Short abstract
Increased expressions of miR-21 have been detected in ankylosing spondylitis (AS) patients. The current study was performed to examine the serum miR-21 expression with radiographic severity in AS patients, which was determined based on the modified New York (NY) criteria for sacroiliac joints assessment and modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) system for spine involvement. Bone mineral density at lumbar 1–4 and femoral neck were examined by dual-energy absorptiometry (DXA). Serum miR-21 expressions were determined by quantitative real-time PCR, and receiver operating characteristic curve analysis was performed to identify the diagnostic value of miR-21 expression levels regarding the NY criteria. Elevated levels of serum miR-21 expressions were detected in AS patients compared with healthy controls. AS patients with modified NY grade 4 showed significantly higher miR-21 expression than grade 3 and grade 2. AS patients with spinal syndesmophytes had significantly higher serum miR-21 expressions than non-syndesmophyte patients. Increased miR-21 expressions were significantly related to the disease radiographic severity. In addition, serum miR-21 expressions were negatively associated with lumbar 1–4 and femoral neck bone mineral density. In summary, serum miR-21 expressions were related to structural damage and radiological progression in AS, indicating that miR-21 may act as a switch between inflammation and new bone information and regulate different signal ways between lesioned enthesis and trabecular bone.
Keywords: miR-21, ankylosing spondylitis, radiographic progression, low bone mineral density
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory joint disease that predominantly affects the sacroiliac joints and spine, with a prevalence that ranges from 0.1% to 1.4% worldwide, with males affected 2–4 times more frequently than females.1 AS mainly comprises two phases: inflammation and abnormal bone formation. Inflammation processes associated with AS can lead to bone erosion, new bone formation, and ankylosis occurring in the spine, which leads to severe pain, a reduction in spinal mobility, and stiffness.2 There are two main problems that remain to be resolved in AS: (1) only one-third of AS patients develop syndesmophytes and ankylosis;3 (2) in the later stage of AS, there is a paradox phenomenon that two opposite processes of bone remodeling occur simultaneously in different AS patients. One represents an abnormal new bone formation in the enthesis point, while another comprises a gradual loss of trabecular bone mass in the vertebral column, resulting in osteoporosis.4
In recent years, epigenetic studies have been performed which provided a crucial step forward in our understanding of the pathogenesis of AS.5 It was supposed that HLA-B27(+) plays a decisive role in the etiopathogenesis of AS. However, in recent years, many AS patients also demonstrated a HLA-B27(−) phenotype,6 indicating that an epigenetic factor plays an important role in AS development. MicroRNAs (miRNAs) belong to the most important epigenetic regulators. They are small ∼22 nucleotide long non-coding RNAs which post-transcriptionally regulate gene expression by targeting the 3′-untranslated region (3′-UTR) for degradation or translational repression.7 Recent advances in miRNA biology have exposed their critical influence in regulating a wide array of cellular processes both in normal physiological status and diseases.8
miR-21 is one of the most highly expressed members of the miRNA family in many mammalian cell types and plays a critical role in mediating cell proliferation, differentiation, and migration.9 miR-21 has been reported to be associated with a broad range of pathophysiological process including inflammation development, injury, fibrosis, and others.10–12 Recent studies have confirmed a key role for miR-21 in the resolution of inflammation and in negatively regulating the pro-inflammatory response induced by many of the same stimuli that trigger miR-21 induction itself.13
Effects of miR-21 on bone metabolism seem to be dual and are tightly related to the inflammatory process. miR-21 is highly expressed in osteoclastogenesis and plays an important role during osteoclastic activity.14,15 On the other hand, miR-21 is also involved in osteogenesis activity,16,17 and appears to be necessary for osteogenic differentiation.18,19
The link between miR-21 and inflammatory or immune activity has been widely explored before. TNF-α has been regarded as a key inflammatory cytokine which is decisive in AS onset and progression. In a previous study, TNF-α led to impaired bone formation both in vivo and in vitro, which is related to the suppression of miR-21.20 In addition, administration of anti-TNF-α in ovariectomized mice increased bone formation by up-regulating miR-21.20 It has been demonstrated that the expression of miR-21 increased gradually at low concentration of TNF-α while being suppressed at high concentration of TNF-α.21 miR-21 could also promote Th17 cell differentiation which is stimulated by IL-17 and IL-22,22 whereas the latter two cytokines are considered to lead to both bone loss and abnormal new bone formation in AS.23 In a recent study, whole blood miR-21 in patients with AS was significantly higher than in paired controls.24
All these previous studies indicated that the role of miR-21 in the process of “inflammation triggering–bone structure damage–inflammation resolution–pathological repair–ankylosis” is in accordance with the pathophysiological AS disease model and may play an important role in AS progression. Our present study was put forward to explore whether serum expressions of miR-21 are correlated with the radiographic progression and bone mineral density (BMD) of AS.
Patients and methods
From February 2017 to February 2019, 69 consecutive AS patients were recruited to this study. All enrolled patients fulfilled the Assessment of SpondyloArthritis international Society (ASAS) axSpA criteria.25 All clinical values were confirmed by two experienced physicians. Clinical data included age, gender, duration of disease, family history of AS, and peripheral arthritis. In addition, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) were evaluated to check disease activity and functional status. To exclude the effects of age, patients aged 50 yr or older were excluded. Further exclusion criteria included patients with thyroid or parathyroid disorders, the presence of chronic renal or liver disease, cancer, coeliac disease, or concurrent rheumatoid arthritis, and the use of corticosteroids. Meanwhile, 65 sex- and age-matched healthy individuals receiving regular medical examination were enrolled as controls. The study protocol was approved by our hospital. Informed consent was obtained from all participants included in the study.
Definition of radiographic severity
Radiographic severity for sacroiliitis and spinal spondyloarthritis were assessed according to the radiological changes of modified New York (NY) criteria and the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS).26 The radiographic evaluation of modified NY scale is defined as follows: grade 0, normal; grade 1, suspicious changes; grade 2, minimal abnormality (small localized areas with erosion or sclerosis, without alteration in the joint width); grade 3, unequivocal abnormality (moderate or advanced sacroiliitis with one or more of erosions, evidence of sclerosis, widening, narrowing or partial ankylosis); grade 4, severe abnormality (total ankylosis). Patients with modified NY grade ≥ 2 at least one sacroiliac joint were included. The modified NY scale used for measurement was the one found higher upon comparison between both sacroiliac joints. In the mSASSS, a lateral view of the anterior parts of the cervical and lumbar spine is scored for squaring and/or erosion and/or sclerosis (1 point), syndesmophytes (2 points), and bridging syndesmophytes (3 points).27 The total scores ranged from 0 to 72. The results were read and analyzed by two trained and experienced radiologists who were blinded to the patients’ clinical data. A kappa value was calculated to test the consistency of the results.
Evaluation of BMD
BMD of the lumbar spine and left hip was assessed using dual-energy absorptiometry (DXA; Lunar Prodigy densitometer, Madison, WI) at the time of enrolment. All measurements were taken by experienced operators using the same machine and standardized procedures for participant positioning. BMD was measured at the lumbar spine 1–4 using an anteroposterior projection at L1–L4, and at the left hip from the femoral neck. The values were expressed as the number of grams of bone mineral per square centimeter (g/cm2).
Laboratory examination
Venous blood was obtained at overnight fast time between 8:00 and 9:00 a.m. and located at room temperature for 2–3 h, and then centrifuged for 20 min at 1900 g. The upper layer of serum was transferred to a new RNA se-free tube and immediately stored at −80°C until use. The miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) was used to isolate total RNA from 200 μL serum according to the manufacturer’s instructions. So far, no housekeeping miRNA has been established and validated to normalize for the miRNA content in serum/plasma. Therefore, after addition of miRNeasy, we supplemented the samples with 5 nmol/L U6 RNA as the spiked-in RNA.
The extracted total RNA was eluted in 14 μL of RNAse-free water. The concentration and purity of the RNA samples were measured using the Nanodrop-1000 (Thermo Fisher Scientific, Waltham, MA). The RNA samples were immediately stored at −80°C for further use. Thirty ng of total RNA were reverse transcribed into complementary DNA using specific primers for miR-21 using the PrimeScript™RT reagent Kit (Takara Biotechnologies Inc, Tokyo, Japan) according to the manufacturer’s instructions. The quantitative RT-PCR (qRT-PCR) was performed using the SYBR® Premix Ex Taq™II (Takara Biotechnologies Inc.) on the Stratagene Mx3000P Real-Time qPCR System (Agilent Technologies, Santa Clara, CA). The qRT-PCR reaction was performed as follows: 95°C for 30 s, then 40 cycles of 95°C for 5 s, and 60°C for 30 s. The primers used to amplify miR-21 and U6 fragments using Primer Premier 5.0 software (Premier, Canada) were: miR-21: RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′; forward primer: 5′-GTGCAGGGTCCGAGGT-3′, reverse primer: 5′-GCCGCTAGCTTATCAGACTGATGT-3′; U6: RT: 5′-AACGCTTCACGAATTTGCGT-3′; forward primer: 5′-CTCGCTTCGGCAGCACA-3′, reverse primer: 5′-AACGCTTCACGAATTTGCGT-3′. The relative miRNA expression levels of interest were calculated using the 2−ΔΔCt method.
Statistical analysis
All data were expressed as the means ± SDs or median and were analyzed with GraphPad 6.0 software. Data normality distribution was analyzed by the Kolmogorov–Smirnov test. The expression of selected miR-21 between two groups was compared using the Student’s t-tests or Mann–Whitney U test, whereas one-way ANOVA or Kruskal–Wallis test was performed among subgroup patients with different modified NY grades, followed by Tukey or Tamhan post-hoc analysis. The correlation of miR-21 levels in serum with radiographic progression or BMD was assessed by Spearman or Pearson correlation analysis. P Values < 0.05 were regarded as statistically significant. Statistical power was calculated by using PASS (Power Analysis and Sample Size) 2008 statistical software (NCSS, Kaysville, UT).
Results
Basic data
Clinical characteristics and laboratory findings of AS patients and healthy controls are listed in Table 1. There were no significant differences of baseline characteristics between AS patients with and healthy controls regarding age, body mass index (BMI), and sex distribution (P > 0.05). Serum C-reactive protein (CRP) levels were significantly increased in AS patients compared with controls (3.07 ± 1.13 mg/dL vs. 0.73 ± 0.11 mg/dL, P < 0.001). Elevated expressions of miR-21 in serum were found in AS patients compared with healthy controls (2.17 ± 0.95 vs. 0.46 ± 0.17 pg/mL, P < 0.001) (Figure 1). The statistical power value was 0.85 after calculation.
Table 1.
Clinical characteristics and laboratory findings of AS patients and health controls.
| AS (n = 69) | Control (n = 65) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 39.5 ± 10.3 | 38.2 ± 11.6 | 0.134 |
| BMI (kg/m2) | 22.1 ± 2.7 | 22.4 ± 2.5 | 0.478 |
| Sex (male/female) | 56/13 | 50/15 | 0.546 |
| Disease duration, median (range) | 5.2 (2.5–13.6) | / | / |
| Family history, n (%) | 36 (52.2%) | / | / |
| Peripheral arthritis, n (%) | 30 (43.5%) | / | / |
| BASDAI | 4.82 ± 2.11 | / | / |
| BASFI | 2.12 ± 1.13 | / | / |
| NSAIDs use, n (%) | 45 (65%) | / | / |
| DMARDs use, n (%) | 23 (33.3%) | / | / |
| TNF-α inhibitor use, n (%) | 20 (28.9%) | / | / |
| CRP, mg/dL | 3.07 ± 1.13 | 0.73 ± 0.11 | < 0.001 |
| HLA-B27(+), n (%) | 59 (85.5%) | / | / |
| Serum miR-21 expression | 2.17 ± 0.95 | 0.46 ± 0.17 | < 0.001 |
BMI, body mass index; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CRP, C-reactive protein; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease-modifying anti-rheumatic drugs.
Figure 1.
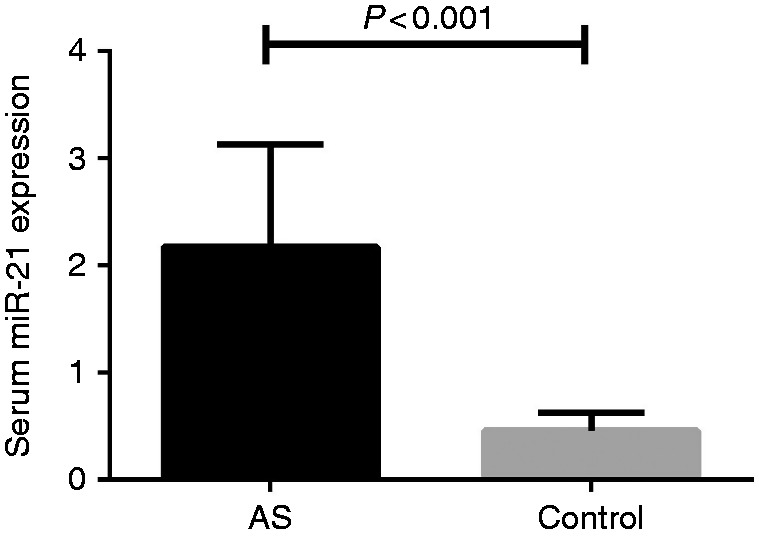
Comparison of miR-21 expression between AS patients and controls.
Serum miR-21 expressions in AS patients with modified NY grade
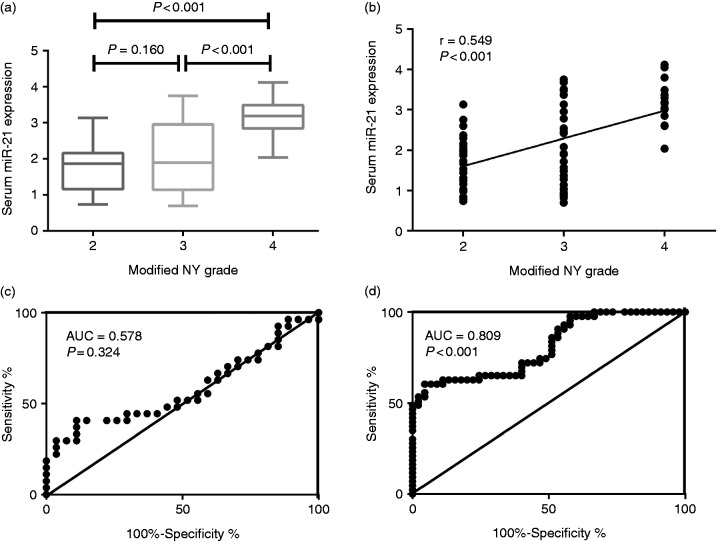
The AS patients were divided into three groups based on their modified NY grades. We included 27 with NY grade 2, 27 with NY grade 3, and 15 with NY grade 4. AS patients with modified NY grade 4 had significantly higher serum miR-21 compared with modified NY grade 3 (3.19 ± 0.55 vs. 2.05 ± 0.99, P < 0.001) and modified NY grade 2 (3.19 ± 0.55 vs. 1.72 ± 0.65, P < 0.001). Besides, although differences between AS patients with NY grade 3 and NY grade 2 did not achieve significance, NY grade 3 patients still show elevated serum miR-21 expressions compared with those with grade 2 (2.05 ± 0.99 vs. 1.72 ± 0.65, P = 0.160). Serum miR-21 levels were positively related to modified NY grades (r = 0.548, P < 0.001) (Figure 2). Further receiver operating characteristic (ROC) curve analysis demonstrated that miR-21 may act as a decent marker in the later stage of radiographic changes AS (area under the curve (AUC) = 0.809, P < 0.001) rather than in the early-mediate stage (AUC = 0.578, P = 0.324) (Figure 2c and d).
Figure 2.
(a) Comparison of serum miR-21 expression among AS patients with different modified NY grades. (b) Correlation of serum miR-21expression with different NY grades. (c) ROC curve analysis of miR-21 as diagnostic marker regarding modified NY grade 2 and modified NY grade 3. (d) ROC curve analysis of miR-21 as diagnostic marker regarding modified NY grade 3 and modified NY grade 4.
Serum miR-21 expressions in AS patients with/without spinal syndesmophytes
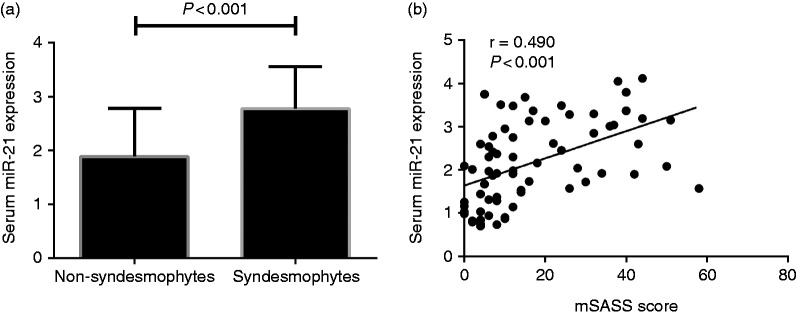
Patients were also classified into another two separate groups depending on whether there were spinal syndesmophytes present or not. Based on the definition given in terms of mSASSS, spinal syndesmophytes were not present in 47 and present in 22 patients. The serum miR-21 expressions in patients without syndesmophytes were significantly lower compared with those with syndesmophytes (1.88 ± 0.90 vs 2.78 ± 0.79, P < 0.001) (Figure 3a). In addition, miR-21 levels in serum were positively related to mSASSS score (r = 0.490, P < 0.001) (Figure 3b).
Figure 3.
(a) Comparison of serum miR-21 expressions between patients with and without spinal syndesmophytes. (b) Correlation of serum miR-21 expressions with mSASSS.
Serum miR-21 expressions in AS patients with BMD
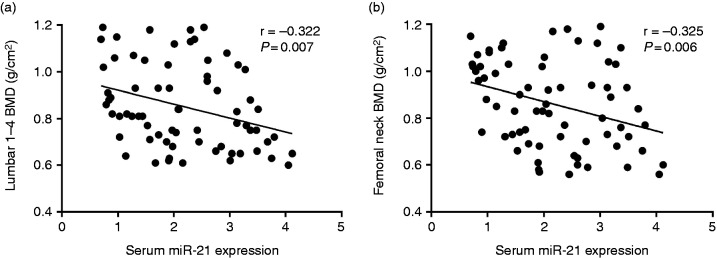
In order to further explore the potential effect of serum miR-21 on the BMD, we also explored the association of serum miR-21 with BMD at lumbar 1–4 and femoral neck. We found increased miR-21 were both related to reduced BMD at lumbar 1–4 (r = −0.322, P = 0.007) (Figure 4a) and femoral neck (r = −0.325, P = 0.006) (Figure 4b).
Figure 4.
(a) Correlation of serum miR-21 expressions with lumbar 1–4 BMD. (b) Correlation of serum miR-21 expressions with femoral neck BMD.
Discussion
Our study explored the correlation of serum miR-21 levels and radiographic severity of AS. We demonstrated for the first time that increased serum miR-21 expressions were positively correlated with radiographic progression and negatively associated with lumbar 1–4 and femoral neck BMD. These findings implicate that serum miR-21 may act as a switch between inflammation and new bone information and regulate different signal ways between lesioned enthesis and trabecular bone.
The major hallmark of AS is syndesmophyte formation that leads to fusion of the spine. This is coupled with osteoporosis of the spine, thereby increasing the risk of vertebral fractures. Chronic inflammation of the spine leads not only to new bone formation in axial joints and vertebral spaces, but also to bone resorption leading to osteoporosis in AS.28 However, there is ongoing debate regarding whether inflammation is the direct trigger of the osteoproliferation or trabecular bone loss seen in AS. One recent study found that in AS, inflammation-driven bone destruction was shown to be a prerequisite, sequential but not parallel process for late new bone formation and ankylosis in the AS mouse model.29 Accordingly, other observational studies concluded that inflammation that thoroughly resolved on follow-up MRI was more likely to form into syndesmophytes in comparison to persistent inflammation in patients taking anti-TNF therapy,30 indicating that resolution of inflammation following treatment is related to new bone formation in AS.31
Several previous works disputed that miR-21 expression to a greater extent, was associated with decreased pro-inflammatory responses and resolution of inflammation.32,33 Meanwhile, its production was found to be also stimulated by inflammatory cytokines including TNF-α, NF-kB, and IL-6.13 TNF-α has been regarded as the most important cytokine contributing to disease progression in AS. However, blocking TNF-α may rather stimulate bone formation, as this hypothesis so-called “TNF-brake” is based on the connection of TNF-α to the Wnt pathway.2 Overexpression of miRNA-21 resulted in decreased TNF-α secretion, whereas suppression of miRNA-21 expression increased the amount of TNF-α.33 Bone marrow-derived macrophages are believed to be the major precursor cells for osteoclasts, which play critical roles in the structure damage in AS,34 and miR-21 is beneficial in modulating the macrophage response to LPS, indicating the anti-inflammatory effects of miRNA-21.33 Therefore, we speculate that increase of miR-21 was a result of inflammation stimulation by cytokines including TNF-α rather than being a causative role. The circuit of miR-21 induction may have the potential to feedback and drive later events in the cell.
In a previous study, whole blood miR-21 expressions in patients with AS were associated with bone damage-related mRNA-programmed cell death 4 (PCD4) as well as the biomarker CTX, which is consistent with the fact that miR-21 was related to osteoclastic activity.25 However, no significant correlation between miR-21 and mSASSS scores were found.25 There are several reasons that may explain this controversy, one of which is that the recorded mSASSS scores were all below 20 which means that almost all AS patients enrolled in the study were in the phases of early-mediate stage patients. For mSASSS, a score from 0 to 1 at each lesioned site only reflects erosive/sclerotic changes but not syndesmophyte growth and as mentioned above, only one-third of AS patients develop bony ankylosis.
Additionally, we observed that increased amounts of miR-21 were related to an attenuated BMD, indicating that miR-21 also participates in osteoclastic activity in AS patients. The previous study established that the presence of syndesmophytes was associated with lower trabecular BMD,35 and vice versa, BMD at baseline was independently associated with the development of new syndesmophytes AS patients.36 This phenomenon indicated that bone formation and bone loss are tightly linked through certain mechanisms that remain to be unveiled, and that miR-21 may also participate the process of osteoclastic activity in AS progression.
There are some limitations that should be taken into consideration. First of all, this is a cross-sectional study carried out with a relatively small number of participants among Chinese people. Therefore, larger samples are needed to further confirm our findings. In addition, cause-and-effect relationships cannot be judged and require prospective longitudinal works to illustrate any relationships. Second, only miR-21 expression was tested, other potential indicators in serum including TNF-α and CTX-1 may provide additional valuable information.
In conclusion, serum miR-21 expressions are correlated with radiographic progression but also lower BMD in AS patients. miR-21 may act as switch between inflammation and new bone information and regulate different signal pathways between lesioned enthesis and trabecular bone. Interventions targeting miR-21 to improve bone metabolism disruption deserve further study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Natural Science Foundation of China (Grant Number 81703883), National Natural Science Foundation of Guangdong Province of China (Grant Number 2017A030310293), China Postdoctoral Science Foundation Funded Project (Grant Number 2018M630971), Southern Medical University Research Start-up Project (Grant Number PY2017N031), and Project of Administration of traditional Chinese Medicine Guangdong Provincial of China (Grant Number 20171176).
References
- 1.Wang R, Ward MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol 2018; 30: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009; 60: 93–102. [DOI] [PubMed] [Google Scholar]
- 3.Baraliakos X, Listing J, Rudwaleit M, et al. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008; 10: R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magrey MN, Khan MA. The paradox of bone formation and bone loss in ankylosing spondylitis: evolving new concepts of bone formation and future trends in management. Curr Rheumatol Rep 2017; 19: 17. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Aslani S, Nicknam MH, et al. New insights toward the pathogenesis of ankylosing spondylitis; genetic variations and epigenetic modifications. Mod Rheumatol 2017; 27: 198–209. [DOI] [PubMed] [Google Scholar]
- 6.Tamai K, Mashitori H, Saotome K, et al. HLA-B27-negative ankylosing spondylitis resulting in panclavicular ligament ossification: a 28-year follow-up. Acta Orthop Scand 1998; 69: 323–325. [DOI] [PubMed] [Google Scholar]
- 7.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136: 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 2007; 23: 175–205. [DOI] [PubMed] [Google Scholar]
- 9.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009; 13: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A, Ganesh K, Khanna S, et al. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 2014; 192: 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji W, Jiao J, Cheng C, et al. MicroRNA-21 in the pathogenesis of traumatic brain injury. Neurochem Res 2018; 43: 1863–1868. [DOI] [PubMed] [Google Scholar]
- 12.Sun C, Tian J, Liu X, et al. MiR-21 promotes fibrosis and hypertrophy of ligamentum flavum in lumbar spinal canal stenosis by activating IL-6 expression. Biochem Biophys Res Commun 2017; 490: 1106–1111. [DOI] [PubMed] [Google Scholar]
- 13.Sheedy FJ. Turning. 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol 2015; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood 2011; 117: 3648–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu CH, Sui BD, Du FY, et al. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci Rep 2017; 7: 43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguchi T, Watanabe K, Hara ES, et al. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One 2013; 8: e58796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei Y, Bian C, Li J, et al. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem 2013; 114: 1374–1384. [DOI] [PubMed] [Google Scholar]
- 18.Wei F, Yang S, Guo Q, et al. MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci Rep 2017; 7: 16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng YB, Li X, Li ZY, et al. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J Orthop Res 2015; 33: 957–964. [DOI] [PubMed] [Google Scholar]
- 20.Yang N, Wang G, Hu C, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 2013; 28: 559–573. [DOI] [PubMed] [Google Scholar]
- 21.Xu K, Xiao J, Zheng K, et al. MiR-21/STAT3 signal is involved in odontoblast differentiation of human dental pulp stem cells mediated by TNF-α. Cell Reprogram 2018; 20: 107–116. [DOI] [PubMed] [Google Scholar]
- 22.Murugaiyan G, da Cunha AP, Ajay AK, et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest 2015; 125: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammatþ CD3þCD4-CD8- entheseal resident T cells. Nat Med 2012; 18: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 24.Huang CH, Wei JC, Chang WC, et al. Higher expression of whole blood microRNA-21 in patients with ankylosing spondylitis associated with programmed cell death 4 mRNA expression and collagen cross-linked C-telopeptide concentration. J Rheumatol 2014; 41: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 25.Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009; 68(Suppl 2): ii1–44. [DOI] [PubMed] [Google Scholar]
- 26.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 27.Wanders AJ, Landewe RB, Spoorenberg A, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004; 50: 2622–2632. [DOI] [PubMed] [Google Scholar]
- 28.Schett G. Structural bone changes in spondyloarthritis: mechanisms, clinical impact and therapeutic considerations. Am J Med Sci 2011; 341: 269–271. [DOI] [PubMed] [Google Scholar]
- 29.Tseng HW, Pitt ME, Glant TT, et al. Inflammation-driven bone formation in a mouse model of ankylosing spondylitis: sequential not parallel processes. Arthritis Res Ther 2016; 18: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009; 60: 93–102. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen SJ, Chiowchanwisawakit P, Lambert RG, et al. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol 2011; 38: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 32.Das A, Ganesh K, Khanna S, et al. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 2014; 192: 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnett RE, Conklin DJ, Ryan L, et al. Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J Leukoc Biol 2016; 99: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S, Qiu M, Chen J. IL-4 modulates macrophage polarization in ankylosing spondylitis. Cell Physiol Biochem 2015; 35: 2213–2222. [DOI] [PubMed] [Google Scholar]
- 35.Klingberg E, Lorentzon M, Göthlin J, et al. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res Ther 2013; 15: R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HR, Hong YS, Park S-H, et al. Low bone mineral density predicts the formation of new syndesmophytes in patients with axial spondyloarthritis. Arthritis Res Ther 2018; 20: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]