Short abstract
Despite the paramount role of TLRs in the induction of innate immune and inflammatory responses, there is a paucity of studies on the role of TLRs in Schistosoma japonicum infection. Here, we observed obvious infiltration of inflammatory cells in S. japonicum-infected C57BL/6 mouse lungs. Expression and release of IFN-γ, IL-4, and IL-17 were significantly higher in pulmonary lymphocytes from infected mice compared with control mice in response to anti-CD3 plus anti-CD28 mAbs. Higher percentages of TLR2, TLR3, TLR4, and TLR7 were expressed on such lymphocytes, and the TLR agonists PGN, Poly I:C, LPS, and R848 induced a higher level of IFN-γ. However, a higher level of IL-4 was found in the supernatant of pulmonary lymphocytes from infected mice stimulated by these TLR agonists plus CD3 Ab. Only R848 plus anti-CD3 mAb could induce a higher level of IFN-γ in such lymphocytes. TLR expressions were then compared on different pulmonary lymphocytes after infection, including T cells, B cells, NK cells, NKT cells, and γδT cells. The expression levels of TLR3 on T cells, B cells, NK cells, and γδT cells were increased in the lungs after infection. NK cells also expressed higher levels of TLR4 after infection of control mice. Collectively, these findings highlight the potential role of TLR expression in the context of S. japonicum infection.
Keywords: Schistosoma japonicum, lung, TLRs, cytokines, ligands
Introduction
Schistosoma japonicum is one of the major infectious agents of schistosomiasis, which causes significant morbidity and mortality, especially in developing countries.1,2 Schistosomula and its egg migration in the lung can trigger immune pathological reactions, including the formation of granulomas, fibrosis, and interstitial pulmonary disease.3 The outcome of schistosome infection relies on both innate and adaptive immune responses,4 including immune cell cytokines, adhesion molecules, and chemokines.5 Additionally, granulomatous inflammation against parasite eggs is the hallmark of schistosome infection.
TLRs, which are a class of PRRs, are germline-encoded innate immune receptors.6,7 Mammalian TLRs, central innate receptors, are able to distinguish distinct pathogen-associated molecular patterns (PAMPs) from viruses, bacteria, and parasites. Binding of TLRs with their specific ligands induces a signaling cascade resulting in the induction of type I IFNs and other cytokines, which drive an inflammatory response and activate the adaptive immune system.8,9 The central role is to protect the host by perceiving danger and detecting the presence of invading pathogens. They are expressed on extensive immune cell types, such as T and B cells, NK cells, macrophages, and dendritic cells (DCs), and also by a number of non-immune cells.7–9 Although in vivo functions of TLRs during bacterial, viral, and, to a lesser extent, during fungal and protozoan parasitic infections have been extensively reported,10 finite studies have addressed the role of TLRs in the immune response to S. japonicum infections.11
In mice and humans combined there are 13 paralogous TLRs; 10 in humans and 12 in mice.12 They are trans-membrane receptors that are found either on the cell membrane (TLR1, 2, 4, 5, and 9) or on intracellular organelles (TLR3, 7, and 8).13,14 It is well known that each TLR family member recognizes a specific pathogen component and, upon activation, triggers a signaling cascade leading to cytokine production and adaptive immune response.15,16 Among potential signaling PRRs, TLR2, TLR3, and TLR4 have been identified as central sensors of parasite and egg components during S. mansoni infection by some scholars.17–19 Moreover, Wang et al. have demonstrated that TLR7/8 ligands could enhance the protective efficacy of DNA vaccines against schistosomiasis.8 In our study, we tested the response to TLR ligands PGN, Poly I:C, LPS, and R848 as measured by IFN-γ and IL-4 secretion in vitro.
In this study, the expressions of TLRs in different kinds of innate immune cells were compared by flow cytometry between normal and S. japonicum-infected mice, and TLR agonists were used to detect the function of TLRs in the progress of S. japonicum infection.
Materials and methods
Mice
Female C57BL/6 mice (6–8 wk old) were acquired from Zhongshan University Animal Center (Guangzhou, China) and fed in a specific-pathogen-free facility at Guangzhou Medical University. All animal experiments were performed in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (1988.11.1). The animal protocols were approved by the Committee on the Ethics of Animal Experiments of Guangzhou Medical University.
Parasite infection
S. japonicum cercariae were shed from naturally infected Oncomelania hupensis snails, which were purchased from Jiangsu Institute of Parasitic Disease (Wuxi, China). Female C57BL/6 mice were divided into two groups, 30 as control (normal group) and 30 infected with 40 ± 5 cercariae of S. japonicum per mouse (infected group) and killed at 6 wk after infection.
Abs
The FITC-conjugated anti-mouse CD3 (17A2), PE-Cy7-conjugated anti-mouse NK1.1 (PK136), FITC-conjugated anti-mouse TCR-cdCR (17A2), Alexa Fluor-conjugated anti-mouse TLR2 (6C2), APC-conjugated anti-mouse TLR3 (118F), PE-conjugated anti-mouse TLR4 (TF901), PE-conjugated anti-mouse TLR7 (A94B10), and isotype-matched control mAb (X39, G155-178) were purchased from BD/Pharmingen (San Diego, CA). The neutralizing rat anti-mouse TLR2 (clone TC11-18H10.1) and an isotype-matched rat IgG2a mAb (clone RTK2758) were purchased from BioLegend (San Diego, CA).
Histology studies
Lungs were removed from the mice and perfused with 0.01 M PBS (pH 7.4) for three times, fixed in 10% formalin, paraffin embedded, and serially sectioned. Standard hematoxylin and eosin staining was done. The slices were examined by light microscopy under 100× magnification.
Lymphocyte isolation
Mice were anesthetized and fixed from wk 5 and 7 after infection. The excised lung was cut to small pieces and incubated in 5 mL of digestion buffer (collagenase IV/DNase I mix, Invitrogen Corporation) for 30 min, at 37 and 5% carbon dioxide. The digested lung tissue was pressed through 200-gauge stainless-steel mesh, and then was suspended in Hank’s balanced salt solution (HBSS). Lymphocytes were isolated by Ficoll-Hypaque (DAKEWE, China) density gradient centrifugation. Isolated cells were washed twice in HBSS and re-suspended in complete RPMI 1640 medium supplemented.
Cytometric bead array (CBA)
Single-cell suspensions were cultured in 96-well microtiter plates at 4 × 105 cells/200 μl medium per well in the presence of anti-CD3 mAb plus anti-CD28 mAb and supernatants were collected 72 h later. Levels of the released cytokines in supernatants were determined by using Mouse Th1/Th2 Kit FlowCytomix (eBioscience). CBA kit was performed in accordance with the manufacturer’s instructions. The samples were analyzed on flow cytometry (BD Calibur and Aria II).
RNA preparation for real-time PCR
Total RNA of pulmonary cells was isolated by using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). 1 μg of total RNA was transcribed to cDNA by using a SuperScript III Reverse Transcriptase Kit (Qiagen, Valencia, CA). Primers were synthesized by Invitrogen (Shanghai, China) and were TLR2 sense 5-CTCTCCGTCCCAACTGATGA-3, antisense 5-GGTCTGGTTGCATGGCTTTT-3; TLR3 sense 5-ATTCGCCCTCCTCTTGAACA-3, antisense 5-TCGAGCTGGGTGAGATTTGT-3; TL4 sense 5-AGGTTGAGAAGTCCCTGCTG-3, antisense 5-GGTCCAAGTTGCCGTTTCTT-3; TLR7 sense 5-GCATTCCCACTAACACCACC-3, antisense 5-ACACACATTGGCTTTGGACC-3 (reverse); β-antisense 5-CCGTAAAGACCTCTATGCCAAC-3, antisense 5-GGGTGTAAAACGCAGCTCAGTA-3. mRNA expression was analyzed with RT-qPCR by using Takara SYBR Premix Ex Taq II (RR820A). Reaction mixtures were incubated for 95°C for 30 s, followed by 95°C for 5s and 60°C for 30s (40 cycles) . In all cases, amplification was tested in triplicate. Amplification was performed by using the CFX96 touch qPCR system (Bio-Rad, Hercules, CA, USA), and the levels of TLR transcripts were normalized to β-actin transcripts by using the relative quantity (RQ) = 2−ΔΔCt method.
ELISA detection
Single-cell suspensions were cultured in 96-well microtiter plates at 4 × 105 cells/200 μl medium per well and challenged with either PGN, Poly I:C, LPS, R848 or together with anti-CD3 Ab, respectively. Then, supernatants were collected, and concentrations of the different cytokines were determined by using mouse cytokine kits for IFN-γ (BD Pharmingen) and IL-4 (BD Biosciences). ELISAs were performed in accordance with the manufacturer’s instructions. The optical density of each well was read at 450 nm by using a microplate reader (Model ELX-800, BioTek).
FACS detection
Single cell suspensions from the lungs of control mice and mice infected with S. japonicum were collected as described before. Cells were stained with conjugated Abs specific for the cell surface Ags CD3, CD19, NK1.1, and γδTCR, respectively. After washing in PBS, cells were fixed with 4% paraformaldehyde, permeabilized overnight at 4°C in PBS buffer containing 0.1% saponin (Sigma), 0.1% BSA, and 0.05% NaN3, then stained with conjugated Abs specific for mouse TLRs, including TLR2, TLR3, TLR4, and TLR7. Ab-Labeled lymphocytes (200,000–300,000 cells per run) were acquired on flow cytometry (BD Calibur and Aria II) and data were analyzed by using Cell Quest software (BD Biosciences). Isotype-matched controls were included in each staining protocol.
Statistics
Data was expressed as mean ± SD and statistical evaluation of difference between means was performed by unpaired, two-tailed t tests. All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) and P < 0.05 was considered significant.
Results
Pathological inflammation in the infected mice
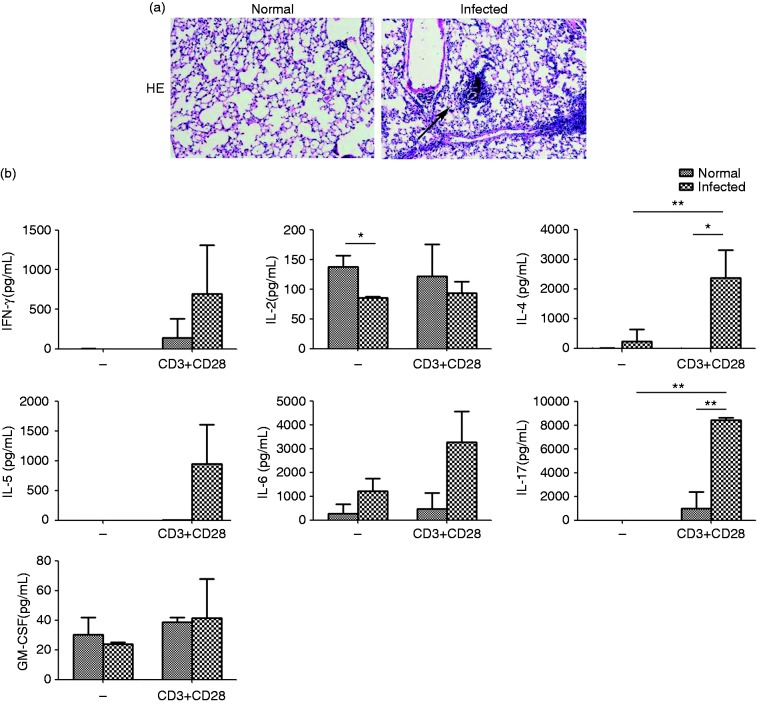
Granulomatous and fibrosing inflammation against parasite eggs are the pathological characters of schistosome infection. To examine the pathological changes in the lungs of S. japonicum-infected mice, 6–8-wk-old female C57BL/6 mice were infected with S. japonicum and were sacrificed 6 wk after infection. Lung tissues were cut into 5 μm sections and stained with hematoxylin and eosin to observe the effects of infection on lung microstructure. The pathologic damage and the infiltration of large amounts of inflammatory cells were observed in infected lung compared to normal control mice (Figure 1a). IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-17, and GM-CSF in the supernatant, which were detected by CBA, were associated with schistosomiasis-associated lung pathology. The releases of IFN-γ, IL-4, IL-5, and IL-17 were barely detectable in cultures of unstimulated lymphocytes from normal and infected lung. However, the levels of these cytokines were substantially higher by anti-CD3 mAb plus anti-CD28 mAb stimulation from normal infected lung than that in normal mice (IFN-γ: 695.4 ± 611.6 pg/mL versus 142.8 ± 236.0 pg/mL, P > 0.05; IL-4: 2392.4 ± 928.4 pg/mL versus 11.2 ± 0.8 pg/mL, P < 0.05; IL-5: 944.6 ± 657.1 pg/mL versus 3.8 ± 6.6 pg/mL, P > 0.05;IL-17: 8443.4 ± 206.1 pg/mL versus 1000.4 ± 1404.6 pg/mL, P < 0.05, Figure 1b). Consistent with them, a higher level of IL-6 from infected lung was observed, although not significantly (P > 0.05). Taken together, these results suggest that infection by S. japonicum markedly altered the histological structure of the mouse lung and induced the cytokines production.
Figure 1.
The histopathological changes in the lung of infected C57BL/6 mice. (a) Sections of the lung of normal mice (left panels) and infected mice (right panels) were examined by H&E staining (×100). The multi-cellular granuloma could be observed in the infected group. (b) The levels of IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-17, and GM-CSF were detected by CBA. The data are representative of six experiments, each with three or four replicates per group (*P < 0.05, **P < 0.01; the error bars indicate SD).
TLR expression on pulmonary cells
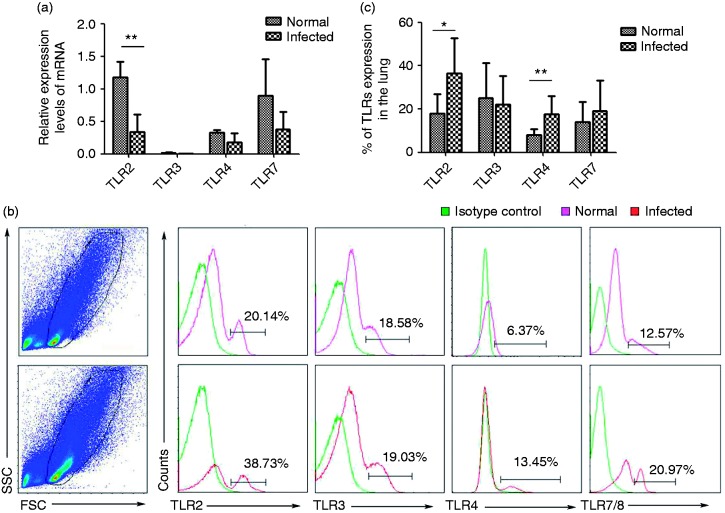
To explore the expression of TLR mRNA and protein in pulmonary cells, cells were isolated from normal and infected C57BL/6 mice lungs and were examined by qPCR and FACS, respectively. As shown in Figure 2a, the results demonstrated that the expression of TLR2 mRNA decreased after S. japonicum infection (P < 0 05), whereas there was no significant difference in the expression of TLR3, TLR4, and TLR7 mRNA between normal and infected mice. Of the normal pulmonary mononuclear cells, the percentages of cells expressing TLR2 and TLR4 protein comprised 18.04 ± 8.99% and 8.10 ± 2.67%, respectively. After infection, their protein expressions were significantly elevated compared with uninfected lungs (TLR2: 36.40 ± 16.01%, TLR4: 17.55 ± 8.52%; *P < 0.05, **P < 0.01, Figure 2c). Likewise, the cells expressing TLR3 and TLR7 showed an increase in the infected group, although not significantly compared to normal mice (TLR3: 22.06 ± 13.32% versus 25.11 ± 16.15%, TLR7: 19.08 ± 14.26% versus 14.14 ± 9.16%, P > 0.05). Thus, we concluded that the infection might induce the expression of TLR2, TLR3, TLR4, and TLR7 protein in infected lung.
Figure 2.
TLR expressions in lymphocytes isolated from control or infected mouse lung. (a) The accumulation of TLR2, TLR3, TLR4, and TLR7 mRNA was quantified by using qPCR. (b) The expression of TLR2, TLR3, TLR4, and TLR7 protein from control or infected lung were analyzed by flow cytometry. Flow cytometric analysis from one representative experiment. (c) Average percentages of TLRs in normal and infected mice were calculated from FACS data (n = 6) (*P < 0.05, **P < 0.01; the error bars indicate SD).
TLR expression regulates IFN-γ and IL-4 secretion
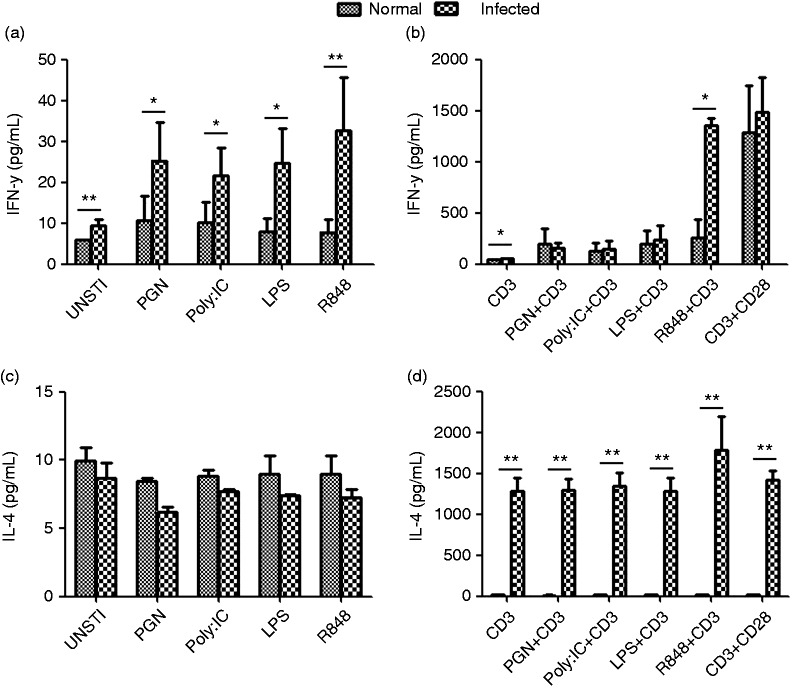
These PRRs recognize PAMPs in various cell compartments and trigger the release of inflammatory cytokines and type I IFNs for host defense. Therefore, we sought to analyze whether TLRs upon stimulation by specific ligands (PGN for TLR2, Poly I:C for TLR3, LPS for TLR4, and R848 for TLR7/8) initiate downstream signaling events that induce secretion of IFN-γ and IL-4. The single mononuclear lung cell suspensions of normal and infected mice were cultured in the presence of different ligands and anti-CD3 plus ligand. 72 h later, the culture supernatants were collected, and IFN-γ and IL-4 levels were detected by ELISA. As shown in Figure 3, we noted that increased IFN-γ secretion from infected lung in response to PGN, Poly I:C, LPS, and R848 (*P < 0.05, **P < 0.01), while there was no significant difference for IL-4 (Figure 3a and c). We noted that the levels of these two cytokines in the supernatant were considerably low in cultures of ligands-stimulated lymphocytes from normal and infected lungs, which was in the range 10–30 pg/mL. However, release of IFN-γ and IL-4 from pulmonary lymphocytes was significantly increased in normal and S. japonicum infected group by anti-CD3 plus specific ligands stimulation, especially IL-4. Thus, we concluded that PGN, Poly I:C, LPS, and R848 in infected lung could induce higher levels of IL-4 compared to the normal control (PGN: 1289.82 ± 295.86 pg/mL versus 13.80 ± 2.98 pg/mL; Poly I:C: 1340.63 ± 342.53 pg/mL versus 13.80 ± 2.98 pg/mL; LPS: 1281.41 ± 323.51 pg/mL versus 22.81 ± 8.46 pg/mL; R848: 1780.65 ± 830.27 pg/mL versus 15.40 ± 1.84 pg/mL; **P < 0.01) and infected mice exhibited significantly elevated IFN-γ+ cells in response to R848 plus CD3 compared with control mice.
Figure 3.
TLRs regulate IFN-γ and IL-4 secretion. Single lung cell suspensions of normal and infected mice were prepared and then cultured in the presence of different ligands plus anti-CD3 mAb. The culture supernatants were collected after 72 h of incubation for detection of IFN-γ and IL-4 by ELISA. The data are representative of four experiments, each with three or four replicates per group (*P < 0.05; the error bars indicate SD).
The percentage and absolute numbers of T cells, B cells, NK cells, NKT cells, and γδT cells
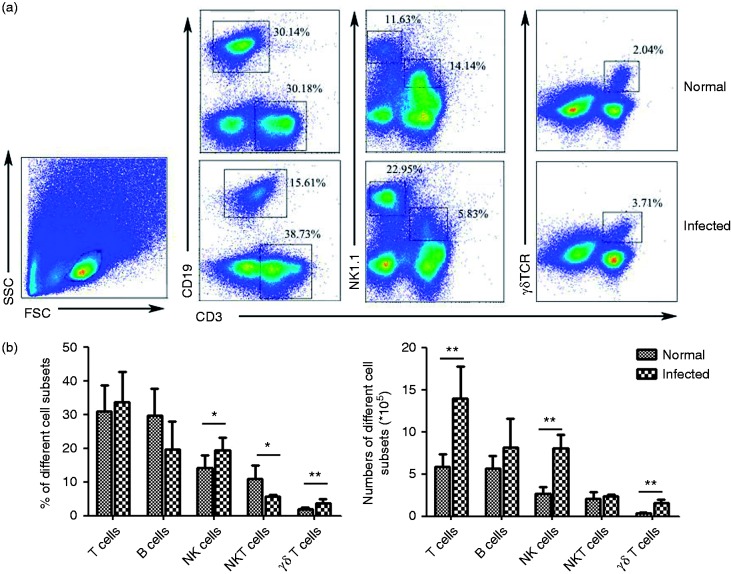
Accumulating evidence indicates that many cells of the adaptive immune system, including T and B cells, but also the innate immune cells, including NK cells and so on, may contribute to immunoregulatory effects during the infection. Therefore, we sought to investigate the effect of S. japonicum infection on the percentage of T cells, B cells, NK cells, NKT cells, and γδT cells in the lungs of C67BL/6 female mice by FACS. As shown in Figure 4, there was no significant increase the proportion of T cells (33.56 ± 9.17%) in the total infected lymphocyte compare to the normal, whereas the absolute number was higher in infected lung (13.92 ± 3.81 × 105 versus 5.88 ± 1.48 × 105, P < 0.01). Moreover, it indicated that the percentages of NK cells and γδT cells from infected mice lung were 19.53 ± 3.72% and 3.71 ± 1.09%, respectively, which were obviously higher than that from normal mice (14.18 ± 3.76% and 2.04 ± 0.32%, respectively) (P < 0.05, Figure 4). Conversely, the percentages of B cells and NKT cells were 19.60 ± 8.35% and 5.76 ± 0.42%, respectively, in infected mice, which were less than that of normal mice (29.71 ± 7.93%, P > 0.05; 10.80 ± 4.13%, P < 0.05). In addition, considering the dramatically increased number of pulmonary mononuclear cells in response to infection (4.15 ± 0.99 × 106 to 1.91 ± 0.23 × 106), the differences between the number of B cells and NKT cells in the normal and infected lungs were not obvious.
Figure 4.
The percentages and absolute numbers of T cells, B cells, NK cells, and γδT cells. (a) Flow cytometric analysis of CD3, CD19, NK1.1, and γδTCR expression on normal and infected mice lung lymphocytes is shown. Representative FACS plots are shown (n = 6). The numbers represent the percentage of cells in each subset. (b) Average percentages of T cells, B cells, NK cells, NKT cells, and γδT cells were calculated from FACS with the number of lymphocytes counted under microscope. Cell numbers are from different cell subsets (*P < 0.05; the error bars indicate SD).
TLR expression in different lymphocytes isolated from infected and uninfected mouse lung
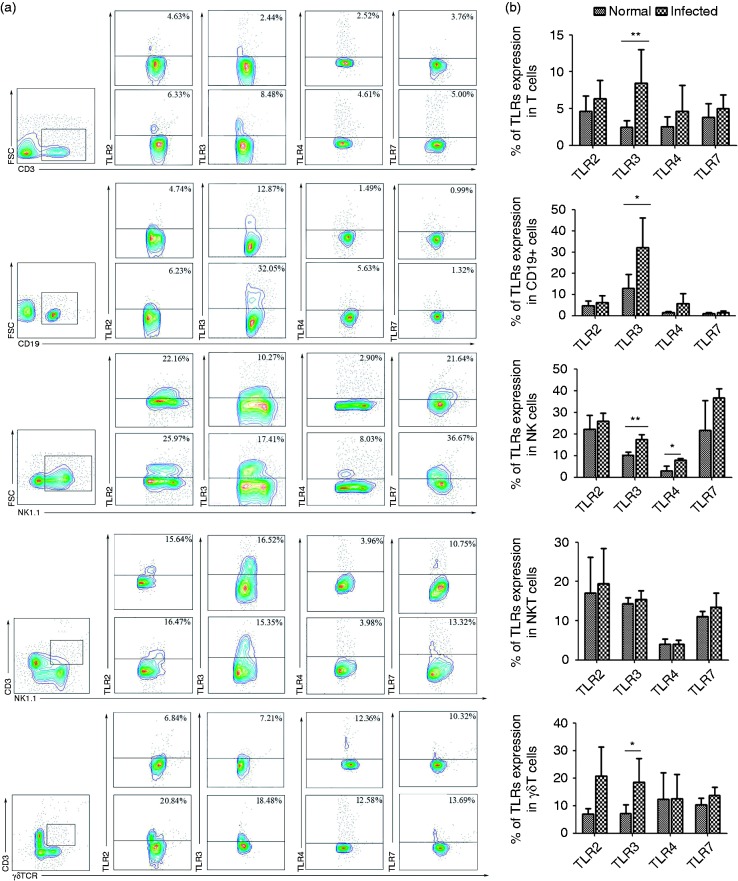
To characterize TLR distribution in different lymphocytes, changes in TLR expression were determined after infection. Lymphocytes were isolated from normal and infected C57BL/6 mice lungs. Firstly, cells were stained with different fluorophore conjugated Abs for CD3, CD19, NK1.1, and γδTCR for FACS analysis. T cell, B cell, NK cell, and γδT cell populations all significantly expressed higher levels of TLR3 (2.45 ± 0.90%, 12.87 ± 6.63%, 10.27 ± 1.37%, and 7.20 ± 3.04%, respectively) in the infected lung than that in the infected cell population (8.48 ± 4.57%, 32.05 ± 14.18%, 17.41 ± 2.30%, and 18.48 ± 8.58%, respectively; Figure 5b, *P < 0.05, **P < 0.01). In contrast, there was no significant difference in NKT cells between normal and infected mice (14.39 ± 1.44% versus 15.35 ± 2.81%). Additionally the proportion of TLR4+ NK1.1+ cells in the lymphocyte population isolated from infected lung was significantly higher compared with uninfected lung (8.02 ± 0.67% versus 2.90 ± 2.37%, P < 0.01, Figure 5).
Figure 5.
TLR expressions in different lymphocytes isolated from infected and uninfected mouse lung. (a) The percentages of TLR2+, TLR3+, TLR4+, and TLR7+ expressed on T cells, B cells, NK cells, NKT cells, and γδT cells, respectively. Flow cytometric analysis from one representative experiment. (B) The percentages of different TLRs expressed on T cells, B cells, NK cells, NKT cells, and γδT cell in the lung were calculated. The results represent for ten independent experiments (*P < 0.05, **P < 0.01; the error bars indicate SD).
Discussion
S. japonicum infections follow direct contact with water cercariae and penetrate the skin of human, then shed their bifurcated tails.20 The resulting schistosomula enter capillaries and lymphatic vessels en route to the lung. The academics pointed out that even after the parasites have exited the lungs and entered the small intestine, systemic immune response in the lung is sustained.21 In S. japonicum-infected mouse lung, the histopathological changes appear compared with healthy model in this study, as reported. Granuloma formation is the immediate product of complex cellular interactions with the participation of adhesion molecules, cytokines and chemokines. The early phase of schistosomiasis is characterized by a moderate Th1 response. The schistosome egg Ags could induce an immediate and robust inflammatory response after egg deposition. As the disease progressing, Th1 response switches to a dominant Th2 response in the host. Thus, we systemically detected the levels of these cytokines and their relationships with infection. Our results showed that S. japonicum infections are effective inducers of the above Th1 (IFN-γ), Th2 (IL-4, IL-5), and Th17 (IL-17) type cytokines response in the lung, which is consistent with our previous report.20 Although some experiments indicated that the results generated from the model differ from those of infection, the different results with cytokines seem to reflect their correlation with granuloma formation.
The potential contribution of TLRs in fighting parasitic infections has gained much attention in the last decade.22 Among numerous TLRs, TLR2, and TLR4 are the most important sensors of parasite components during S. japonicum infection.23 TLR3 interacts with dsRNA from Schistosoma eggs.24 Ashour demonstrated that other TLR-related genes are generally decreased during the course of Schistosoma infection.25 Consistent with it, we observed that the significantly reduced expression of TLR2 mRNA in the lung after Schistosoma infection. Our results showed that the higher levels of TLR2 and TLR4 protein existed in S. japonicum-infected lung tissue compared to normal mice, whereas levels of TLR3 and TLR7 were not changed significantly (P > 005). The results indicated that infection might induce the expression of TLR2 and TLR4 protein in the lung. TLRs are classic pattern recognition receptors, which bind virulent factors from pathogens and initiate synthesis of pro-inflammatory cytokines (e.g. CXCL8). Therefore, irrespective of tissue type, levels of TLR expression have profound impacts on cytokines secretion, no matter produce or impress. The higher expression of TLR2 and TLR4 in the infected lung may induce some kind of cytokines but further investigation is required. In agreement with that, in studies involving pulmonary inflammation in response to bacterial endotoxins in rabbits, blocking TLR4 reduced CXCL8 expression in bronchoalveolar lavage fluid.26
The infection by S. japonicum induces the production of multiple cytokines that mediate the immune response, which is a multi-cellular parasite with an extremely diverse repertoire of Ags.27 It is known that TLRs are classic pattern recognition receptors that bind virulent factors from pathogens and initiate synthesis of pro-inflammatory cytokines.28,29 In studies involving pulmonary inflammation in response to bacterial endotoxins in rabbits, blocking TLR4 could reduce CXCL8 expression in bronchoalveolar lavage fluid.30 We observed that in the presence of TLR2, TLR3, TLR4, and TLR7/8 ligands, IFN-γ was induced in the infected lung compared to the control. Stimulation by anti-CD3 mAb plus R848 induced higher IFN-γ release from lymphocytes of infected mice than from lymphocytes of uninfected mice (P < 005). Release of IL-4 into the supernatant was barely detectable in cultures of different types of TLR ligands stimulating lymphocytes from normal and infected lung, but was significantly induced in cell populations by anti-CD3 mAb plus TLR ligands stimulation (P < 0.01). In vitro studies have shown that anti-CD3 mAb can also be potent activators of T cells. Consistently, we showed that secretion of Th1 cytokine (IFN-γ) by infected lung lymphocytes was significantly enhanced upon challenge with anti-CD3 mAb stimulation plus R848 in vitro. Moreover, in the infected lung, TLR ligands with anti-CD3 mAb could significantly induce Th2 cytokine (IL-4). Consequently, almost four kinds of TLRs (TLR2, TLR3, TLR4, and TLR7) were involved in immune response to induce Th2 cytokine, but the secretion of Th1 cytokine only could be effectively stimulated by R848.
B lymphocytes, as positive and negative regulators of immunity, have critical roles in both autoimmune and infectious diseases.31 NK cells and NKT cells are classic innate immunologic lymphocytes, which participate in immunity to infective diseases, tumors,32 and transplantation.33 Our previous study reported that γδT cells play a considerable role in fighting S. japonicum infection in the liver and mesenteric lymph nodes of C57BL/6 mice.34,35 Numerous studies on the immunology of schistosomiasis have clearly pointed out the immune cells, such as T cells, B cells, NK cells, NKT cells, and γδT cells, participate in the S. japonicum infection. In this report, we observed a significant accumulation of NK cells and γδT cells in lung infected with S. japonicum (Figure 4). Combined, it is likely that NK cells and γδT cells exert significant effects during the infection. These results are consistent with our previous reports, which implicated NK cells and γδT cells in the lung during S. japonicum infection.
B Cells exert suppressive activity after activation and TLR are critical in this process.31 Becker et al. reported that NK cells could be activated by Leishmania lipophosphoglycan through TLR2.36 γδT cells respond to conserved structures, such as PAMPs released during infection and danger-associated molecular patterns (DAMPs), generated in the context of cell damage and stress through their TCR, NK cell receptors (NKRs), and TLRs.37–39 Fang et al. described TLR2, TLR3, TLR4, and TLR7 mRNA expression in splenic γδT cells.40 Expression of TLR1, TLR2, TLR4, and TLR6 can be up-regulated in γδT cells in response to mitochondrial DAMPs, following tissue burn injury.41 Our results showed that TLR3 expressed on the T cells, B cells, NK cells, and γδT cells in the lung is up-regulated after infection compare to the control mice and TLR4 expression is higher in the NK cells. In addition, TLR3 activates Ag-presenting cells and bridges innate and adaptive immunity by coordinating responses of T cells, B cells, NK cells, and γδT cells. TLR4 stimulation by infection might also lead to positive inflammatory signals on NK cells. Taken together, we can speculate that T cells, B cells, NK cells, and γδT cells through different types of TLRs contribute to the inflammatory immune response.
In conclusion, our findings demonstrated that TLRs contribute to S. japonicum infection, which might provide basic scientific knowledge for the development of new therapeutic approaches for the treatment of schistosomiasis patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Natural Science Foundation of China (grant number 81771696), the Guangdong provincial education department (grant number 2016KZDXM033), and the Science and Technology Planning Project of Guangdong province (grant number 2016A020215133).
References
- 1.Steinmann P, Keiser J, Bos R, et al. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 2.Tang J, Huang H, Ji X, et al. Involvement of IL-13 and tissue transglutaminase in liver granuloma and fibrosis after Schistosoma japonicum infection. Mediators Inflamm 2014; 2014: 753483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha H, Qin W, Yang Q, et al. Differential pulmonic NK and NKT cell responses in Schistosoma japonicum-infected mice. Parasitol Res 2017; 116: 559–567. [DOI] [PubMed] [Google Scholar]
- 4.Smith PM, Shainheit MG, Bazzone LE, et al. Genetic control of severe egg-induced immunopathology and IL-17 production in murine schistosomiasis. J Immunol 2009; 183: 3317–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gause WC, Urban JJ, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol 2003; 24: 269–277. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 2010; 9: 293–307. [DOI] [PubMed] [Google Scholar]
- 7.Panchapakesan U, Pollock C. The role of Toll-like receptors in diabetic kidney disease. Curr Opin Nephrol Hypertens 2018; 27: 30–34. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Dong L, Ni H, et al. Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine. PLoS Negl Trop Dis 2013; 7: e2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 2006; 6: 895–906. [DOI] [PubMed] [Google Scholar]
- 11.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol 2008; 10: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol 2011; 21: R488–R493. [DOI] [PubMed] [Google Scholar]
- 13.Sun S, Wang X, Wu X, et al. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors 2011; 4: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Zhang G, Hayden MS, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004; 303: 1522–1526. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi T, Sado T, Naruse K, et al. Evidence for activation of Toll-like receptor and receptor for advanced glycation end products in preterm birth. Mediators Inflamm 2010; 2010: 490406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasu MR, Devaraj S, Park S, et al. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010; 33: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas PG, Carter MR, Atochina O, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol 2003; 171: 5837–5841. [DOI] [PubMed] [Google Scholar]
- 18.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem 2005; 280: 277–283. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte F, Breuilh L, Fontaine J, et al. Toll-like receptor (TLR)2 and TLR3 sensing is required for dendritic cell activation, but dispensable to control Schistosoma mansoni infection and pathology. Microbes Infect 2007; 9: 1606–1613. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Xie H, Luo X, et al. Roles of Th17 cells in pulmonary granulomas induced by Schistosoma japonicum in C57BL/6 mice. Cell Immunol 2013; 285: 149–157. [DOI] [PubMed] [Google Scholar]
- 21.Mishra PK, Palma M, Bleich D, et al. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014; 7: 753–762. [DOI] [PubMed] [Google Scholar]
- 22.Faria MS, Reis FC, Lima AP. Toll-like receptors in leishmania infections: guardians or promoters? J Parasitol Res 2012; 2012: 930257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen Z Ji X, andTang J et al. Positive feedback regulation between transglutaminase 2 and Toll-like receptor 4 signaling in hepatic stellate cells correlates with liver fibrosis post Schistosoma japonicum infection. Front Immunol 2017; 8: 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhoutte F Breuilh L, andFontaine J et al. Toll-like receptor (TLR)2 and TLR3 sensing is required for dendritic cell activation, but dispensable to control Schistosoma mansoni infection and pathology. Microbes Infect 2007; 9: 1606–1613. [DOI] [PubMed] [Google Scholar]
- 25.Ashour DS. Toll-like receptor signaling in parasitic infections. Expert Rev Clin Immunol 2015; 11: 771–780. [DOI] [PubMed] [Google Scholar]
- 26.Mendez-Samperio P, Belmont L, Miranda E. Mycobacterium bovis BCG Toll-like receptors 2 and 4 cooperation increases the innate epithelial immune response. Arch Med Res 2008; 39: 33–39. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Luo X, Xie H, et al. Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology 2013; 139: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Cheng Y, Li C. The role of TLRs in cervical cancer with HPV infection: a review. Signal Transduct Target Ther 2017; 2: 17055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi T, Sado T, Naruse K, et al. Evidence for activation of Toll-like receptor and receptor for advanced glycation end products in preterm birth. Mediators Inflamm 2010; 2010: 490406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habibzay M, Saldana JI, Goulding J, et al. Altered regulation of Toll-like receptor responses impairs antibacterial immunity in the allergic lung. Mucosal Immunol 2012; 5: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol 2010; 10: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ando T, Ito H, Arioka Y, et al. Combination therapy with alpha-galactosylceramide and a Toll-like receptor agonist exerts an augmented suppressive effect on lung tumor metastasis in a mouse model. Oncol Rep 2015; 33: 826–832. [DOI] [PubMed] [Google Scholar]
- 33.DeKruyff RH, Yu S, Kim HY, et al. Innate immunity in the lung regulates the development of asthma. Immunol Rev 2014; 260: 235–248. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Luo X, Xie H, et al. Characteristics of gammadelta T cells in Schistosoma japonicum-infected mouse mesenteric lymph nodes. Parasitol Res 2014; 113: 3393–3401. [DOI] [PubMed] [Google Scholar]
- 35.Xie H, Chen D, Li L, et al. Immune response of gammadelta T cells in Schistosoma japonicum-infected C57BL/6 mouse liver. Parasite Immunol 2014; 36: 658–667. [DOI] [PubMed] [Google Scholar]
- 36.Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through Toll-like receptor-2. Mol Biochem Parasitol 2003; 130: 65–74. [DOI] [PubMed] [Google Scholar]
- 37.Derkow K, Kruger C, Dembny P, et al. Microglia induce neurotoxic IL-17+ gammadelta T cells dependent on TLR2, TLR4, and TLR9 activation. PLoS One 2015; 10: e135898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478. [DOI] [PubMed] [Google Scholar]
- 39.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 2014; 32: 121–155. [DOI] [PubMed] [Google Scholar]
- 40.Fang H, Welte T, Zheng X, et al. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol 2010; 59: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwacha MG, Rani M, Zhang Q, et al. Mitochondrial damage-associated molecular patterns activate gammadelta T-cells. Innate Immun 2014; 20: 261–268. [DOI] [PubMed] [Google Scholar]