutta AK, Chacko A. Influence of environmental factors on the onset and course of inflammatory bowel disease. World J Gastroenterol 2016; 22: 1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szilagyi A, Xue X. Comparison of geographic distributions of Irritable Bowel Syndrome with Inflammatory Bowel Disease fail to support common evolutionary roots. Med Hypotheses 2018; 110: 31–37. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen JH, Etzerodt A, Svendsen P, et al. The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid Med Cell Longev 2013; 2013: 523652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinrich F, Lehmbecker A, Raddatz BB, et al. Morphologic, phenotypic, and transcriptomic characterization of classically and alternatively activated canine blood-derived macrophages in vitro. PloS one 2017; 12: e0183572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreira PRR, Fernando FS, Montassier HJ, et al. Polarized M2 macrophages in dogs with visceral leishmaniasis. Vet Parasitol 2016; 226: 69–73. [DOI] [PubMed] [Google Scholar]
- 43.Ciccia F, Alessandro R, Rizzo A, et al. Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology 2014; 53: 104–113. [DOI] [PubMed] [Google Scholar]
- 44.Franze E, Caruso R, Stolfi C, et al. Lesional accumulation of CD163-expressing cells in the gut of patients with inflammatory bowel disease. PloS one 2013; 8: e69839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott TR, Rayment NB, Hudspith BN, et al. Lamina propria macrophage phenotypes in relation to Escherichia coli in Crohn’s disease. BMC Gastroenterol 2015; 15: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011; 41: 381–398. [DOI] [PubMed] [Google Scholar]
- 47.Rubio CA, Orrego A, Nesi G, et al. Frequency of epithelioid granulomas in colonoscopic biopsy specimens from paediatric and adult patients with Crohn’s colitis. J Clin Pathol 2007; 60: 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barman S, Kayama H, Okuzaki D, et al. Identification of a human intestinal myeloid cell subset that regulates gut homeostasis. Int Immunol 2016; 28: 533–545. [DOI] [PubMed] [Google Scholar]
- 49.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol 2010; 47: 1650–1660. [DOI] [PubMed] [Google Scholar]
- 50.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014; 15: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Changes in duodenal CD163-positive cells in dogs with chronic enteropathy after successful treatment by Julien RS Dandrieux, Lina Maria Martinez Lopez, Andrew Stent, Albert Jergens, Karin Allenspach, Cameron J Nowell, Simon M Firestone, Wayne Kimpton and Caroline S Mansfield in Innate Immunity