Abstract
Rotator cuff tears are common musculoskeletal injuries that can cause significant pain and disability. While the clinical results of rotator cuff repair can be good, failure of tendon healing remains a significant problem. Molecular mechanisms underlying structural failure following surgical repair remain unclear. Histologically, enhanced inflammation, disorganization of the collagen fibers, calcification, apoptosis and tissue necrosis affect the normal healing process. Mesenchymal stem cells (MSCs) have the ability to provide improved healing following rotator cuff repair via the release of mediators from secreted 30–100nm extracellular vesicles called exosomes. They carry regulatory proteins, mRNA and miRNA and have the ability to increase collagen synthesis and angiogenesis through increased expression of mRNA and release of proangiogenic factors and regulatory proteins that play a major role in proper tissue remodeling and preventing extracellular matrix degradation. Various studies have shown the effect of exosomes on improving outcome of cutaneous wound healing, scar tissue formation, degenerative bone disease and Duchenne Muscular Dystrophy. In this article, we critically reviewed the potential role of exosomes in tendon regeneration and propose the novel use of exosomes alone or seeded onto biomaterial matrices to stimulate secretion of favorable cellular factors in accelerating the healing response following rotator cuff repair.
Keywords: Biceps tendon, Collagen disorganization, Exosomes, Mesenchymal stem cells, Microvesicles, Rotator cuff injury, Stem cell therapy
Introduction
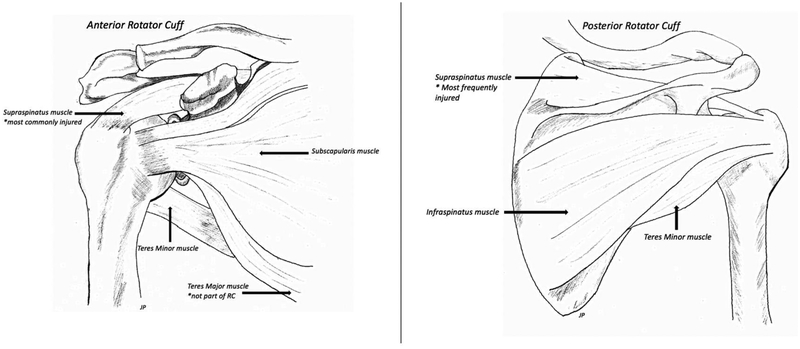
The rotator cuff is a group of 4 muscles and their tendons that are essential for the strength and stability of the shoulder joint (Figure 1) [1]. Rotator cuff (RC) tears are one of the most common shoulder injuries that impede daily activity, cause pain, and reduce functionality [1,2]. In the United States, over 270,000 rotator cuff repairs are performed annually [3]. Tears are caused by a number of intrinsic and extrinsic factors [4]. Ruptured tendons show collagen degeneration and a disordered arrangement of collagen [4]. In larger tears, there is a greater degree of reduction in fibroblast population which contributes to reduced healing [4]. A high percentage of tendons fail to heal back to bone properly, and often resulting in worse outcomes [5]. Due to the hypocellular nature of tendon, tissue regeneration is limited following repair. After surgery, scar tissue is laid down at the site of healing and is a major contributing factor to the high re-tear rate due to its weak mechanical properties.
Figure 1:
Schematic representation of the four muscles comprising the rotator cuff in both anterior and posterior views. These include Supraspinatus (most frequently torn), Infraspinatus, Teres Minor, and Subscapularis.
Tendon to bone union occurs at a specialized structure called the enthesis. It marks the transition between the soft tendinous tissue and hard bony tissue [6,7]. There are four continuous but distinct regions: tendon, non-mineralized fibrocartilage, mineralized fibrocartilage, and bone. These regions increase in mineralization and decrease in collagen fiber organization in the transition from tendon to bone. The enthesis distributes the mechanical strain along the transition to strengthen the bond between tendon and bone [8]. Following RC repair, healing of the enthesis occurs through scar tissue formation as opposed to regeneration of the transitional tissue [9]. The scar tissue lacks the gradient of mineral distribution and collagen fiber organization leading to weaker tissue and a greater re-tear rate [10]. There is currently no strategy to promote anatomic regeneration of the enthesis because it generally heals with scar-like tissue. However, recent literature has begun to focus on mesenchymal stem cells (MSCs) to promote proper regeneration. Regenerative medicine in orthopedic surgery is a young and challenging field. We have yet to completely understand the healing pathway of RC injuries. In particular, we need to achieve a greater understanding of stem cell biology and growth factor pathways to minimize the re-tear rate associated with RC repair.
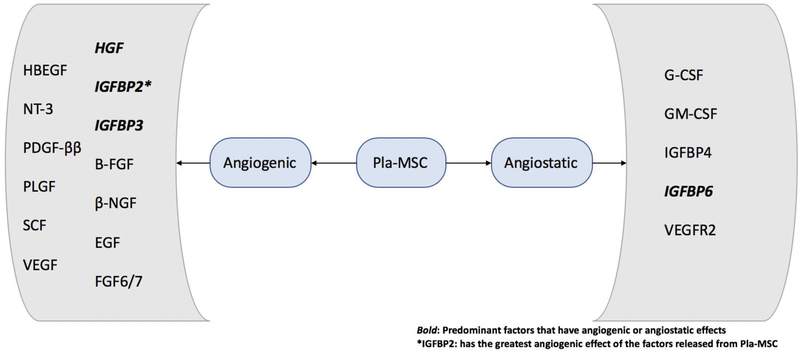
Stem cells are found in nearly every adult tissue and are multipotent in nature. Mesenchymal stem cells are capable of self-renewal and differentiation into a wide variety of cell phenotypes including chondrocytes, tenocytes and osteoblasts. In addition, MSCs have the ability to secrete growth factors to induce soft and hard tissue growth (Figure 2) [11]. While Figure 2 shows factors released specifically from placental MSCs, further investigations are warranted to examine if MSCs of different origins release shared factors or if they are tissue specific and to what extent they vary. The traditional approach of in vitro stem cell expansion on a plastic media provides difficulty in clinical translation due to phenotypic drift and senescence [12]. In vivo, stem cells are located within specialized microenvironments maintaining a balance between quiescence, self-renewal and differentiation.
Figure 2:
Schematic diagram showing proangiogenic factors and angiostatic factors released from Placental Mesenchymal Stem Cells (Pla-MSC) via exosomes, and the effect of these mediators on revascularization of the tissue contributed to wound healing. It is not currently understood if MSCs from different tissue origins release shared factors, or if the secretions are tissue specific. The bolded factors have a predominant effect on vacularization31 Angiogenic factors include: heparin binding EGF like growth factor (HBEGF), neurotrophin-3 (NT-3), platelet derived growth factor ββ(PDGF-ββ), placental growth factor (PLGF), stem cell factor (SCF), vascular endothelial growth factor (VEGF), human growth factor (HGF), insulin like growth factor binding protein 2 and 3 (IGFBP2 and IGFBP3), basic fibroblast growth factor (B-FGF), beta nerve growth factor (β-NGF), epidermal growth factor (EGF), and fibroblast growth factor 6 and 7 (FGF6/7). The angiostatic growth factors include: granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), insulin like growth factor binding protein 4 and 6 (IGFBP4 and IGFBP6), and vascular endothelial growth factor receptor 2 (VEGFR2).
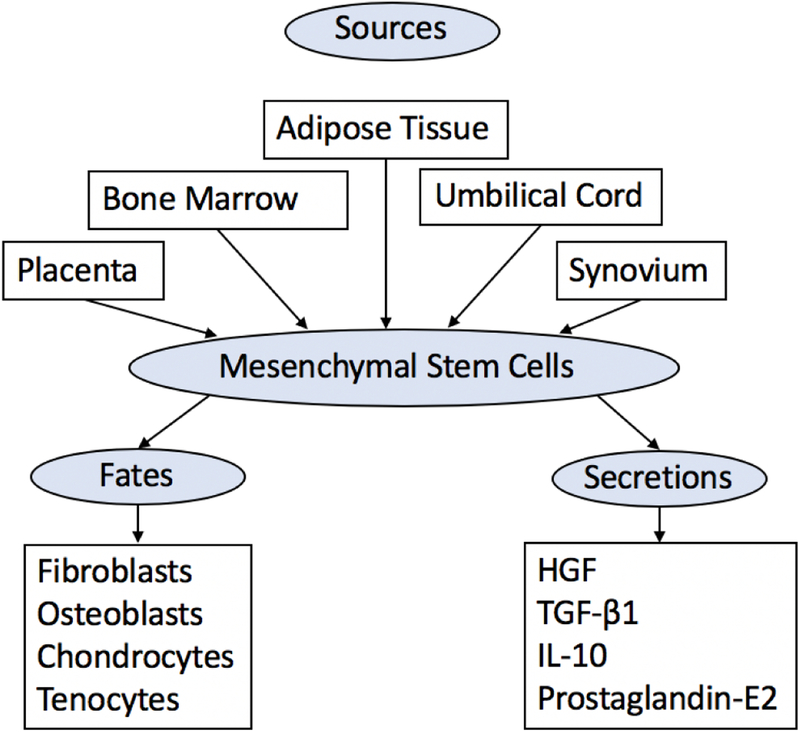
MSCs may be obtained from bone marrow, adipose tissue, umbilical cord blood, Wharton’s jelly, and synovial fluid (Figure 3) [13]. Bone marrow-derived MSCs are easily obtained during anchor hole placement in human arthroscopic rotator cuff repair [14]. Human studies using bone marrow-derived MSCs indicate improved functionality when applied to rotator cuff tear repair [15].
Figure 3:
Schematic diagram showing the sources, fates of differentiation, and primary mediators responsible for paracrine effect of mesenchymal stem cells that are responsible for improved tissue healing. Mesenchymal stem cells release Human Growth Factor (HGF), Transforming Growth Factor β1 (TGF-β1), Interleukin 10 (IL-10), and prostaglandin-E2.
Sevivas et al. [16] demonstrated the benefit of bone marrow derived MSC’s in RC repair by culturing tenocytes in a human MSC secretome that contains cytokines, growth factors and vesicles released from the MSCs. The cultured tenocytes increased in density and viability in vitro. However, in vivo tenocytes also displayed greater tendon maturing, elongation to rupture and lower stiffness, as evidenced by improved biochemical and histological properties. A study by Kim et al. [17] compared conventional RC repair vs. those who received RC repair with an injection of adipose-derived MSCs. The 12-month follow up demonstrated a retear rate of 28.5% in the conventional group and 14.3% in the MSC treated group. These two studies demonstrate that MSCs do potentially have a role in improved RC repair healing and that the substances secreted from MSCs may lead to increased tenocyte proliferation and differentiation via paracrine mechanisms. It is also likely that MSCs lead to increased mechanical properties and improved tissue morphology by modulating the local immune and bioactivity environment. However, this hypothesis warrants careful studies. Further exploration into how the MSC secretome may cause cellular differentiation could prove useful. Do MSCs differentiate into tendon cells or do they signal some other unknown local cell to do so? Perhaps there are several different kinds of cells involved in the enthesis healing, such as bone, cartilage and ligament, that can be used as a therapeutic tool to improve outcomes. However, the published reports demonstrate that MSCs have the potential to be used as a therapeutic tool yet the exact mechanism has not been elucidated.
Anatomy of the healthy tendon
Tendon is an anatomical structure connecting muscle to bone and transmitting the force from the muscle to the bone allowing movement of the structure. The basic elements of tendons are collagen (primarily type I collagen), cells and ground substance. Collagen provides tensile strength to the tendon and are arranged into primary, secondary, and tertiary bundles to form the tendon. In a normal healthy tendon, collagen type I is the most abundant, however collagen type III can be found in trace amounts. As the tendon degenerates, there is an increase in collagen type III [4].
The ground substance of the tendon provides structural support for the collagen fibers and is composed of glycosaminoglycans (GAG), proteoglycans and glycoproteins. In rotator cuff tendons, there is a higher amount of GAG and proteoglycans compared to purely tensional tendons. Rotator cuff tendons are also found to have higher concentration of hyaluronic acid.
Normal tendon tissue undergoes continuous remodeling. This process is under the control of matrix metalloproteinases (MMPs) that degrade collagen and the extracellular matrix (ECM). Subsequently, tenocytes will synthesize collagen for the remodeling process. No studies to date have identified definite stem or progenitor cells for tendon tissues. However, Bi et. al [18] isolated cells from mouse and human tendons and found that they possess criteria of stem cells but the population showed heterogeneity. Zhang et. al [19] demonstrated that tendons under stress from exercise increase collagen production and proliferation rates of tendon stem cells. It can be inferred that highly stressed tendons, such as the supraspinatus tendon, show higher levels of collagen remodeling than those under low stress.
The rotator cuff is unique and is composed of 4 muscles, each with a tendon that forms a collagen aponeurosis over the humeral head. It has been identified that each different tendon fuses to form one structure near the insertion to the humeral tuberosities [4].
Tendon Healing
The pathogenesis of rotator cuff tears is considered to be a combination of extrinsic impingement from structures surrounding the shoulder cuff and intrinsic degeneration from within the tendon [4]. Following a rotator cuff tear, the rotator cuff undergoes either intrinsic or extrinsic healing. Intrinsic healing occurs by the proliferation of tenocytes and results in better biomechanics. In the case of rotator cuff tears, extrinsic healing dominates. This involves the invasion of cells from the surrounding sheath and synovium resulting in scar tissue formation [4]. Following injury, collagen type III is prevalent in the healing process. Collagen type III is composed of smaller diameter fibrils than collagen I, resulting in weaker tensile strength. Eventually, collagen III switches to collagen I to complete the healing response [4]. However, this process usually takes about 6–12 months and the chance of re-tear during the first postoperative year is 15%−45% [11]. Additionally, co-morbidities often significantly delay the healing process.
Exosome Implication
Exosomes are a class of extracellular vesicles that are released by various cell types and have been found in cell cultures and body fluids [20,21]. Exosomes are 30–100 nm vesicles that are released by cancer cells, endothelial cells, immune cells and various other cells of the body including skeletal muscle [22,23]. Exosomes are recognized as potent vehicles for intracellular communication and can transfer messages encoded in protein, lipids, mRNA and miRNA enclosed within a lipid bilayer acting in an autocrine and paracrine manner. The contents of exosomes are regulated by the cells producing them in response to activation and stress inducing stimuli. [21] Exosomes derived from different tissues of origin influence various cellular properties due to their unique contents. Therefore, it is crucial to understand the tissue specific contents of exosomes to elucidate the manner in which they trigger a target cell effect. MSC-derived exosomes (MSC-exos) carry regulatory proteins (Figures 4–5), mRNA and miRNA which are delivered to various cells including fibroblasts altering the phenotype and function [24]. While research into exosomes role in post-operative rotator cuff enthesis is lacking, the indirect role of exosomes via MSC action (as discussed above) and the function of exosomes in other processes have been examined. Recent studies demonstrated that exosomes can regulate many biological processes including cancer progression, immune response, cell proliferation, cell migration and angiogenesis [25]. As a result, exosomes have become a novel topic for wound healing.
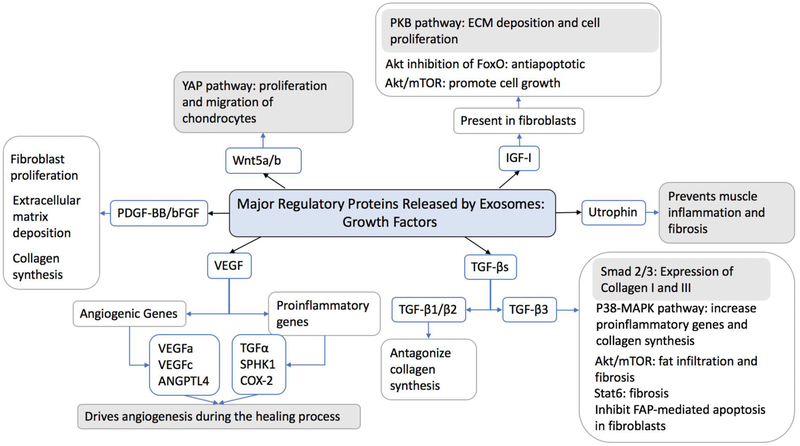
Figure 4:
Schematic diagram showing the major regulatory growth factors released by exosomes and the changes in tendon that occur aiding the healing process. Blue boxes indicate critical mediators for normal healing and tissue remodeling.23 Growth factors released by exosomes include: insulin like growth factor 1 (IGF1) which mediates the protein kinase B (PKB/AKT) pathway, forkhead box (FoxO) and mammalian target of rapamycin (mTOR). Transforming growth factor (TGF) activates mothers again decapentaplegic homolog 2 and 3 (SMAD 2/3), P38 mitogen-activated protein kinases (P38-MAPK), signal transducer and activator of transcription 6 (STAT6) and inhibit fibroblast activation protein (FAP) mediated apoptosis. Vascular endothelial growth factor (VEGF) alters transcription of VEGFa, VEGFc, angiopoietin-like 4 (ANGPTL4), TGFα, sphingosine kinase 1 (SPHK1) and cyclooxygenase 2 (COX2). Platelet derived growth factor BB (PDGF-BB) and basic fibroblast growth factor (bFGF). Wnt family member 5 a/b (Wnt5a/b) activate the yes associated protein pathway (YAP).
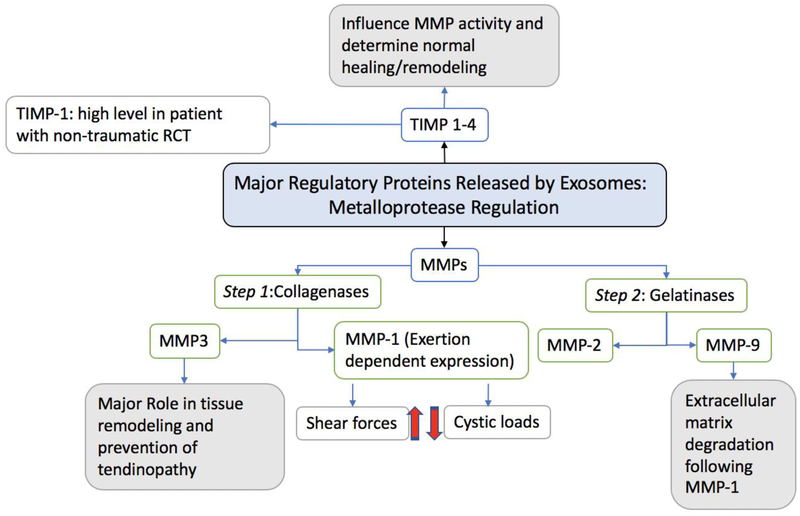
Figure 5:
Schematic diagram showing the major factors involved in metalloproteinase regulation released by exosomes and the changes in tendon that occur aiding the healing process. Blue boxes indicate critical mediators for normal healing and tissue remodeling.23 Exosomes release matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinase (TIMP).
As demonstrated in Figure 2, MSCs release proangiogenic and angiostatic factors via exosomes. Due to the hypocellular nature of native RC healing, scar tissue formation occurs leading to the high re-tear rate. The mediators contained in exosomes will in theory lead to revascularization of the tissue following RC repair resulting in better tissue perfusion, wound healing and ultimately lower chance of retear.
Several studies have been conducted to analyze the effect of MSC-exos on cutaneous wound healing in murine models. Studies with adipose derived MSC exosomes and induced pluripotent MSC exosomes have demonstrated the ability of MSC-exos to facilitate cutaneous wound healing through promotion of collagen synthesis and angiogenesis in vivo and in vitro [26–32]. Exosomes were found to promote fibroblast proliferation in a dose dependent manner and increase mRNA expression of collagen 1 and elastin leading to significantly faster wound healing [27]. MSC-exos increase the mRNA expression of the stemness-related gene OCT4, in fibroblasts suggesting they also regulate the responsiveness of endothelial cells to proangiogenic factors and cause vasodilation in existing blood vessels [33]. It is believed that the underlying mechanism of stem cell transplantation therapy is due to the paracrine activity of exosomes [28]. If so, exosomes could provide similar outcomes to stem cell therapy without the ethical concerns. Indeed, MSCs-derived exosomes act as paracrine mediators in the vicinity of transplantation site and recapitulate the regenerative functions of parental MSCs. This suggests that MSCs and MSC-exo display similar regenerative functions.
Even though studies have shown improved or similar regenerative effects of MSC-exo as with MSCs, the limiting factors such as lack of appropriate control, lack of appropriate dose, alterations in the end point of diseases, batch-to-batch variations of exosomal contents and the lack of knowledge regarding the off target effects of the exosomal mediators offers serious challenges to draw meaningful comparisons [34]. Thus, it is reasonable to consider MSC-exo as therapeutic biomaterial, however, warrants a more vivid definition for exosome-based biomaterials.
Further, scar tissue results from loss of function in cutaneous wounds and is due to disorganization and misalignment of collagen fibers [35]. Wang et al. [32] analyzed the effect of intravenous injection of adipose exosomes on scar tissue healing in a murine model. MSC-exos decreased the size of scars and increased the ratio of collagen III to collagen I. Additionally, exosomes increased that ratio of TGF-β3 to TGF-β1, important cell signals for collagen formation. There was also upregulation of matrix metalloproteinases-3 (MMP3) and tissue inhibitor of matrix metalloproteinases-1 leading to remodeling of the ECM and decreased scar formation. Further studies into the effect of MMPs and exosomes found that many exosomes carry MMP9 which transforms the collagen matrix from laminar to a fibrillar architecture [36]. This study also found that activation of MMP9 is more efficient on a 3D culturing medium compared to a 2D medium. A study on scleroderma dermal fibroblasts also found that there are reduced serum exosomes in diseased fibroblasts leading to down regulation of collagen and increased susceptibility to pitting scars, further confirming the effects of exosomes on scar formation [37].
Degenerative bone conditions are a major challenge in orthopedic surgery. MSC’s have been extensively investigated in regenerative medicine but further insight into the role exosomes play in degenerative bone diseases is needed. In osteoporotic rat models, MSC-exos enhanced cell proliferation and alkaline phosphatase activity as well as up-regulated mRNA and protein expression of osteoblast related genes, in vitro. In vivo, MSC-exos dramatically stimulated bone regeneration and angiogenesis in ovariectomized rats in a concentration-dependent manner [38]. Another study focused on the role of exosomes in alleviating osteoarthritis in mice following double medial meniscus destabilization surgery. Intra-articular injection of exosomes 4 weeks post-surgery-maintained chondrocyte phenotype by increasing collagen type II synthesis and decreasing ADAMTS5 expression in the presence of IL-1β, showing a beneficial therapeutic effect [39]. Tao et al. [40] utilized exosomes derived from miR-140–5p-overexpressing synovial mesenchymal stem cells in the treatment of osteoarthritis. This study found that Wnt5a and Wnt5b are carried by the exosomes and activate YAP via the alternative Wnt signaling pathway to enhance proliferation and migration of chondrocytes, in turn slowing the progression of early osteoarthritis and preventing severe damage to the knee articular cartilage [40].
Exosomes have also proved useful in the alleviation of muscular degeneration. Bier et al. [41] studied the effects of placenta derived MSCs and exosomes on Duchenne Muscular Dystrophy (DMD) patient’s myoblasts and mouse myoblasts. It was found that the MSCs increased differentiation of the myoblasts soaked in MSC medium, decreased the fibrinogenic gene expression typical of DMD myoblasts and increased expression of utrophin, which is expressed in high levels in developing muscle [42]. Intramuscular transplantation of MSCs in mice resulted in decreased creatinine kinase, significantly decreased expression of TGF-β and fibrosis in cardiac and diaphragm muscles, inhibited inflammation and increased utrophin expression [41]. Further, using a quantitative miR-29c reporter demonstrated that the effect of the MSCs was partly due to the transfer of miR-29c via secreted exosomes.
It is known that exosomes contain numerous miRNAs with diverse effects. Feng et al. [43] demonstrated that MSCs release exosomes containing miR-22 to protect cardiomyocytes from ischemia by reducing apoptosis and fibrosis. A recent study by Ferguson et al. [44] further explored the use of MSC exosomes and miRNA in the use of cardiac regeneration following myocardial infarction. Using nanoparticle analysis, they found that many miRNAs are released in exosomes from MSCs but the top 23 are responsible for 79.1% of miRNA found in exosomes. The miRNAs are involved in several processes in cardiovascular development, angiogenesis, cellular proliferation, and fibrosis. The exosomes mediate angiogenic, antifibrotic and antiapoptotic phenotypes and can be altered with a virus-free approach to enhance these phenotype effects. This data demonstrates that the miRNA found in MSC-exos play a major role in the regenerative property post-myocardial infarction. Further exploration into the use of these miRNA and the role they may play in angiogenesis, antifibrosis and anti-apoptosis following RC repair may provide avenues for therapeutic manipulation utilizing their favorable effects.
Several different origins of MSCs have been explored including placental, adipose, bone marrow, synovial fluid, etc. It is not currently understood if MSCs of different tissue origins release shared factors or if the secretions are tissue specific. Perhaps isolation of MSCs and exosomes from specific tissues can alter cellular functioning and manifest in better healing outcomes. Additionally, MSCs and therefore exosomes are secreted by the cells of muscle, tendon, and surrounding tissue when a rotator cuff tear has occurred. Xu et. al [45] found that MSCs in proximity to tenocytes would undergo tenogenic differentiation via paracrine mechanisms involving TGF-β existing in tenocyte derived exosomes. Further, Tokunaga et al. [46,47] also demonstrated that fibroblast growth factor-2 (FGF-2) promotes growth of tenogenic progenitor cells resulting in biomechanical and histologic improvement of RC repair. Perhaps utilization of tenocyte derived exosomes in RC repair would lead to better outcomes if used in conjunction with MSCs. Inquiry into the use of tendon and muscle cell derived exosomes as therapy following RC repair may prove beneficial due to the shared tissue origin allowing for more tissue specific wound regeneration. Currently, there is no optimal tissue of origin yielding better outcomes in RC repair, however further insight into origin specific differences in secretion pattern may provide a superior source of isolation.
A recently published article by Cui et al. [48] demonstrates that not all exosome function is beneficial to wound healing, and some may actually contribute to fibrotic tendon healing. This study found that macrophage depleted mice had less peritendinous fibrosis following hand tendon injury. When these mice were then treated with bone marrow macrophage derived exosomes fibrogenesis of tendons occurred. Exosomal miR-21–5p leads to fibrogenesis of tendons, demonstrating that miR-21–5p contained in exosomes controls the fibrotic healing response.
While research into the effect exosomes have on rotator cuff healing is currently lacking, exosomes have been demonstrated to play a significant role in cutaneous wound healing and scar formation, alleviating osteoporosis and osteoarthritis, and increasing healthy myoblast expression in DMD patients. Many studies suggest that the function of MSCs is partly executed through exosome secretion. Further research is needed into the application of exosomes in rotator cuff healing to gain a more complete understanding of possible implications of exosomes for future therapeutic treatments, including a more detailed analysis of which miRNA carry out the function of exosomes.
Exosomes with Biomaterials
There is limited literature regarding the use of stem cells seeded onto various biomaterial matrices and how this can improve tissue healing compared to a single method used in isolation. A recent article discussed the use of endothelial progenitor cells (EPCs) and MSCs as cell therapy in ischemic heart disease [49,50]. In a rat model, the group was able to increase engraftment and retention of EPCs in the myocardium and improve left ventricular end diastolic function (LVEF). While there isn’t current data on the effect of this methodology in a more clinically relevant model, success in larger mammals could lead to extension into human trials. It also raises the question if the use of exosomes seeded in matrices will produce a similar effect as EPCs and MSCs in the heart tissue and if this can be translated to utilization with rotator cuff matrices.
A limited number of studies have explored the use of stimuli and the role it plays in the maintenance of human tissues [51,52]. Further studies were conducted to analyze the effects of uniaxial stretching on MSCs and found that increased strain improved MSC differentiation and enhancement of tissue [53]. This brings up the question of what specific mediators are released from MSCs when stretched and if these factors can be loaded into exosomes and directly implanted into the rotator cuff repair to improve tissue regeneration. Additionally, is it possible to manipulate MSCs to release exosomes containing desired cytokines and growth factors that will aid the healing process?
Further exploration is needed regarding the best method of delivery of exosomes to rotator cuff healing. Is it directly injected to the site or loaded on a biomaterial to enhance healing? What other methods of delivery can prove useful? Additionally, can exosome effect be enhanced with the addition of various medications? Is it possible to manipulate exosomes by adding specific cytokines and growth factors to improve enthesis healing and limit re-tear? Greater investigation and understanding of exosomal function will answer these questions and lead to improved efficacy of tissue healing and regenerative medicine.
Conclusions
At this current, early stage of exosome studies, we do not have a complete understanding of the potential therapeutic applicability. However, since the function of MSCs are carried out by paracrine mechanisms, exosomes provide a promising avenue to achieve similar outcomes. Due to the exosome’s properties of increased stability in circulation, biocompatibility, low immunogenicity and toxicity, they are an attractive delivery system for therapeutics [54]. Additionally, exosomes do not require engraftment, which is a common barrier for classic cell-based therapies, and they have the potential to undergo large scale production and manufacturing [55]. These characteristics provide an advantage over traditional stem cell-based therapies, providing a more complete understanding of exosomal function can be achieved.
Rotator cuff tears are a common problem that account for a significant amount of morbidity in the adult population. By furthering our understanding into the process of enthesis healing, we can reduce the high recurrence rate. Various studies have been conducted using MSCs and their effect on wound healing, however, there are various obstacles to their use. Recent insight has shown that exosomes may carry out the wound healing benefit of MSCs. Exosomes are found to increase collagen synthesis and angiogenesis through increased expression of mRNA of collagen 1 and elastin as well as proangiogenic factors, resulting in reduced scar formation and improved tissue regeneration. Limited research has been conducted into exosome function and the role they can play in wound healing, but they may be responsible for the underlying mechanisms of MSC healing properties. Therefore, further examination of their ability to improve healing of the enthesis in rotator cuff models is needed.
Key points.
Ruptured supraspinatus tendons show collagen degeneration and disordered arrangement of collagen.
In larger tears of the supraspinatus, there is a reduction in fibroblast production resulting in decreased healing and scar tissue formation.
Treatment of rotator cuff with MSCs is safe, increases the rate of healing, and decreases the rate of re-tear, but are accompanied with ethical concerns.
Exosomes cause increased collagen synthesis and angiogenesis through increased expression of mRNA of collagen 1, elastin and release of proangiogenic factors.
Exosomes reduce scar formation via upregulation of TGF-β and matrix metalloproteinases.
Exosomes enhanced cell proliferation and alkaline phosphatase activity and upregulate expression of osteoblast genes in osteoporotic rats.
Exosomes dramatically stimulated bone regeneration and angiogenesis in ovariectomized rats.
Exosomes enhance proliferation and migration of chondrocytes to slow progression of early osteoarthritis and prevent severe knee articular cartilage damage.
Intramuscular transplantation of MSCs in DMD mice resulted in decreased fibrosis in cardiac and diaphragm muscles and inflammation via exosome function.
Exosomes may be the underlying mechanism of stem cell therapy, providing similar results with reduced ethical concerns.
Acknowledgements
This work was supported primarily by the State of Nebraska LB506 grant to DKA and LB692 grant to MFD by Creighton University. The research work of DKA is also supported by grants R01HL120659 and R01HL144125 from the National Institutes of Health (NIH). The content of this original research article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the State of Nebraska.
Footnotes
Competing interests’ disclosure
All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no other relevant affiliations or financial or non-financial involvement with any organization or entity with financial or non-financial interest or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- 1.Zhao S, Su W, Shah V, et al. Biomaterials based strategies for rotator cuff repair. Colloids and Surfaces B: Biointerfaces. 2017;157:407–416. doi: 10.1016/j.colsurfb.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Gold JE, Hallman DM, Hellström F, et al. Systematic review of quantitative imaging biomarkers for neck and shoulder musculoskeletal disorders. BMC Musculoskeletal Disorders. 2017;18:395. doi: 10.1186/s12891-017-1694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskeletal Disorders. 2014;15:4. doi: 10.1186/1471-2474-15-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longo UG, Berton A, Khan WS, Maffulli N, Denaro V. Histopathology of Rotator Cuff Tears: Sports Medicine and Arthroscopy Review. 2011;19(3):227–236. doi: 10.1097/JSA.0b013e318213bccb [DOI] [PubMed] [Google Scholar]
- 5.Rashid MS, Cooper C, Cook J, et al. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthopaedica. 2017;88(6):606–611. doi: 10.1080/17453674.2017.1370844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahishnu Patel, Caldwell Jon-Michael, Doty Stephen B., et al. Integrating soft and hard tissues via interface tissue engineering. Journal of Orthopaedic Research. 2017;0(0). doi: 10.1002/jor.23810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruyssen-Witrand A, Jamard B, Cantagrel A, et al. Relationships between ultrasound enthesitis, disease activity and axial radiographic structural changes in patients with early spondyloarthritis: data from DESIR cohort. RMD Open. 2017;3(2):e000482. doi: 10.1136/rmdopen-2017-000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hideaki Takahashi, Hiroyuki Tamaki, Mineo Oyama, Noriaki Yamamoto, Hideaki Onishi. Time‐Dependent Changes in the Structure of Calcified Fibrocartilage in the Rat Achilles Tendon–Bone Interface With Sciatic Denervation. The Anatomical Record. 2017;300(12):2166–2174. doi: 10.1002/ar.23684 [DOI] [PubMed] [Google Scholar]
- 9.Hexter AT, Pendegrass C, Haddad F, Blunn G. Demineralized Bone Matrix to Augment Tendon-Bone Healing: A Systematic Review. Orthop J Sports Med. 2017;5(10). doi: 10.1177/2325967117734517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa H, Morihara T, Fujiwara H, et al. Effect of Footprint Preparation on Tendon-to-Bone Healing: A Histologic and Biomechanical Study in a Rat Rotator Cuff Repair Model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2017;33(8):1482–1492. doi: 10.1016/j.arthro.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. International Orthopaedics. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1 [DOI] [PubMed] [Google Scholar]
- 12.Zitnay JL, Reese SP, Tran G, Farhang N, Bowles RD, Weiss JA. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomaterialia. 2018;65:76–87. doi: 10.1016/j.actbio.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Frontiers in Genetics. 2016;7. doi: 10.3389/fgene.2016.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Arciero RA, Drissi H. Rapid Isolation of Human Stem Cells (Connective Tissue Progenitor Cells) From the Proximal Humerus During Arthroscopic Rotator Cuff Surgery. The American Journal of Sports Medicine. 2010;38(7):1438–1447. doi: 10.1177/0363546509360924 [DOI] [PubMed] [Google Scholar]
- 15.Honda H, Gotoh M, Kanazawa T, et al. Hyaluronic Acid Accelerates Tendon-to-Bone Healing After Rotator Cuff Repair. The American Journal of Sports Medicine. 2017;45(14):3322–3330. doi: 10.1177/0363546517720199 [DOI] [PubMed] [Google Scholar]
- 16.Sevivas N, Teixeira FG, Portugal R, et al. Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear. Am J Sports Med. 2018;46(2):449–459. doi: 10.1177/0363546517735850 [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863 [DOI] [PubMed] [Google Scholar]
- 18.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine. 2007;13(10):1219–1227. doi: 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Pan T, Liu Y, Wang JH-C. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. Journal of Orthopaedic Research. 2010;28(9):1178–1183. doi: 10.1002/jor.21123 [DOI] [PubMed] [Google Scholar]
- 20.Crescitelli R, Lässer C, Szabó TG, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. Journal of Extracellular Vesicles. 2013;2(1):20677. doi: 10.3402/jev.v2i0.20677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zonneveld MI, Brisson AR, van Herwijnen MJC, et al. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. Journal of Extracellular Vesicles. 2014;3(1):24215. doi: 10.3402/jev.v3.24215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kida Y, Morihara T, Matsuda K-I, et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. Journal of Shoulder and Elbow Surgery. 2013;22(2):197–205. doi: 10.1016/j.jse.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Gasperi RD, Hamidi S, Harlow LM, Ksiezak-Reding H, Bauman WA, Cardozo CP. Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Scientific Reports. 2017;7(1):12888. doi: 10.1038/s41598-017-13105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell–derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol Ther Nucleic Acids. 2013;2(10):e126. doi: 10.1038/mtna.2013.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Than UTT, Guanzon D, Leavesley D, Parker T. Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing. International Journal of Molecular Sciences. 2017;18(5):956. doi: 10.3390/ijms18050956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fouda MB, Thankam FG, Dilisio MF, Agrawal DK. Alterations in tendon microenvironment in response to mechanical load: potential molecular targets for treatment strategies. Am J Transl Res. 2017;9(10):4341–4360. [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports. 2016;6(1). doi: 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of Translational Medicine. 2015;13(1). doi: 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Experimental Dermatology. November 2017. doi: 10.1111/exd.13451 [DOI] [PubMed] [Google Scholar]
- 30.El-Tookhy OS, Shamaa AA, Shehab GG, Abdallah AN, Azzam OM. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int J Stem Cells. 2017;10(2):144–153. doi: 10.15283/ijsc17043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hocking AM. Mesenchymal Stem Cell Therapy for Cutaneous Wounds. Adv Wound Care (New Rochelle). 2012;1(4):166–171. doi: 10.1089/wound.2011.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports. 2017;7(1):13321. doi: 10.1038/s41598-017-12919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komaki M, Numata Y, Morioka C, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Research & Therapy. 2017;8(1). doi: 10.1186/s13287-017-0660-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phinney DG, Pittenger MF. MSC-derived exosomes for cell-free therapy. Stem Cells 2017; 35: 851–858. [DOI] [PubMed] [Google Scholar]
- 35.Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015;4(3):119–136. doi: 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laghezza Masci V, Taddei AR, Gambellini G, Giorgi F, Fausto AM. Microvesicles shed from fibroblasts act as metalloproteinase carriers in a 3-D collagen matrix. J Circ Biomark. 2016;5. doi: 10.1177/1849454416663660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura K, Jinnin M, Harada M, et al. Altered expression of CD63 and exosomes in scleroderma dermal fibroblasts. Journal of Dermatological Science. 2016;84(1):30–39. doi: 10.1016/j.jdermsci.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 38.Qi X, Zhang J, Yuan H, et al. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. International Journal of Biological Sciences. 2016;12(7):836–849. doi: 10.7150/ijbs.14809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Yu D, Liu Z, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Research & Therapy. 2017;8:189. doi: 10.1186/s13287-017-0632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao S-C, Yuan T, Zhang Y-L, Yin W-J, Guo S-C, Zhang C-Q. Exosomes derived from miR-140–5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bier A, Berenstein P, Kronfeld N, et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018;174:67–78. doi: 10.1016/j.biomaterials.2018.04.055 [DOI] [PubMed] [Google Scholar]
- 42.McNally E. Chapter81 - Novel Targets and Approaches to Treating Skeletal Muscle Disease In: Hill JA, Olson EN, eds. Muscle. Boston/Waltham: Academic Press; 2012:1095–1103. doi: 10.1016/B978-0-12-381510-1.00081-8 [DOI] [Google Scholar]
- 43.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via miR-22. PLOS ONE. 2014;9(2):e88685. doi: 10.1371/journal.pone.0088685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson SW, Wang J, Lee CJ, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8. doi: 10.1038/s41598-018-19581-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu T, Xu M, Bai J, et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71(1):57–65. doi: 10.1007/s10616-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokunaga T, Shukunami C, Okamoto N, et al. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am J Sports Med. 2015;43(10):2411–2422. doi: 10.1177/0363546515597488 [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga T, Karasugi T, Arimura H, et al. Enhancement of rotator cuff tendon–bone healing with fibroblast growth factor 2 impregnated in gelatin hydrogel sheets in a rabbit model. Journal of Shoulder and Elbow Surgery. 2017;26(10):1708–1717. doi: 10.1016/j.jse.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 48.Cui H, He Y, Chen S, Zhang D, Yu Y, Fan C. Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21–5p/Smad7 Pathway. Mol Ther Nucleic Acids. 2018;14:114–130. doi: 10.1016/j.omtn.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaffey AC, Chen MH, Venkataraman CM, et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. The Journal of Thoracic and Cardiovascular Surgery. 2015;150(5):1268–1277. doi: 10.1016/j.jtcvs.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal DK, Siddique A. Rejuvenation of “broken heart” with bioengineered gel. The Journal of Thoracic and Cardiovascular Surgery. doi: 10.1016/j.jtcvs.2018.08.076 [DOI] [PubMed] [Google Scholar]
- 51.Nam HY, Pingguan-Murphy B, Abbas AA, Merican AM, Kamarul T. The proliferation and tenogenic differentiation potential of bone marrow-derived mesenchymal stromal cell are influenced by specific uniaxial cyclic tensile loading conditions. Biomech Model Mechanobiol. 2015;14(3):649–663. doi: 10.1007/s10237-014-0628-y [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto H, Aoki M, Hidaka E, Fujimiya M, Uchiyama E. Measurement of strain and tensile force of the supraspinatus tendon under conditions that simulates low angle isometric elevation of the gleno-humeral joint: Influence of adduction torque and joint positioning. Clinical Biomechanics. 2017;50:92–98. doi: 10.1016/j.clinbiomech.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 53.Nam HY, Raghavendran HRB, Pingguan-Murphy B, Abbas AA, Merican AM, Kamarul T. Fate of tenogenic differentiation potential of human bone marrow stromal cells by uniaxial stretching affected by stretch-activated calcium channel agonist gadolinium. PLOS ONE. 2017;12(6):e0178117. doi: 10.1371/journal.pone.0178117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacologica Sinica. 2018;39(4):501–513. doi: 10.1038/aps.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tracy SA, Ahmed A, Tigges JC, et al. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: Bone marrow and amniotic fluid. Journal of Pediatric Surgery. 2019;54(1):86–90. doi: 10.1016/j.jpedsurg.2018.10.020 [DOI] [PubMed] [Google Scholar]