Abstract
Platelets are the main players in thrombosis and hemostasis; however they also play important roles during inflammation and infection. Through their surface receptors, platelets can directly interact with pathogens and immune cells. Platelets form complexes with neutrophils to modulate their capacities to produce reactive oxygen species or form neutrophil extracellular traps. Furthermore, they release microbicidal factors and cytokines that kill pathogens and influence the immune response, respectively. Platelets also maintain the vascular integrity during inflammation by a mechanism that is different from classical platelet activation. In this review we summarize the current knowledge about how platelets interact with the innate immune system during inflammation and infection and highlight recent advances in the field.
Keywords: Platelet, inflammation, infection, Kupffer cell, neutrophils, macrophages
Introduction
Platelets are small anucleated cell fragments patrolling the vasculature, and immediately respond to vessel breaches and restore hemostasis. In recent decades it has become clear that they play roles beyond hemostasis and also contribute to (thrombo-)inflammatory processes like those unfolding after stroke.1 Furthermore, they also play an important role during infection by either directly interacting with pathogens or by recruiting and stimulating immune cells.2 More recently, we have come to understand that platelets also maintain vascular integrity in inflamed vessels in a process different from classical hemostasis.3,4 Acknowledging their multifaceted capabilities, platelets have lately been described as autonomous drones for hemostatic and immune surveillance.5
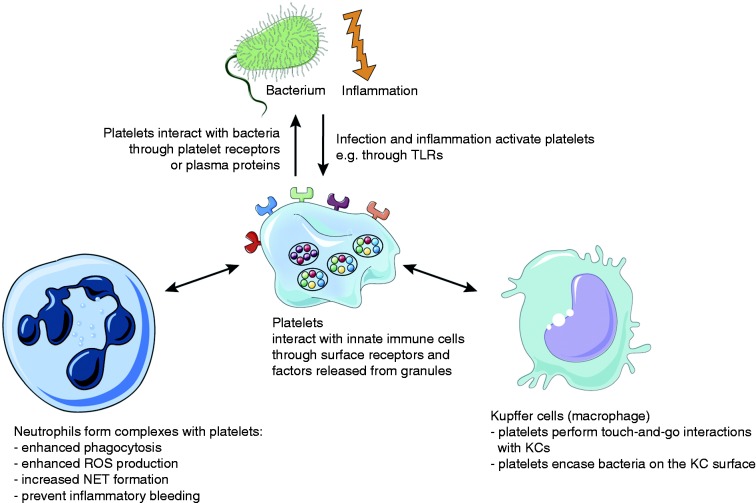
The finding that platelets form aggregates around bacteria is not new. In fact, one of the first descriptions of this process dates back to 19016 when Levaditi showed that platelets aggregated upon incubation with Vibrio cholerae; however, more systematic investigations on how bacteria cause platelet aggregation were only performed in the 1970s.7,8 Platelet surface receptors enable direct platelet–bacteria or platelet–immune cell interactions.9 Factors stored in platelet granules that are released upon activation include cytokines, inflammatory mediators and antimicrobial peptides.10,11 In this review we discuss how platelets, being among the first cells to respond to vessel injury, are at the front line of antimicrobial host defense which allows them to orchestrate the innate immune response (Figure 1).
Figure 1.
Platelets interact with bacteria and cells of the innate immune system. Platelets interact with bacteria directly through their surface receptors or indirectly through plasma proteins. Platelets orchestrate the immune reaction to inflammation and infection by direct interactions with cells of the innate immune system (neutrophils and Kupffer cells) or through the secretion of mediators.
A lot of the findings presented here were discovered using experimental mouse models or knockout mouse lines. It is therefore worth to mention that there are some distinct characteristics between murine and human platelets such as their size, number and some histological features. However, they also share a lot of similarities, and mice offer an excellent model to study various aspects of platelet biology in vivo.12
Platelets and sepsis
Severe sepsis—defined as infection in combination with acute organ dysfunction—is the leading cause of in-hospital death in the United States (US).13,14 Estimations suggest there are 750,000 cases of severe sepsis every year in the US, with most of them requiring intensive care. Case numbers for patients with severe sepsis admitted to a hospital have seen a significant rise in recent years,14 with the highest incidence rates reported for newborns younger than 12 months (5.3 per 1000) and senior patients older than 85 years (26.2 per 1000).15 In the 1960s, some studies reported mortality rates of up to 80% for septic shock patients. Thanks to improvement in monitoring and therapy, this number has decreased significantly; however, it still remains at around 25% today.13,16 In addition, total hospital costs for patients with severe sepsis have seen a steady increase, and it is now a major burden for the public health system with estimated annual costs of more than 24 billion USD in the US.14
During severe sepsis both pro- and anti-inflammatory responses occur simultaneously. The two processes aim at eliminating the pathogen and at the same time try to restrict the immune reaction to prevent excessive damage. Both responses have to be delicately balanced to provide a response that is powerful enough to clear the pathogen and prevent secondary infections but also to minimize collateral tissue damage.13
Severe sepsis is often accompanied by disseminated intravascular coagulation and thrombocytopenia; however, the underlying mechanisms are incompletely understood.13,17 It is well established, however, that there is extensive cross-talk between the inflammatory and coagulation pathways. Tissue factor (TF) is up-regulated by leukocytes, platelets, endothelial and smooth muscle cells, which triggers thrombin generation leading to a sustained pro-inflammatory and thrombotic response while at the same time dampening anticoagulant mechanisms and fibrinolysis.18,19
A recent study showed that thrombocytopenia led to severely impaired survival and enhanced bacterial growth in blood and lungs in a mouse model of pneumonia-derived sepsis.20 Thrombocytopenia also caused hemorrhage at the site of infection, in line with previous results demonstrating that inflammation induces hemorrhage during thrombocytopenia.20,21 A clinical study including more than 900 sepsis patients grouped them according to their platelet counts upon admission to the intensive care unit. Remarkably, patients with very low or intermediate–low platelet counts showed significant increases in both 30-day mortality as well as cytokine levels and enhanced endothelial cell activation.17 These results show that platelet count might be used as a prognostic marker during sepsis.
Platelet receptors enable interactions with bacteria
Several platelet surface receptors are also involved in inflammation and infection in addition to thrombosis and hemostasis. Platelet–bacteria interactions can be either direct (bacterial surface proteins binding to a platelet receptor) or indirect (bacteria binding to a plasma protein, for example von Willebrand factor (vWF) or fibrinogen, which then binds to the respective platelet receptor).
Glycoprotein Ib (GPIb)
GPIb is exclusively expressed on the surface of platelets and precursor megakaryocytes. The interaction with its main ligand vWF is especially important for platelet adhesion under high shear conditions, for example in stenosed vessels or capillaries.22,23 GPIb can also bind to serine-rich repeat proteins like the Streptococcus sanguis platelet adhesin called serine-rich protein A (SrpA) which enables binding to GPIb in a sialic acid-dependent manner.24 Staphylococcus aureus protein A (Spa) facilitates indirect interaction with platelets through both soluble and immobilized vWF which then binds to platelets via GPIb.25,26
Integrin αIIbβ3 (GPIIb/IIIa)
The platelet-specific integrin αIIbβ3 is the most abundant glycoprotein on the platelet surface and binds ligands that contain an arginine-glycine-aspartic acid (RGD) sequence such as fibrinogen, vWF, fibronectin and vitronectin. αIIbβ3 enables stable platelet–platelet interactions and adhesion to the extracellular matrix (ECM). Firm binding only occurs after a conformational change that puts αIIbβ3 in an activated or high-affinity state in which the RGD binding site is uncovered.27 Binding of Borrelia burgdorferi to human platelets was shown to be mediated by αIIbβ3 and could be blocked by a synthetic RGD peptide.28 Another bacterial protein that contains a RGD-like motif to interact with platelet αIIbβ3 is SdrG (Fbe) from Staphylococcus epidermis, which is present in many clinical strains and causes platelet aggregation which can be blocked using the αIIbβ3 antagonists abciximab or tirofiban, but also aspirin. SdrG also mediates indirect interactions through fibrinogen which in turn binds αIIbβ3 and the IgG receptor FcγRIIa.29,30 Another well-characterized indirect interaction between platelets and bacteria is through fibrinogen and clumping factors (Clf) on S. aureus. Like the Sdr proteins, ClfA and B both contain serine and aspartic acid dipeptide repeats (SD repeats) and bind fibrinogen to induce platelet aggregation via αIIbβ3.31,32
TLR2 / TLR4
TLRs recognize PAMPs, for example the bacterial cell wall components lipoteichoic acid (LTA) and LPS.33 Platelets express TLR2 and TLR4 on their surface.34,35 Streptococcus pneumoniae triggers platelet aggregation through TLR2, and αIIbβ3, independent of pneumolysin toxin, causes activation of the phosphoinositide 3-kinase (PI3-K) pathway and provokes dense-granule release.36 In mice, LPS injection was shown to induce an increase in platelet binding to fibrinogen under flow. Furthermore, LPS administration caused thrombocytopenia through P-Selectin-independent neutrophil-mediated pulmonary platelet sequestration in wildtype but not in TLR4-deficient mice.37 Another study demonstrated that human platelets responded to LPS stimulation with release of soluble CD62p, epidermal growth factor, TGFb, IL-8, platelet activating factor 4 (PAF4) and platelet-derived growth factor (PDGF) α and β.37 During hemolytic-uremic syndrome (HUS) caused by infection with enterohemorrhagic Escherichia coli (EHEC), platelets were activated by EHEC-LPS binding to TLR4.38 Platelet TLR4 detects TLR4 ligands (LPS, but also others like high mobility group B1 (HMGB1) and heat-shock proteins) in the blood and causes platelets to bind to adherent neutrophils and the formation of neutrophil extracellular traps (NETs) in liver sinusoids and pulmonary capillaries, which facilitate bacterial capture during sepsis39 but can also have detrimental effects such as causing vascular occlusion.40,41
(hem)ITAM receptors GPVI and CLEC-2
Platelets are activated by collagen through their main collagen receptor GPVI, which signals through an immunoreceptor tyrosine-based activation motif (ITAM) in the Fc receptor (FcR) γ-chain it forms a complex with.42,43 The platelet C-type lectin receptor 2 (CLEC-2) binds podoplanin, which is highly expressed in type 1 lung alveolar cells, lymphatic endothelial cells and kidney podocytes, but absent from endothelial cells and platelets. CLEC-2 contains a single cytosolic YXXL motif known as a hemITAM that becomes phosphorylated upon receptor multimerization to enable signaling.44,45 GPVI and CLEC-2 play important roles in thrombosis and hemostasis;42,46–48 however, they are also involved in maintaining vascular integrity in inflamed vessels thus preventing inflammatory bleeding,3,49,50 formation of cerebral blood vessels51 and mediating blood/lymphatic vessel separation.52 A recent report showed that CLEC-2 mediates inflammation-triggered thrombosis after salmonella infection in the liver53 through platelets interacting with podoplanin-expressing monocytes and Kupffer cells. Lately, a thrombosis-independent role for CLEC-2 during sepsis was described: Platelet CLEC-2 interacts with podoplanin expressed on inflammatory macrophages to regulate immune cell recruitment as well as the cytokine/chemokine storm following infection to limit organ damage.54 In another study, S. aureus α-toxin bound ADAM10 on the platelet surface to trigger platelet activation and platelet–neutrophil complex formation that enhanced neutrophil activity during sepsis.55 ADAM10 is a metalloproteinase that cleaves the ectodomain of GPVI,56 and in fact soluble GPVI (sGPVI) was released from platelets following incubation with S. aureus α-toxin. Whether this demonstrates a direct interaction of ADAM10 with S. aureus or a potential role for sGPVI needs further investigation. Moreover, GPVI-deficient mice showed increased bacterial growth in lungs and distant body sites after pneumonia-derived sepsis as well as reduced platelet activation and platelet–leukocyte complex formation in the bronchoalveolar space.57 Interestingly, in a recent clinical study, sGPVI was identified as a marker for platelet activation and predictive for the occurrence of sepsis and overall survival in patients with thermal injury.58 GPVI is also critically involved in the formation of platelet microparticles—submicrometer vesicles shed from activated platelets—that can have pro-inflammatory effects, for example in patients with rheumatoid arthritis.59 Recently it was shown that microparticles shed from activated platelets lose GPVI expression while maintaining CLEC-2,60 contributing to sGPVI levels. Indeed, sGPVI levels in plasma from patients with rheumatoid arthritis were significantly increased compared with healthy controls.
Platelet granule-derived factors and their impact on innate immunity
Upon activation, platelets release a plethora of different factors stored in two major types of granules: α-granules and dense granules. α-Granules are highly abundant, with 50–80 granules per mouse platelet,61,62 while dense granules are considerably less abundant with 5–6 granules per platelet. α-Granules contain more than 300 different membrane and soluble proteins, which are recruited to the plasma membrane or secreted upon platelet activation, respectively, and are involved in processes such as platelet adhesion, coagulation, thrombo-inflammation, wound healing, tumor growth, angiogenesis and antimicrobial host defense.61,63,64
There is increasing evidence that platelets contribute to the onset and spread of inflammation.65 Platelets adhere to the activated endothelium or form complexes with immune cells to activate, attract or differentiate other immune cells by several mechanisms. Many platelet-derived factors contribute to shaping the inflammatory response, and one of the most important ones is P-Selectin, which is exposed on the platelet surface upon activation and mediates interactions of platelets with immune cells and the endothelium. Platelet P-Selectin binds to P-Selectin glycoprotein ligand-1 (PSGL1) on endothelial or immune cells, thereby enabling platelets to bind to the inflamed endothelium, to recruit circulating monocytes, neutrophils and lymphocytes and to initiate an inflammatory response at the site of injury. Importantly, blocking P-Selectin or PSGL-1 using antibodies almost completely abolished platelet tethering, rolling and adhesion on activated endothelium.66 In a mouse model of acute lung injury, blocking platelet P-Selectin reduced the number of platelet–neutrophil complexes, improved gas exchange, reduced neutrophil recruitment and permeability and prolonged survival of the animals.67
Upon activation, platelets secrete numerous chemokines, including CXCL1, CXCL4, CXCL5, CXCL7, CXCL8, CXCL12, CCL2, CCL3 and CCL5.10 The most abundant platelet chemokine CXCL7 is present in several variants: platelet basic protein, connective tissue-activating peptide III (CTAP-III), β-thromboglobulin (β-TG) and neutrophil-activating peptide-2 (NAP-2), and all of them are generated by proteolytic cleavage from a precursor protein.10,61 However, the only variant that possesses chemotactic activity is NAP-2.68 It was shown that both CTAP-III and NAP-2 induce neutrophil adhesion to human umbilical vein endothelial cells (HUVECs); however, only NAP-2 triggered neutrophil transendothelial migration.69 Interestingly, CXCL7 is also involved in the recruitment of circulating endothelial progenitor cells after arterial injury through its receptor CXCR2, indicating that CXCL7 secreted by platelets may contribute to revascularization after vessel injury.70
Platelets are a major source of CCL5 (RANTES) in the circulation, as its concentration was highly correlated to platelet counts in a study of patients with hematological malignancy undergoing chemotherapy.71 Platelet-derived CCL5 contributes to recruiting monocytes to the vessel wall. In a mouse model of atherosclerosis, activated platelets were shown to deliver CCL5 and CXCL4 (platelet factor 4, PF4) to the surface of both monocytes and endothelial cells in atherosclerotic lesions in a P-Selectin-dependent manner.72 It was further shown that CXCL4 facilitates CCL5 oligomerization and amplifies its effect on monocyte recruitment.73
Platelets (and their precursors megakaryocytes) are the exclusive source of CXCL4 (PF4). That platelets have an abundance of CXCL4 is strikingly demonstrated by a 1000-fold increase in the serum concentration after thrombin stimulation.61,74 Although CXCL4 lacks chemotactic activity,75 it causes firm neutrophil adhesion to endothelial cells and degranulation. While the first process is a direct effect of Src kinase activation, the latter requires costimulation, for example by TNF through p38 MAP kinase and PI3 kinase.76 Furthermore, CXCL4 triggers several functions in monocytes, including phagocytosis, respiratory burst, survival and cytokine secretion. CXCL4-initiated respiratory burst was shown to depend on rapid activation of the PI3 kinase, Syk and p38 MAP kinase. By contrast, monocyte differentiation and survival is mediated by CXCL4-mediated delayed Erk activation approximately 6 h after stimulation.77,78 CXCL4 released from platelets is also capable of inducing differentiation of monocytes into macrophages and prevents them from undergoing spontaneous apoptosis in culture.79 In combination with IL-4, CXCL4 induces a rapid differentiation of monocytes into specialized APCs that stimulates lymphocyte proliferation and lytic NK activity while inducing only moderate cytokine release.80
CXCL4 also acts on other cell types such as endothelial cells. Platelet-derived CXCL4 inhibited fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor function by blocking the binding to their respective receptors. Furthermore, both CXCL4 and its variant CXCL4L1 potently inhibit chemotaxis and proliferation of endothelial cells as well as in vitro and in vivo angiogenesis.81,82 CXCR3-B, a splice variant of CXCR3, was shown to be a receptor for CXCL4 and might be involved in the angiostatic activity of CXCL4 released by platelets83 that causes inhibition of endothelial cell growth.
Platelets as phagocytes and platelet-derived antimicrobial peptides
We know that platelets express TLR436 and other receptors which enable them to detect and bind to bacteria, which raises the question of their capability of phagocyting bacteria. Indeed, electron microscopic studies demonstrated that activated platelets engulf S. aureus in vacuoles and appear to secrete granule content into the vacuole.84 However, whether platelets actually killed the bacteria or transferred them to professional phagocytes was not clarified in this study. Interestingly, in an earlier report, Yeaman and colleagues isolated and characterized cationic proteins from rabbit platelets that displayed in vitro microbiostatic or microbicidal activity against S. aureus, Escherichia coli and Candida albicans.85 In another study, thrombin-induced platelet microbicidal protein (tPMP-1) potently lysed S. aureus during logarithmic-phase growth.86 Releasate from thrombin-activated platelets reduced the number of adherent bacteria in a rabbit model of infective endocarditis using Streptococcus sanguis.87 Using the same model, experimentally induced thrombocytopenia led to higher densities of streptococci within vegetations as well as higher total number of bacteria per valve.88 Another group of antibacterial proteins found in platelets is called thrombocidins (TCs). TC-1 and TC-2 were able to kill Bacillus subtilis, E. coli, S. aureus and Lactococcus lactis89 using a mechanism that did not lyse the bacterial cell wall, indicating that they act differently than tPMP-1. Platelets were also shown to bind Plasmodium falciparum-infected erythrocytes and kill the parasite inside the cell.90 Treating the platelets with aspirin or a P2Y1 antagonist rendered them incapable of killing the parasite. Accordingly, treating mice with antiplatelet Abs or aspirin resulted in lower overall survival after infection with Plasmodium chabaudi. Lately, E. coli were found to be killed by human platelets through a process involving CXCL4 and FcγRIIA: Anti-CXCL4/polyanion Abs opsonized E. coli coated with platelet-derived CXCL4. The Ab complex was then detected by platelet FcγRIIA,91 causing platelets to cover the opsonized bacteria and release antimicrobial factors in a concerted way to effectively kill them.
Platelet interactions with innate immune cells in infection and inflammation
Platelets interact with a number of key players during infection and inflammation, namely macrophages (e.g. Kupffer cells in the liver), neutrophils, monocytes, NK cells, dendritic cells and components of the complement system. Here, we focus on reviewing their interactions with Kupffer cells, neutrophils, the complement system and how they maintain vascular integrity during inflammation and infection.
Kupffer cells
The liver is not only the largest internal organ with important roles in detoxification and metabolism, it is also the first line of defense against pathogens present in the blood stream. It filters about a third of the body’s total blood volume each minute and contains the largest population of phagocytes in the body.92 The liver-resident macrophage population known as Kupffer cells (KCs) are very large, immobile cells that reside in the sinusoidal space where they scan for foreign objects in the blood. More than 25 years ago, Endo and colleagues discovered that LPS injection into mice caused an increase in hepatic serotonin levels which was independent of mast cells and correlated with a drop in the number of circulating platelets. They found that LPS application caused accumulation of non-activated platelets in the sinusoidal space where they frequently interacted with KCs.93 Subsequent studies showed that while aspirin or heparin did not affect serotonin accumulation in the liver, KC depletion using clodronate liposomes almost completely abolished platelet and serotonin accumulation.94 This suggested that LPS stimulation causes platelet retention in the liver through a process that is different from classical platelet aggregation and that involves KCs.
We now know that KCs rapidly capture a striking quantity of pathogens from the bloodstream in a process that often involves platelets. KCs were shown to bind B. burgdorferi and prevent them from using sinusoidal endothelial cells to gain access to the extravascular space.95 It was also demonstrated that methicillin-resistant S. aureus (MRSA) is primarily sequestered and killed by KCs through an interaction of the complement receptor of the immunoglobulin superfamily (CRIg) with LTA on the bacterial surface.96 A minority of staphylococci, however, can overcome the antimicrobial activity of the KCs to survive and proliferate inside this intracellular niche.97 Importantly, KCs were found to collaborate with platelets to eradicate blood-borne infections with Bacillus cereus and MRSA:98 In the naïve mouse liver, platelets performed touch-and-go interactions through GPIb with vWF constitutively expressed on KCs. Upon infection, KCs captured bacteria and platelets rapidly adhered and formed aggregates around them in an integrin αIIbβ3-dependent way to contain the bacterium. This suggested an important role for platelets in KC-mediated bacterial clearance. Indeed, platelet depletion or lack of GPIb resulted in severely increased mortality in mice following infection. Importantly, opsonization with complement factor C3 was necessary for successful bacterial clearance, indicating a complex interplay between KCs, platelets and the complement system during bacterial clearance in the liver. Another group investigating the clearance of Listeria monocytogenes bloodstream infections by KCs discovered a dual-track mechanism consisting of a slow clearance of bacteria–platelet complexes that requires platelet GPIb, CRIg and C3 opsonization as well as a fast clearance of free bacteria independent of complement and platelets that requires scavenger receptors.99,100 The authors hypothesized that the slow clearance allows a small number of platelet–bacteria complexes to remain in the circulation long enough to be detected by splenic CD8α+ dendritic cells to launch an antibacterial cytotoxic T cell response.
Neutrophils
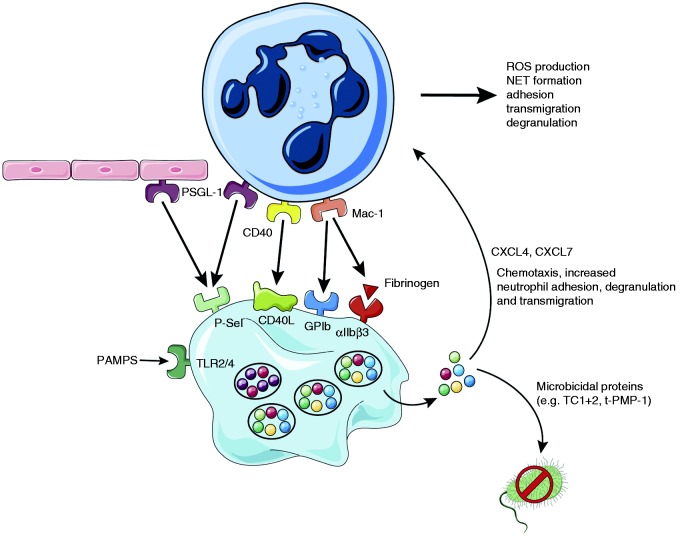
It is well established that platelets interact with neutrophils during inflammation and infection. Indeed, circulating platelet–neutrophil complexes (PNC) have been found in a variety of diseases such as asthma, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, stroke and severe sepsis.101–102 The effect of platelets binding to neutrophils includes increased neutrophil adhesion to the endothelium, increased reactive oxygen (ROS) production and NET formation (Figure 2). P-Selectin, which upon activation is recruited to the platelet surface from α-granular stores, seems to be the most important platelet surface receptor for platelet–neutrophil interactions. Platelet P-Selectin binds to the high-affinity counter-ligand PSGL-1 on neutrophils.104,105 P-Selectin-deficient mice show severe leukocyte defects, for example abrogated leukocyte rolling as well as delayed neutrophil recruitment and reduced neutrophil extravasation.106,107 Using P-Selectin-deficient mice or treating mice with an anti-P-Selectin Ab and subjecting them to different models of lung inflammation resulted in reduced neutrophil recruitment and less lung damage.108,109 However, all those studies looked at global P-Selectin deficiency in which the individual contribution of endothelial and platelet P-Selectin are difficult to isolate. In later studies it was shown that platelet depletion significantly inhibits neutrophil recruitment to the site of inflammation in a zymosan-induced peritonitis and LPS-induced lung inflammation model.110 In inflamed glomerular capillaries, platelets are essential for leukocyte adhesion via a non-classical cascade that involves platelet P-Selectin binding to endothelial PSGL-1 as well as β2 integrin/ICAM-1 and nonrolling interactions.111 Depletion of neutrophils and platelets reduced urinary protein excretion induced by anti-glomerular basement membrane Abs, underlining their importance for the development of renal injury. In addition, it was shown that platelet P-Selectin is important for neutrophil recruitment into the outer and inner medulla during acute post-ischemic renal failure.112 In another paper, Sreeramkumar et al. showed that in inflamed vessels, neutrophils scan for the presence of activated platelets using PSGL-1 clusters. Migration and NET formation only occurred once activated platelets had bound.113 In a mouse model of multiple sclerosis called experimental autoimmune encephalomyelitis (EAE), platelet depletion significantly improved the disease state and slowed its progression through reduced recruitment of leukocytes to the inflamed central nervous system and attenuated inflammation. More specifically, targeting GPIb or αIIbβ3 led to a pronounced improvement of EAE outcome.114
Figure 2.
Platelets form complexes with neutrophils to potentiate their activity. Platelets interact with neutrophils through multiple receptors: Activated platelets express P-Selectin on their surface which binds PSGL-1 on neutrophils and endothelial cells, CD40L is expressed on the platelet surface upon activation and binds CD40, Neutrophil Mac-1 binds platelet GPIb as well as αIIbβ3. Together these interactions promote ROS production, NET formation, adhesion, transmigration and degranulation of neutrophils. Upon activation (e.g. through PAMPS binding to TLRs), platelets release microbicidal proteins like TC1+2 which can kill bacteria. They also secrete large quantities of CXCL4 and CXCL7 that promote neutrophil adhesion, degranulation and transmigration.
Interestingly, platelets not only express P-Selectin, but also its counter-receptor PSGL-1 which mediates platelet–endothelium interaction.115,116 Furthermore, platelet GPIb was also shown to bind to (endothelial) P-Selectin,117 thereby allowing platelets to scan for the activation status of the vasculature. In addition, platelets can stimulate the secretion of Weibel–Palade bodies from endothelial cells and leukocyte rolling through P-Selectin.118 Therefore, it seems that platelets can interact with the endothelium to pave the way for leukocytes to bind to both platelets and the endothelium.
P-Selectin/PSGL-1 interactions between activated platelets and neutrophils in the inflamed vasculature promote further interactions through β2 integrins (CD18), especially Mac-1 (αMβ2, CD11b/CD18) which follows a three-step process that involves binding to fibrinogen/GPIb and outside-in-signaling through Src family kinases.119,120 In addition, activated platelets express intercellular adhesion molecular-2 (ICAM-2, CD102) which enables the formation of firm and shear resistant platelet-neutrophil-complexes under flow conditions by binding to β2 integrins.121,122
Neutrophil accumulation on activated platelets under flow conditions also involves interactions of Mac-1 with fibrinogen bound to platelet αIIbβ3, as found by using blocking Abs against the major platelet integrin. Furthermore, platelets from patients with Glanzmann thrombasthenia harboring genetic defects in their ITGA2B or ITGB3 genes that cause impaired αIIbβ3 function or expression123 demonstrated significantly reduced neutrophil adhesion to platelets under flow compared with healthy controls.124
Platelets are a major source of CD40 ligand (CD40L), which they express on their surface upon activation.125 Patients with diabetes, ischemic stroke or acute coronary syndromes often show elevated levels of circulating soluble CD40L. Stimulation of endothelial cells through CD40 by platelet CD40L induces recruitment of neutrophils, likely via platelet P-Selectin and neutrophil Mac-1 expression. Furthermore, CD40L increased neutrophil oxidative burst and degranulation.126,127
Indeed, neutrophils in PNCs show increased activation, CD11b expression, phagocytosis and ROS production compared with free neutrophils.128 Upon stimulation of platelets, the activation state of neutrophils in PNCs was even more pronounced.128,129 Interestingly, incubation with resting platelets was able to restrict neutrophil activation, indicating that there is a strong interconnection between the activation state of platelets and neutrophils in PNCs, which is most likely mediated through P-Selectin.129,130
Besides phagocytosis, neutrophils possess another clever way to capture bacteria through the formation of NETs. During NET formation, neutrophils expel large amounts of chromatin and granular proteins (e.g. elastase and myeloperoxidase (MPO)), thereby forming extracellular fibers to immobilize and kill bacteria.131,132 Initially, it was thought that all neutrophils die during NETosis; however, early in infection, live neutrophils in fact release NETs to prevent bacterial dissemination.133 While this may sound counter-intuitive at first, we know that red blood cells or platelets live without a nucleus for several days and that neutrophils whose nucleus has been removed retain their ability to crawl, transmigrate, phagocytose and kill bacteria at least for a short time.134
Platelets are critically involved in NET formation through their TLR4 receptor that can detect PAMPs in the bloodstream. Platelet activation through TLR4 causes them to bind to neutrophils adhering primarily to liver sinusoids and pulmonary capillaries, causing neutrophil activation and NET formation.39 Significantly, adding plasma from patients with severe sepsis to platelets and neutrophils from healthy donors triggered TLR4-dependent PNC formation. Moreover, neutrophils migrate to liver sinusoids during sepsis to release NETs and prevent bacterial dissemination to other organs by a mechanism that requires PNC formation through LFA-1 (CD11a/CD18).135 Platelets were shown to form aggregates around S. aureus thereby limiting their growth. In addition, human platelets also release an antimicrobial peptide called human β-defensin-1 (hBD-1) after stimulation with S. aureus α-toxin. hBD-1 significantly impairs bacterial growth but was also shown to induce robust NET formation.136
Interestingly, NETs can also be found under sterile inflammation conditions in the lungs and plasma of patients with transfusion-related acute lung injury (TRALI). Targeting platelet activation using aspirin or an αIIbβ3 inhibitor decreased NET formation and lung injury in a mouse model of TRALI.137 Blocking Mac-1 but not LFA-1 during acute lung injury also significantly reduced the amount of NET formation and lung injury.138 Platelets are critically involved in the propagation of deep vein thrombosis (DVT) by promoting leukocyte accumulation and NET formation, which provides a prothrombotic surface through its decoration with TF.40 Importantly, treating mice with DNase significantly reduced NET formation and DVT growth. A recent publication showed that both mice and humans harbor endogenous DNases that help to contain NET formation in the host.41 Serum from mice deficient in DNase1 or DNase1L3 was able to degrade NETs; however, deficiency in both DNases completely abrogated NET-degrading capacity. Double-deficient animals subjected to chronic neutrophilia or septicemia showed a high mortality due to vascular occlusion through “pure” NET clots independent of classical hemostasis or thrombus formation.
Platelets were recently found to be capable of actively probing their local environment and migrating using actomyosin-generated forces.139 While migrating they act as cellular scavengers that collect bacteria both in vitro and in vivo. Platelet–bacteria bundles generated this way facilitate phagocytosis by neutrophils as well as NET formation.
Complement
The complement system facilitates lysis of pathogens and damaged cells by forming a pore in the target cell membrane through the membrane attack complex. Platelets are capable of activating both the classical and alternative complement pathway; however, the mechanism is still incompletely understood.140 It is known that activated human platelets express gC1qR—a receptor for C1q and the first factor of the classical complement pathway141,142 which also binds to other ligands such as SpA expressed on the surface of S. aureus.143,144 Platelet granules store complement C3 and C4 precursor but also C1 inhibitor, which indicates that platelets might in fact regulate the complement response.61 Chondroitin sulfate released from activated platelets causes complement activation through interactions with C1q.145 In addition, platelet P-Selectin can bind C3b and trigger the alternative complement pathway.140
S. sanguis induces platelet aggregation in a complement-dependent way with a lag time that can be explained by the time needed for the assembly of the C5b-9 complex on the bacterial surface.146 S. aureus ClfA exhibits an alternative route to bind platelets that is fibrinogen-independent and involves FcγRIIa and the assembly of complement proteins as well as a complement receptor.147
The complement factors C1q, C4, C3, and C9 bind TRAP (thrombin receptor-activating peptide)-activated platelets without, however, activating the complement cascade, indicating that under physiological conditions there is no activation of the complement system on the platelet surface.148 Assembly of the lytic terminal complement complex C5b-9 on the platelet plasma membrane can activate platelets and induce platelet procoagulant activity.149 Patients suffering from HUS display hemolytic anemia, acute kidney failure, complement system activation and microvascular thrombosis leading to thrombocytopenia.150 During HUS, endothelial and complement system activation lead to vWF release from endothelial Weibel–Palade bodies as well as P-Selectin and TF recruitment in a C3a or C5a-dependent way that leads to platelet adhesion and establish the prothrombotic state.150–152
Platelets maintain vascular integrity during inflammation
In patients with immune thrombocytopenia (ITP), the immune system wreaks havoc on endogenous platelets through antiplatelet Abs against major platelet receptors, in most cases GPIb and αIIbβ3.153 This results in a significantly reduced number of circulating platelets (<100 × 109/L), causing varying degrees of bleeding culminating—in some cases—in intracranial hemorrhages.154 Often the degree of thrombocytopenia observed in ITP patients does not predict the severity of bleeding,155 indicating that thrombocytopenia alone is not sufficient to cause bleeding and that an additional trigger—such as inflammation—is needed.
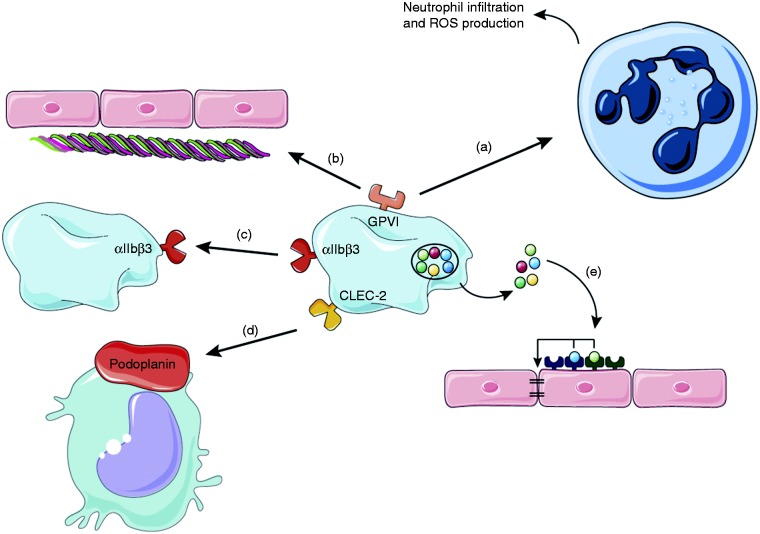
In 2008, Goerge et al. showed that inflammation induces hemorrhage in thrombocytopenic mice.21 Using multiple models to induce local inflammation in the skin, brain and lung, they observed no signs of bleeding in mice with normal platelet counts. In striking contrast, when using thrombocytopenic mice, they observed massive hemorrhage at the site of inflammation. Remarkably, as little as 5% of the baseline platelet count was sufficient to significantly reduce bleeding. Interestingly, similar results were obtained in a model of pneumonia-derived sepsis using Klebsiella pneumoniae: Thrombocytopenic mice displayed increased bacterial growth and hemorrhage in the lung.20 Later, the importance of platelet ITAM signaling downstream of GPVI and CLEC-2 in maintaining vascular integrity during skin and lung inflammation was demonstrated.49 Recent findings show that the CLEC-2 ligand podoplanin is upregulated on macrophages and other extravascular cells during skin inflammation, indicating that in the absence of GPVI, binding of platelet CLEC-2 to podoplanin-expressing cells contributes to limiting bleeding in the inflamed skin (Figure 3).156,157 The study also showed that vascular integrity during lung inflammation was partially dependent on GPIb.
Figure 3.
Platelets maintain vascular integrity during inflammation. During local inflammation (e.g. in the skin), platelet GPVI plays a dual role: On the one hand it promotes neutrophil infiltration and ROS production which causes tissue damage (a) and on the other hand it binds to the extracellular matrix protein collagen which gets exposed and facilitates platelet adhesion to restore vascular integrity (b). In the ischemic brain αIIbβ3 facilitates platelet–platelet interactions to prevent intracranial hemorrhage (c). Platelets can also bind to podoplanin, expressed on inflammatory macrophages via CLEC-2 (d). Factors secreted from platelet granules support cerebral hemostasis after stroke, for example by acting on endothelial cell receptors that stabilize cellular junctions (e).
It was suggested that platelet granule secretion following platelet activation through the aforementioned pathways could be part of the mechanism that maintains vascular integrity.21,49,50,158 However, recent findings demonstrate that platelet α- and dense-granule contents are dispensable for maintaining vascular integrity during acute inflammation in the skin and lung.159 Strikingly, when mice lacking platelet α- and dense-granule secretion were subjected to transient middle cerebral artery occlusion (tMCAO), this resulted in significantly impaired hemostasis in the ischemic brain, causing increased intracranial hemorrhage and 50% mortality comparable to that observed in mice treated with αIIbβ3-blocking Abs.160 This is especially interesting, since mice with a single deficiency in either platelet α- or dense-granule secretion did not show signs of spontaneous hemorrhage during thrombosis,64,161 thrombo-inflammation after stroke63,64 or tumor metastasis.162,163 This indicates that factors from both α- and dense granules are necessary to maintain cerebral hemostasis after tMCAO; for example platelet-derived angiopoietin-1 or serotonin—which were previously shown to prevent intra-tumor hemorrhage165—might play a role in this setting. Of note, angiopoietin-1 was also shown to be critical for the maintenance of vascular integrity and survival in a mouse model of cerebral malaria.165 In addition, brain endothelial cells express the P2Y2 receptor that binds nucleotides such as ADP and ATP, which could also contribute to permeability.166 Indeed, platelet-derived ATP was shown to enable tumor cell transendothelial migration and metastasis via P2Y2.163
During inflammatory bleeding in the skin, neutrophil extravasation and RBC loss colocalize,167 and inhibiting neutrophil capturing, adhesion and crawling on the endothelial cell layer significantly reduced hemorrhage. Furthermore, neutrophil diapedesis opens endothelial junctions via dephosphorylation of VE-cadherin during skin inflammation. Interestingly, platelet GPVI on the one hand enhances neutrophil infiltration and ROS production, thereby causing more endothelial damage,50 while on the other hand it enables platelets to adhere to binding sites exposed by neutrophils. GPVI therefore has a Janus face in this process: It contributes to the pro-inflammatory role of platelets while at the same time helps to repair the damage inflicted by neutrophils and thereby maintains vascular integrity.168
Conclusion
The roles of platelets besides those in thrombosis and hemostasis have long been neglected, but today we know that they also contribute to inflammation during sepsis, thrombo-inflammation, atherosclerosis and stroke. In most of these cases, platelets present themselves as a most versatile actor: They can form complexes with neutrophils and enhance their phagocytosis, ROS production and NET formation capacity, encase bacteria on the surface of KCs to assist with their destruction or confront pathogens on their own by acting like a wannabe-phagocyte.
First reports of platelets aggregating around bacteria are more than 100 years old; however, only recently we have started to understand the complex interplay between platelets and the cells of the innate immune response during inflammation and infection. Platelets interact with bacteria by direct interactions between platelet receptors and proteins on the bacterial surface. Upon activation platelets release a plethora of factors, for example microbicidal agents but also factors that modulate the innate immune response.
NET formation by neutrophils is a powerful tool to capture and destroy bacteria and it has become clear that platelets critically contribute to this process. A very new concept is that platelets assist in bacterial clearance in the liver through KCs and the complement system, and we will probably see a lot of exciting new findings in this field in the near future.
Another emerging role for platelets is in maintaining vascular integrity during inflammation through an organ-specific process that is independent of classical activation and involves platelet receptors as well as the content of α- and dense granules.
Acknowledgements
We thank Servier for providing Servier Medical Art which was used for creation of figures.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.D. is supported by Deutsche Forschungsgemeinschaft (DFG) Research Fellowship DE 2654/1-1. P.K. is supported by grants from the Canadian Institute of Health Research (CIHR), Alberta Innovates Health Solutions (AIHS), the Heart and Stroke Foundation of Canada and the Canada Research Chairs programme.
References
- 1.Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: A thrombo-inflammatory disease? J Physiol 2011; 589: 4115–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deppermann C, Kubes P. Platelets and infection. Semin Immunol 2016; 28: 536–545. [DOI] [PubMed] [Google Scholar]
- 3.Ho-Tin-Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018; 131: 277–288. [DOI] [PubMed] [Google Scholar]
- 4.Deppermann C. Platelets and vascular integrity. Platelets 2018; 00: 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Li JL, Zarbock A, Hidalgo A. Platelets as autonomous drones for hemostatic and immune surveillance. J Exp Med 2017; 214: 2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levaditi C. Et des organisms vaccines contre le vibron cholerique. Ann Inst Pasteur 1901; 15: 894–924. [Google Scholar]
- 7.Clawson CC, White JG. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol 1971; 65: 367–380. [PMC free article] [PubMed] [Google Scholar]
- 8.Clawson CC, White JG. Platelet interaction with bacteria. II. Fate of the bacteria. Am J Pathol 1971; 65: 381–397. [PMC free article] [PubMed] [Google Scholar]
- 9.Kerrigan SW. The expanding field of platelet-bacterial interconnections. Platelets 2015; 26: 293–301. [DOI] [PubMed] [Google Scholar]
- 10.Gleissner CA, Von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol 2008; 28: 1920–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair P, Flaumenhaft R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev 2009; 23: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt A, Guichard J, Massé JM, et al. Of mice and men: Comparison of the ultrastructure of megakaryocytes and platelets. Exp Hematol 2001; 29: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 13.Angus (Pitt) DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 840–851. [DOI] [PubMed] [Google Scholar]
- 14.Lagu T, Rothberg MB, Shieh M-S, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40: 754–761. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 16.Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time? Crit Care Med 1998; 26: 2078–2086. [DOI] [PubMed] [Google Scholar]
- 17.Claushuis TAM, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 2016; 127: blood-2015-11-680744–blood-2015-11-680744. [DOI] [PubMed] [Google Scholar]
- 18.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 2016; 118: 1392–1408. [DOI] [PubMed] [Google Scholar]
- 19.Levi M, Van Der Poll T. Inflammation and coagulation. Crit Care Med 2010; 38: S26–S34. [DOI] [PubMed] [Google Scholar]
- 20.de Stoppelaar SF, van ’t Veer C, Claushuis TAM, et al. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 2014; 124: 3781–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008; 111: 4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez JA. The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis 1994; 5: 97–119. [PubMed] [Google Scholar]
- 23.Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost 2013; 11: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer C, Wu H, Kerrigan SW, et al. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br J Haematol 2005; 129: 101–109. [DOI] [PubMed] [Google Scholar]
- 25.Hartleib J, Köhler N, Dickinson RB, et al. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 2000; 96: 2149–2156. [PubMed] [Google Scholar]
- 26.O’Seaghdha M, Van Schooten CJ, Kerrigan SW, et al. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J 2006; 273: 4831–4841. [DOI] [PubMed] [Google Scholar]
- 27.Bennett JS. Structure and function of the platelet integrin αIIbβ3. J Clin Invest 2005; 115: 3363–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coburn J, Leong JM, Erban JK. Integrin aIIbb3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci USA 1993; 90: 7059–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arciola CR, Campoccia D, Gamberini S, et al. Presence of fibrinogen-binding adhesin gene in Staphylococcus epidermidis isolates from central venous catheters-associated and orthopaedic implant-associated infections. Biomaterials 2004; 25: 4825–4829. [DOI] [PubMed] [Google Scholar]
- 30.Brennan MP, Loughman A, Devocelle M, et al. Elucidating the role of Staphylococcus epidermidis serine-aspartate repeat protein G in platelet activation. J Thromb Haemost 2009; 7: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 31.Siboo IR, Cheung AL, Bayer AS, et al. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun 2001; 69: 3120–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien L, Kerrigan SW, Kaw G, et al. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: Roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol 2002; 44: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 34.Cognasse F, Hamzeh H, Chavarin P, et al. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol 2005; 83: 196–198. [DOI] [PubMed] [Google Scholar]
- 35.Andonegui G, Kerfoot SM, McNagny K, et al. Platelets express functional Toll-like receptor-4. Blood 2005; 106: 2417–2423. [DOI] [PubMed] [Google Scholar]
- 36.Keane C, Tilley D, Cunningham A, et al. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. J Thromb Haemost 2010; 8: 2757–2765. [DOI] [PubMed] [Google Scholar]
- 37.Cognasse F, Hamzeh-Cognasse H, Lafarge S, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol 2008; 141: 84–91. [DOI] [PubMed] [Google Scholar]
- 38.Ståhl AL, Svensson M, Mörgelin M, et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood 2006; 108: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469. [DOI] [PubMed] [Google Scholar]
- 40.von Brühl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209: 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017; 358: 1202–1206. [DOI] [PubMed] [Google Scholar]
- 42.Nieswandt B, Brakebusch C, Bergmeier W, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J 2001; 20: 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieswandt B, Watson SP. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003; 102: 449–461. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki-Inoue K, Fuller GLJ, García A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 2006; 107: 542–549. [DOI] [PubMed] [Google Scholar]
- 45.Watson SP, Herbert JMJ, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost 2010; 8: 1456–1467. [DOI] [PubMed] [Google Scholar]
- 46.Nieswandt B, Schulte V, Bergmeier W, et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med 2001; 193: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massberg S, Gawaz M, Grüner S, et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med 2003; 197: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May F, Hagedorn I, Pleines I, et al. CLEC-2 is an essential platelet activating receptor in hemostasis and thrombosis. Blood 2009; 114: 3464–3473. [DOI] [PubMed] [Google Scholar]
- 49.Boulaftali Y, Hess PR, Getz TM, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest 2013; 123: 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gros A, Syvannarath V, Lamrani L, et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood 2015; 126: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 51.Lowe KL, Finney BA, Deppermann C, et al. Podoplanin and CLEC-2 drive cerebrovascular patterning and integrity during development. Blood 2015; 125: 3769–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010; 116: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hitchcock JR, Cook CN, Bobat S, et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Invest 2015; 125: 4429–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rayes J, Lax S, Wichaiyo S, et al. The podoplanin-CLEC-2 axis inhibits inflammation in sepsis. Nat Commun 2017; 8 : 1: 2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers ME, Becker REN, Sailer A, et al. Synergistic action of Staphylococcus aureus α-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe 2015; 17: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bender M, Hofmann S, Stegner D, et al. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood 2010; 116: 3347–3355. [DOI] [PubMed] [Google Scholar]
- 57.Claushuis TAM, de Vos AF, Nieswandt B, et al. Platelet glycoprotein VI aids in local immunity during gram-negative pneumonia-derived sepsis. Blood 2017; 131: 864–876. [DOI] [PubMed] [Google Scholar]
- 58.Montague SJ, Delierneux C, Lecut C, et al. Soluble GPVI is elevated in injured patients: Shedding is mediated by fibrin activation of GPVI. Blood Adv 2018; 2: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010; 327: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gitz E, Pollitt AY, Gitz-Francois JJ, et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 2014; 124: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev 2009; 23: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frojmovic MM, Milton JG. Human platelet size, shape, and related functions in health and disease. Physiol Rev 1982; 62: 185–261. [DOI] [PubMed] [Google Scholar]
- 63.Stegner D, Deppermann C, Kraft P, et al. Munc13-4 mediated secretion is essential for infarct progression but not intracranial haemostasis in acute stroke. J Thromb Haemost 2013; 11: 1430–1433. [DOI] [PubMed] [Google Scholar]
- 64.Deppermann C, Cherpokova D, Nurden P, et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J Clin Invest 2013; 123: 3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.May AE, Seizer P, Gawaz M. Platelets: Inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol 2008; 28: s5–s10. [DOI] [PubMed] [Google Scholar]
- 66.Braun OÖ, Slotta JE, Menger MD, et al. Primary and secondary capture of platelets onto inflamed femoral artery endothelium is dependent on P-selectin and PSGL-1. Eur J Pharmacol 2008; 592: 128–132. [DOI] [PubMed] [Google Scholar]
- 67.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 2006; 116: 3211–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walz A, Dewald B, Tscharner V, et al. Nap-2, Platelet Basic Protein, Connective Platelet Factor 4 on human neutrophils. J Exp Med 1989; 170: 1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schenk BI, Petersen F, Flad H-D, et al. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J Immunol 2002; 169: 2602–2610. [DOI] [PubMed] [Google Scholar]
- 70.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res 2007; 100: 590–597. [DOI] [PubMed] [Google Scholar]
- 71.Ellis M, Al-Ramadi B, Hedström U, et al. Significance of the CC chemokine RANTES in patients with haematological malignancy: Results from a prospective observational study. Br J Haematol 2005; 128: 482–489. [DOI] [PubMed] [Google Scholar]
- 72.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med 2003; 9: 61–67. [DOI] [PubMed] [Google Scholar]
- 73.Von Hundelshausen P, Koenen RR, Sack M, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005; 105: 924–930. [DOI] [PubMed] [Google Scholar]
- 74.Slungaard A. Platelet factor 4: A chemokine enigma. Int J Biochem Cell Biol 2005; 37: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 75.Petersen F, Ludwig A, Flad HD, et al. TNF-alpha renders human neutrophils responsive to platelet factor 4. Comparison of PF-4 and IL-8 reveals different activity profiles of the two chemokines. J Immunol 1996; 156: 1954–1962. [PubMed] [Google Scholar]
- 76.Kasper B, Brandt E, Bulfone-Paus S, et al. Platelet factor 4 (PF-4)-induced neutrophil adhesion is controlled by src-kinases, whereas PF-4-mediated exocytosis requires the additional activation of p38 MAP kinase and phosphatidylinositol 3-kinase. Blood 2004; 103: 1602–1610. [DOI] [PubMed] [Google Scholar]
- 77.Kasper B, Brandt E, Brandau S, et al. Platelet factor 4 (CXC chemokine ligand 4) differentially regulates respiratory burst, survival, and cytokine expression of human monocytes by using distinct signaling pathways. J Immunol 2007; 179: 2584–2591. [DOI] [PubMed] [Google Scholar]
- 78.Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev 2015; 29: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheuerer B, Ernst M, Dürrbaum-Landmann I, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000; 95: 1158–1166. [PubMed] [Google Scholar]
- 80.Fricke I, Mitchell D, Petersen F, et al. Platelet factor 4 in conjunction with IL-4 directs differentiation of human monocytes into specialized antigen-presenting cells. FASEB J 2004; 18: 1588–1590. [DOI] [PubMed] [Google Scholar]
- 81.Jouan V, Canron X, Alemany M, et al. Inhibition of in vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood 1999; 94: 984–993. [PubMed] [Google Scholar]
- 82.Struyf S, Burdick MD, Proost P, et al. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res 2004; 95: 855–857. [DOI] [PubMed] [Google Scholar]
- 83.Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 2003; 197: 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Youssefian T, Drouin A, Massé JM, et al. Host defense role of platelets: Engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 2002; 99: 4021–4029. [DOI] [PubMed] [Google Scholar]
- 85.Yeaman MR, Tang YIQ, Shen AJ, et al. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun 1997; 65: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koo SP, Yeaman MR, Nast CC, et al. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun 1997; 65: 4795–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dankert J, Van der Werff J, Zaat SAJ, Joldersma W, Klein D, Hess J. Involvement of bactericidal factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect Immun 1995; 63: 663–671. [DOI] [PMC free article] [PubMed]
- 88.Sullam PM, Frank U, Yeaman MR, et al. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis 1993; 168: 910–914. [DOI] [PubMed] [Google Scholar]
- 89.Krijgsveld J, Zaat SA, Meeldijk J, et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem 2000; 275: 20374–20381. [DOI] [PubMed] [Google Scholar]
- 90.McMorran BJ, Marshall VM, De Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 2009; 323: 797–800. [DOI] [PubMed] [Google Scholar]
- 91.Palankar R, Kohler TP, Krauel K, et al. Platelets kill bacteria by bridging innate and adaptive immunity via PF4 and FcγRIIA. J Thromb Haemost 2018; 38: 42–49. [DOI] [PubMed] [Google Scholar]
- 92.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- 93.Endo Y, Nakamura M. The effect of lipopolysaccharide, interleukin-1 and tumour necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. Br J Pharmacol 1992; 105: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakamura M, Shibazaki M, Nitta Y, et al. Translocation of platelets into Disse spaces and their entry into hepatocytes in response to lipopolysaccharides, interleukin-1 and tumour necrosis factor: The role of Kupffer cells. J Hepatol 1998; 28: 991–999. [DOI] [PubMed] [Google Scholar]
- 95.Lee W-Y, Moriarty TJ, Wong CHY, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol 2010; 11: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng Z, Surewaard BGJ, Wong CHY, et al. CRIg Functions as a macrophage pattern recognition receptor to directly bind and capture blood-borne gram-positive bacteria. Cell Host Microbe 2016; 20: 99–106. [DOI] [PubMed] [Google Scholar]
- 97.Surewaard BGJ, Deniset JF, Zemp FJ, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 2016. jem.20160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wong CHY, Jenne CN, Petri B, et al. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 2013; 14: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verschoor A, Neuenhahn M, Navarini AA, et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8alpha+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol 2011; 12: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 100.Broadley SP, Plaumann A, Coletti R, et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe 2016; 20: 36–48. [DOI] [PubMed] [Google Scholar]
- 101.Gawaz M, Fateh-Moghadam S, Pilz G, et al. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest 1995; 25: 843–851. [DOI] [PubMed] [Google Scholar]
- 102.Page C, Pitchford S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int Immunopharmacol 2013; 17: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 103.Htun P, Fateh-Moghadam S, Tomandl B, et al. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke 2006; 37: 2283–2287. [DOI] [PubMed] [Google Scholar]
- 104.Moore KL, Varki A, McEver RG. GMP-140 binds to a glycoprotein receptor on human neutrophils: Evidence for a lectin-like interaction. J Cell Biol 1991; 112: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larsen E, Palabrica T, Sajer S, et al. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15). Cell 1990; 63: 467–474. [DOI] [PubMed] [Google Scholar]
- 106.Mayadas TN, Johnson RC, Rayburn H, et al. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 1993; 74: 541–554. [DOI] [PubMed] [Google Scholar]
- 107.Ley K, Bullard DC, Arbonés ML, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med 1995; 181: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Sanctis GT, Wolyniec WW, Green FH, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol 1997; 83: 681–687. [DOI] [PubMed] [Google Scholar]
- 109.Mulligan MS, Polley MJ, Bayer RJ, et al. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest 1992; 90: 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kornerup KN, Salmon GP, Pitchford SC, et al. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol 2010; 109: 758–767. [DOI] [PubMed] [Google Scholar]
- 111.Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: A critical role for platelet-derived P-selectin in the absence of rolling. J Immunol 2006; 176: 6991–6999. [DOI] [PubMed] [Google Scholar]
- 112.Singbartl K, Forlow SB, Ley K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J 2001; 15: 2337–2344. [DOI] [PubMed] [Google Scholar]
- 113.Sreeramkumar V, Adrover J, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (80-) 2014; 346: 1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Langer HF, Choi EY, Zhou H, et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res 2012; 110: 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frenette PS, Denis CV, Weiss L, et al. P-selectin glycoprotein ligand 1 (Psgl-1) is expressed on platelets and can mediate platelet–endothelial interactions in vivo. J Exp Med 2000; 191: 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frenette PS, Johnson RC, Hynes RO, et al. Platelets roll on stimulated endothelium in vivo: An interaction mediated by endothelial P-selectin. Proc Natl Acad Sci USA 1995; 92: 7450–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romo GM, Dong JF, Schade AJ, et al. The glycoprotein Ib-IX-v complex is a platelet counterreceptor for P-selectin. Blood 1999; 190: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dole VS, Bergmeier W, Mitchell HA, et al. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: Role of P-selectin. Blood. 23005; 106: 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evangelista V, Pamuklar Z, Piccoli A, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood 2007; 109: 2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med 2000; 192: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diacovo TG, DeFougerolles AR, Bainton DF, et al. A functional integrin ligand on the surface of platelets: Intercellular adhesion molecule-2. J Clin Invest 1994; 94: 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuijper PH, Gallardo Tores HI, Lammers JW, et al. Platelet associated fibrinogen and ICAM-2 induce firm adhesion of neutrophils under flow conditions. Thromb Haemost 1998; 80: 443–448. [PubMed] [Google Scholar]
- 123.Nurden AT, Nurden P. Congenital platelet disorders and understanding of platelet function. Br J Haematol 2014; 165: 165–178. [DOI] [PubMed] [Google Scholar]
- 124.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest 1997; 100: 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henn V, Slupsky JR, Gräfe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998; 391: 591–594. [DOI] [PubMed] [Google Scholar]
- 126.Li G, Sanders JM, Bevard MH, et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am J Pathol 2008; 172: 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Büchner K, Henn V, Gräfe M, et al. CD40 ligand is selectively expressed on CD4+ T cells and platelets: Implications for CD40-CD40L signalling in atherosclerosis. J Pathol 2003; 201: 288–295. [DOI] [PubMed] [Google Scholar]
- 128.Peters MJ, Dixon G, Kotowicz KT, et al. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol 1999; 106: 391–399. [DOI] [PubMed] [Google Scholar]
- 129.Nagata K, Tsuji T, Todoroki N, et al. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62). J Immunol 1993; 151: 3267–3273. [PubMed] [Google Scholar]
- 130.Bengtsson T, Zalavary S, Stendahl O, et al. Release of oxygen metabolites from chemoattractant-stimulated neutrophils is inhibited by resting platelets: Role of extracellular adenosine and actin polymerization. Blood 1996; 87: 4411–4423. [PubMed] [Google Scholar]
- 131.Brinkmann V. Neutrophil extracellular traps kill bacteria. Science (80.) 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 132.Carestia A, Kaufman T, Schattner M. Platelets: New bricks in the building of neutrophil extracellular traps. Front Immunol 2016; 7: 271–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012; 18: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malawista SE, Van Blaricom G, Breitenstein MG. Cryopreservable neutrophil surrogates. Stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J Clin Invest 1989; 83: 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McDonald B, Urrutia R, Yipp BG, et al. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012; 12: 324–333. [DOI] [PubMed] [Google Scholar]
- 136.Kraemer BF, Campbell RA, Schwertz H, et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog 2011; 7: e1002355–e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 2012; 122: 2661–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rossaint J, Herter JM, Van Aken H, et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 2014; 123: 2573–2584. [DOI] [PubMed] [Google Scholar]
- 139.Gaertner F, Ahmad Z, Rosenberger G, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 2017; 171: 1368–1382. e23. [DOI] [PubMed] [Google Scholar]
- 140.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol Immunol 2010; 47: 2170–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peerschke EIB, Murphy TK, Ghebrehiwet B. Activation-dependent surface expression of gC1qR / p33 on human blood platelets. Thromb Haemost 2003; 89: 331–339. [PubMed] [Google Scholar]
- 142.Ghebrehiwet B, Lim BL, Peerschke EIB, et al. Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular “heads” of C1q. J Exp Med 1994; 179: 1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ghebrehiwet B, Lim BL, Kumar R, et al. gC1q-R/p33, a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection. Immunol Rev 2001; 180: 65–77. [DOI] [PubMed] [Google Scholar]
- 144.Nguyen T, Ghebrehiwet B, Ellinor IB. Staphylococcus aureus Protein A recognizes platelet gC1qR / p33: A novel mechanism for staphylococcal interactions with platelets. Infect Immun 2000; 68: 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamad OA, Ekdahl KN, Nilsson PH, et al. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost 2008; 6: 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ford I, Douglas CW, Heath J, et al. Evidence for the involvement of complement proteins in platelet aggregation by Streptococcus sanguis NCTC 7863. Br J Haematol 1996; 94: 729–739. [DOI] [PubMed] [Google Scholar]
- 147.Loughman A, Fitzgerald JR, Brennan MP, et al. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol Microbiol 2005; 57: 804–818. [DOI] [PubMed] [Google Scholar]
- 148.Hamad OA, Nilsson PH, Wouters D, et al. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol 2010; 184: 2686–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 1986; 68: 875–880. [PubMed] [Google Scholar]
- 150.Verschoor A, Langer HF. Crosstalk between platelets and the complement system in immune protection and disease. Thromb Haemost 2013; 110: 910–919. [DOI] [PubMed] [Google Scholar]
- 151.Tedesco F, Pausa M, Nardon E, et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med 1997; 185: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morigi M, Galbusera M, Gastoldi S, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol 2011; 187: 172–180. [DOI] [PubMed] [Google Scholar]
- 153.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood 2017; 129: 2829–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cohen YC, Djulbegovic B, Shamai-Lubovitz O, et al. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med 2000; 160: 1630–1638. [DOI] [PubMed] [Google Scholar]
- 155.Arnold DM. Platelet count or bleeding as the outcome in ITP trials. Am J Hematol 2012; 87: 945–946. [DOI] [PubMed] [Google Scholar]
- 156.Lax S, Rayes J, Wichaiyo S, et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am J Physiol Lung Cell Mol Physiol 2017; 44: ajplung.00023.2017–ajplung.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rayes J, Jadoui S, Lax S, et al. The contribution of platelet glycoprotein receptors to inflammatory bleeding prevention is stimulus and organ dependent. Haematologica 2018; 103 submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boulaftali Y, Hess PR, Kahn ML, et al. Platelet Immunoreceptor Tyrosine-Based Activation Motif (ITAM) signaling and vascular integrity. Circ Res 2014; 114: 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deppermann C, Kraft P, Volz J, et al. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood 2017; 129: 1702–1707. [DOI] [PubMed] [Google Scholar]
- 160.Kleinschnitz C, Pozgajova M, Pham M, et al. Targeting platelets in acute experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 2007; 115: 2323–2330. [DOI] [PubMed] [Google Scholar]
- 161.Savage JS, Williams CM, Konopatskaya O, et al. Munc13-4 is critical for thrombosis through regulating release of ADP from platelets. J Thromb Haemost 2013; 11: 771–775. [DOI] [PubMed] [Google Scholar]
- 162.Guerrero JA, Bennett C, van der Weyden L, et al. Gray platelet syndrome: Pro-inflammatory megakaryocytes and α-granule loss cause myelofibrosis and confer resistance to cancer metastasis in mice. Blood 2014; 124: 3624–3635. [DOI] [PubMed] [Google Scholar]
- 163.Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013; 24: 1–8. [DOI] [PubMed] [Google Scholar]
- 164.Ho-Tin-Noé B, Goerge T, Cifuni SMS, et al. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res 2008; 68: 6851–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Higgins SJ, Purcell LA, Silver KL, et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci Transl Med 2016; 8: 358ra128–358ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 2000; 20: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hillgruber C, Pöppelmann B, Weishaupt C, et al. Blocking neutrophil diapedesis prevents hemorrhage during thrombocytopenia. J Exp Med 2015; 212: 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rayes J, Watson SP. Platelet GPVI repairs its own damage. Blood 2015; 126: 933–934. [DOI] [PubMed] [Google Scholar]