Short abstract
The experimental human endotoxemia model is used to study the systemic inflammatory response in vivo. The previously used lot of endotoxin, which was used for over a decade, is no longer approved for human use and a new Good Manufacturing Practices-grade batch has become available. We compared the inflammatory response induced by either bolus or continuous administration of either the previously used lot #1188844 or new lots of endotoxin (#94332B1 and #94332B4). Compared with lot #1188844, bolus administration of lot #94332B1 induced a more pronounced systemic inflammatory response including higher plasma levels of pro-inflammatory cytokines and more pronounced clinical signs of inflammation. In contrast, continuous infusion of lot #94332B4 resulted in a slightly less pronounced inflammatory response compared with lot #1188844. Furthermore, we evaluated whether lot #1188844 displayed in vivo potency loss by reviewing inflammatory parameters obtained from 17 endotoxemia studies performed in our centre between 2007 and 2016. Despite inter-study variability in endotoxemia-induced effects on temperature, heart rate, symptoms, and leukocyte counts, the magnitude of these effects did not decrease over time. In conclusion, although all lots of endotoxin induce a pronounced inflammatory response, the magnitude differs between lots. We observed no potency loss of endotoxin over time.
Keywords: Endotoxemia, in vivo, lipopolysaccharide, potency, systemic inflammation
Introduction
A dysregulated systemic inflammatory response plays a role in a plethora of pathological conditions, such as sepsis,1 trauma,2 burns,3 and major surgery.4 An overzealous response may harm the patient by inducing tissue damage. However, a suppressed inflammatory response can be equally detrimental, as it compromises the host’s ability to combat infections. The experimental human endotoxemia model has contributed considerably to our understanding of the systemic inflammatory response. In this model, intravenous administration of bacterial endotoxin, mostly Escherichia coli derived, to healthy volunteers results in a transient systemic inflammatory response.5,6 This response is characterized by increased cytokine mRNA expression and plasma levels, haemodynamic alterations, fever, and flu-like symptoms.7–10 Compared with the uncertainties regarding the cause, time of onset and extent of inflammation,11 and large inter-individual differences in age, comorbidities and medication use, for example in patients with sepsis, the experimental human endotoxemia is considered a standardized, controlled and safe model of systemic inflammation.5 Over time, different lots of E. coli endotoxin have been in use. The EC-5 lot, derived from non-Good Manufacturing Practices (GMP) bulk material, was vialled in 1976 and distributed by the Food and Drug Administration (FDA), but use of this lot was ceased in the late 1990s, as it did not meet regulatory standards. In 1997 and 2006, two new lots (#67801 and #1188844, respectively) derived from the same non-GMP bulk material used for EC-5, were vialled under GMP conditions by the National Institutes of Health (NIH). These lots were named Clinical Center Reference Endotoxin (CCRE). Back then, researchers were under the impression that the EC-5 lot had lost its potency to elicit a systemic inflammatory response,12 which was confirmed in a head-to-head comparison of EC-5 with CCRE.13 From 1997 to 2016, the CCRE lots were provided to researchers by the Clinical Center Endotoxin Repository of the NIH, who also coordinated potency and sterility testing. Recently, the FDA required the use of full GMP pharmaceutical-grade endotoxin. Therefore, use of the CCRE lot was no longer permitted and production of a novel GMP-grade CCRE batch was commissioned by the NIH, and produced by List Biologicals Laboratories Inc. (Campbell CA, USA).
For the comparison of data obtained in different experimental human endotoxemia studies, it is important to know whether the systemic inflammatory response induced by lot #1188844 and the new GMP-grade lots are comparable. In addition, although it was previously concluded that the EC-5 lot lost potency over time, the question remains whether this also applies to the #1188844 lot, which may be relevant for new batches and lots.
Herein, we performed a head-to-head comparison of the systemic inflammatory response induced by different lots of endotoxin using bolus as well as continuous administration. First, we compared the response to bolus administration of the #1188844 lot and the novel #94332B1 lot. Second, we compared the response to continuous infusion of the #1188844 lot and another new lot, named #94332B4. We assessed plasma cytokine/chemokine levels, haemodynamic alterations, symptoms, and changes in leukocyte counts. Furthermore, to assess potential potency loss of the #1188844 lot over time, we reviewed clinical parameters of systemic inflammation obtained in 17 endotoxemia studies performed at our department between 2007 and 2016.
Material and methods
Study design
For head-to-head comparison of different lots of endotoxin, we compared clinical parameters of systemic inflammation and cytokine/chemokine data obtained from two studies in which subjects received a bolus administration of 2 ng/kg endotoxin from either the lot #1188844 (vialled in 2006) or lot #94332B1 (new GMP-grade lot), and from two studies in which bolus administration of 1 ng/kg of endotoxin was followed by continuous infusion of endotoxin at a dose of 1 ng/kg/h for 3 h using either lot #1188844 or lot #94332B4 (another new GMP-grade lot). To assess potential potency loss of lot #1188844 over time, we compared clinical parameters of systemic inflammation obtained from subjects randomized to the control groups (no intervention besides administration of endotoxin) of 17 human endotoxemia studies performed at the department of Intensive Care Medicine of the Radboud university medical center using the bolus model (administration of 2 ng/kg endotoxin, lot #1188844) from 2007 to 2016. A list of included studies is provided in Table 1. To compare these responses with those observed after bolus administration of a new GMP-grade batch, we used data obtained in the above described study employing bolus administration of 2 ng/kg #94332B1.
Table 1.
Experimental human endotoxemia studies included for analyses.
| yr | n | Registration number | Mode of administration | Lot | |
|---|---|---|---|---|---|
| 23 | 2007 | 10 | NL17473.091.07 | bolus | #1188844 |
| 24 | 2007 | 7 | NL20388.091.07 | bolus | #1188844 |
| 25 | 2008 | 10 | NL24017.091.08 | bolus | #1188844 |
| submitted | 2009 | 10 | NL27052.091.09 | bolus | #1188844 |
| 26 | 2009 | 10 | NL29171.091.09 | bolus | #1188844 |
| 27 | 2009 | 6 | NL27963.091.09 | bolus | #1188844 |
| 28 | 2009 | 10 | NL30625.091.09 | bolus | #1188844 |
| 29 | 2011 | 6 | NL36068.091.11 | bolus | #1188844 |
| 30 | 2011 | 12 | NL38438.091.11 | bolus | #1188844 |
| 31 | 2012 | 12 | NL42337.091.12 | bolus | #1188844 |
| 32 | 2013 | 10 | NL43020.091.12 | bolus | #1188844 |
| 33 | 2013 | 10 | NL44630.091.13 | bolus | #1188844 |
| submitted | 2015 | 12 | NL49674.091.14 | bolus | #1188844 |
| submitted | 2015 | 10 | NL53584.091.15 | bolus* | #1188844 |
| 35 | 2015 | 10 | NL51923.091.14 | continuous* | #1188844 |
| 34 | 2016 | 15 | NL54870.091.15 | bolus | #1188844 |
| in preparation | 2016 | 10 | NL53411.091.15 | bolus | #1188844 |
| submitted | 2016 | 12 | NL56686.091.16 | bolus | #1188844 |
| in press | 2016 | 20 | NL57410.091.16 | continuous* | #94332B4 |
| in preparation | 2017 | 8 | NL61136.091.17 | bolus* | #94332B1 |
Registration number represents registration at the Dutch national registry (toetsingonline.nl) for medical research involving human subjects. Lot #1188844 was produced in 2006 and lots #94332B1 and #94332B4 in 2015. Bolus administration was performed at a dose of 2 ng/kg, continuous administration was performed by infusion of 1 ng/kg, followed by 1 ng/kg/h for 3 h.*studies included in the head-to-head comparison.
Apart from the use of different batches of endotoxin, the study procedures were identical and in accordance with the declaration of Helsinki, including the latest revisions. All studies were approved by the local ethics committee (CMO Arnhem-Nijmegen), and registration numbers are listed in Table 1. Data of most studies described in this work have been published previously (references provided in Table 1).
In all studies, eligible subjects were healthy, non-smoking males aged 18–35 yr. Subjects were included after providing written informed consent and when declared eligible based on a full medical history, a normal physical examination, electrocardiography, and routine laboratory values. Exclusion criteria were pre-existent disease, drug use, and febrile illness in the past 4 wk. The experiments were performed in accordance to our standardized protocol described in detail elsewhere.6 In short, subjects refrained from caffeine and alcohol for 24 h and from food and drinks for 12 h before the experiment. Continuous blood pressure monitoring and blood withdrawal were facilitated by placement of an arterial cannula. Endotoxin and intravenous hydration were administered through a venous cannula. Heart rate was registered with a three-lead electrocardiogram, and all haemodynamic data were recorded. Every 30 min, temperature was measured using a tympanic thermometer and flu-like symptoms (headache, nausea, shivering, muscle and back pain) were scored on a six-point Likert scale (0 = no symptoms, 5 = worst ever experienced) with addition of 3 points for vomiting, resulting in a total symptom score ranging from 0–28. Furthermore, subjects were pre-hydrated by infusion of 1.5 l Glc 2.5%/NaCl 0.45% during the 1-h-period prior to start of endotoxin administration, because this decreases the chances of a vasovagal reaction during endotoxemia.14 Hereafter, hydration was continued with 150 ml/h for 8 h.
Endotoxin
All endotoxin lots were derived from an E. coli Type O113:H10:K-negative strain of bacteria. Endotoxin was stored at the investigational site in a dedicated fridge at 2–8°C, and reconstituted prior to administration, both according to the manufacturer’s instructions. The temperature of the fridge was constantly monitored and logged using an automated system. Lot #1188844 was derived from bulk material produced by the National Institute of Allergy and Infectious Diseases and FDA in 1967 and vialled in 2006 under GMP conditions by the Pharmaceutical Development Section of the NIH (Bethesda, MD, USA). The lots #94332B1 and #94332B4 were both derived from the same new GMP-grade bulk material and vialled under GMP conditions by List Biological Laboratories, Inc (Campbell, California, USA). This GMP-grade endotoxin has previously shown to be safe for administration in humans.15
Cytokine and chemokine analysis
EDRTA-anticoagulated blood was collected at various time points up to 8 h after start of endotoxin administration. After centrifugation (2000 g, 4°C, 10 min), plasma was stored at –80°C until further analysis. Concentrations of TNF-α, IL-6, IL-8, IL-10, Monocyte Chemoattractant Protein (MCP)-1 and Monocyte Inflammatory Protein (MIP)-1β were measured using a simultaneous Luminex assay (Milliplex, Merck Millipore, Billerica, MA, USA). To enable head-to-head comparison of studies using different endotoxin lots, cytokine/chemokine analysis was performed batchwise (in one run). For all measured mediators, the lower detection limit was 3.2 pg/ml.
Leukocyte counts
Analysis of leukocyte (subset) counts was performed using standardized routine methods at the Laboratory of Clinical Chemistry of the Radboud university medical center.
In vitro endotoxin potency
All endotoxin vials described in this study contained 1 µg of endotoxin, which is claimed to correspond to a potency of 10.000 endotoxin units (EU) per vial. In vitro potency measurements of lot #1188844 were commissioned by the NIH to determine the actual potency, and were performed on randomly selected vials, by commercial entities with extensive experience in the conduct of endotoxin assays. List Biologicals performed in vitro potency measurements for the #94332 batch. In vitro potency was quantified as EU/vial using the Limulus amebocyte lysate (LAL) test. This test is based upon a clotting reaction of amoebocytes form the Limulus polyphemus with endotoxin, and estimates the endotoxin content with an uncertainty range of 50–200%.16
Statistical analysis
Distribution of data was tested for normality using the Shapiro–Wilk test. Data are presented as mean with SEM. Between-group differences over time were analysed using repeated measures two-way ANOVA (group * time interaction term). Linear regression analysis was performed to assess changes over time in the studies performed between 2007 and 2016 and coefficients of variation were calculated to assess inter-study variation. Clinical parameters of systemic inflammation obtained from the study using bolus administration of lot #94332B1 were compared with values obtained from all studies with lot #1188844 using unpaired Student’s t-tests. Statistical analyses were performed using Graphpad Prism version 5.03 (Graphpad Software, San Diego, CA, USA). P-Values <0.05 were considered statistically significant.
Results
Head-to-head comparison of the systemic inflammatory response induced by different lots of endotoxin
Demographic characteristics and safety
Demographic characteristics of the subjects participating in the studies employing bolus and continuous endotoxin administration are listed in Tables 2a and 2b, respectively. Apart from a higher body mass index (BMI), subjects that received a bolus administration of lot #94332B1 were comparable to those that received the #1188844 lot. All subjects were well at time of discharge and no serious adverse events occurred in any of the studies.
Table 2.
Demographic characteristics of subjects receiving (A) bolus and (B) continuous administration of different lots of endotoxin.
|
A | |||
|---|---|---|---|
| Bolus | Lot #1188844n = 10 | Lot #94332B1n = 8 | P-Value |
| Age [yr] | 22.1 ± 1.0 | 24.1 ± 1.4 | 0.24 |
| Height [cm] | 183 ± 4 | 184 ± 1 | 0.96 |
| Mass [kg] | 73 ± 2 | 86 ± 1 | <0.001 |
| BMI [kg/m²] | 21.8 ± 0.6 | 25.5 ± 0.4 | <0.001 |
|
B | |||
|
Continuous |
Lot #1188844n = 10 |
Lot #94332B4n = 20 |
P-Value |
| Age [yr] | 23.6 ± 1.6 | 22.4 ± 0.5 | 0.36 |
| Height [cm] | 182 ± 2 | 182 ± 1 | 0.77 |
| Mass [kg] | 77 ± 4 | 82 ± 2 | 0.23 |
| BMI [kg/m²] | 23.2 ± 0.7 | 24.5 ± 0.9 | 0.16 |
Data were obtained during screening visit and are presented as mean ± SEM. P-Values were calculated with unpaired Student’s t-tests.
Plasma cytokine and chemokine levels
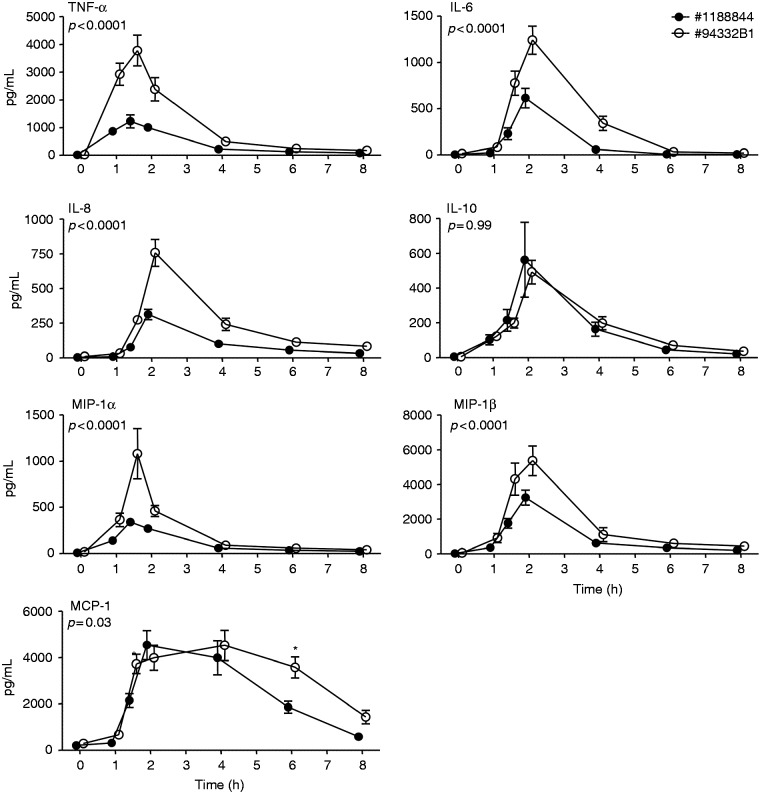
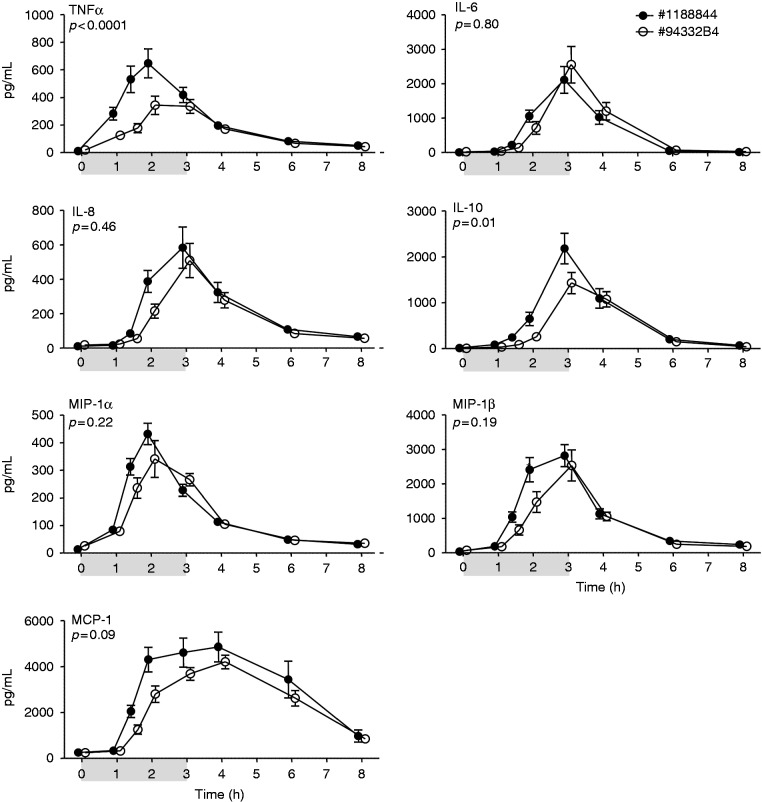
Bolus as well as continuous administration of endotoxin resulted in a profound increase in plasma levels of TNF-α, IL-6, IL-8, IL-10, MCP-1, MIP-1α, and MIP-1β (Figures 1 and 2). In the bolus model, concentrations of the pro-inflammatory mediators TNF-α, IL-6, IL-8, MCP-1, MIP-1α, and MIP-1β were significantly higher in response to administration of #94332B1 compared with administration of lot #1188844 (Figure 1). There were no differences in levels of the anti-inflammatory cytokine IL-10. The cytokine/chemokine responses induced by continuous infusion with either lot #1188844 or #94332B4 were highly comparable for IL-6, IL-8, MCP-1, MIP-1α, and MIP-1β, whereas plasma concentrations of TNF-α and IL-10 were significantly higher in subjects who received lot #1188844 (Figure 2).
Figure 1.
Plasma cytokine/chemokine levels upon bolus administration of endotoxin of lot #1188844 (n = 10) and lot #94332B1 (n = 8). Plasma concentrations (pg/ml) of TNF-α, IL-6, IL-8, IL-10, MCP-1, MIP-1α, and MIP-1β are depicted over time. At t = 0 endotoxin was administered at a dose of 2 ng/kg. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA on log transformed data and interaction term (time × group) P-values are displayed.
Figure 2.
Plasma cytokine/chemokine levels upon continuous administration of endotoxin of lot #1188844 (n = 10) and lot #94332B4 (n = 20). Plasma concentrations (pg/ml) of TNF-α, IL-6, IL-8, IL-10, MCP-1, MIP-1α, and MIP-1β are depicted over time. At t = 0 endotoxin was administered at a dose of 1 ng/kg, followed by a continuous infusion of 1 ng/kg/h for 3 h, as indicated by the grey bar. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA on log transformed data and interaction term (time × group) P-values are displayed.
Flu-like symptoms, temperature and haemodynamic changes
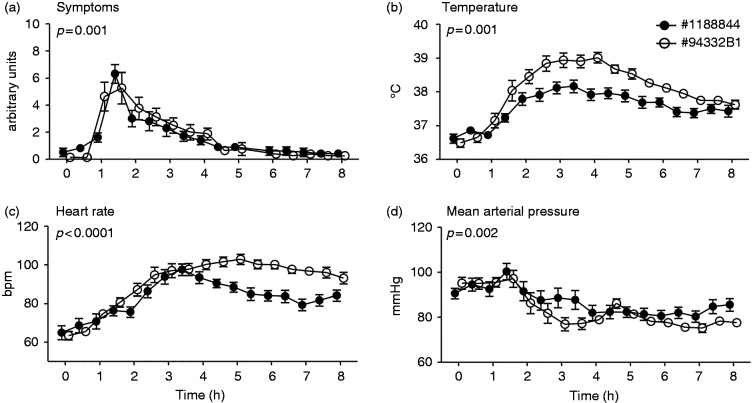
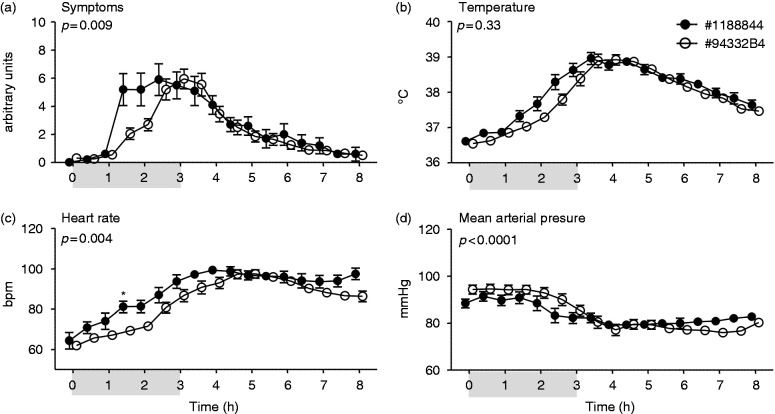
Administration of endotoxin resulted in flu-like symptoms in all subjects, mainly consisting of headache, shivering, muscle and back pain, occasionally nausea and, incidentally, vomiting (Figures 3a and 4a). As expected, systemic inflammation was characterized by an increase in body temperature and heart rate, and a decrease in mean arterial pressure (Figure 3b–d and 4b–d). Bolus administration of lot #94332B1 resulted in an earlier onset of symptoms (Figure 3a), with distinctly higher temperature and heart rate, and a lower mean arterial pressure compared with administration of lot #1188844 (Figure 3b–d). Continuous infusion of lot #94332B4 resulted in a more gradual development of symptoms compared with infusion of the #1188844 lot, with lower symptom scores at 1.5 and 2 h after start of endotoxin infusion (Figure 4a). Correspondingly, the increase in heart rate was more pronounced in subjects that received lot #1188844, accompanied by a slightly, but significantly, more pronounced decrease in mean arterial pressure (Figure 4c, d). There was no significant difference in the course of temperature between the groups (Figure 4b).
Figure 3.
Clinical parameters of systemic inflammation upon bolus administration endotoxin of lot #1188844 (n = 10) and lot #94332B1 (n = 8). Changes over time of (a) symptoms (arbitrary units), (b) temperature (°C), (c) heart rate (beats per min [bpm]), and (d) mean arterial pressure (mmHg) are depicted. At t = 0 endotoxin was administered at a bolus dose of 2 ng/kg. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA and interaction term (time × group) P-values are displayed.
Figure 4.
Clinical parameters of systemic inflammation upon continuous administration of endotoxin of lot #1188844 (n = 10) and lot #94332B4 (n = 20). Changes over time of (a) symptoms (arbitrary units), (b) temperature (°C), (c) heart rate (beats per min [bpm]), and (d) mean arterial pressure (mmHg) are depicted. At t = 0 endotoxin was administered at a dose of 1 ng/kg, followed by a continuous infusion of 1 ng/kg/h for 3 h, as indicated by the grey bar. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA and interaction term (time × group) P-values are displayed.
Haematological parameters
Bolus administration resulted in a transient decrease in neutrophils, lymphocytes and monocytes, followed by a distinct neutrophilia (Figure 5a–c). Compared with lot #1188844, neutrophilia was more pronounced (Figure 5a), there was a trend towards prolonged monocytopenia (Figure 5b), and the initial decrease in lymphocyte numbers (Figure 5c) was more marked after administration of lot #94332B1. Continuous infusion of both lots of endotoxin caused a transient mono- and lymphocytopenia, followed by a neutrophilia and monocytosis, without any differences between lots (Figure 6a–c).
Figure 5.
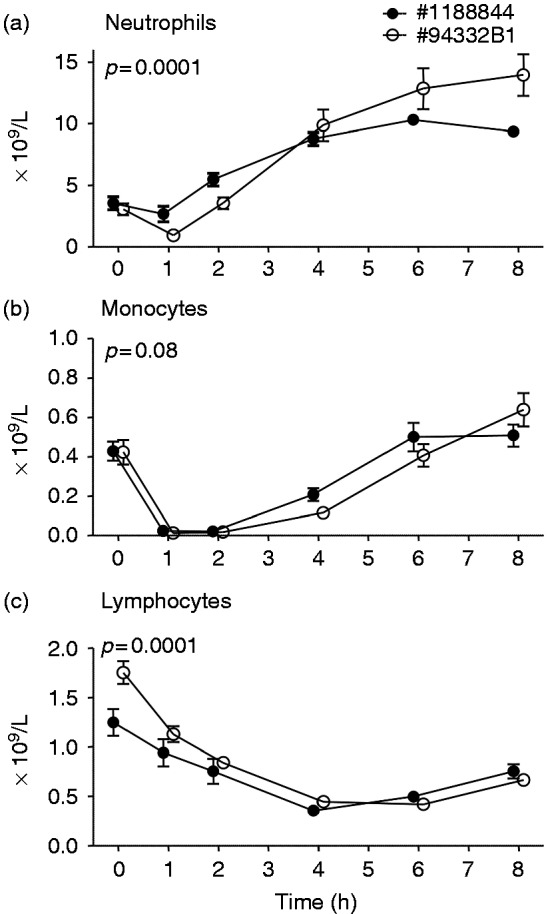
Circulating neutrophil (a), monocyte (b), and lymphocyte (c) numbers upon bolus administration of endotoxin of lot #1188844 (n = 10) and #94332B1 (n = 8). At t = 0 endotoxin was administered at a bolus dose of 2 ng/kg. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA and interaction term (time × group) P-values are displayed.
Figure 6.
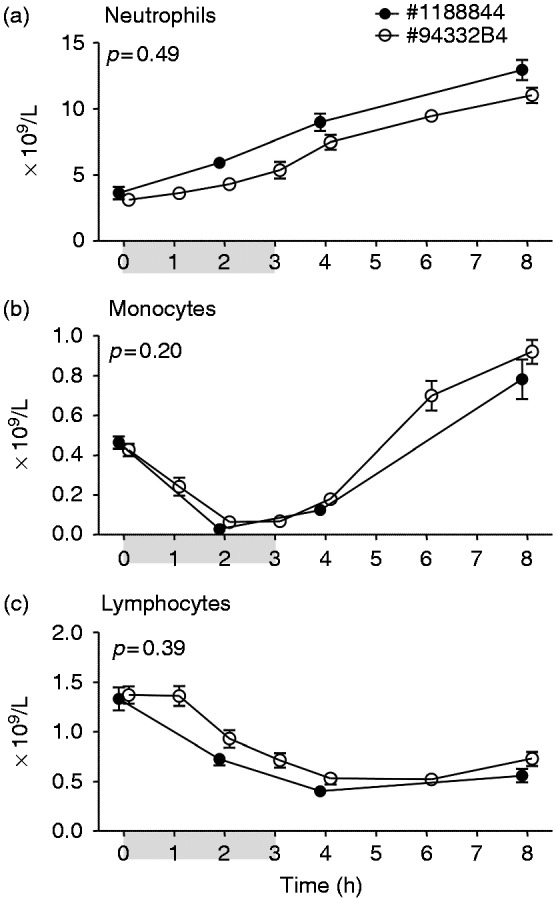
Circulating neutrophil (a), monocyte (b), and lymphocyte (c) numbers upon continuous administration of endotoxin of lot #1188844 (n = 10) and #94332B4 (n = 20). At t = 0 endotoxin was administered at a dose of 1 ng/kg, followed by a continuous infusion of 1 ng/kg/h for 3 h, as indicated by the grey bar. Data are expressed as mean ± SEM. Differences between groups were evaluated using two-way ANOVA and interaction term (time × group) P-values are displayed.
Assessment of potential potency loss of lot #1188844 over time
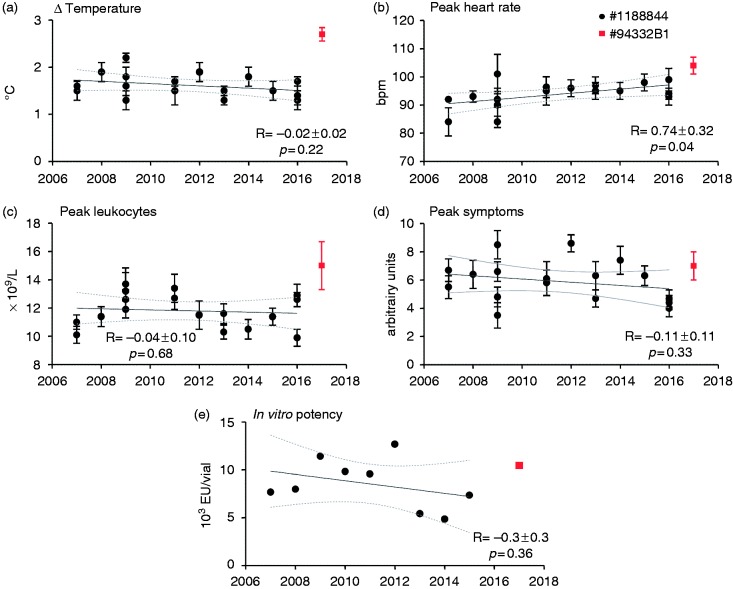
Between 2007 and 2016, 17 experimental human endotoxemia studies were performed at our centre using bolus administration of 2 ng/kg of lot #1188844 (Table 1). Linear regression revealed no significant changes over time in temperature, leukocyte count, and symptom score (Figure 7a, 7c and 7d, respectively). There was a slight, but significant, increase in peak heart rate over the years (Figure 7b). Inter-study variability for the endotoxin-induced effects on heart rate and leukocyte counts was relatively small (variation coefficients of 4.9% and 10.2%, respectively), whereas inter-study variation in temperature changes (15.3%) and peak symptom scores (24.7%) was more substantial. Furthermore, the in vitro endotoxin potency was highly variable over the years (30.4%) but did not significantly decrease over time (Figure 7e).
Figure 7.
In vivo potency of endotoxin administration of lot #1188844 over time. Clinical parameters of systemic inflammation induced by bolus administration of 2 ng/kg lot #1188844 in studies performed in our center from 2007 to 2016 (in black) and novel lot #94332B1 in 2017 (in red) are depicted. Maximum temperature increase (Δ temperature) (a), peak heart rate (b), peak leukocytes (c), and peak symptoms (d). In vitro potency as determined by LAL tests between 2007 and 2015 for lot #1188844, and for the #94332B1 lot in 2017 (e). Data are expressed as mean±SEM. Linear regression analysis was performed, and the slopes (R) with 95% confidential intervals and P-values are reported.
To provide an additional assessment of the in vivo potency of the new lot #94332B1 versus the previously used #1188844 lot, we compared clinical parameters of systemic inflammation induced by bolus administration of #94332B1 (n = 8) with the aggregate mean of these parameters obtained from the previous 17 studies using bolus administration of #1188844 (n = 172). The maximum temperature increase was significantly higher after administration of #94332B1 compared with lot #1188844 (2.7 ± 0.1 vs. 1.6 ± 0.1°C, respectively, P = 0.0001, Figure 7a), as was the maximum heart rate (104 ± 3 vs. 94 ± 1 beats per min, P < 0.0001, Figure 7b). Peak leukocyte numbers (15.0 ± 1.7 vs. 11.0 ± 0.3 × 109/ml, P = 0.10, Figure 7c) and maximum symptom scores (7.0 ± 1 vs. 5.9 ± 0.3, P = 0.31, Figure 7d) were comparable between lots.
Discussion
In the present work, we compare the systemic inflammatory response induced by two lots derived from a new GMP-grade batch of endotoxin with the response induced by the previously used lot #1188844 in humans in vivo. Compared with lot #1188844, bolus administration of the new #94332B1 lot resulted in a more pronounced inflammatory response, with higher levels of pro-inflammatory cytokines and chemokines, an earlier onset of symptoms, higher temperatures and heart rate, and more profound neutrophilia. In contrast, continuous infusion of another new lot (#94332B4) resulted in a slightly less pronounced inflammatory response compared with continuous infusion of #1188844, with lower levels of TNF-α and IL-10 and a more gradual onset of symptoms and increase in heart rate, but no differences in other cytokines/chemokines, temperature, or leukocyte numbers. In addition, despite considerable inter-study variability especially in flu-like symptoms and fever, no decline in endotoxin-induced clinical parameters of systemic inflammation were observed over a period of 10 yr in our centre, indicating that the #1188844 lot did not lose potency.
A previous study compared the in vivo potency of the EC-5 lot (which was used until 1999) with that of the CCRE lot vialled in 1997 (#67801). Both of these lots were derived from the same bulk material. This head-to-head comparison, using bolus administration (4 ng/kg) in four subjects per group,13 showed that administration of the older EC-5 lot resulted in lower peak temperatures, leukocyte counts, cytokine responses, cortisol production, and C-reactive protein levels. Based on this, the authors concluded that endotoxin may lose its potency over the years. In the current work, we have not only evaluated cytokine responses and inflammatory parameters of lot #1188844 versus two lots derived from novel GMP-grade bulk material, but we also performed an over-time analysis of clinical parameters of systemic inflammation induced by lot #1188844 in a large group of subjects. These in vivo data did not show a time-dependent decrease in effect size. Although we observed a slight increase in peak heart rate over the years, these changes were not related to differences in body temperature, and despite being statistically significant, the rather small increase likely has limited relevance.
The in vitro quantification of endotoxin potency is notoriously difficult, and various methods have been described over the years.17 The LAL assay is the most well-known method, and this was also employed for the in vitro potency data reported in the current work.16 In this assay, specimens and controls are diluted serially and these dilutions are added to the haemolymph of the horseshoe crab. A coagulation reaction takes place for as long as the endotoxin concentration is sufficient. Due to the 1:1 dilution methodology used in the assay, the true endotoxin concentration lies between 50% and 200% of the reported concentration. This is a plausible explanation for the highly variable in vitro potency results of the #1188844 lot described in the present study. An alternative endotoxin measurement method is the endotoxin activity assay (EAA). This assay involves chemiluminescent measurement on the oxidative burst reaction of activated neutrophils to complement-coated LPS-IgM immune complexes.18 As such, this assay does not measure endotoxin content directly. The use of endogenous neutrophils in the assay represents a potential limitation, as there may exist large inter-individual differences in neutrophils, especially under pathological conditions, which might not be fully corrected by the use of positive and negative controls in the assay.18 Furthermore, the EAA assay can only be performed in real time using whole blood, not on stored material, and the results are semi-quantitative (i.e. the assay reports low, intermediate, or high endotoxin levels). Recently, a reversed HPLC/MS method to detect endotoxin in biological samples was developed, which is based on quantifying 3HM, the most abundant hydroxylated fatty acid of the lipid A moiety.14 Plasma endotoxin levels measured using this new method were shown to correlate with disease severity in patients suffering from systemic inflammatory response syndrome.19 This method appears to be promising, but requires additional validation.
Our results show that there exists considerable variation in inflammation parameters between individual studies, which for example can be attributed to differences in diet, genetic background, and previous illnesses. Especially the variation in symptoms was substantial, but it has to be emphasized that symptom scores are notoriously subjective.20 Endotoxin experiments in our department were carried out with one, two, or three subjects simultaneously in the same room. Therefore, it is possible that symptoms reported are affected by the presence of symptoms in the other subjects.
Lot #1188844 was large and was used in over 17 studies over a period of 10 yr in our centre alone. New lots from the new GMP-grade batch are produced in smaller quantities. Herein, we report results obtained using the new lots #94332B1 and #94332B4, and these lots already have several successors. Although lot #94332B1 was more potent than #1188844 using the bolus administration model, the other new lot (#94332B4) showed to be less potent than lot #1188844 in the continuous infusion model. These findings suggest that there are potency differences between these two new lots, despite the fact that they are derived from the same bulk material. However, because we used different modes of endotoxin administration and did not compare these two new lots head-to-head, no definite conclusions can be drawn pertaining to this issue. For now, it is only safe to conclude that directly comparing responses induced by different lots of endotoxin is not recommended, and that a control group (e.g. a group that only receives endotoxin) should always be included in intervention studies using the experimental human endotoxemia model.
This work has several limitations. First, subjects who received a bolus of lot #94332B1 had a 14% higher body mass, but similar length compared with subjects that received a bolus of the #1188844 lot. Therefore, they received 14% more endotoxin as it is dosed per kg body mass, which theoretically could partially explain the increased cytokine production and symptoms. However, we have previously shown that BMI does not correlate with the endotoxin-induced increase in plasma cytokine levels, nor with the increase in body temperature.21 Furthermore, taking into account that previous work did not reveal a proportional increase in the cytokine response with higher dosages of endotoxin,6 the 200–300% increase in cytokine levels observed in the #94332B1 group is unlikely to be explained by the 14% higher endotoxin dose. Another drawback is that we only present a limited set of inflammatory parameters for the in vivo potency analysis over time. It would have been valuable to include cytokine responses as well. Unfortunately, this is not possible, as original cytokine data were obtained using various assays which are not sufficiently comparable due to inter-assay variability, and plasma samples are no longer available for batchwise reanalysis. Furthermore, cytokines show substantial degradation after 5 yr, even when stored at –80°C.22 A final limitation is that the LAL tests were performed by several commercial parties, which adds an extra source of variation.
In conclusion, similar to the previous lots, administration of lots derived fromthe novelGMP-grade endotoxin batch elicit a transient, controlled, and safe systemic inflammatory response in humans in vivo. However, newer lots of endotoxin are neither necessarily equipotent to previous lots, nor to other new lots. Although we found no evidence for loss of potency of the #1188844 lot over time, there is considerable inter-study variability in inflammatory parameters, despite using the same lot of endotoxin and experimental protocol. As such, we propose that for interventional studies with, for example, immunomodulatory compounds, direct comparison of cytokine responses or clinical parameters of systemic inflammation is only valid using a control group within the same study, using the same lot of endotoxin.
Acknowledgements
The authors would like to thank Anthony Suffredini for his assistance pertaining to the experimental human endotoxemia model throughout the years, and for his input and feedback on the manuscript. The authors thank Annelies Draisma, Lucas van Eijk, Mirrin Dorresteijn, Bart Ramakers, Jenneke Leentjens, Roger Groenendael, and Rebecca Koch for sharing the data of previous experimental human endotoxemia studies.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was financed by the Department of Intensive Care Medicine of the Radboud university medical center in Nijmegen, the Netherlands.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lord JM, Midwinter MJ, Chen YF, et al. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014; 384: 1455–1465. 2014/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock (Augusta, GA) 2006; 26: 13–19. [DOI] [PubMed] [Google Scholar]
- 4.Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: A systematic review. Surgery 2015; 157: 362–380. [DOI] [PubMed] [Google Scholar]
- 5.van Lier D, Geven C, Leijte GP, et al. Experimental human endotoxemia as a model of systemic inflammation. Biochimie 2018. 2018/06/25. [DOI] [PubMed]
- 6.Kiers D, Koch RM, Hamers L, et al. Characterization of a model of systemic inflammation in humans in vivo elicited by continuous infusion of endotoxin. Scientific Rep 2017; 7: 40149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahador M, Cross AS. From therapy to experimental model: A hundred years of endotoxin administration to human subjects. J Endotoxin Res 2007; 13: 251–279. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen AS, Krabbe KS, Krogh-Madsen R, et al. Human endotoxemia as a model of systemic inflammation. Curr Med Chem 2008; 15: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 9.Lelubre C, Medfai H, Akl I, et al. Leukocyte phosphodiesterase expression after lipopolysaccharide and during sepsis and its relationship with HLA-DR expression. J Leuk Biol 2017; 101: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 10.Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature 2005; 437: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: A roadmap for future research. Lancet Infect Dis 2015; 15: 581–614. [DOI] [PubMed] [Google Scholar]
- 12.Lowry SF, Fong Y. Determine whether 1 week of total parenteral nutrition (TPN) in healthy subjects alters systemic responses to a subsequent dose of endotoxin . Ann Surg 1996; 223: 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: Evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis 1999; 179: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 14.van Eijk LT, Pickkers P, Smits P, et al. Severe vagal response after endotoxin administration in humans. Intens Care Med 2004; 30: 2279–2281. [DOI] [PubMed] [Google Scholar]
- 15.Noveck RJ, Guptill JT, Cohen-Wolkowiez M, et al. Dose-related inflammatory effects of intravenous endotoin in humans: Evaluation of a new clinical lot (CCRE Lot 94332B1) of Escherichia coli O:113 endotoxin (LPS). Clin Pharmacol Ther 2018; 103 S82–S83. [Google Scholar]
- 16.Iwanaga S. Biochemical principle of Limulus test for detecting bacterial endotoxins. Proc Japan Acad Series B Phys Biol Sci 2007; 83: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munford RS. Endotoxemia-menace, marker, or mistake? J Leuk Biol 2016; 100: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto N, Takahashi G, Kojika M, et al. Interleukin-8 induces an elevation in the endotoxin activity assay (EAA) level: Does the EAA truly measure the endotoxin level? J Infect Chemother 2013; 19: 825–832. [DOI] [PubMed] [Google Scholar]
- 19.Pais de Barros J-P, Gautier T, Sali W, et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res 2015; 56: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosendal M, Jarbol DE, Pedersen AF, et al. Multiple perspectives on symptom interpretation in primary care research. BMC Fam Pract 2013; 14: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Eijk LT, van der Pluijm RW, Ramakers BP, et al. Body mass index is not associated with cytokine induction during experimental human endotoxemia. Innate Immun 2014; 20: 61–67. [DOI] [PubMed] [Google Scholar]
- 22.de Jager W, Bourcier K, Rijkers GT, et al. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 2009; 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakers BP, Riksen NP, van den Broek P, et al. Circulating adenosine increases during human experimental endotoxemia but blockade of its receptor does not influence the immune response and subsequent organ injury. Crit Care (London, England) 2011; 15: R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kox M, Pompe JC, Gordinou de Gouberville MC, et al. Effects of the alpha7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock (Augusta, GA) 2011; 36: 5–11. [DOI] [PubMed] [Google Scholar]
- 25.Dorresteijn MJ, Visser T, Cox LA, et al. C1-esterase inhibitor attenuates the inflammatory response during human endotoxemia. Crit Care Med 2010; 38: 2139–2145. [DOI] [PubMed] [Google Scholar]
- 26.van Eijk LT, Heemskerk S, van der Pluijm RW, et al. The effect of iron loading and iron chelation on the innate immune response and subclinical organ injury during human endotoxemia: A randomized trial. Haematologica 2014; 99: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubbers T, Kox M, de Haan JJ, et al. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit Care Med 2013; 41: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 28.Ramakers BP, Riksen NP, Stal TH, et al. Dipyridamole augments the antiinflammatory response during human endotoxemia. Crit Care (London, England) 2011; 15: R289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leentjens J, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomized pilot study. Am J Resp Crit Care Med 2012; 186: 838–845. [DOI] [PubMed] [Google Scholar]
- 30.van Eijk LT, John AS, Schwoebel F, et al. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood 2014; 124: 2643–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kox M, van Eijk LT, Zwaag J, et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A 2014; 111: 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kox M, van Eijk LT, Verhaak T, et al. Transvenous vagus nerve stimulation does not modulate the innate immune response during experimental human endotoxemia: A randomized controlled study. Arthritis Res Ther 2015; 17: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiers D, Wielockx B, Peters E, et al. Short-term hypoxia dampens inflammation in vivo via enhanced adenosine release and adenosine 2B receptor stimulation. EBioMedicine 2018; 33: 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch RM, Kox M, Thijs EJM, et al. Development of endotoxin tolerance does not influence the response to a challenge with the mucosal live-attenuated influenza vaccine in humans in vivo. Front Immunol 2017; 8: 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiers D, van der Heijden WA, van Ede L, et al. A randomised trial on the effect of anti-platelet therapy on the systemic inflammatory response in human endotoxaemia. Thromb Haemostasis 2017; 117: 1798–1807. [DOI] [PubMed] [Google Scholar]