Short abstract
The correlation of serum and synovial fluid (SF) pituitary adenylate cyclase-activating polypeptide (PACAP) levels with disease progression of primary knee osteoarthritis (OA) was explored. Radiographic severity of OA was determined by Kellgren–Lawrence (K-L) grades. PACAP levels were measured by ELISA before treatment, and 4 and 8 wk following hyaluronic acid (HA) injection. Levels of IL-1β and MMP-3 were also detected. The numeric pain scale (NPS), revised Oxford Knee Score (OKS), and American Knee Society Score (AKSS) were employed to evaluate to symptomatic severity. Receiver-operating-characteristic (ROC) curve analysis was carried out to compare the diagnostic value of PACAP, IL-1β, and MMP-3 for the K-L grade. PACAP concentrations in SF but not serum were significantly lower in OA patients compared with controls. SF PACAP levels were negatively associated with K-L grades and higher NPS as well as worse AKSS and OKS. Further analysis demonstrated that PACAP concentration in SF was negatively correlated with expressions of IL-1β as well as MMP-3 and may act as a marker for radiographic progression along with MMP-3. Last, we found SF PACAP levels exhibited an incremental trend after HA injection. These findings confirmed the crucial role of PACAP deficiency in the development of primary knee OA.
Keywords: Pituitary adenylate cyclase-activating polypeptide, knee osteoarthritis, disease progression, hyaluronic acid injection
Introduction
Osteoarthritis (OA) is characterized by loss and damage of articular cartilage and other structures surrounding the joint: ligaments, synovial membrane, and subchondral bone.1 OA is one of the most frequently diagnosed diseases worldwide, which is especially pronounced reaching prevalence of about 30% in patients aged over 50 yr.2 Primary OA mainly affects the knee compared with other joints. Although the pathogenesis of knee OA remains poorly known, some factors that play a role in synoviocytes inflammation and altered biomechanical conditions have been identified to contribute to the development and progression of OA.3 The synovium produces specific materials that nourish the joint which plays a very important part in articular cartilage metabolism.4 Also, the loss of synovial lubricating ability was suggested in the pathogenesis of OA.5
Until now, the most common diagnosis protocol for primary knee OA assessment comprises complaints of knee joint pain and functional impairment in addition to joint space width measurement using radiography.6 However, since primary knee OA starts long before it can be detected by plain X-ray, irreversible joint damages have often already occurred at the time radiological diagnosis is performed. Therefore, more sensitive techniques for early diagnosis of OA are needed.7 Magnetic resonance imaging (MRI) and direct arthroscopic examination are both well-established for this purpose. However, these methods are limited because of high costs, disputes regarding critical standards, and traumatic defects.8
In recent years, molecular biomarkers that are secreted into biological fluids during matrix metabolism of articular cartilage, subchondral bone, and synovial tissue, have received increased research attention for the diagnosis of knee OA.9 The synovial fluid (SF) is comprised of an ultrafiltrate of blood plasma and is primarily composed of hyaluronic acid (HA), lubricin, collagenases, etc., which have been regarded as important regulators in the cartilage metabolism.10–12 Biomarkers have the capacity to detect early joint degradation and provide useful diagnostic and prognostic information by reflecting disease relevant biological activity in the joint and predict the course of OA.13
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a peptide that has been demonstrated to exert various bio-effects.14 PACAP exerts several functions in a large array of physiological and pathophysiological processes related to inflammation and immune responses by mainly activating three receptors including G protein-coupled receptors mainly associated with the adenylate cyclase and phospholipase C, the PAC1 receptor which specifically binds PACAP and the VPAC1/VPAC2 receptors.15 PACAP has been shown to dramatically alleviate the clinical symptoms and down-regulate the pathogenic and cytokine secretion in animal models of immune diseases including septic shock, rheumatoid arthritis, multiple sclerosis, and Crohn’s disease.16
Recent studies have also pointed to the role that PACAP plays on cartilage protection. PACAP has been detected at moderate-high expression in the articular cartilage as well as in SF.17 In an animal experimental OA, PACAP levels remarkably attenuated in cartilage tissue and SF.17 In isolated chondrocyte cultures, PACAP inhibited the pro-inflammatory cytokine IL-1β, and induced apoptosis in vitro.17 PACAPs also rescued cartilage formation during H2O2-induced oxidative stress,18 indicating the chrondro-protective role that PACAP plays during inflammatory joint diseases.
All these studies indicated that PACAP may play an important role to protect cartilage lesion in knee OA. However, the relationship between serum and SF levels of PACAP and OA has never been fully illuminated. Therefore, we aimed to investigate the potential correlation of serum and SF with the disease severity knee OA and whether HA injection affects the expressions of PACAP.
Patients and methods
Study patients
From January 2018 to January 2019, 101 knee OA patients diagnosed in our hospital were enrolled in our study. All primary OA patients met the American College of Rheumatology clinical symptomatic and radiographic criteria for OA.19 Patients were excluded from the present study if they had previous knee injury or joint infection, systemic inflammatory or autoimmune disorders, malignant disease, advanced renal disease, and histories of corticosteroids medication. Meanwhile, a total of age and sex matched 62 patients who underwent patellar dislocation receiving surgery in our hospital were also drafted as control. These patellar dislocation patients had healthy joints with normal cartilage under therapy. Participants of the control group have neither history of secondary OA, knee injury, intra-articular fracture, nor history of steroid injection within 6 mo. The study was performed according to the recommendation of the Declaration of Helsinki and was approved by the ethics committee of our hospital. Written informed consent was obtained prior to their participation in this study.
Definition of radiographic severity
The radiographic progression of knee OA was assessed using weight-bearing anteroposterior radiographs of the affected knee. Knee radiographs were determined according to the Kellgren–Lawrence (K-L) classification based on osteophyte formation, joint space narrowing, sclerosis, and joint deformity characteristics according to the five-level scale defined as follows:20 Grade 1, doubtful narrowing of joint space and possible osteophytic lipping; Grade 2, definite osteophytes and possible narrowing of joint space; Grade 3, moderate multiple osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of bone contour; Grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour. The grading scale used for analysis was the higher one of the two knees. The results of K-L grading were evaluated by two independent and blinded radiologists in our hospital. In our study, patients with K-L ≥ 2 were enrolled with the severe knee included that both knees had OA.
Definition of clinical and functional severity
The clinical and functional severity was assessed by numeric pain scale (NPS), Oxford Knee Score (OKS) and American Knee Society (AKS) knee score and AKS knee function score. The NPS has 11 levels of pain ranging from 0 for no pain to 10 indicating worst possible pain.21 The OKS of each item ranges from 0 (severe problems) to 4 (no problems), with a summary score ranging from 0 (worst outcome) to 48 (best outcome).22 The AKS scale is comprised of two aspects. The first evaluates the knee clinically through the physical examination (clinical AKS knee score), and the second assesses the individual's functionality (AKS function score), while both reach a total of 100 points each with the higher scores indicating better outcomes.23 All the NPS, OKS, and AKS scale have been shown to be valid, responsive, and reliable for measuring patients’ perceptions of their knee disorder.
Treatment by HA injection
Patients were placed in a supine position and sterile drapes were placed around the surrounding area. Next, each patient’s symptomatic knee was cleaned with chlorhexadine and iodine solution and the knee was anaesthetized using an intra-articular injection of 5 mL of 1% xylocaine. Following activation, 3 mL of HA (Synvisc® Hylan G-F 20, Genzyme Biosurgery, Ridgefield, NJ, USA) were injected under ultrasound guidance (Logic i, GE Healthcare) into the symptomatic region using an anteromedial approach. Following the injection, the knee was passive flexed and extended 10 times. Then the patients were asked to lie in the supine position for approximately 10 min. Patients were advised to take paracetamol if they felt any pain, and to limit their mass bearing activities for the subsequent 24 h, followed by gradual resumption of normal activities. The HA injection was conducted three times once a week.
ELISA
Blood samples from all patients and controls were collected after overnight fast in plain tubes containing a separation gel. SF was aspirated from the affected knee before HA injection in OA patients and prior to the surgery in patellar dislocation patients. The samples were then centrifuged to remove cells and joint debris and stored immediately at −80°C until the day of measurement. Double-blinded quantitative detection of PACAP in serum and SF were performed using commercial ELISA (ELISA Genie, Dublin, Ireland) according to the manufacturers' instructions. The detected range of PACAP was 7.8–500 pg/mL. Each data was evaluated by three independent experiments. The assays had intra-assay coefficients of variation < 5% and inter-assay coefficients of variation < 6%. Levels of IL-1β and MMP-3 were also tested using a commercial ELISA kit (Abcam, Cambridge, UK). The intra-assay coefficients for IL-1β and MMP-3 were 4.5% and 4.1%, and inter-assay coefficients for IL-1β and MMP-3 were 6.7% and 5.4%, respectively.
Statistical analysis
Tests of data normality were performed using the Kolmogorov–Smirnov test. Data were described as mean value ± SD, median (interquartile range) when appropriate. Differences between the two groups were analyzed using unpaired t-test, Mann–Whitney U test, Chi-square test when appropriate. Differences among groups were analyzed by one-way ANOVA followed by Tukey post hoc analysis or Kruskal–Wallis test as indicated. Differences between PACAP concentrations in serum and SF were analyzed by the Wilcoxon signed rank test for paired samples. Pearson or Spearman correlation coefficient was calculated to determine the relationship between PACAP concentrations with other parameters. All of the statistical analyses were performed by using GraphPad 6.0. A P value less than 0.05 (two-tailed) was considered as statistically significant.
Results
Demographic and clinical data
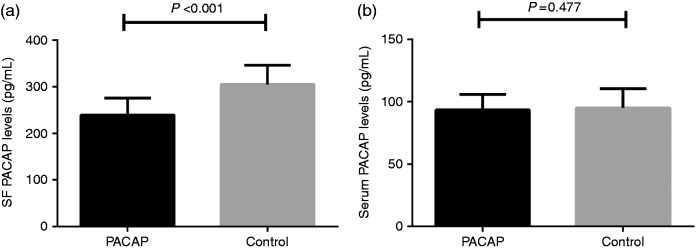
The demographic and clinical characteristics of all participants are depicted in Table 1. No significant differences were found in age, gender, and body mass index (BMI) between OA patients and controls (P > 0.05). Compared with controls, OA patients had a significantly lower PACAP concentration in SF in comparison with controls (239.5 ± 36.4 pg/mL vs 305.1 ± 41.2 pg/mL, P < 0.001). As shown in Figure 1, there was no significant difference in serum PACAP levels between OA patients and controls (93.2 ± 12.5 pg/mL vs 94.9 ± 15.5 pg/mL, P = 0.477). In OA patients, PACAP levels in SF were significantly higher than those in paired serum samples (239.5 ± 36.4 pg/mL vs 93.2 ± 12.5 pg/mL, P < 0.001).
Table 1.
Baseline statistics.
| Knee OA patients (n = 101) | Controls (n = 62) | P Value | |
|---|---|---|---|
| Age (yr) | 66.2 ± 5.3 | 64.5 ± 6. | 0.216 |
| Gender (F/M) | 60/41 | 40/22 | 0.317 |
| BMI (kg/m2) | 24.2 ± 2.1 | 23.8 ± 2.0 | 0.141 |
| K-L grade (2/3/4) | 33/37/31 | / | |
| NPS | 5.3 ± 1.9 | / | |
| OKS | 21.5 ± 7.9 | / | |
| AKS knee score | 55.2 ± 8.2 | / | |
| AKS function score | 49.1 ± 7.9 | / | |
| Serum PACAP levels (pg/mL) | 93.2 ± 12.5 | 94.9 ± 15.5 | 0.477 |
| SF PACAP levels (pg/mL) | 239.5 ± 36.4 | 305.1 ± 41.2 | < 0.001 |
Basic indices and PACAP levels are given as the mean value ± SD.
Figure 1.
(a) Comparison of SF PACAP levels between knee OA and controls and (b) Comparison of serum PACAP levels between knee OA and controls.
Correlation of SF PACAP levels with K-L grades
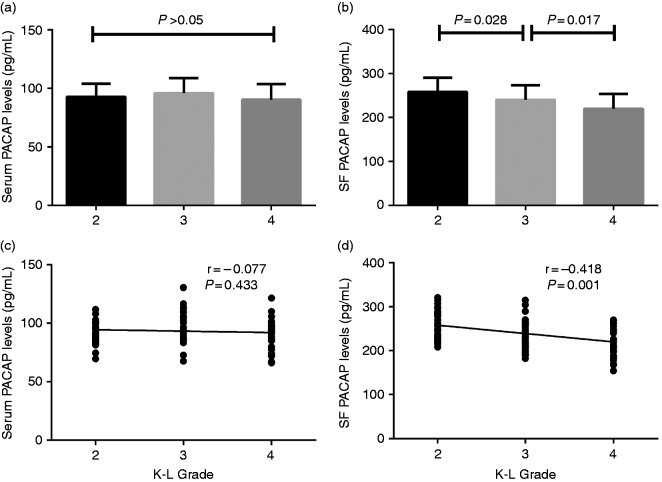
According to the K-L grading scale, 33 patients were K-L Grade 2, 37 patients K-L Grade 3, and 31 patients K-L Grade 4. There was no significant difference in serum PACAP levels among patients with different K-L grades (P > 0.05) (Figure 2a), and the correlation of serum PACAP levels with K-L grade did not reach significant differences (r = −0.077, P = 0.433) (Figure 2c). SF PACAP concentrations were significantly lower in knee OA patients with K-L Grade 3 than those with Grade 2 (239.8 ± 33.7 pg/mL vs 257.7.0 ± 32.7 pg/mL, P = 0.028) (Figure 2b). Moreover, SF PACAP concentrations were significantly decreased in K-L Grade 4 patients compared with K-L Grade 3 (219.6 ± 33.9 pg/mL vs 239.8±33.7 pg/mL, P = 0.017) (Figure 2b). SF PACAP levels were negatively correlated with K-L grading (r = −0.418, P < 0.001) (Figure 2d).
Figure 2.
(a) Comparison of serum PACAP levels among different K-L grades in knee OA patients. (b) Comparison of SF PACAP levels among different K-L grades in knee OA patients. (c) Correlation of serum PACAP levels with K-L Grades in knee OA patients and (d) Correlation of SF PACAP levels with K-L grades in knee OA patients.
Correlation of PACAP levels with clinical and functional severity
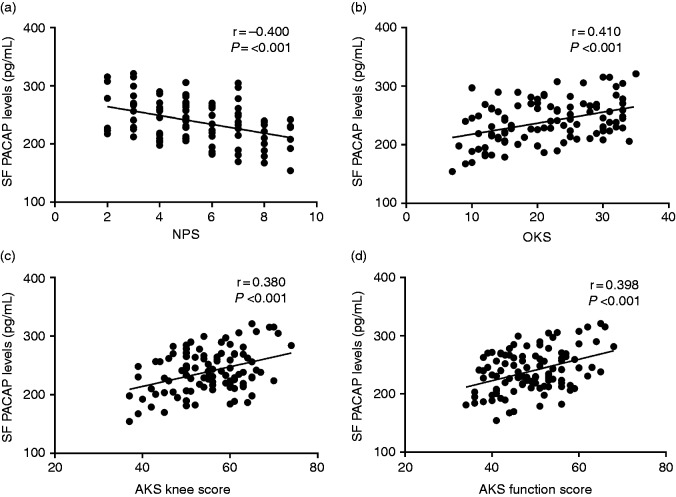
We next carried out the association analysis between SF PACAP levels with symptomatic severity and functional disability. As illustrated in Figure 3, SF PACAP levels were negatively correlated with self-reported pain by NPS (r = −0.400, P < 0.001) (Figure3a).With regard to the functional severity, we observed that SF PACAP levels were positively related to OKS (r = 0.410, P < 0.001) (Figure 3b), AKS knee score (r = 0.380, P < 0.001) (Figure 3c), and AKS function score (r = 0.398, P < 0.001) (Figure 3d).
Figure 3.
(a) Correlation of SF PACAP levels with NPS in knee OA patients. (b) Correlation of SF PACAP levels with WOMAC OKS in knee OA patients. (c) Correlation of SF PACAP levels with AKS knee score in knee OA patients and (d) Correlation of SF PACAP levels with AKS function score in knee OA patients.
Correlation of PACAP levels with biochemical indices and receiver-operating-characteristic (ROC) analysis
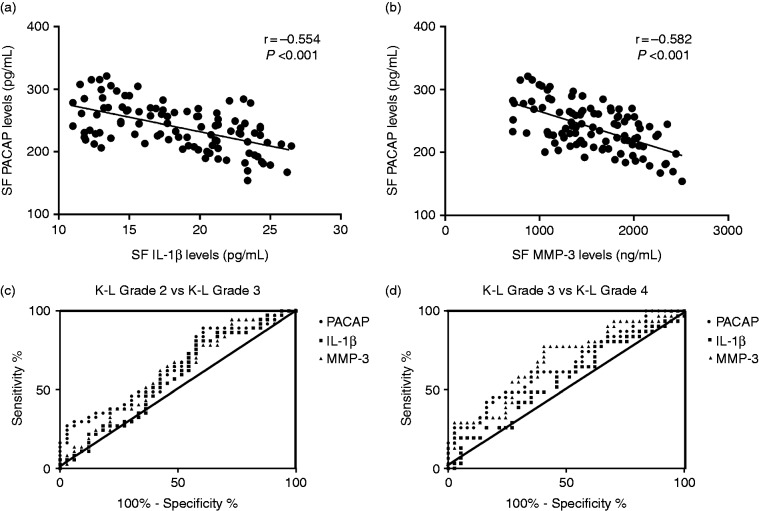
In order to figure out the potential mechanisms of PACAP treating OA, we also tested the levels of inflammatory cytokine IL-1β and cartilage damage marker MMP-3, which are two important factors during OA progression. Interestingly, we found that SF PACAP levels were significantly and negatively related to IL-1β (r = −0.554, P < 0.001) (Figure 4a) and MMP-3 (r = −0.582, P < 0.001) (Figure 4b).
Figure 4.
(a) Correlation of SF PACAP levels with IL-1β levels. (b) Correlation of SF PACAP levels with MMP-3 levels. (c) ROC curve analysis for K-L Grade 2 vs Grade 3 and (d) ROC curve analysis for K-L Grade 3 vs Grade 4.
We next performed ROC curve analysis to compare PACAP with IL-1β and MMP-3 to explore the diagnostic value of these factors with regard to K-L grade. As demonstrated in Figure 4, for Grade 2 vs Grade 3, PACAP had a larger area under the curve (AUC) (AUC = 0.642, P = 0.041) than IL-1β (AUC = 0.579, P = 0.259) and MMP-3 (AUC = 0.609, P = 0.118) (Figure 4c, Table 2). However, for K-L Grade 3 vs Grade 4, PACAP had a larger AUC (AUC = 0.642, P = 0.041) than IL-1β (AUC = 0.562, P = 0.385), but smaller than MMP-3 (AUC = 0.681, P = 0.011) (Figure 4d, Table 3). The findings of ROC analysis suggested that decreased PACAP expression along with up-regulation of MMP-3 in SF during OA might act as two potential markers for the K-L grade at different stages.
Table 2.
Statistics of area under curve (AUC) among PACAP, IL-1β, and MMP-3 (K-L Grade 2 vs Grade 3).
| AUC | SE | 95% CI | P Value | |
|---|---|---|---|---|
| PACAP | 0.642 | 0.066 | 0.513–0.771 | 0.041 |
| IL-1β | 0.579 | 0.069 | 0.442–0.715 | 0.259 |
| MMP-3 | 0.609 | 0.068 | 0.475–0.743 | 0.118 |
SE: standard error; 95% CI: 95% confidence interval.
Table 3.
Statistics of area under curve (AUC) among PACAP, IL-1β, and MMP-3 (K-L Grade 3 vs Grade 4).
| AUC | SE | 95% CI | P Value | |
|---|---|---|---|---|
| PACAP | 0.651 | 0.067 | 0.520–0.783 | 0.033 |
| IL-1β | 0.562 | 0.071 | 0.423–0.700 | 0.385 |
| MMP-3 | 0.681 | 0.066 | 0.553–0.809 | 0.011 |
SE: standard error; 95% CI: 95% confidence interval.
Alternation of SF PACAP levels following HA injection
We finally investigated whether knee joint HA injection would affect SF PACAP levels. We missed the follow-up of 36 patients due to various reasons, thus remaining 75 patients finishing the final treatment. We found SF PACAP concentrations exhibited a global incremental trend after HA injection (Figure 5), although the differences of PACAP between 4 wk and baseline (248.6 ± 35.4 pg/mL vs 239.5 ± 34.4 pg/mL, P = 0.115) did not reach significance. The PACAP levels were significantly elevated at 8 wk compared to the baseline and 4 wk (296.5 ± 43.1 pg/mL vs 239.5 ± 34.4 pg/mL, P < 0.001; 296.5 ± 43.1 pg/mL vs 248.6 ± 35.4 pg/mL, P = 0.013) (Figure 5).
Figure 5.
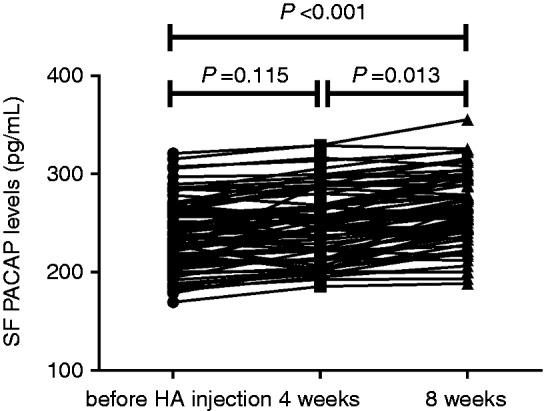
Change of SF PACAP levels before HA injection and at 4 and 8 wk.
Discussion
This study investigated the association of serum and SF PACAP concentrations with primary knee OA. To our best knowledge, this is the first study implicating that SF PACAP concentrations were attenuated in OA patients and that they were related to OA progression through assessing the correlation of SF PACAP levels with the radiographic as well as clinical severity and also the biochemical analysis. Further ROC analysis indicated that decreased PACAP expression along with up-regulation of MMP-3 in SF during OA might act as two potential markers for the K-L grade at different stages. Finally, we observed that SF PACAP levels were gradually increased following HA injection. These findings suggest that attenuated PACAP expression might act as a biomarker in knee OA.
So far, accumulating studies reported that there are no definite therapies available to effectively treat or cure OA.24 Moreover, no evidence has been established pointing to the efficacy of an agent or disease-modifying drug that is actually capable of arresting disease progression in an appreciable manner.25 Therefore, to find alternative interventions in accord with the OA progression is important.
PACAP is an endogenous bioactive neuropeptide eliciting a broad spectrum of biological functions, including the modulation of innate and adaptive immunity, and shows a predominant anti-inflammatory action.26,27 In addition, PACAP also could prevent harmful cellular effects of ischemic conditions and oxidative stress.28,29 Growing evidences have implicated the important role of PACAP in different pathological conditions, and PACAP has been found in different kinds of SFs.30 PACAP has been utilized as a protective factor in several models of disease including amyotrophic lateral sclerosis,31 diabetes,32 Alzheimer's disease, and Parkinson's disease.33
Recently, PACAP has been implicated as a protector of cartilage and to inhibit arthritis. PACAP inhibited cytokine expressions and protein secretion via suppression of NF-κB translocation,34 which triggers the expression of a series of genes guiding destruction of the articular joint, contributing to OA onset and progression.35 In a recent study, PACAP attenuates the oxidative and mechanical stress induced matrix degradation in chondrocytes.36
Close monitoring of radiographic development may facilitate the design of corresponding therapies. We first found that SF PACAP levels were decreased in knee OA patients compared with the control which is consistent with a previous study which showed that SF PACAP expressions were present in a knee OA animal model.11 The K-L grade was negatively associated with SF instead of serum PACAP levels, indicating that PACAP may perform its function on protecting OA cartilage from damage and suppressing inflammation locally. SF originates from the synovial membrane, which is a thin cellular layer that lines the joint cavity acting as a semipermeable filter to regulate the transfer of molecules in and out of the joint. In normal status, the SF components, including nutrients, hormones and lubricant factors, contribute to the unique low-friction properties of the articular surface.37 Under physiological conditions, extracellular matrix production, cytokine secretion, host defense and immunomodulation are present.38
We also found that SF PACAP was negatively related to pain and clinical severity. Pain is the most common complaint among knee OA patients. In addition to the inflammatory factors, the importance of the central integration of repeated nociceptive input is prominent in pain conditions with peripheral nociceptive drivers,39 leading to the pain sensitization in OA patients.40 PACAP and its receptors are broadly located at different levels of the pain transmitting pathway.41 The action of PACAP at the peripheral sensory nerve terminals has been found to be divergent. It can exert both pro- and anti-nociceptive effects, depending on the mode of administration (local or systemic) and the mechanism of the pain process (acute or chronic, inflammatory or neuropathic).42 It has been reported that PACAP may play an important role in inhibiting the transmission of the nociceptive information evoked by inflammation,43 indicating that PACAP may inhibit pain signal transmitting under inflammatory environment.
We next found PACAP levels were negatively associated with IL-1β and the damage marker MMP-3. IL-1β and MMP-3 have been identified as two key factors in cartilage destruction of OA.44 In an OA model, the up-regulation of expressions of the IL-1β in the SF was associated with the dramatic attenuation in PACAP immunoreactivity in the articular cartilage and its concentration in SF.17 It was also reported that expression and activation of the majority of cartilage matrix specific MMPs were decreased by PACAP.36 We further compared PACAP with IL-1β and MMP-3 to determine their roles in OA radiographic progression diagnosis, and we found that early decreased PACAP together with later increment of MMP-3 could be decent for K-L grade diagnosis.
Finally, we found that SF PACAP concentrations were increased following HA injection both at 4 and 8 wk compared with the baseline level. HA is an integral component in SF, mostly utilized in clinical practice for treating knee OA.45 The relation between TLR and an inflammatory response is demonstrated as HA degradation products induced an inflammatory response through CD44 and TLR interaction.46 On the other hand, PACAP has been shown to down-regulate the pro-inflammatory cytokines, including monocyte chemoattractant protein-1 (MCP-1) and IL-6, which had been induced by the activation of TLR.47
This study has several limitations. First, the sample size was not large enough and we investigated only those patients of knee OA who attended in our hospital. Further multi-center and larger samples are needed in the future. Secondly, the cross-sectional design of our study precluded addressing whether the analyzed level of PACAP predicted alteration in severity in patients with knee OA. Thirdly, we did not examine the expressions of PACAP in cartilage tissues in that we argued that SF examination is convenient for the purpose of early diagnosis. Last, we did not explore the possible mechanisms of HA injection on upgrading the PACAP levels, which will be discussed in our future study.
In summary, patients with primary knee OA had lower levels of SF PACAP compared with controls. PACAP levels in SF negatively correlated with the severity of knee OA and were increased following HA injection. Local use of PACAP may act as a potential therapy for treating knee OA. Further studies are in progress to elucidate the contribution of PACAP as a protective marker during the process of OA.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Foshan Medical Science and Technology Project (grant number 2018AB002473) and Shandong Provincial Natural Science Foundation (grant number BS2014YY0260).
References
- 1.Carmona-Terés V, Moix-Queraltó J, Pujol-Ribera E, et al. Understanding knee osteoarthritis from the patients' perspective: a qualitative study. BMC Musculoskelet Disord 2017; 18: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinds MB, Welsing PMJ, Vignon EP, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthr Cartil 2011; 19: 768–778. [DOI] [PubMed] [Google Scholar]
- 3.Mora JC, Przkora R, Cruz-Almeida Y, et al. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 2018; 11: 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castrogiovanni P, Di Rosa M, Ravalli S, et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci 25; 20: E511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Rosa M, Castrogiovanni P, Musumeci G, et al. The synovium theory: can exercise prevent knee osteoarthritis? The role of “mechanokines”, a possible biological key. J Funct Morphol Kinesiol 2019; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidari B. Knee osteoarthritis diagnosis, treatment and associated factors of progression: part II . Caspian J Intern Med 2011; 2: 249–255. [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi M, Naito K, Abe M, et al. Relationship between radiographic grading of osteoarthritis and the biochemical markers for arthritis in knee osteoarthritis . Arthritis Res Ther 2004; 6: R208–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding C, Cicuttini F and, Jones G. How important is MRI for detecting early osteoarthritis? Nat Clin Pract Rheumatol 2008; 4: 4–5. [DOI] [PubMed] [Google Scholar]
- 9.McIlwraith CW, Kawcak CE, Frisbie DD, et al. Biomarkers for equine joint injury and osteoarthritis. J Orthop Res. 2018; 36: 823–831. [DOI] [PubMed] [Google Scholar]
- 10.Musumeci G, Loreto C, Leonardi R, et al. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab 2013; 31: 274–284. [DOI] [PubMed] [Google Scholar]
- 11.Musumeci G, Trovato FM, Pichler K, et al. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: an in vivo and in vitro study on lubricin expression. J Nutr Biochem 2013; 24: 2064–2075. [DOI] [PubMed] [Google Scholar]
- 12.Musumeci G, Aiello FC, Szychlinska MA, et al. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci 2015; 16: 6093–6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosnijeh FS, Runhaar J, van Meurs JB, et al. Biomarkers for osteoarthritis: can they be used for risk assessment? A systematic review. Maturitas 2015; 82: 36–49. [DOI] [PubMed] [Google Scholar]
- 14.Maugeri G, Longo A, D'Amico AG, et al. Trophic effect of PACAP on human corneal endothelium. Peptides 2018; 99: 20–26. [DOI] [PubMed] [Google Scholar]
- 15.Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 2009; 61: 283–357. [DOI] [PubMed] [Google Scholar]
- 16.Abad C, Gomariz RP, Waschek JA, et al. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: focus on VIP and PACAP. Curr Top Med Chem 2006, 6; 151–163. [DOI] [PubMed] [Google Scholar]
- 17.Giunta S, Castorina A, Marzagalli R, et al. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci 2015; 16: 5922–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musumeci G, Castrogiovanni P, Trovato FM, et al. Moderate physical activity ameliorates cartilage degeneration in a rat model of aging: a study on lubricin expression. Scand J Med Sci Sports 2015; 25: e222–e230. [DOI] [PubMed] [Google Scholar]
- 19.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 1986; 29: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthosis. Ann Rheum Dis.1957; 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marco CA, Marco AP, Plewa MC, et al. The verbal numeric pain scale: effects of patient education on self-reports of pain. Acad Emerg Med 2006; 13: 853–859. [DOI] [PubMed] [Google Scholar]
- 22.Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007; 89: 1010–1014. [DOI] [PubMed] [Google Scholar]
- 23.Martimbianco ALC, Calabrese FR, Iha LAN, et al. Reliability of the “American Knee Society Score” (AKSS). Acta Ortop Bras 2012; 20: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musumeci G, Castrogiovanni P, Leonardi R, et al. New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J Orthop 2014; 5: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev 2018; 14: 108–116. [DOI] [PubMed] [Google Scholar]
- 26.Giunta S, Castorina A, Bucolo C, et al. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides 2012; 37: 32–39. [DOI] [PubMed] [Google Scholar]
- 27.Castorina A, Scuderi S, D’Amico AG, et al. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp Cell Res. 2014; 322: 108–121. [DOI] [PubMed] [Google Scholar]
- 28.Laszlo E, Varga A, Kovacs K, et al. Ischemia/reperfusion-induced kidney injury in heterozygous PACAP-deficient mice. Transplant Proc 2015; 47: 2210–2215. [DOI] [PubMed] [Google Scholar]
- 29.Mester L, Kovacs K, Racz B, et al. Pituitary adenylate cyclase-activating polypeptide is protective against oxidative stress in human retinal pigment epithelial cells. J Mol Neurosci 2011; 43: 35–43. [DOI] [PubMed] [Google Scholar]
- 30.Brubel R, Reglodi D, Jambor E, et al. Investigation of pituitary adenylate cyclase activating polypeptide in human gynecological and other biological fluids by using MALDI TOF mass spectrometry. J Mass Spectrom 2011; 46: 189–194. [DOI] [PubMed] [Google Scholar]
- 31.Maugeri G, D'Amico AG, Rasà DM, et al. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J Cell Physiol 2019; 234: 5203–5214. [DOI] [PubMed] [Google Scholar]
- 32.Banki E, Kovacs K, Nagy D, et al. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J Mol Neurosci 2014; 54: 300–309. [DOI] [PubMed] [Google Scholar]
- 33.Yang R, Jiang X, Ji R, et al. Therapeutic potential of PACAP for neurodegenerative diseases. Cell Mol Biol Lett 2015; 20: 265–278. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XY, Hayasaka S, Chi ZL, et al. Effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on IL-6, IL-8, and MCP-1 expression in human retinal pigment epithelial cell line. Curr Eye Res 2005; 30: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 35.Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 2013; 45: 2580–2584. [DOI] [PubMed] [Google Scholar]
- 36.Szentléleky E, Szegeczki V, Karanyicz E, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces oxidative and mechanical stress-evoked matrix degradation in chondrifying cell cultures. Int J Mol Sci 2019; 20: E168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martel-Pelletier J, Barr A.J, Cicuttini F.M, et al. Osteoarthritis. Nat Rev Dis Prim 2016; 2: 16072. [DOI] [PubMed] [Google Scholar]
- 38.De Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr Cartil 2012; 20: 1484–1499. [DOI] [PubMed] [Google Scholar]
- 39.Arendt-nielsen L, Skou ST, Nielsen TA, et al. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep 2015; 13: 225–234. [DOI] [PubMed] [Google Scholar]
- 40.Fingleton C, Smart K, Moloney N, et al. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil 2015; 23: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 41.Ohsawa M, Brailoiu GC, Shiraki M, et al. Modulation of nociceptive transmission by pituitary adenylate cyclase activating polypeptide in the spinal cord of the mouse. Pain 2002; 100: 27–34. [DOI] [PubMed] [Google Scholar]
- 42.Tajti J, Tuka B, Botz B, et al. Role of pituitary adenylate cyclase-activating polypeptide in nociception and migraine. CNS Neurol Disord Drug Targets 2015; 14: 540–553. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T and, Tatsuno I. Antinociceptive effect of intrathecally administered pituitary adenylate cyclase activating polypeptide (PACAP) on the rat formalin test. Neurosci Lett 1995; 184: 32–35. [DOI] [PubMed] [Google Scholar]
- 44.Kunisch E, Kinne RW, Alsalameh RJ, et al. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: in situ hybridization studies on a single cell level. Int J Rheum Dis 2016; 19: 557–566. [DOI] [PubMed] [Google Scholar]
- 45.Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician 2000; 62: 565–570. [PubMed] [Google Scholar]
- 46.Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 2015; 16: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto K, Kuno K, Takemoto M, et al. Pituitary adenylate cyclase-activating polypeptide protects glomerular podocytes from inflammatory injuries. J Diabetes Res 2015; 2015: 727152. [DOI] [PMC free article] [PubMed] [Google Scholar]