Short abstract
This study mainly investigated the effects of matrine on cell apoptosis and the effects of anticancer drugs in non-small cell lung cancer (NSCLC) cell lines (A549 and LK2 cells). The results showed that matrine (≥10 μM) caused a significant inhibition on cell viability and 10 and 100 μM matrine induced cell apoptosis via influencing p53, bax, casp3, and bcl-2 expressions in A549 cells. In addition, matrine significantly down-regulated C-C chemokine receptor type 7 (CCR7) expression, and blocking the down-regulation of CCR7 by exogenous chemokine ligand 21 (CCL21) treatment alleviated matrine-caused effects of apoptosis genes in A549 cells. The results were further validated in LK2 cells that matrine regulated apoptosis gene expressions, which were reversed by CCL21 treatment. Furthermore, matrine enhances the effects of cisplatin, 5-fluorouracil, and paclitaxel in A549 cells, and the anticancer effects exhibit a dosage-dependent manner. In summary, matrine induced cell apoptosis and enhanced the effects of anticancer drugs in NSCLC cells; the mechanism might be associated with the CCR7 signal.
Keywords: Matrine, C-C chemokine receptor type 7, apoptosis, non-small cell lung cancer
Introduction
Lung cancer is a common tumor characterized by unlimited cell proliferation in the lung tissues.1 Non-small cell lung cancer (NSCLC) accounts for about 85% of development of all lung cancers.2,3 Previous reports suggest that natural plants serve as potential anticancer drugs for NSCLC and various natural compounds have been identified to be effective in inhibiting NSCLC in the lab and in the clinic.4–6 Matrine, a kind of alkaloid component isolated from the roots of Sophora species, shows various pharmacological activities, such as anti-inflammation, anti-oxidative stress, anti-infection, and anti-cancer.7–9 For example, Li et al. reported that matrine inhibits the growth and induces apoptosis in breast carcinoma MCF-7 cells via controlling bax/bcl-2 signaling.10
C-C chemokine receptor type 7 (CCR7) has been identified as an aberrant expression on certain tumor types and has been linked to pro-survival and invasive pathways. In NSCLC, CCR7 is up-regulated through its upstream ligand, exogenous chemokine ligand 21 (CCL21), and inhibits cell apoptosis via up-regulating bax/bcl-2 signaling in A549 and H460 cells of NSCLC.11 Currently, CCR7 has been considered to be a novel prognostic biomarker and therapeutic target for NSCLC. In this study, we hypothesize that matrine can regulate CCR7 expression and enhance the effects of anticancer drugs in A549 cells, which further mediates cell apoptosis.
Materials and methods
Cell culture
A549 cells (human lung cancer cell lines) and LK2 cells (human lung squamous cell carcinoma cell lines) were seeded in DMEM-F12 culture and supplemented with 10% FBS (Gibco, USA) and 1% penicillin in a humidified 5% CO2 atmosphere at 37°C.
Cell viability
Cell viability after drugs treatment was tested by the CKK-8 assay (Sigma–Aldrich). Briefly, cells were seeded in a 96-well plate (1 × 104 cells/well). Next day, cells were incubated with different concentrations of matrine (0, 10, and 100 µM) plus cisplatin (0.1, 1.0, and 5.0 mM), 5-fluorouracil (0.1, 1.0, and 10.0 mM), or paclitaxel (0.1, 1.0, and 5.0 mM) for 48 h. After treatment with drugs, cells were treated with CKK-8 for 2 h incubation. Then, the optical density (OD) of each well was tested using a microplate reader at 570 nm and the cell proliferation inhibitory ratio was calculated basing on the formula: (ODcontrol − ODtreatment/ODcontrol) × 100%.
Cell apoptosis
After treatment with drugs, cells were collected and centrifuged at 12,000 g for 5 min. Then the cells were fixed by 70% pre-cold ethanol and cells were washed with ice-cold PBS and re-suspended in the staining solution for 0.5 h in the dark, which contained 50 mg/ml propidium iodide (PI) and 100 mg/ml RNase. Harvested cells were further stained with PI/Annexin-V-FITC and cell cycle arrest and apoptosis were determined by flow cytometry at 630 nm and 525 nm, respectively.
Western blot
Total proteins from 10-cm dishes (106–108 cells) were extracted (Thermo Fisher Scientific Inc., Waltham, MA, USA). Protein abundances from each sample were tested by BCA Kit (Beyotime, Jiangsu, China) and denatured by adding 5X SDS-PAGE Sample Loading Buffer (Beyotime, Jiangsu, China) and heating for 5 min. Protein samples were separated by a SDS-PAGE electrophoresis system, and then the proteins at the gel were transferred into a PVDF membrane (Millipore, MA, USA). Then the membrane was blocked in milk for 1.5 h. The primary Abs (anti-GAPDH Ab (ab8245) and anti-CCR7 Ab (ab32527)) were incubated overnight at 4°C and then the relative secondary Abs were incubated for 2 h at room temperature. We digitally quantified the resultant signals and normalized the data to GAPDH.
Real-time PCR
Gene expressions after drugs treatment were determined by real-time PCR. Approximately 1 × 106 cells/ml cells from each sample were collected and RNA was extracted, and reverse transcribed into cDNA. Primers used in this study can be found at Table 1. Expression of β-actin was used as house-keeping gene and the relative expression of target genes was normalized relative to the expression of β-actin. The 2-ΔΔCt cycle threshold formula was used to calculate the relative abundance of mRNA.
Table 1.
Primers used in this study.
| Genes | Sequence 5′–3′ | Length (bp) |
|---|---|---|
| β-Actin | F: CGACAGGATGCAGAACGAGA | 145 |
| R: AGTGAGGACCCTGGATGTGA | ||
| p53 | F: TGGATTGGCCAGACTGCCTTC | 235 |
| R: CTGGCATTCTGGGAGCTTCAT | ||
| Casp3 | F: GCGGTTGTAGAAGTTAATAAAGGT | 251 |
| R: TGAGGTTTGCTGCATCGACA | ||
| Casp8 | F: GAGTGAGTCATCTCTGTTCTGCTT | 186 |
| R: TGAGTTGACTAGCAAATTCAGCA | ||
| Casp9 | F: GCTCAGACCAGAGATTCGCA | 100 |
| R: GTCTTTCTGCTCGACATCACCA | ||
| Bcl-2 | F: GTGAAGTCAACATGCCTGCC | 106 |
| R: ACAGCCTGCAGCTTTGTTTC | ||
| Bax | F: CCAGAGGCGGGGTTTCAT | 206 |
| R: GGAAAAAGACCTCTCGGGGG | ||
| Fas | F: ATGGGGATGAACCAGACTGC | 288 |
| R: TTCTCTTCACTTCCTCTTTGCAC | ||
| Fas-L | F: ACCTCAAGGGGGACTGTCTT | 168 |
| R: AGTTTCACCGATGGCTCAGG | ||
| CCR7 | F: CTTCACTTGTGGCATCGCAG | 266 |
| R: AAGTTCCGCACGTCCTTCTT |
Statistical analysis
All data were analyzed using IBM SPSS 21.0 software. Difference between groups was determined by Ducan’s multiple comparison tests. Data are expressed as the mean ± SEM. Values in the same row with different superscripts are significant (P < 0.05).
Results
Matrine promotes cell apoptosis in A549 cells
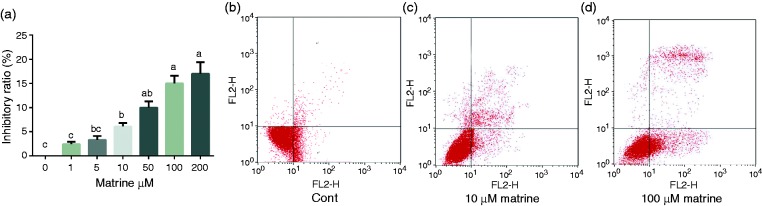
One, 5, 10, 50, 100, and 200 µM matrine were selected to investigate the inhibitory percent of matrine in A549 cells (Figure 1) and the results showed that matrine ≥10 μM exhibited a significant inhibitory effect on cell viability in A549 cells (P < 0.05). Ten and 100 µM were further used in the apoptosis assay and the results showed that matrine treatment significantly induced cell death compared with the control group (P < 0.05).
Figure 1.
Effects of matrine on cell apoptosis. (a) Cell viability after exposure to matrine. (b–d) Cell apoptosis via flow cytometry: (b) control; (c) 10 μM matrine; (d) 100 μM matrine. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 3 or 6).
Effects of matrine on apoptosis relative genes in A549 cells
Compared with the control group, matrine markedly enhanced cell p53 and bax expression in a dosage-dependent manner (P < 0.05) (Table 2). One hundred µM matrine markedly reduced casp3 mRNA abundance compared with the others groups (P < 0.05). Bcl-2 expression was markedly lower in 50 and 100 µM matrine groups than that in control group (P < 0.05).
Table 2.
Effects of matrine on apoptosis relative genes in A549 cells. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 6).
| Genes | 0 μM Matrine | 10 μM Matrine | 50 μM Matrine | 100 μM Matrine |
|---|---|---|---|---|
| p53 | 1.00 ± 0.08b | 1.18 ± 0.13ab | 1.33 ± 0.17ab | 1.42 ± 0.22a |
| Casp3 | 1.00 ± 0.22a | 0.87 ± 0.15a | 0.91 ± 0.09a | 0.52 ± 0.12b |
| Casp8 | 1.00 ± 0.24 | 0.89 ± 0.16 | 0.85 ± 0.17 | 0.79 ± 0.16 |
| Casp9 | 1.00 ± 0.18 | 0.84 ± 0.16 | 0.92 ± 0.21 | 0.84 ± 0.18 |
| Bcl-2 | 1.00 ± 0.08a | 0.83 ± 0.11ab | 0.77 ± 0.16b | 0.43 ± 0.05b |
| Bax | 1.00 ± 0.05b | 1.37 ± 0.12a | 1.40 ± 0.13a | 1.68 ± 0.25a |
| Fas | 1.00 ± 0.17 | 1.12 ± 0.12 | 1.27 ± 0.23 | 0.92 ± 0.02 |
| Fas-L | 1.00 ± 0.25 | 0.93 ± 0.21 | 1.28 ± 0.30 | 1.23 ± 0.26 |
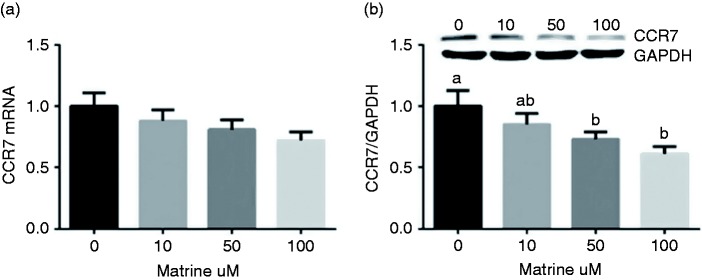
Matrine inhibits CCR7 expression in A549 cells
We determined cellular mRNA and protein abundances of CCR7 after matrine treatment via RT-PCR and Western blot (Figure 2). Matrine tended to down-regulate CCR7 expression, but the difference was insignificant (P > 0.05). However, CCR7 protein was markedly inhibited in 50 and 100 μM matrine groups compared with the compared with the control group (P < 0.05).
Figure 2.
Effects of matrine on CCR7 expression via RT-PCR and Western blot. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 3).
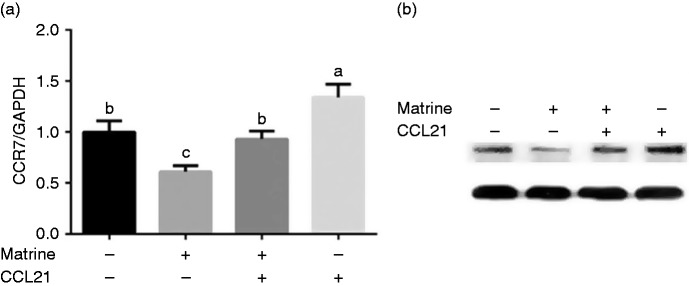
CCR7 mediates matrine-induced cell apoptosis in A549 cells and LK2 cells
CCL21 (100 nM) was used to block the inhibitory effect of matrine (100 µM) on CCR7 expression. The results showed that CCL21 treatment significantly induced CCR7 expression and blocked the down-regulation of matrine on CCR7 (Figure 3) (P < 0.05).
Figure 3.
Effects of CCL21 on CCR7 via Western blot. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 3).
Apoptosis relative genes were further determined, and the results are shown in Table 3. Compared with the control group, CCL21 treatment markedly downregulated p53 and bax expression and up-regulated bcl-2 expression (P < 0.05). Meanwhile, CCL21 blocked the effects of matrine on p53 and bcl-2, suggesting that matrine-induced cell apoptosis involve in CCR7 expression.
Table 3.
Effects of matrine and CCL21 on apoptosis relative genes in A549 cells. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 6).
| Genes | Control | Matrine | Matrine+CCL21 | CCL21 |
|---|---|---|---|---|
| p53 | 1.00 ± 0.06b | 1.55 ± 0.17a | 1.05 ± 0.17b | 0.71 ± 0.11c |
| Casp3 | 1.00 ± 0.12a | 0.58 ± 0.13b | 0.87 ± 0.22ab | 1.13 ± 0.09a |
| Casp8 | 1.00 ± 0.14 | 0.83 ± 0.12 | 0.94 ± 0.16 | 0.88 ± 0.11 |
| Casp9 | 1.00 ± 0.08 | 0.87 ± 0.15 | 1.14 ± 0.18 | 0.46 ± 0.26 |
| Bcl-2 | 1.00 ± 0.18b | 0.39 ± 0.07c | 0.73 ± 0.11b | 1.56 ± 0.08a |
| Bax | 1.00 ± 0.10b | 1.77 ± 0.22a | 1.34 ± 0.23ab | 0.62 ± 0.11c |
| Fas | 1.00 ± 0.07 | 1.12 ± 0.13 | 1.22 ± 0.02 | 1.21 ± 0.42 |
| Fas-L | 1.00 ± 0.05 | 1.19 ± 0.16 | 1.03 ± 0.26 | 1.29 ± 0.07 |
The effect of CCR7 and matrine on cell apoptosis was further validated in LK2 cells (Table 4) and the results exhibited a similar trend compared with the A549 cells. Matrine treatment (100 µM) markedly up-regulated p53 and bax expressions (P < 0.05), which were blocked by CCL21 exposure (100 nM) (P < 0.05). Compared with the control, matrine+CCL21, and CCL21 groups, Casp3 expression was significantly lower in matrine-treated LK2 cells (P < 0.05).
Table 4.
Effects of matrine and CCL21 on apoptosis relative genes in LK2 cells. Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 6).
| Genes | Control | Matrine | Matrine+CCL21 | CCL21 |
|---|---|---|---|---|
| p53 | 1.00 ± 0.12b | 1.35 ± 0.09a | 0.96 ± 0.08b | 0.87 ± 0.08c |
| Casp3 | 1.00 ± 0.09a | 0.76 ± 0.09b | 0.94 ± 0.12a | 1.03 ± 0.11a |
| Casp8 | 1.00 ± 0.17 | 0.94 ± 0.22 | 1.14 ± 0.23 | 1.07 ± 0.19 |
| Casp9 | 1.00 ± 0.12 | 0.97 ± 0.14 | 1.05 ± 0.13 | 0.88 ± 0.14 |
| Bcl-2 | 1.00 ± 0.16 | 0.99 ± 0.12 | 0.99 ± 0.08 | 1.12 ± 0.14 |
| Bax | 1.00 ± 0.11c | 1.41 ± 0.17a | 1.25 ± 0.06b | 0.89 ± 0.09c |
| Fas | 1.00 ± 0.15 | 1.23 ± 0.18 | 1.27 ± 0.17 | 1.09 ± 0.11 |
| Fas-L | 1.00 ± 0.13 | 1.21 ± 0.13 | 1.32 ± 0.16 | 1.24 ± 0.28 |
Matrine enhances the effects of anticancer drugs in A549 cells
Cisplatin, 5-fluorouracil, and paclitaxel were used to investigate the effect of matrine on the anticancer drugs (Table 5). Matrine treatment markedly enhanced the inhibitory effect of cisplatin, 5-fluorouracil, and paclitaxel on A549 cells (P < 0.05), and the effects were dosage-dependent.
Table 5.
Effect of matrine combined with anticancer drugs on cell inhibitory ratio in A549 cells (%). Data are presented as mean ± SEM. The values having different superscript letters were significantly different with other groups (P < 0.05; n = 6).
| Item | 0 μM Matrine | 10 μM Matrine | 50 μM Matrine | 100 μM Matrine |
|---|---|---|---|---|
| Cisplatin mM | ||||
| 0.1 | 9.3 ± 3.4b | 13.5 ± 4.2b | 19.1 ± 4.8ab | 24.8 ± 5.1a |
| 1.0 | 21.7 ±3.7b | 29.5 ± 3.1b | 39.3 ± 4.5a | 48.2 ± 6.1a |
| 5.0 | 36.2 ± 4.2b | 44.7 ± 5.9b | 57.2 ± 3.1a | 70.7 ± 6.3a |
| 5-Fluorouracil mM | ||||
| 0.1 | 6.4 ± 1.3b | 9.7 ± 2.7b | 15.6 ± 3.7ab | 21.3 ± 3.2a |
| 1.0 | 17.9 ± 2.9b | 24.5 ± 3.1a | 33.3 ± 3.7a | 42.1 ± 4.8a |
| 10.0 | 28.4 ± 3.9b | 34.8 ± 5.9b | 49.7 ± 5.0a | 64.3 ± 6.8a |
| Paclitaxel mM | ||||
| 0.1 | 8.7 ± 1.9b | 15.3 ± 3.7a | 24.8 ± 2.8a | 29.1 ± 4.9a |
| 1.0 | 19.4 ± 2.3c | 27.3 ± 3.4bc | 37.8 ± 4.3b | 47.5 ± 4.5a |
| 5.0 | 33.2 ± 5.3b | 47.9 ± 5.9a | 60.1 ± 5.7a | 75.3 ± 5.1a |
Discussion
Sophora root is a traditional herb medicine and distributes mainly in China, Japan, and some European countries. Sophora root is the dried root of Sophora flavescens Aiton (Leguminosae) and includes matrine and oxymatrine, two major tetracyclo-quinolizindine alkaloids.12 Recently, matrine has been demonstrated to exhibit anticancer potentials, such as inhibiting cancer cell proliferation, inducing cell cycle arrest, accelerating apoptosis, restraining angiogenesis, inducing cell differentiation, inhibiting cancer metastasis and invasion, reversing multidrug resistance, and preventing or reducing chemotherapy or radiotherapy induced toxicity when combined with other chemotherapeutic drugs.12–14 However, the functions and underlying mechanisms of matrine in NSCLC is still largely obscure. Thus, in this study, we investigated the effects of matrine on the apoptosis and the molecular mechanism in A549 cells.
In this study, we found that matrine induces cell apoptosis in A549 cells, which may be mediated by p53, bax, casp3, and bcl-2. p53, a tumor suppressor, has established functions in cancer, such as causing cell-cycle arrest and apoptosis.15 Similarly, previous report indicates that matrine activates p53 signal and plays a beneficial role in human hepatoma cells.16 Bax is a pro-apoptotic gene of the Bcl-2 protein family and is inhibited after matrine exposure in A549 cells. Bcl-2 is specifically considered as an important anti-apoptotic protein and tends to increase in matrine groups.17 Liang et al. reported that matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through influencing bax/bcl-2 signal.18
Also, CCR7 has been widely demonstrated to involve in carcinogenesis.19,20 In NSCLC, CCR7 is activated and contributes to cell proliferation and metastasis.21 In this study, we found that matrine significantly inhibits CCR7 expression, which may mediate cell apoptosis in A549 cells. Similarly, matrine has been reported to alleviate LPS-induced intestinal inflammation and oxidative stress in mice and Caco-2 cells, while CCR7-siRNA transfection blocked the protective effects of matrine and exacerbated LPS caused injury.22 Thus, CCL21, a ligand of CCR7, has been suggested to upregulate CCR7 expression,11,23 and used to block the inhibitory effect of matrine on CCR7. We found that CCL21 blocked the effects of matrine on p53, bax, and bcl-2, suggesting that matrine induced cell apoptosis by regulating CCR7 expression. Previous reports suggested that matrine enhances the effects of anticancer drugs.24,25 In this study, cisplatin, 5-fluorouracil, and paclitaxel were used to investigate the effect of matrine on the anticancer drugs and the results showed that matrine treatment markedly enhanced the inhibitory effect of cisplatin, 5-fluorouracil, and paclitaxel on A549 cells, and the effects were dosage-dependent.
In conclusion, matrine induces cell apoptosis in A549 cells via targeting CCR7. Blocking the inhibitory effect of matrine on CCR7 via CCL21 treatment alleviates matrine induced cell apoptosis. In addition, matrine enhances effects of anticancer drugs in A549 cells, such as cisplatin, 5-fluorouracil, and paclitaxel.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Chen D, Guo W, Qiu Z, et al. MicroRNA-30d-5p inhibits tumour cell proliferation and motility by directly targeting CCNE2 in non-small cell lung cancer. Cancer Lett 2015; 362: 208–217. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee S, Rhee YH and Ahn JC. Sulforaphene-Carboplatin Combination Synergistically Enhances Apoptosis by Disruption of Mitochondrial Membrane Potential and Cell Cycle Arrest in Human Non-Small Cell Lung Carcinoma. J Med Food 2016; 19: 860–869. [DOI] [PubMed] [Google Scholar]
- 3.Facchinetti F, Marabelle A, Rossi G, et al. Moving immune checkpoint blockade in thoracic tumors beyond non-small cell lung cancer. J Thorac Oncol 2016; 11: 1819–1836. [DOI] [PubMed] [Google Scholar]
- 4.Ancuceanu RV, Istudor V. Pharmacologically active natural compounds for lung cancer. Altern Med Rev 2004; 9: 402–419. [PubMed] [Google Scholar]
- 5.Kolac UK, Ustuner MC, Tekin N, et al. The Anti-Inflammatory and Antioxidant Effects of Salvia officinalis on Lipopolysaccharide-Induced Inflammation in Rats. J Med Food 2017; 20: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YL, Xi MZ, Choi YB, et al. Antithrombotic Effect of Fermented Ophiopogon japonicus in Thrombosis-Induced Rat Models. J Med Food 2017; 20: 637–645. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Xu Y, Ji W, et al. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol 2014; 35: 5111–5119. [DOI] [PubMed] [Google Scholar]
- 8.Cordero-Herrera I, Martin MA, Goya L, et al. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: relevance of MAPKs. Mol Nutr Food Res 2015; 59: 597–609. [DOI] [PubMed] [Google Scholar]
- 9.Yan C, Sun W, Wang X, et al. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol Nutr Food Res 2016; 60: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Li X, Bai M, et al. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int J Clin Exp Pathol 2015; 8: 14793–14799. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Liu L, Qiu X, et al. CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS One 2012; 7: e33262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Liu S, Yao M, et al. Effects of Vietnamese Sophora Root on growth, adhesion, invasion and motility of melanoma cells. Afr J Tradit Complement Altern Med 2014; 11: 62–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Xie S, Liu X, et al. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol Rep 2014; 32: 2118–2126. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Du H, Geng G, et al. Matrine inhibits proliferation and induces apoptosis via BID-mediated mitochondrial pathway in esophageal cancer cells. Mol Biol Rep 2014; 41: 3009–3020. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Jeong Y and Kim M. Mammea longifolia Planch. and Triana Fruit Extract Induces Cell Death in the Human Colon Cancer Cell Line, SW480, via Mitochondria-Related Apoptosis and Activation of p53. J Med Food 2017; 20: 485–490. [DOI] [PubMed] [Google Scholar]
- 16.Xie SB, He XX, Yao SK. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int J Oncol 2015; 47: 517–526. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo DB, Phulukdaree A, Anand K, et al. Centella asiatica Fraction-3 Suppresses the Nuclear Factor Erythroid 2-Related Factor 2 Anti-Oxidant Pathway and Enhances Reactive Oxygen Species-Mediated Cell Death in Cancerous Lung A549 Cells. J Med Food 2017; 20: 959–968. [DOI] [PubMed] [Google Scholar]
- 18.Liang CZ, Zhang JK, Shi ZL, et al. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemoth Pharm 2012; 69: 317–331. [DOI] [PubMed] [Google Scholar]
- 19.Tutunea-Fatan E, Majumder M, Xin X, et al. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer 2015; 14: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Zhou Y, Yang Y. CCR7 pathway induces epithelial-mesenchymal transition through up-regulation of Snail signaling in gastric cancer. Med Oncol 2015; 32: 467. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Zhang Q, Li Y, et al. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int J Clin Exp Pathol 2015; 8: 15729–15738. [PMC free article] [PubMed] [Google Scholar]
- 22.Wu GJ, Zhou WH, Zhao JF, et al. Matrine alleviates lipopolysaccharide-induced intestinal inflammation and oxidative stress via CCR7 signal. Oncotarget 2017; 8: 11621–11628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Cai W, Tao J, Zhang X, et al. Contribution of homeostatic chemokines CCL19 and CCL21 and their receptor CCR7 to coronary artery disease. Arterioscler Thromb Vasc Biol 2014; 34: 1933–1941. [DOI] [PubMed] [Google Scholar]
- 24.Rong B, Zhao C, Gao W, et al. Matrine promotes the efficacy and safety of platinum-based doublet chemotherapy for advanced non-small cell lung cancer. Int J Clin Exp Med 2015; 8: 14701–14717. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HQ, Jin JJ, Wang J. Matrine induces mitochondrial apoptosis in cisplatin-resistant non-small cell lung cancer cells via suppression of beta-catenin/survivin signaling. Oncol Rep 2015; 33: 2561–2566. [DOI] [PubMed] [Google Scholar]