Abstract
Levels of bacterial LPS, pro-inflammatory cytokines and IL-10 are related to the severity of meningococcal septicaemia. Patients infected with a Neisseria meninigitidis lpxL1 mutant (Nm-mutant) with penta-acylated lipid A present with a milder meningococcal disease than those infected with hexa-acylated Nm wild type (Nm-wt). The aim was to compare the pro-inflammatory responses after ex vivo incubation with the heat-inactivated Nm-wt or the Nm-mutant in citrated whole blood, and the modulating effects of IL-10. Concomitantly, we measured intracellular IL-6, IL-8 and TNF-α to elucidate which cell types were responsible for the pro-inflammatory responses. Incubation with Nm-wt (106/ml;107/ml;108/ml) resulted in a dose-dependent increase of the MyD88-dependent pro-inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α), which were mainly derived from monocytes. In comparison, only 108/ml of the Nm-mutant significantly increased the concentration of these cytokines. The MyD88-independent cytokines (IP-10, RANTES) were evidently increased after incubation with the Nm-wt but were unaffected by the Nm-mutant. Co-incubation with IL-10 significantly reduced the concentrations of the MyD88-dependent cytokines induced by both the Nm-wt and the Nm-mutant, whereas the MyD88-independent cytokines were almost unaffected. In summary, the Nm-mutant is a weaker inducer of the MyD88-dependent/independent cytokines than the Nm-wt in whole blood, and IL-10 attenuates the Nm-stimulated increase in MyD88-dependent pro-inflammatory cytokines.
Keywords: IL-10, Neisseria meningitidis, lpxL1, whole blood, MyD88, pro-inflammatory cytokines
Introduction
Neisseria meningitidis (N. meningitidis) is a Gram-negative diplococcus and the causative agent of worldwide meningococcal meningitis and sepsis.1 The bacteria are carried asymptomatically in the nasopharynx in approximately 10% of the world's population, varying from 4–5% in infants to 20–24% in young adults to 7–8% in 50-yr-old adults.2,3 However, in rare occasions, the bacteria pass the mucosal barrier and enter the bloodstream. A high bacterial load in the circulation is associated with an increased risk of developing shock, organ dysfunction, thrombus formation, bleeding and, in many cases, death.4,5
The lipid A part of the lipopolysaccharides (LPS) in the outer membrane of the N. meningitidis wild type (wt) is a strong inducer of inflammation, and high LPS levels in plasma from patients with meningococcal sepsis are associated with an increased severity of the disease and decreased survival.4–6 The severity of systemic meningococcal sepsis also correlates to the plasma concentrations of IL-10.7 IL-10 is a major anti-inflammatory regulator of myeloid cells that are activated by bacteria or LPS,8 and has been identified as the single most important plasma component suppressing the ability of monocytes to produce pro-inflammatory cytokines and to induce pro-coagulant activity.9 Therefore, IL-10 is a physiologic inhibitor that may counterbalance many LPS responses.10
The cellular inflammatory response is initiated by the soluble LPS binding protein that transfers an LPS molecule to the membrane-bound CD14 receptor on monocytes and macrophages.11 CD14 presents the LPS molecule to the TLR4/myeloid differentiation protein-2 (MD-2) complex,12 which, in turn, activates the MyD88-dependent and the MyD88-independent pathways. The activation of the MyD88-dependent pathway results in rapid activation of NF-κB and MAPKs, and the subsequent release of pro-inflammatory cytokines like IL-1β, IL-6, IL-8 and TNF-α.13 LPS activate the MyD88-dependent pathway, but other bacterial components are also able to activate this pathway through TLR2.14 However, only LPS, and not any other parts of the outer membrane of N. meningitidis, are able to activate the MyD88-independent pathway.13 Here, IFN regulatory factor 3 is activated,15 which induces the production of IFN-β and also a late-phase activation of NF-κB and MAPKs.12 The IFN-β binds to the type 1 IFN-α/β receptor, which activates STAT1 and leads to type 1 IFN-α/β, IFN-γ-induced protein 10 (IP-10; CXCL10) and regulated on activation, normal T cell expressed and secreted (RANTES; CCL5).15
The lipid A part of LPS is essential for recognition by the TLR4 receptor complex, and the acylation pattern of lipid A determines, to a large part, the biological activity of LPS. In N. meningitidis wt, the lipid A is hexa-acylated. In the laboratory, a N. meningitidis mutant has been made by insertional inactivation of the lpxL1 gene, which is required for addition of a secondary acyl chain to the lipid A molecule.16 N. meningitidis with a mutated lpxL1 gene has five, instead of six, acyl chains in the lipid A moiety, and this mutant activates human TLR4 less efficiently than the wt.13 The N. meningitidis lpxL1 mutant is also found naturally occurring in patients, and compared with patients infected with the wt meningococcus, these patients appear to have a milder meningococcal disease with less systemic inflammation and coagulopathy.13,17,18 Fransen et al. have previously shown that the spontaneously occurring lpxL1 mutant induced low levels of the pro-inflammatory cytokines IL-6, TNF-α and IL-1β in peripheral blood mononuclear cells (PBMCs).13 The effect of the lpxL1 mutant on the pro-inflammatory response has also been studied using human cell lines19,20 or cell lines originated from rodents.21,22 However, to our knowledge, the effect of the N. meningitidis lpxL1 mutant on the pro-inflammatory response in human whole blood has not yet been studied. Ex vivo incubation in whole blood is an experimental model closer to the in vivo environment than in vitro incubation of isolated cells,23 and whole blood contains soluble factors that may influence cytokine release.24
In this study, the aim was to compare the pro-inflammatory responses, and the modulating effects of recombinant human IL-10 (rhIL-10), in citrated whole blood after ex vivo incubation with heat-inactivated N. meningitidis wt or heat-inactivated N. meninigitidis lpxL1 mutant. In addition to plasma cytokine levels, intracellular cytokine levels were measured to elucidate which cell types were responsible for the inflammatory responses.
Material and methods
Ethics statement
The project was approved by the Regional Committee for Medical Research Ethics, South-Eastern Norway Regional Health Authority (2011/1413).
Materials
Stock solution (10 µg/ml) of rhIL-10 (R&D Systems, Abingdon, UK) was reconstituted in Tris-NaCl buffer (TBS; Sigma, St Louis, MO, USA). Brefeldin A (10 mg/ml) was purchased from Sigma Aldrich (Oslo, Norway). The monoclonal antibodies (abs) anti-CD45 fluorescein isothiocyanate (FITC) (clone J33), anti-CD3-phycoerythrin-cyanine 7 (PE-Cy7) (clone UCHT1), anti-CD14-allophycocyanin (APC) (clone RMO52), anti-TNF-α phycoerythrin (PE) (clone IPM2) and the PerFix-nc kit used for intracellular staining were purchased from Beckman Coulter (Indianapolis, IN, USA). Anti-IL-6 PE abs (clone MQ2-13A5) and anti-IL-8 PE abs (clone B-K8) were purchased from Nordic Biosite (Oslo, Norway).
The N. meningitidis wt and the N. meningitidis lpxL1 mutant
N. meningitidis strain H44/76 is a serogroup B (B:15:P1.7,16 with immunotype L,3,7,9) reference strain initially isolated from a Norwegian patient with lethal meningococcal septicaemia.25 The N. meningitidis wt has six acyl chains in the lipid A portion of the LPS molecule (hexa-acylated), whereas the lpxL1 mutant has five acyl chains (penta-acylated). The N. meningitidis lpxL1 mutant was obtained by insertional inactivation of the lpxL1 gene in the N. meningitidis strain (H44/76), and kindly provided by Arie van der Ende (Academic Medical Centre, Amsterdam, the Netherlands) and Peter van der Ley (National Institute of Public Health and Environment, Bilthoven, the Netherlands) to the National Institute of Public Health, Oslo, Norway, for research purposes. It has previously been reported that the N. meningitidis lpxL1 mutant makes normal amounts of LPS and, compared with the wt, it is only the lipid A part that is altered.16,26 It has also been shown that the laboratory-engineered lpxL1 mutant has an identical ability to induce pro-inflammatory cytokines compared with similar strains isolated from patients.13 The meningococci were grown and heat-inactivated (56℃, 30 min) as previously described.13,27 To quantify the number of N. meningitidis (either wt or lpxL1), bacterial DNA was isolated with the MagNA Pure LC DNA isolation Kit I (Roche Diagnostics, Mannheim, Germany) and the DNA copy number, equivalent to the number of bacteria, was estimated by spectroscopic measurements at 260 nm (NanoDrop ND-1000; NanoDrop Technologies, Wilmington, DE, USA) combined with a genome calculator (http://cels.uri.edu/gsc/cndna.html) using a N. meningitidis genome size of 2,272,351 base pairs.28
Incubation of the N. meningitidis wt and the N. meningitidis lpxL1 mutant in citrated whole blood
After signed consent was obtained, whole blood from healthy volunteers was drawn into 3.5 ml citrate tubes (Vacuette 3.5 ml; Greiner Bio One Gmbh, Kremsmünster, Austria), and the first tube was discarded. Whole blood was immediately incubated with heat-inactivated N. meningitidis wt, N. meningitidis lpxL1 mutant (both 107/ml) or vehicle (TBS) [37℃, 12 rpm Hulamixer (Invitrogen Dynal AS, Oslo Norway)].
To determine the optimal time point to measure pro-inflammatory cytokines, whole blood from four donors was centrifuged [2500 g, 15 min at room temperature (RT)] after 0, 4, 6 and 12 h, and plasma was collected for measurements of cytokines.
After these initial experiments, an incubation period of 4 h was chosen, and whole blood from six donors was incubated with increasing concentrations of N. meningitidis wt or N. meningitidis lpxL1 mutant (106/ml, 107/ml or 108/ml). In some cases, rhIL-10 (25 ng/ml) or vehicle (TBS) was added to whole blood 15 min prior to the addition of the bacteria. Similar IL-10 concentrations have previously been used in in vitro studies,29 and have also been observed in patients with severe fulminant meningococcal sepsis.30
Quantification of cytokines in plasma
Citrated whole blood was prepared by centrifugation at 2500 g for 15 min at RT. Then, 1 ml plasma was collected in a 1.5-ml Eppendorf tube and centrifuged at 10,000 g for 10 min at 4℃. The top supernatant (800 µl) was collected prior to freezing, and stored at –80℃. Measurements of cytokines were performed using a microsphere-based multiplexing bioassay system with Xmap technology (Austin, TX, USA) with a Luminex IS 100 instrument (Bio-Rad, Hercules, CA, USA), powered with the Bio-Plex manager Software version 6.0 (build 617). The anti-inflammatory cytokine IL-10, the MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α,13 and the MyD88-independent cytokines IP-10 and RANTES,15 were measured (Bio-Rad, Oslo, Norway) in duplicates.
The intracellular cytokine expression of different cell types
To determine which cell type expresses IL-6, IL-8 and TNF-α, whole blood from eight healthy volunteers was pre-incubated with Brefeldin A (10 µg/ml) for 15 min. Brefeldin A inhibits the release of cytokines by blocking the transport of proteins from the endoplasmic reticulum to the Golgi apparatus. Whole-blood samples were stimulated with either N. meningitidis wt (108/ml), N. meningitidis lpxL1 mutant (108/ml) or vehicle for 4 h. In order to perform intra- and extracellular staining of the white blood cells (WBCs), the low fixation procedure of the PerFix-nc kit was performed to fix and permeabilize cells, as well as to lyse the red blood cells. The cells were stained with the following final concentrations: 8.3 ng/ml anti-CD45 abs, 40 ng/ml anti-CD3 abs and 5.0 ng/ml anti-CD14 abs. The intracellular staining was performed with either 8.3 ng/ml of anti-IL-6 abs, 4.2 ng/ml of anti-IL-8 abs or 5 ng/ml of anti-TNF-α abs. All abs were titrated prior to analysis. Cells were stained for 30 min sheltered from light, and then washed with 3 ml of the final 1 × reagent (Perfix-nc kit buffer 3). After washing, the cells were pelleted by centrifugation at 500 g (5 min), the supernatant removed, and the cells re-suspended in 300 μl final 1 × reagent. Samples were analysed on a Accuri C6 (Becton Dickinson, San Jose, CA, USA). The intracellular expression of IL-6, IL-8 and TNF-α was presented as PE median fluorescence intensity (MFI). For the three intracellular cytokines, the delta MFI (ΔMFI) was calculated as: MFI of cells (incubated with N. meningitidis wt or N. meningitidis lpxL1 mutant) – MFI of cells (incubated with vehicle only).
Statistical analysis
Data are presented as median (range) throughout. The non-parametric Friedman test without correction for multiple comparisons (GraphPad Prism version 7.02; GraphPad, La Jolla, CA, USA) was used to statistically test the differences in cytokine expression and release between vehicle and N. meningitidis wt and between vehicle and N. meningitidis lpxL1 mutant. Comparisons of medians were also performed between the N. meningitidis wt and the N. meningitidis lpxL1 mutant and between the presence and absence of rhIL-10. For these comparisons we used the non-parametric Wilcoxon rank sum test. To compare the levels of intracellular cytokines between different types of cells (monocytes, granulocytes and lymphocytes), we calculated the ΔMFI (both after incubation with the N. meningitidis wt and the N. meningitidis lpxL1 mutant) and performed a non-parametric Kruskal–Wallis test. P < 0.05 was considered statistically significant.
Results
The time course of cytokine plasma levels after incubation with the N. meningitidis wt or the N. meningitidis lpxL1 mutant
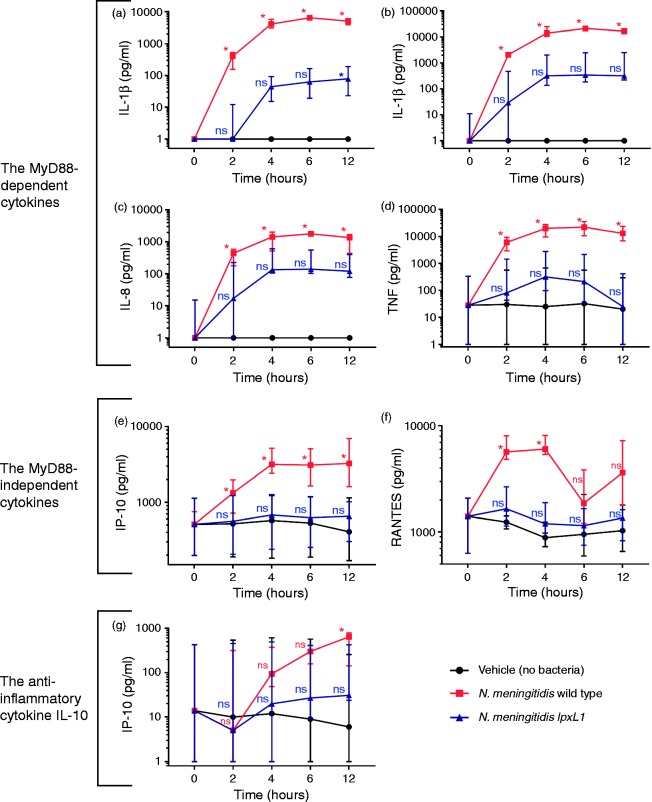
After incubation with the N. meningitidis wt, compared with vehicle, the plasma levels of the MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α were significantly increased at 2 h, peaked at 6 h and remained at a high level also at 12 h (Figure 1a–d, red and black lines; P < 0.05). After incubation with the N. meningitidis lpxL1 mutant, only IL-1β was significantly increased compared with vehicle, and only at 12 h (Figure 1a–d, blue and black lines). All cytokines peaked/almost peaked at 4–6 h (Figure 1a–d),
Figure 1.
Time course of cytokine plasma levels. Whole blood from four donors was incubated (0, 2, 4, 6 and 12 h) with N. meningitidis wt (107/ml) (red line), N. meningitidis lpxL1mutant (107/ml) (blue line) or vehicle (no bacteria) (black line), plasma collected and measurements of cytokines performed. The concentrations [median (range)] (pg/ml) of the (a–d) MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α (e, f), the MyD88-independent cytokines IP-10 and RANTES, and (g) the anti-inflammatory cytokine IL-10 are presented. The y-axis is presented in a log scale. *Significant differences in cytokine levels between the N. meningitidis wt and vehicle or between the N. meningitidis lpxL1 mutant and vehicle (P < 0.05). ns: non-significant differences.
After incubation with the N. meningitidis wt, compared with vehicle, the MyD88-independent cytokines IP-10 and RANTES significantly increased at 2 h and peaked at 4 h (Figure 1e and f, red and black lines; P < 0.05). IP-10 remained at the same level at 6 and 12 h, whereas the level of RANTES decreased and was similar to vehicle only, at these time points. After incubation with the N. meningitidis lpxL1 mutant, the plasma levels of IP-10 and RANTES remained low between 0 to 12 h, similar to the levels observed for vehicle only (Figure 1e and f, blue and black lines).
After incubation with the N. meningitidis wt, IL-10 was significantly increased at 12 h compared with vehicle only, but not at the other time-points (Figure 1g, red and black lines). Compared with incubation with vehicle only, IL-10 was not increased after incubation with the N. meningitidis lpxL1 mutant at any time-point (Figure 1g, blue and black lines).
The cytokine plasma levels after incubation with the N. meningitidis wt or the N. meningitidis lpxL1 mutant in increasing concentrations
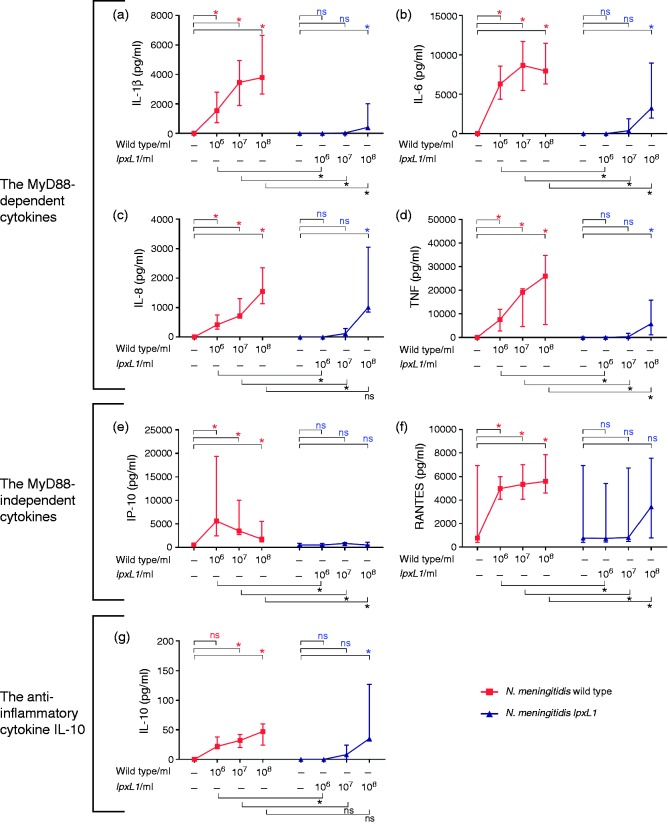
After incubation with 106/ml, 107/ml and 108/ml of the N. meningitidis wt, the MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α were significantly increased compared with vehicle only (Figure 2a–d; P < 0.05).
Figure 2.
Plasma cytokine levels released upon increasing concentrations of bacteria. Whole blood from six donors was incubated for 4 h with increasing concentrations of N. meningitidis wt (wild type), N. meningitidis lpxL1 mutant (lpxL1) or vehicle (no bacteria), plasma collected and measurements of cytokines performed. The concentrations [median (range)] (pg/ml) of (a–d) the MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α (e, f), the MyD88-independent cytokines IP-10 and RANTES, and (g) the anti-inflammatory IL-10 are presented. *(Top of charts) indicates significant differences in cytokine levels between the wt and vehicle or between the mutant lpxL1 and vehicle (P < 0.05). *(Below charts) indicates significant differences in cytokine levels between the wt and the mutant lpxL1, P < 0.05. ns: non-significant differences.
With the N. meningitidis lpxL1 mutant, only the 108/ml concentration induced significant differences in the plasma levels of the MyD88-dependent cytokines, compared with vehicle only, and IL-8 was the only cytokine that reached the same level as after incubation with the N. meningitidis wt, with concentrations of [1015 (853–3054)] and [1545 (1128–2350)] pg/ml, respectively (Figure 2a–d; P < 0.05).
After incubation with the N. meningitidis wt (106/ml, 107/ml and 108/ml), the MyD88-independent cytokines IP-10 and RANTES, were significantly increased compared with vehicle only (Figure 2e and f; P < 0.05).
After incubation with the N. meningitidis lpxL1 mutant (106/ml, 107/ml and 108/ml), the levels of IP-10 and RANTES were not significantly increased compared with vehicle only and IP-10 and RANTES never reached the same levels as after incubation with the N. meningitidis wt (Figure 2e and f; P > 0.05).
After incubation with the N. meningitidis wt (107/ml and 108/ml), IL-10 was significantly increased compared with vehicle only (Figure 2g; P < 0.05).
After incubation with the N. meningitidis lpxL1 mutant, IL-10 was only significantly increased at 108/ml compared with vehicle only (Figure 2g; P < 0.05).
After incubation with the N. meningitidis lpxL1 mutant at 107/ml and 108/ml, IL-10 reached the same level as after incubation with N. meningitidis wt.
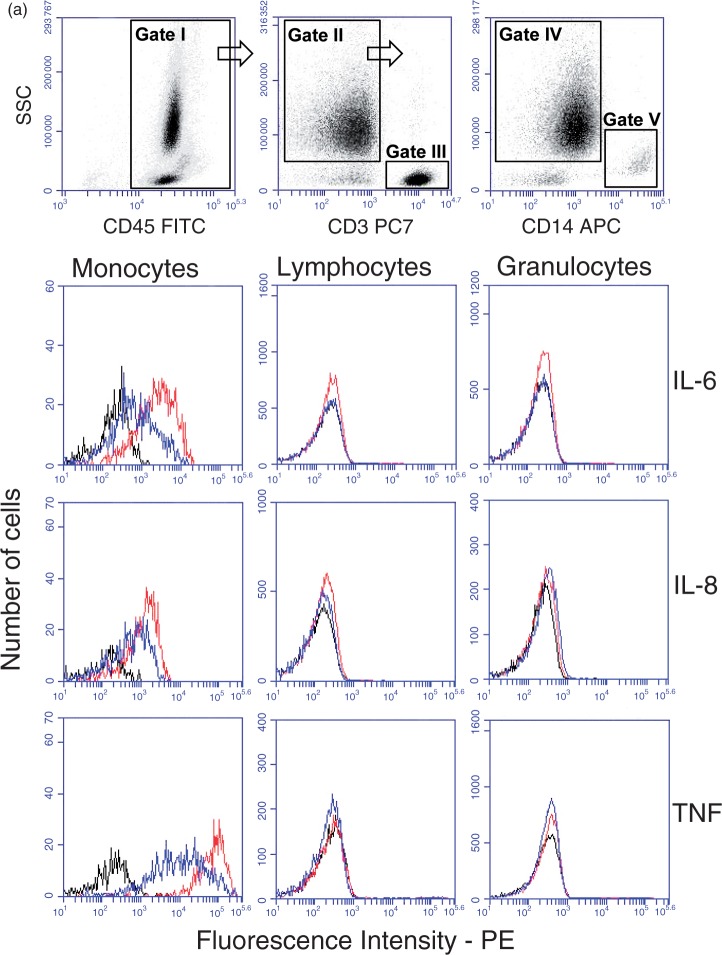
The intracellular cytokine expression of different cell types after whole blood incubation with the N. meningitidis wt or the N. meningitidis lpxL1 mutant
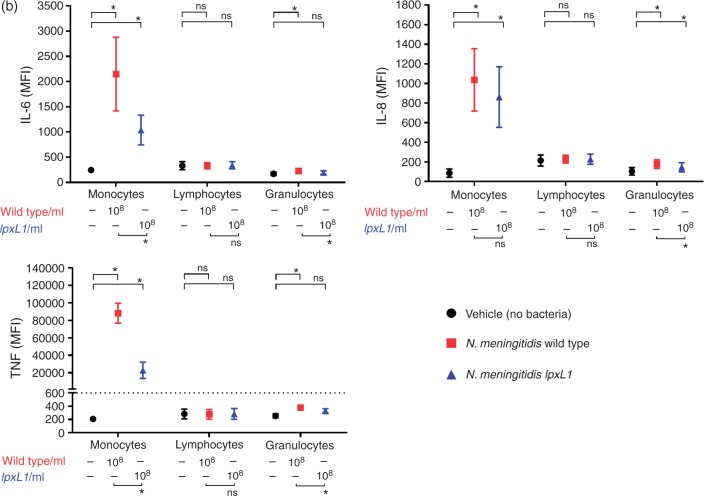
To determine which cell type expresses IL-6, IL-8 and TNF-α, whole blood was pre-incubated with Brefeldin A prior to the addition of N. meningitidis wt or lpxL1 mutant (both 108/ml). Subsequently, whole blood was subjected to flow cytometry analysis (Figure 3a). The WBCs (gate I) were separated from erythrocytes in a CD45/side scatter (SSC) dot plot and the WBCs (gate I) were further displayed in a CD3/SSC dot plot with the CD3+ lymphocytes in gate III. The CD3–cells in the CD3/SSC dot plot (gate II) were then further analysed in a CD14/SSC dot plot. Monocytes were selected as CD14+ (gate V), and granulocytes as CD14– cells with high SSC (gate IV). Representative overlay histograms of IL-6, IL-8 and TNF-α for monocytes, lymphocytes and granulocytes are shown (Figure 3a). The intracellular expression of IL-6, IL-8 and TNF-α was measured as PE MFI. CD14+ monocytes showed significantly increased intracellular concentrations of IL-6, IL-8 and TNF-α after incubation with either the N. meningitidis wt or the N. meningitidis lpxL1 mutant compared with vehicle only (Figure 3b; P < 0.05). Granulocytes showed significantly increased intracellular concentrations of IL-6, IL-8 and TNF-α after incubation with the N. meningitidis wt, and increased IL-8 after incubation with the N. meningitidis lpxL1 mutant, compared with vehicle only (Figure 3b; P < 0.05). In contrast, CD3+ lymphocytes did not show any significant increase in intracellular IL-6, IL-8 or TNF-α, neither after incubation with the N. meningitidis wt nor with the N. meningitidis lpxL1 mutant, compared with vehicle only. The remaining cells, i.e. all cells that did not appear within the gates of the three selected populations, were not positive for IL-6, IL-8 or TNF-α (data not shown).
Figure 3.
Intracellular cytokine levels in different cell types. Whole blood from eight donors was incubated for 4 h with N. meningitidis wt (108/ml), N. meningitidis lpxL1mutant (lpxL1) (108/ml) or vehicle (no bacteria), and flow cytometry measurements of intracellular cytokines performed. In (a) the gating strategy used to identify the different types of WBCs is shown. WBCs are shown in gate I, CD3+ lymphocytes in gate III, granulocytes in gate IV and CD14+ monocytes in gate V. The cytokine fluorescence intensity (FI) levels of IL-6, IL-8 and TNF-α for monocytes, lymphocytes and granulocytes are displayed in overlay histograms of one representative donor, with lines in black (vehicle), red (108/ml N. meningitidis wt) and blue (108/ml N. meningitidis lpxL1 mutant). In (b) intracellular levels of IL-6, IL-8 and TNF-α in monocytes, lymphocytes and granulocytes are presented as median (range) of MFI. *(Top of charts) indicates significant differences in intracellular cytokine MFI between the wt and vehicle or between the lpxL1 mutant and vehicle (P < 0.05). *(Below charts) indicates significant differences between the wt and the lpxL1 mutant (P < 0.05). ns: non-significant differences.
The intracellular levels of IL-6 and TNF-α were also significantly higher in monocytes incubated with N. meningitidis wt compared with the N. meningitidis lpxL1 mutant, but, in contrast, the cytokine IL-8 had the same level with both bacteria. ΔMFI was calculated and the ΔMFI of IL-6, IL-8 and TNF-α were all significantly higher in monocytes compared with granulocytes and compared with lymphocytes in whole blood incubated with either N. meningitidis wt or the N. meningitidis lpxL1 mutant (data not shown in figure) (Kruskal–Wallis test, P < 0.05).
The cytokine plasma levels after incubation with the N. meningitidis wt or the N. meningitidis lpxL1 mutant in the absence and presence of rhIL-10
The inhibitory effect of rhIL-10 on cytokine production was measured in plasma obtained from whole blood after 4 h incubation with increasing concentrations of N. meningitidis wt, N. meningitidis lpxL1 mutant or vehicle, and only data from 108/ml are presented in Table 1.
Table 1.
The effect of rhIL-10 on the MyD88-dependent and -independent cytokines.
| IL-1β | IL-6 | IL-8 | TNF-α | IP-10 | RANTES | |
|---|---|---|---|---|---|---|
| Vehicle (no bacteria) | 0 | 0 | 0 | 0 | 515 | 773 |
|
|
(0–0) |
(0–0) |
(0–0) |
(0–179) |
(274–855) |
(405–6943) |
| N. meningitidis wt | 3790 (2666–6654) | 7945 (6317–11,481) | 1545 (1128–2350) | 25,929 (5377–34,772) | 1769 (1152–5555) | 5601 (4615–7871) |
| N. meningitidis wt + rhIL-10 (25 ng/ml) | 61 (36–172) | 1011 (862–2029) | 256 (173–355) | 1060 (332–2773) | 1031 (617–2040) | 5062 (1388–6900) |
| Fold reduction |
62.1*
|
7.9*
|
6.0*
|
24.5*
|
1.7*
|
1.1
ns
|
| N. meningitidis lpxL1 mutant | 407 (245–2015) | 3214 (1947–8956) | 1015 (853–3053) | 5766 (1155–15,816) | 583 (482–1169) | 3443 (776–7576) |
| N. meningitidis lpxL1 mutant + rhIL-10 (25 ng/ml) | 6 (0–30) | 89 (37–513) | 119 (63–246) | 68 (23–567) | 663 (388–1425) | 1171 (1057–6718) |
| Fold reduction | 67.8* | 36.1* | 8.5* | 84.8* | 0.9 ns | 2.9 ns |
Whole blood from six donors was incubated for 4 h with N. meningitidis wt (108/ml), N. meningitidis lpxL1mutant (108/ml) or vehicle, in the absence or presence of recombinant IL-10 (rhIL-10), plasma collected, and measurements of cytokines performed. The concentrations [median (range)] (pg/ml) of the cytokines, and fold reduction between ± rhIL-10 are shown. *Indicates significant differences in cytokine levels with and without rhIL-10 in whole blood incubated with either the N. meningitidis wt, or the N. meningitidis lpxL1 mutant (P < 0.05). ns: non-significant differences.
After incubation with the N. meningitidis wt, the plasma levels of the MyD88-dependent cytokines IL-1β, Il-6, IL-8 and TNF-α were significantly reduced by the presence of rhIL-10 with the following fold reductions: 62.1 (IL-1β), 7.9 (IL-6), 6.0 (IL-8) and 24.5 (TNF-α) (Table 1; P < 0.05).
Also, after incubation with the N. meningitidis lpxL1 mutant, the release of the MyD88-dependent cytokines in whole blood was significantly reduced in the presence of rhIL-10, with fold reductions of 67.8 (IL-1β), 36.1 (IL-6), 8.5 (IL-8) and 84.8 (TNF-α) (Table 1; P < 0.05).
After incubation with the N. meningitidis wt, the levels of the MyD88-independent cytokines IP-10 and RANTES only showed a modest fold reduction of 1.7 and 1.1, respectively (only IP-10 was significantly decreased), in the presence of rh-IL10.
After incubation with the N. meningitidis lpxL1 mutant in the presence of rhIL-10, IP-10 and RANTES were not significantly reduced with fold reductions of only 0.9 (IP-10) and 2.9 (RANTES).
Discussion
Ex vivo incubation of N. meningitidis wt in citrated whole blood resulted in a dose-dependent increase of the MyD88-dependent cytokines IL-1β, IL-6, IL-8 and TNF-α. In contrast, only the highest concentration of the N. meningitidis lpxL1 mutant (108/ml) was able to significantly increase the plasma levels of the MyD88-dependent cytokines. At this concentration, the lpxL1 mutant induced the same IL-8 level as found for the wt, but for IL-1β, IL-6 and TNF-α the levels were significantly lower. These data are in accordance with previous publications where the N. meningitidis lpxL1 mutant bacterium has been shown to have a reduced capacity to induce TLR-4 mediated NF-κB activation and cytokine production in human PBMCs and cell lines as compared with the N. meningitidis wt.13,21 Fransen et al. concluded that the discrepancy in the concentrations of MyD88-dependent cytokines induced by the wt and the lpxL1 mutant was only present at a low concentration of bacteria.13 However, we found that in whole blood, the lpxL1 mutant was a weaker inducer of all cytokines even at high concentrations (except for IL-8). Whole blood contains soluble factors that may influence cytokine release,24 and we do not know if the observed discrepancy in the levels of MyD88-dependent cytokines between the wt and the lpxL1 mutant would have disappeared at higher bacteria concentrations than 108/ml. However, concentrations of 106/ml–108/ml for the N. meningitidis wt or the lpxL1 mutant are clinically relevant concentrations found in patients with meningococcal sepsis.27,31
We observed that both the extracellular and intracellular level of IL-8 was similar after addition of 108/ml N. meningitidis wt and 108/ml N. meningitidis lpxL1 mutant (but not for 107/ml bacteria). It is interesting that the monocytes were able to express IL-8, an important signalling molecule for recruitment of other immune cells, in a less LPS-dependent manner. In a lepirudin-anticoagulated human whole-blood model, Hellerud et al. compared the levels of IL-8 after incubation (for 2 h) with a N. meningitidis wt or a lpxA-mutant (LPS deficient),32 and in agreement with our study they also found similar IL-8 levels at high bacterial concentrations (108/ml). The similar levels of IL-8 in plasma after stimulation with either the N. meningitidis wt or the N. meningitidis lpxL1 mutant may suggest that other components than LPS may favour the expression of IL-8, and less for IL-1β, IL-6 and TNF-α. Hellerud et al. suggested that the production of IL-8 is more dependent on complement than IL-6 and TNF-α.32,33 To study complement activation, lepirudin is the preferred anticoagulant in the tube. The use of citrated whole blood in our study is not optimal for activation of complement, but we cannot exclude that also in our study the IL-8 expression was more dependent on the activation of complement than of LPS.
Mollnes et al. have systematically examined to what extent time and temperature influence the degree of in vitro complement activation in plasma anticoagulated with heparin, citrate and EDTA.34 The authors showed that EDTA inhibited C3 and terminal complement activation in plasma more efficiently than citrate and heparin, but increasing the temperature to 37℃ in vitro overcame inhibitory effects present in EDTA plasma but even more in citrated plasma. We incubated citrated whole blood for 4 h at 37℃, which therefore are conditions that probably will allow activation of the complement system to some degree.
To our knowledge, measurements of the MyD88-independent cytokines (IP-10 and RANTES) have not previously been performed in human PBMCs, cell lines or whole blood after incubation with the N. meningitidis lpxL1 mutant. However, after incubation with purified penta-acylated lipid A, extracted from the N. meningitidis lpxL1 mutant, in concentrations ranging from 0.1 pg/ml to 1 ng/ml, Pupo et al. did not find an increase in IP-10 in the culture supernatant obtained from a human monocyte macrophage cell line (MM6).20 In accordance with these results, we found that the N. meningitidis lpxL1 mutant with penta-acylated lipid A did not significantly induce the MyD88-independent cytokines IP-10 and RANTES, not even after incubation with the highest concentration (108/ml).
In the present study we found that the monocytes were the main producers of IL-6, IL-8 and TNF-α in whole blood, and incubation with the N. meningitidis wt gave significantly higher intracellular levels of IL-6 and TNF-α (but not IL-8) than incubation with the N. meningitidis lpxL1 mutant. Our findings that the monocyte is a key orchestrator of LPS responsiveness are in accordance with a study by Sabroe et al.35 The authors examined the expression of TLR2, TLR4 and CD14, and found that the monocytes expressed relatively high levels of cell surface TLR2, TLR4 and CD14, whereas neutrophils expressed all three molecules at low levels.35 A quantitative cell-surface CD14 antigen study by Antal-Szalmast et al. showed that the neutrophils expressed 30 times fewer CD14 copies on the surface compared with the monocytes.36 CD14 plays a crucial part in introducing the LPS molecules to the TLR4/MD-2 complex and it is possible that a different expression of CD14 on the surface of monocytes and neutrophils may explain the different concentrations of intracellular IL-6 and TNF-α, and possibly for IL-8.
In our study, we observed a great donor to donor variation in the levels of all the pro-inflammatory cytokines released in whole blood stimulated with either the N. meningitidis wt or the N. meningitidis lpxL1 mutant. This phenomenon has been described by others,37 and the variation may reflect the individual biological diversity in response to bacterial invasion. Netea et al. have proposed that patients may develop systemic meningococcal septicaemia due to innate moderate production of pro-inflammatory cytokines (low responder) that allows uncontrolled growth of bacteria.38 This is supported by Westendorp et al. who showed that individuals with a low production of TNF-α and a high production of IL-10 were at risk of developing fulminant meningococcal septicaemia because they were more susceptible to the development of a large bacterial burden.39
In our study, the presence of rhIL-10 significantly reduced the concentrations of the MyD88-dependent cytokines (IL-1β, IL-6, IL-8 and TNF-α) when whole blood was either stimulated with the N. meningitidis wt or the lpxL1 mutant. However, the presence of rh-IL10 did almost not affect the cytokine levels of the MyD88-independent pathway (IP-10 and RANTES). Our results in whole blood are in accordance with Chang et al.,40 who demonstrated that IL-10 inhibited the MyD88-dependent signalling mechanisms and cytokine release in dendritic cells. They also showed that IL-10 did not exert any inhibition on the MyD88-independent signalling pathway proteins.40 IL-10 is identified as the single most important plasma component suppressing the ability of monocytes to produce pro-inflammatory cytokines and to induce monocyte-associated procoagulant activity.9 However, it is clear that despite elevated levels of IL-10, sepsis often progress rapidly leading to multiple organ failure and death within 24 h. Evidently, the high anti-inflammatory level in sepsis patients is not sufficient to prevent uncontrolled pro-inflammatory activation. In our study, the median plasma levels of IL-10 after incubation with N. meningitidis wt (107/ml) for 12 h was moderate, with a median (range) concentration of 0.642 (0.144–0.784) ng/ml. This is in strong contrast to Lehmann et al.,30 who observed 15 patients with meningococcal shock where the mean (range) level of IL-10 was much higher; 21.2 (0.25–64.5) ng/ml. The modest levels of IL-10 reached in our whole-blood model, may suggest that circulating IL-10 in meningococcal patients may derive from sources other than WBCs, such as lung, liver and spleen tissues.41,42
Taken together, in this study we evaluated the pro-inflammatory response of the N. meningitidis wt and the lpxL1 mutant, with and without the presence of rhIL-10, in an ex vivo citrated whole blood model, i.e. an experimental model closer to the in vivo environment than the in vitro incubation of isolated cells.23,24
We demonstrated that after incubation of whole blood with low doses of the N. meningitidis lpxL1 mutant, the induction of both the MyD88-dependent and -independent pro-inflammatory cytokines were reduced or absent. Higher concentrations of the N. meningitidis lpxL1 mutant were required to obtain significant levels of the MyD88-dependent cytokines; IL-1β, IL-6, IL-8 and TNF-α, but the MyD88-independent cytokines IP-10 and RANTES were not significantly increased.
We also showed that co-incubation of rhIL-10 with N. meningitidis with hexa- or penta-acylated LPS lipid A reduced the MyD88-dependent cytokines, but the MyD88-independent cytokine release was rather unaffected. In conclusion, the ex vivo whole-blood cytokine release assay contributes to a better understanding of the pro-inflammatory responses exerted by hexa- and penta-acylated LPS components of the N. meningitidis bacteria, and also the major regulatory role of IL-10.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007; 369: 2196–2210. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001; 344: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 3.Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10: 853–861. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis 1989; 159: 195–204. [DOI] [PubMed] [Google Scholar]
- 5.Darton T, Guiver M, Naylor S, et al. Severity of meningococcal disease associated with genomic bacterial load. Clin Infect Dis 2009; 48: 587–594. [DOI] [PubMed] [Google Scholar]
- 6.Steeghs L, Tommassen J, Leusen JH, et al. Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J Endotoxin Res 2004; 10: 113–119. [DOI] [PubMed] [Google Scholar]
- 7.Friedman G, Jankowski S, Marchant A, et al. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care 1997; 12: 183–187. [DOI] [PubMed] [Google Scholar]
- 8.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008; 180: 5771–5777. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P, Osnes L, Ovstebo R, et al. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med 1996; 184: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derkx B, Marchant A, Goldman M, et al. High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis 1995; 171: 229–232. [DOI] [PubMed] [Google Scholar]
- 11.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem 1996; 271: 4100–4105. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 13.Fransen F, Heckenberg SG, Hamstra HJ, et al. Naturally occurring lipid A mutants in neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLOS Pathog 2009; 5: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingalls RR, Lien E, Golenbock DT. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J Endotoxin Res 2000; 6: 411–415. [PubMed] [Google Scholar]
- 15.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 2003; 198: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Ley P, Steeghs L, Hamstra HJ, et al. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun 2001; 69: 5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Plessis M, Wolter N, Crowther-Gibson P, et al. Meningococcal serogroup Y lpxL1 variants from South Africa are associated with clonal complex 23 among young adults. J Infect 2014; 68: 455–461. [DOI] [PubMed] [Google Scholar]
- 18.Rodenburg GD, Fransen F, Bogaert D, et al. Prevalence and clinical course in invasive infections with meningococcal endotoxin variants. PLOS ONE 2012; 7: e49295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zughaier SM, Lindner B, Howe J, et al. Physicochemical characterization and biological activity of lipooligosaccharides and lipid A from Neisseria meningitidis. J Endotoxin Res 2007; 13: 343–357. [DOI] [PubMed] [Google Scholar]
- 20.Pupo E, Hamstra HJ, Meiring H, van der Ley P. Lipopolysaccharide engineering in Neisseria meningitidis: structural analysis of different pentaacyl lipid A mutants and comparison of their modified agonist properties. J Biol Chem 2014; 289: 8668–8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprong T, Ley P, Abdollahi-Roodsaz S, et al. Neisseria meningitidis lipid A mutant LPSs function as LPS antagonists in humans by inhibiting TLR 4-dependent cytokine production. Innate Immun 2011; 17: 517–525. [DOI] [PubMed] [Google Scholar]
- 22.Fransen F, Hamstra HJ, Boog CJ, et al. The structure of Neisseria meningitidis lipid A determines outcome in experimental meningococcal disease. Infect Immun 2010; 78: 3177–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva D, Ponte CG, Hacker MA, Antas PR. A whole blood assay as a simple, broad assessment of cytokines and chemokines to evaluate human immune responses to Mycobacterium tuberculosis antigens. Acta Trop 2013; 127: 75–81. [DOI] [PubMed] [Google Scholar]
- 24.Jagger MP, Huo Z, Riches PG. Inflammatory cytokine (interleukin 6 and tumour necrosis factor alpha) release in a human whole blood system in response to Streptococcus pneumoniae serotype 14 and its capsular polysaccharide. Clin Exp Immunol 2002; 130: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol 1979; 9: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Ley P, van den Dobbelsteen G. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants. Hum Vaccin 2011; 7: 886–890. [DOI] [PubMed] [Google Scholar]
- 27.Ovstebo R, Brandtzaeg P, Brusletto B, et al. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J Clin Microbiol 2004; 42: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tettelin H, Saunders NJ, Heidelberg J, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 2000; 287: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 29.Gopinathan U, Brusletto BS, Olstad OK, et al. IL-10 immunodepletion from meningococcal sepsis plasma induces extensive changes in gene expression and cytokine release in stimulated human monocytes. Innate Immun 2015; 21: 429–449. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann AK, Halstensen A, Sornes S, et al. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun 1995; 63: 2109–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackett SJ, Guiver M, Marsh J, et al. Meningococcal bacterial DNA load at presentation correlates with disease severity. Arch Dis Child 2002; 86: 44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellerud BC, Stenvik J, Espevik T, et al. Stages of meningococcal sepsis simulated in vitro, with emphasis on complement and Toll-like receptor activation. Infect Immun 2008; 76: 4183–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lappegard KT, Christiansen D, Pharo A, et al. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc Natl Acad Sci U S A 2009; 106: 15861–15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol 1988; 73: 484–488. [PMC free article] [PubMed] [Google Scholar]
- 35.Sabroe I, Jones EC, Usher LR, et al. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 2002; 168: 4701–4710. [DOI] [PubMed] [Google Scholar]
- 36.Antal-Szalmas P, Strijp JA, Weersink AJ, et al. Quantitation of surface CD14 on human monocytes and neutrophils. J Leukoc Biol 1997; 61: 721–728. [DOI] [PubMed] [Google Scholar]
- 37.Osterud B, Hansen JB. Fatty acids, platelets and monocytes. Something to do with atherogenesis. Ann Med 1989; 21: 47–51. [DOI] [PubMed] [Google Scholar]
- 38.Netea MG, van der Meer JW, van Deuren M, Kullberg BJ. Pro-inflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing?. Trends Immunol 2003; 24: 254–258. [DOI] [PubMed] [Google Scholar]
- 39.Westendorp RG, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production in meningococcal disease. Lancet 1997; 349: 1912–1913. [DOI] [PubMed] [Google Scholar]
- 40.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci U S A 2009; 106: 18327–18332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frosch M. Brandtzaeg P. Handbook of meningococcal disease: infection biology, vaccination, clinical management. Chapter 23 Pathogenesis and Pathophysiology of Invasive Meningococcal Disease, 2006 Wiley-VCH Verlag GmbH & Co. KGaA, 2006. [Google Scholar]
- 42.Brusletto BS, Hellerud BC, Loberg EM, et al. Traceability and distribution of Neisseria meningitidis DNA in archived post mortem tissue samples from patients with systemic meningococcal disease. BMC Clin Pathol 2017; 17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]