Short abstract
Lipoteichoic acid (LTA) of Enterococcus faecalis is a potent stimulator of inflammatory responses, but the effects of E. faecalis LTA on osteoclastogenesis remains far from well understood. This study showed that E. faecalis LTA significantly inhibited osteoclastogenesis of wild type murine bone marrow-derived macrophages (BMMs) in the presence of a high dose of RANKL, while the inhibition of osteoclastogenesis by E. faecalis LTA was significantly removed in BMMs with deficient expression of the transcription factor RBP-J. In addition, a few small osteoclasts were generated in BMMs with only E. faecalis LTA stimulation, presumably due to the production of TNF-α and IL-6. Furthermore, both p38 and ERK1/2 MAPK signaling pathways were activated after 24 h of E. faecalis LTA treatment, but these signaling pathways were not activated after 6 d of treatment with RANKL in mature osteoclasts. In conclusion, E. faecalis LTA, which induces inflammatory response, could inhibit RANKL-induced osteoclastogenesis via RBP-J in BMMs.
Keywords: Enterococcus faecalis, lipoteichoic acid, bone marrow-derived macrophages, osteoclastogenesis, RBP-J
Introduction
Lipoteichoic acid (LTA) is an amphiphile which is located at the interface of the cytoplasmic membrane and cell wall of pathogenic and non-pathogenic Gram-positive bacteria and is released during growth.1 LTA, as a major virulence factor, plays an important role in stimulation of inflammatory responses.2 The predominant virulent attributes of Enterococcus faecalis consist of lytic enzymes, cytolysin, aggregation substance, peptidoglycan and LTA which can promote colonization, invasion of host tissues and evasion of host defense mechanisms.3 E. faecalis LTA can induce inflammatory responses by stimulating macrophages to release cytokines and mediators,4 and contributes to biofilm formation that enables bacteria survival in adverse environments.5
Bone homeostasis is a dynamic balance between bone resorption and bone formation.6 Disruption of the balance between osteoblasts and osteoclasts will result in osteopenia/osteoporosis or other metabolic bone diseases.7 Bone resorptive osteoclasts are multinucleated cells derived from monocyte/macrophage precursors.8 The receptor activator of RANKL and M-CSF are two essential cytokines for osteoclast differentiation.9 When pro-inflammatory cytokines stimulate osteoclast precursors, the transcription factors NF-κB, c-Fos and NFATc1, which play essential roles during osteoclast differentiation, will be further activated.10–12 NFATc1 modulates osteoclast-specific genes including cathepsin K, TRAP and matrix metalloproteinase-9 (MMP-9).13–15
The transcription factor recombinant recognition sequence binding protein at the Jκ site (RBP-J) is expressed in most cells and is a nuclear DNA-binding protein that can repress or activate transcription when acting in conjunction with different proteins.16 RBP-J is involved in cell proliferation, differentiation and cell fate determination.16 RBP-J plays an important role in pro-inflammatory M1 macrophage polarization.17 Both NK-κB and Notch signaling pathways are associated with osteoclastogenesis.18 The induction of NFATc1 is dependent on NF-κB and c-Fos pathways resulting in osteoclast differentiation. RBP-J negative regulates the expression and function of NFATc1 via inhibition of NF-κB and c-Fos and further suppresses osteoclast differentiation and bone resorption.18,19 On the other hand, the Notch signaling pathway participates in bone remodeling, and the activation of the Notch intracellular domain 1 (NICD1) significantly activates RBP-J activity.19 When Notch signaling is attenuated, osteoclastogenesis and bone resorption will be aggravated.20 It was reported that RBP-J negatively regulates osteoclast differentiation and bone resorption, particularly in TNF-α-induced osteoclastogenesis and inflammatory bone resorption.18,19 It has also been found that TNF or LPS-mediated osteoclast differentiation and inflammatory bone resorption are drastically suppressed by RBP-J and IFN regulatory factor-8.18,21 These studies show that RBP-J has a strong inhibitory effect on osteoclast differentiation and inflammatory bone resorption.
To date, the effects of E. faecalis LTA on osteoclast differentiation within the inflammatory environment of persistent apical periodontitis caused by E. faecalis is still unclear. Hence, in this study, we explored the modulatory effects and mechanisms of E. faecalis LTA on the differentiation of inflammatory osteoclasts and the relevant underlying mechanisms involved.
Materials and methods
Bacterial culture and LTA preparation
E. faecalis P25RC and P52Sa were isolated, respectively, from patients’ root canal and saliva at the Hospital of Stomatology of Peking University by Dr. Xiaofei Zhu.22 E. faecalis ATCC 29212 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). E. faecalis were cultured anaerobically (N2, 90%, CO2, 5% and H2, 5%) overnight at 37°C in brain heart infusion broth (OXOID, Basingstoke, Hampshire, England). The three highly purified E. faecalis LTAs were extracted using the butanol method followed by hydrophobic interaction chromatography purification. Contaminations were excluded as described in our previous paper.23
Culture of osteoclast precursors
Bone marrow-derived macrophages (BMMs) from wild type (WT) and Rbpj conditional knockout mice (RbpjΔM/ΔM) were used as osteoclast precursors as previously described.18 Cells were cultured to 80% confluence, and then treated with the three E. faecalis LTAs (50 μg/ml), RANKL (80 ng/ml), E. faecalis LTAs (50 μg/ml) plus RANKL (80 ng/ml) for 6 d, or pre-treated with RANKL (20 ng/ml) for 3 d prior to treatment with E. faecalis LTAs (50 ng/ml) for an additional 3 d, respectively.
Cell viability assay
The WT BMMs were seeded in a 96-well plate at a density of 1 × 103 cells/well. After 24 h of culture, the cells were treated with various concentrations of the three E. faecalis LTAs for an additional 24 h. The effects of E. faecalis LTA on cell viability was evaluated using the Cell Counting Kit-8 (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions.
TRAP staining
The WT BMMs and RbpjΔM/ΔM BMMs were seeded in 6-well plates at a density of 2 × 105 cells/well, respectively. The cells were treated with the three E. faecalis LTAs and/or RANKL. TRAP staining was carried out using TRAP kit according to the manufacturer’s instruction (Sigma-Aldrich, St. Louis, MO, USA). The cells were washed twice with PBS and fixed with 4% paraformaldehyde for 30 min. Then the cells were rinsed thoroughly using pre-warmed deionized water. The staining mixing solution was prepared according to the manufacturer’s instructions. The cells were incubated in the staining solution, protected from light, at 37°C for 1 h. The cells were counterstained for 2 min in Hematoxylin solution and rinsed in tap water thoroughly. The TRAP-positive multinucleated cells were observed and counted under a light microscope.
Gene expression analysis
Total RNA was extracted from WT BMMs after E. faecalis LTA treatment. The gene expression analyses were carried out using real-time PCR. Primer sequences were as follows: cathepsin K, 5′(CTGAAGATGCTTTCCCATATGTGGG)3′ and 5′(GCAGGCGTTGTTCTTATTCCGAGC)3′; TRAP, 5′(ACACAGTGATGCTGTGTGGCAACTC)3′ and 5′(CCAGAGGCTTCCACATATATGATGG)3′; MMP-9, 5′(GCCCTGGAACTCACACGACA)3′ and 5′(TTGGAAACTCACACGCCAGAAG)3′;c-Fos, 5′(ACGTGGAGCTGAAGGCAGAAC)3′ and 5′(AGCCACTGGGCCTAGATGATG)3′; Nfatc1, 5′(CAAGTCTCACCACAGGGCTCACTA)3′ and 5′(TCAGCCGTCCCAATGAACAG)3′; Notch1, 5′(GCTCCGAGGAGATCAACGAG)3′ and 5′(TTGACATCACCCTCACACCG)3′; Rbpj, 5′(CGGCCTCCACCCAAACGACT)3′ and 5′(TCCAACCACTGCCCATAAGATACA)3′; GAPDH 5′(ATGTGTCCGTCGTGGATCTGA)3′ and 5′(ATGCCTGCTTCACCACCTTCT)3′.
ELISA assay
The supernatants were collected from WT BMMs after E. faecalis LTA treatment. The expression levels of TNF-α and IL-6 were analyzed with the corresponding ELISA kits (R&D systems, Minneapolis, MN, USA).
Western blotting
The whole cell lysates were extracted from WT BMMs after E. faecalis LTA treatment. The phospho-p38, p38, phospho-ERK1/2 and ERK1/2 Abs (Cell Signaling Technology, Boston, MA, USA) were used to detect MAPK signaling pathways with Western blotting.
Statistical analysis
Each experiment was conducted in triplicate and repeated at least three times. Data were presented as mean ± SD and analyzed by ANOVA. The threshold of statistical significance was set at P < 0.05.
Results
The effect of the three E. faecalis LTAs on the cell viability of BMMs
The WT BMMs were cultured with 40 ng/ml M-CSF and stimulated with the three different E. faecalis LTAs at various concentrations of 1 µg/ml, 10 µg/ml and 50 µg/ml for 24 h. The result showed that E. faecalis LTA could not inhibit the cell viability of osteoclast precursors. LTAs from E. faecalis P25RC (50 µg/ml) and P52Sa could increase the cell viability (Figure 1).
Figure 1.
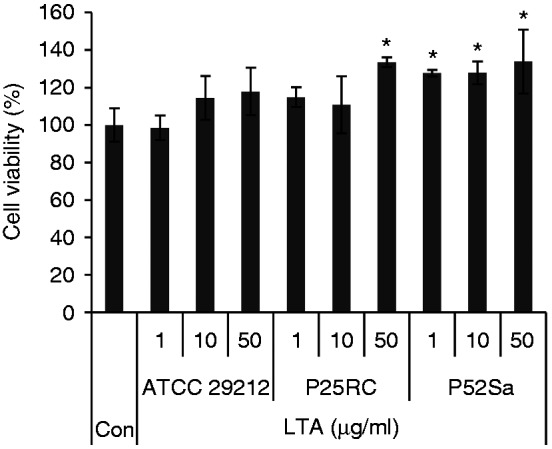
Effects of the three different E. faecalis LTAs on the cell viability of BMMs. WT BMMs were cultured with 40 ng/ml M-CSF and stimulated with the three different E. faecalis LTAs at various concentrations for 24 h. Data are presented as mean ± SD, *P < 0.05 compared with untreated cells. Con, untreated cells were set as control.
E. faecalis LTAs inhibit RANKL-induced osteoclastogenesis
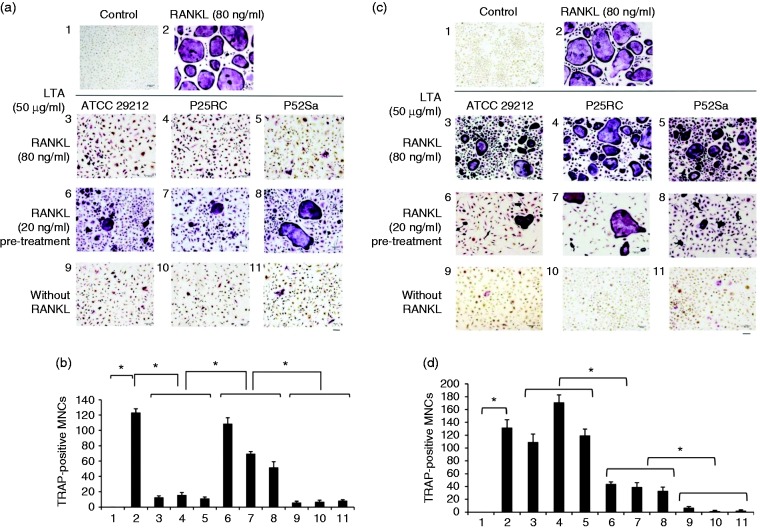
The TRAP staining demonstrated that E. faecalis LTAs effectively inhibited osteoclast differentiation of WT BMMs in the presence of high-dose RANKL (80 ng/ml) and resulted in small, immature TRAP-positive osteoclasts with fewer nuclei, while E. faecalis LTAs could not inhibit osteoclast differentiation when WT BMMs were pre-treated with low-dose RANKL (20 ng/ml) (Figure 2(a) and 2(b)). However, E. faecalis LTAs induced osteoclast differentiation of RbpjΔM/ΔM BMMs and resulted in large osteoclasts with many nuclei in the presence of high-dose RANKL (80 ng/ml). In contrast, E. faecalis LTAs could induce WT BMMs and RbpjΔM/ΔM BMMs to form a few small, immature TRAP-positive osteoclasts independent of RANKL (Figure 2(c) and 2(d)).
Figure 2.
Osteoclast differentiation induced by the three E. faecalis LTAs in osteoclast precursors was evaluated with the TRAP staining assay. (a and b) WT BMMs and (c and d) RbpjΔM/ΔM BMMs were treated for 6 d in the presence of 40 ng/ml M-CSF with the three E. faecalis LTAs, RANKL, E. faecalis LTAs plus RANKL, or pre-treated with RANKL for 3 d prior to treatment with E. faecalis LTAs for an additional 3 d, respectively. The cells were then subjected to TRAP staining. TRAP staining assay was carried out and visualized at 100× magnification under an inverted bright field microscope. Bar, 100 µm. The numbers of TRAP-positive multinucleated cells with more than 3 nuclei were counted from 6 random fields of view at 40× magnification. 1, Untreated cells; 2, RANKL (80 ng/ml); 3, RANKL (80 ng/ml) and E. faecalis ATCC 29212 LTA (50 µg/ml); 4, RANKL (80 ng/ml) and E. faecalis P25RC LTA (50 µg/ml); 5, RANKL (80 ng/ml) and E. faecalis P52Sa LTA (50 µg/ml); 6, Pre-treated with RANKL (20 ng/ml) and E. faecalis ATCC 29212 LTA (50 µg/ml); 7, Pre-treated with RANKL (20 ng/ml) and E. faecalis P25RC LTA (50 µg/ml); 8, Pre-treated with RANKL (20 ng/ml) and E. faecalis P52Sa LTA (50 µg/ml); 9, E. faecalis ATCC 29212 LTA (50 µg/ml); 10, E. faecalis P25RC LTA (50 µg/ml); 11, E. faecalis P52Sa LTA (50 µg/ml). The mean and SD are shown. *P < 0.05 was considered statistically significant compared with the untreated cells or RANKL-only treated cells. MNCs, multinucleated cells.
Gene expression of osteoclast differentiation induced by E. faecalis LTAs with and without RANKL
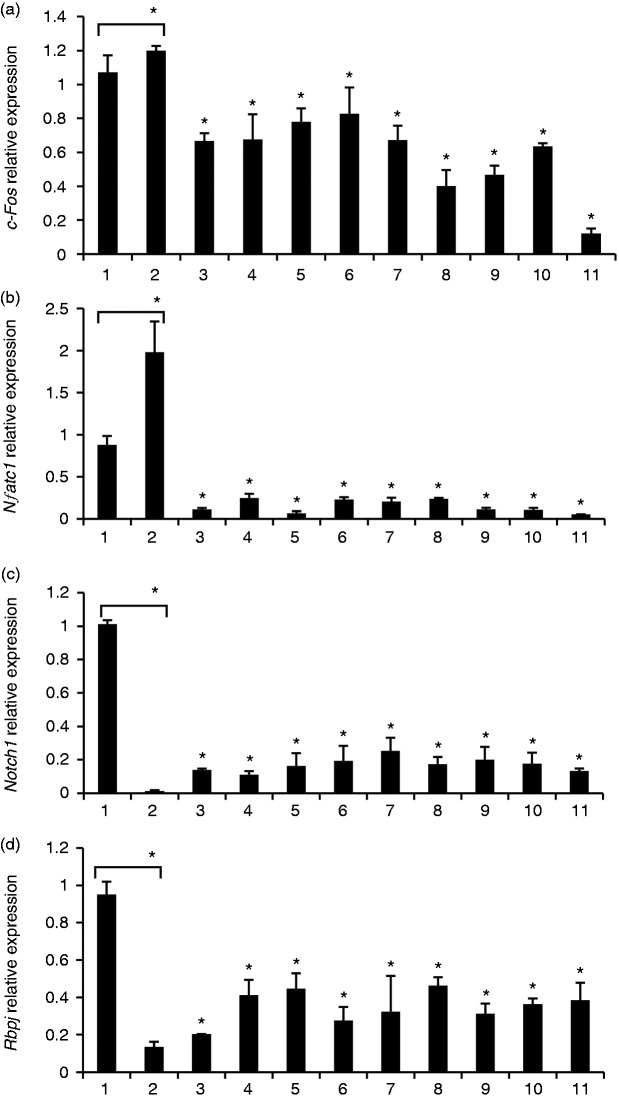
Gene expression levels of cathepsin K, TRAP and MMP-9 were significantly up-regulated to varying degrees when WT BMMs were treated with E. faecalis LTAs upon exposure to RANKL compared with the untreated control. Upon exposure to high-dose RANKL, expressions of the three osteoclast-related genes were markedly down-regulated by E. faecalis LTAs compared with RANKL treatment alone. However, compared with the untreated control, the expression level of cathepsin K was even all down-regulated by E. faecalis LTAs alone in WT BMMs.
Gene expression levels of c-Fos and NFATc1 were significantly up-regulated in WT BMMs only treated with high-dose RANKL compared with untreated control. In addition, gene expression levels of c-Fos and NFATc1 were significantly down-regulated in WT BMM treated with E. faecalis LTAs compared with cells only treated with high-dose RANKL (Figure 3(a) and 3(b)). On the contrary, gene expression levels of Notch1 and RBP-J were markedly down-regulated in WT BMMs treated only with high-dose RANKL compared with untreated control. Furthermore, gene expression levels of Notch1 and RBP-J were up-regulated in WT BMMs treated with E. faecalis LTAs compared with cells only treated with high-dose RANKL (Figure 3(c) and 3(d)).
Figure 3.
Gene expression analysis of osteoclast differentiation induced by E. faecalis LTA in osteoclast precursors. WT BMMs were treated for 6 d in the presence of 40 ng/ml M-CSF with the three E. faecalis LTAs, RANKL, E. faecalis LTAs plus RANKL, or pre-treated with RANKL for 3 d prior to treatment with E. faecalis LTAs for an additional 3 d, respectively. The gene expression levels of (a) c-Fos, (b) NFATc1, (c) Notch1 and (d) RBP-J were assayed using real-time PCR. The numbers of abscissa represent the same treatment groups as those in Figure 2. The mean and SD are shown. *P < 0.05 was considered statistically significant compared with untreated cells or RANKL-only treated cells.
The pro-inflammatory effects of E. faecalis LTAs on osteoclast precursors
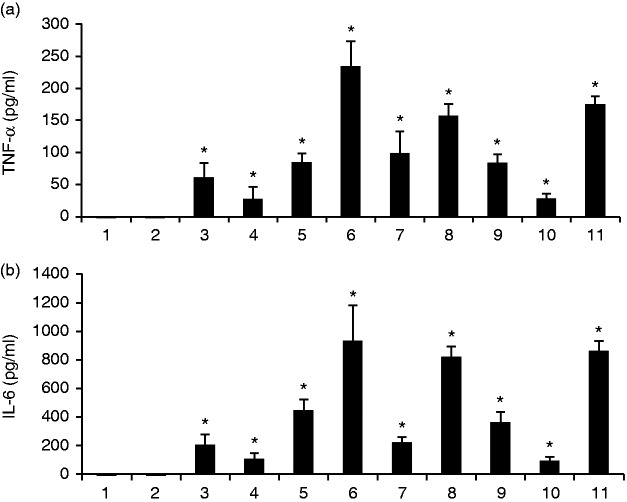
The three E. faecalis LTAs significantly increased the levels of TNF-α and IL-6 in varying degrees compared with untreated control (Figure 4).
Figure 4.
The pro-inflammatory effects of E. faecalis LTAs on osteoclast precursors. WT BMMs were treated for 6 d in the presence of 40 ng/ml M-CSF with the three E. faecalis LTAs, RANKL, E. faecalis LTAs plus RANKL, or pre-treated with RANKL for 3 d prior to treatment with E. faecalis LTAs for an additional 3 d, respectively. The secretory levels of (a) TNF-α and (b) IL-6 were assayed using ELISA. The numbers of abscissa represent the same treatment groups as those in Figure 2. The mean and SD are shown. *P < 0.05 was considered statistically significant compared with untreated cells.
Protein expression of osteoclast differentiation induced by E. faecalis LTAs with and without RANKL
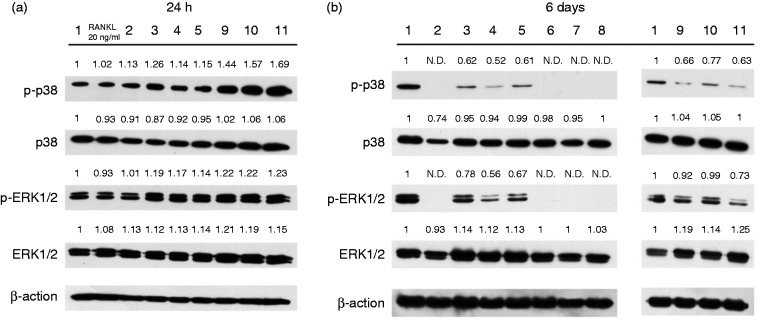
Both p38 and ERK1/2 MAPK signaling pathways were activated after 24 h E. faecalis LTA treatment in WT BMM (Figure 5(a)). However, phosphorylation of both p38 and ERK1/2 MAPK could not be detected after 6 d with only high-dose RANKL treatment and RANKL pre-treatment prior to E. faecalis LTA treatment. Phosphorylation of both p38 and ERK1/2 MAPK were down-regulated by high-dose RANKL and E. faecalis LTA treatment and only E. faecalis LTA treatment (Figure 5(b)).
Figure 5.
Protein expression analysis of osteoclast differentiation induced by E. faecalis LTA in osteoclast precursors. (a) WT BMMs were treated with the three E. faecalis LTAs, RANKL and E. faecalis LTAs plus RANKL for 24 h in the presence of 40 ng/ml M-CSF. (b) WT BMMs were treated for 6 d in the presence of 40 ng/ml M-CSF with the three E. faecalis LTAs, RANKL, E. faecalis LTAs plus RANKL, or pre-treated with RANKL for 3 d prior to treatment with E. faecalis LTAs for an additional 3 d, respectively. The numbers above the bold line represent the same treatment groups as those in Figure 2. The numerical values above the bands denote the relative density values.
Discussion
LTA is a key virulence factor in inflammatory process and is expressed exclusively on the surface of Gram-positive bacteria.24 The structure and function of LTA vary across different species.25 E. faecalis LTA is a typical D-alanyl-LTA with glycerophosphate backbone (Type 1).5,26 Structural microheterogeneity between various LTAs mainly lies in the D-alanylation rates, glycerolphosphate chain length, fatty acid composition and type of glycosyl substitution.1,27 These subtle differences may cause varying levels of inflammatory responses.28
In this study, E. faecalis LTA had no significant detrimental effect on cell viability, which was consistent with previous studies.29,30 On the contrary, LTAs from E. faecalis P25RC and P52Sa were observed to enhance cell viability after 24 h treatment, which may be explained in Figure 5(a). It was demonstrated that E. faecalis LTAs induced phosphorylation of p38 and ERK1/2 MAPKs after 24 h of treatment. MAPK signaling pathways are essential for cell proliferation and development.31 It is likely that the observed enhancement of cell viability might be caused by activation of MAPK signaling pathways.
Interestingly, this study showed that the three E. faecalis LTAs effectively inhibited osteoclast differentiation of WT BMMs in the presence of a high dose of RANKL and only resulted in the formation of some small immature TRAP-positive osteoclasts with fewer nuclei. The efficacy of RANKL on osteoclast differentiation was greatly reduced by E. faecalis LTAs. LTA, as a pro-inflammatory stimulus, could induce M1 polarization of macrophages. It has been demonstrated that M1 macrophages could attenuate osteoclastogenesis.32 A similar phenomenon was also observed in a previous report that S. aureus LTA inhibited osteoclastogenesis upon exposure to M-CSF and a low dose of RANKL (20 ng/ml).30 However, the inhibition of osteoclastogenesis by E. faecalis LTAs was removed in the RbpjΔM/ΔM BMM cell cultures, in which large amounts of osteoclasts with many nuclei were formed in the presence of a high dose of RANKL. It has been reported that RBP-J is responsible for M1 macrophage polarization.17 In this study, the RBP-J-deficiency inhibited M1 polarization of BMMs and revived osteoclastogenesis upon exposure to LTA and a high dose of RANKL. This thus suggests that E. faecalis LTA inhibits RANKL-induced osteoclast differentiation, at least partially via RBP-J. RBP-J plays an important role in the process of E. faecalis LTA inhibiting RANKL-induced osteoclastogenesis. In addition, the inhibitory effect of E. faecalis LTAs on osteoclastogenesis was decreased when WT BMMs were pre-treated with a low dose of RANKL (20 ng/ml), indicating that E. faecalis LTA mainly functions during the early stage of osteoclast differentiation. A few TRAP-positive immature small osteoclasts were formed by WT and RbpjΔM/ΔM BMMs with E. faecalis LTA treatment alone. E. faecalis LTA has a very weak effect in stimulating osteoclast differentiation, presumably through TNF-α, as reported previously.33
Gene expression levels of cathepsin K, TRAP and MMP-9 were suppressed by E. faecalis LTAs upon exposure to a high dose of RANKL, as compared with treatment with RANKL only. At the same time, E. faecalis LTAs had no significant effects in modulating the expression of the three osteoclast-related genes: cathepsin K, TRAP and MMP-9. Because LTAs were derived from different E. faecalis strains and had different structures, a few differences among groups with similar treatments could still be observed. E. faecalis LTAs inhibited the gene expression levels of c-Fos and NFATc1, while enhancing the gene expression levels of Notch1 and the negative regulator RBP-J, as compared with the RANKL treatment group. These results showed that E. faecalis LTAs could inhibit RANKL-induced osteoclast differentiation, which was consistent with the results of the TRAP staining analysis.
The ELISA analysis result showed that treatment with E. faecalis LTAs could significantly increase the production of pro-inflammatory cytokines, TNF-α and IL-6. TNF-α and IL-6 promote osteoclast differentiation.34 Therefore, the weak direct effects of E. faecalis LTAs on osteoclast differentiation might be associated with the secretion of TNF- α and IL-6.
E. faecalis LTAs significantly increased the phosphorylation of p38 and ERK1/2 after 24 h of treatment. MAPK signaling pathways are involved in the regulation of the production of inflammatory cytokines.35,36 In contrast, p38 and ERK1/2 MAPKs were not phosphorylated even after 6 d of treatment with RANKL only and RANKL pretreatment prior to exposure to E. faecalis LTAs. The activation of p38 and ERK1/2 MAPKs greatly decreased compared with the untreated WT BMMs. This might be related to the maturation and activation of osteoclasts. It has been previously reported that phosphorylation of p38 disappears during the differentiation of osteoclast precursors to mature osteoclasts.37 In this study, the phosphorylation of ERK1/2 was gradually reduced in osteoclast precursors during their differentiation to osteoclasts. Therefore, mature osteoclasts lost the capacity for phosphorylation of p38 and ERK1/2.
In conclusion, the present study shows that E. faecalis LTA may be a strong stimulator of inflammatory response, but a weak inducer of osteoclast differentiation, presumably due to the production of TNF-α and IL-6. E. faecalis LTA significantly inhibited RANKL-induced osteoclastogenesis via RBP-J.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by RFCID/HHSRF HMRF Grant No.12110772, a grant from the National Nature Science Foundation of China (NSFC 81271135) and a grant from the Shenzhen Innovation of Science and Technology Commission, Shenzhen key laboratory of otolaryngology disease (ZDSYS201506050935272).
References
- 1.Neuhaus FC, Baddiley J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 2003; 67: 686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morath S, von Aulock S, Hartung T. Structure/function relationships of lipoteichoic acids. J Endotoxin Res 2005; 11: 348–356. [DOI] [PubMed] [Google Scholar]
- 3.Kayaoglu G, Orstavik D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit Rev Oral Biol Med 2004; 15: 308–320. [DOI] [PubMed] [Google Scholar]
- 4.Baik JE, Ryu YH, Han JY, et al. Lipoteichoic acid partially contributes to the inflammatory responses to Enterococcus faecalis. J Endod 2008; 34: 975–982. [DOI] [PubMed] [Google Scholar]
- 5.Fabretti F, Theilacker C, Baldassarri L, et al. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect Immun 2006; 74: 4164–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang H, Wu XH, Fu SL, et al. Co-culture with endothelial progenitor cells promotes survival, migration, and differentiation of osteoclast precursors. Biochem Biophys Res Commun 2013; 430: 729–734. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol 2011; 6: 121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337–342. [DOI] [PubMed] [Google Scholar]
- 9.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone 2007; 40: 251–264. [DOI] [PubMed] [Google Scholar]
- 10.Franzoso G, Carlson L, Xing L, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev 1997; 11: 3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grigoriadis AE, Wang ZQ, Cecchini MG, et al. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994; 266: 443–448. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Lee SH, Ha Kim J, et al. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol 2008; 22: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002; 3: 889–901. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Kogawa M, Wada S, et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem 2004; 279: 45969–45979. [DOI] [PubMed] [Google Scholar]
- 15.Kamio N, Kawato T, Tanabe N, et al. Vaspin attenuates RANKL-induced osteoclast formation in RAW264.7 cells. Connect Tissue Res 2013; 54: 147–152. [DOI] [PubMed] [Google Scholar]
- 16.Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009; 137: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Zhu J, Smith S, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol 2012; 13: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Grimes SN, Li S, et al. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med 2012; 209: 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Liu YL, Hu YY, et al. Disruption of the transcription factor RBP-J results in osteopenia attributable to attenuated osteoclast differentiation. Mol Biol Rep 2013; 40: 2097–2105. [DOI] [PubMed] [Google Scholar]
- 20.Bai S, Kopan R, Zou W, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 2008; 283: 6509–6518. [DOI] [PubMed] [Google Scholar]
- 21.Zhao B, Takami M, Yamada A, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 2009; 15: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Wang Q, Zhang C, et al. Prevalence, phenotype, and genotype of Enterococcus faecalis isolated from saliva and root canals in patients with persistent apical periodontitis. J Endod 2010; 36: 1950–1955. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Liu K, Seneviratne CJ, et al. Lipoteichoic acid from an clinical strain promotes TNF-alpha expression through the NF-kappaB and p38 MAPK signaling pathways in differentiated THP-1 macrophages. Biomed Rep 2015; 3: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park OJ, Han JY, Baik JE, et al. Lipoteichoic acid of Enterococcus faecalis induces the expression of chemokines via TLR2 and PAFR signaling pathways. J Leukoc Biol 2013; 94: 1275–1284. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg JW, Fischer W, Joiner KA. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect Immun 1996; 64: 3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theilacker C, Kropec A, Hammer F, et al. Protection against Staphylococcus aureus by Ab to the polyglycerolphosphate backbone of heterologous lipoteichoic acid. J Infect Dis 2012; 205: 1076–1085. [DOI] [PubMed] [Google Scholar]
- 27.Fischer W. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography. J Microbiol Meth 1996; 25: 129–144. [Google Scholar]
- 28.Villeger R, Saad N, Grenier K, et al. Characterization of lipoteichoic acid structures from three probiotic Bacillus strains: Involvement of D-alanine in their biological activity. Antonie Van Leeuwenhoek 2014; 106: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baik JE, Jang KS, Kang SS, et al. Calcium hydroxide inactivates lipoteichoic acid from Enterococcus faecalis through deacylation of the lipid moiety. J Endod 2011; 37: 191–196. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Ryu YH, Yun CH, et al. Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J Leukoc Biol 2009; 86: 823–831. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Movila A, Kataoka S, et al. Proinflammatory M1 macrophages inhibit RANKL-induced osteoclastogenesis. Infect Immun 2016; 84: 2802–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair SP, Meghji S, Wilson M, et al. Bacterially induced bone destruction: Mechanisms and misconceptions. Infect Immun 1996; 64: 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Yang J, Park OJ, et al. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J Bone Miner Res 2013; 28: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 35.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 2005; 1754: 253–262. [DOI] [PubMed] [Google Scholar]
- 36.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008; 47: 409–414. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Udagawa N, Itoh K, et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 2002; 143: 3105–3113. [DOI] [PubMed] [Google Scholar]