Abstract
Human breast milk has been shown to reduce the incidence of necrotizing enterocolitis (NEC). Breast milk has many components (immunoglobulins, proteins, fat, and, of recent interest, exosomes), but the specific component that affords protection against NEC is not known. Exosomes are small-nanometer vesicles that are rich in protein, lipid, and microRNA. Here, we hypothesized that human breast milk-derived exosomes can protect intestinal epithelial cells (IECs) from cell death. Human breast milk was collected, separated using ultracentrifugation, and quantified using NanoSight tracking analysis. Purified exosomes were added to IECs that had been treated with varying concentrations of H2O2. Cells were then incubated overnight with the human breast milk-derived exosomes and assessed for cell viability. Western blot analysis showed that both clathrin and CD81 were present in the purified sample. Oxidative stress using H2O2 caused a 50% decrease in cell viability and human breast milk-derived exosomes had a protective effect in IECs. In the presence of H2O2, exosomes had a statistically significant protective effect. The protection seen by human breast milk-derived exosomes was not attenuated by cycloheximide. Thus, human breast milk-derived exosomes allow IECs to be protected from oxidative stress, but the mechanism is still not clear. Exosomes derived from human breast milk are an attractive treatment concept for children with intestinal injury.
Keywords: Necrotizing enterocolitis, breast milk, exosomes, intestinal epithelial cells
Introduction
Necrotizing enterocolitis (NEC) is one the leading causes of morbidity and mortality among neonates.1–3 NEC can affect thousands of premature neonates per year, making it the leading cause of gastrointestinal surgical emergencies in neonates. In the United States, the incidence is around two per 1000 live births.4 NEC can occur in up to 10% of babies weighing less than 1500 g.5 The mortality in severe NEC can be as high as 50%, leading to a high number of neonatal deaths per year.4 Many of the surviving neonates of severe NEC are left with devastating morbidity, which can include short bowel syndrome and abnormal neurodevelopmental outcomes.6 The etiology of NEC is not completely known, but possible causes include: pathogenic bacterial colonization;7 immaturity of the circulatory, digestive, and immune system; hypoxia-ischemia; and enteral feedings.8 Therapeutic strategies are desperately needed for this devastating condition.
Breast milk (compared with infant formula) has been shown to be protective against NEC.9–12 Its benefits to newborns include neutralizing and killing luminal pathogens, developing the immune system,13 promoting microbial/host intestinal immunity,14 and reducing the incidence of food and environmental allergies.15 The mechanism by which breast milk protects neonates from NEC is still not well understood.
One of the leading potential protective mechanisms could be related to the high number of exosomes in breast milk. Over the past few years, exosomes have been identified to have an impact on how we understand intercellular communication. Exosomes are small endocytic vesicular bodies (30–200 nm) released from the plasma membrane of cells.16 They play a critical role in many physiological processes such as cell communication, therapeutic applications,17,18 and viral entry.19,20 They are found in numerous body fluids such as saliva, urine, plasma, and breast milk.21 A recent study of proteomics in breast milk-derived exosomes suggested that these vesicles can support the development of the gastrointestinal and immune system.22
In an in vivo model of NEC, exosomes derived from bone marrow-derived mesenchymal stem cells were protective.23 Also, in an in vitro model using intestinal epithelial cells (IECs), it was shown that exosomes derived from murine breast milk increased cell growth and proliferation.24 To date, no studies have looked at human breast milk-derived exosomes to determine if they have a protective effect on the intestinal epithelial compartment. In our present study, we aimed to show the protective effect of human breast milk-derived exosomes. Our hypothesis was that IECs would be protected from oxidative stress by human breast milk-derived exosomes. This study provides useful preclinical data that support investigating human breast milk-derived exosomes as a therapeutic option to decrease the incidence and severity of NEC in at-risk neonates.
Materials and methods
Human breast milk collection
Breast milk was collected in the University of Alabama at Birmingham (UAB) Regional Intensive Care Unit. Scavenged samples were collected after breast milk was thawed for patient use and the patient was not able to take the whole feeding. These feedings are normally discarded but were collected for research purposes. Samples were collected and stored at −80℃ until time for the experiments. Collection was approved by the UAB Internal Review Board Protocol N160203002.
Electromagnetic imaging
A 109 sample of exosomes was prepared for electron microscopy (EM). Samples for analysis were processed at the UAB Cryo-EM facility. The core EM facility uses an FEI Tecnai F20 200 kV field-emission gun transmission electron microscope. Images were collected using a 4 k × 4 k-pixel Gatan charge-coupled device camera.
Exosome isolation
Breast milk was centrifuged twice at 3000 g for 10 min at 25℃. The fat layer was aspirated and supernatant transferred to a new tube. A third spin was performed at 5000 g for 30 min at 25℃. The fat layer was aspirated from the sample and the supernatant transferred to a new tube. Breast milk was then syringe filtered with a 0.22 µm filter collected and prepared for the ultracentrifuge. A final spin at 32,000 g for 70 min at 4℃ was done and the pellet was collected and re-suspended in sterile PBS.
Exosome visualization
A nanoparticle tracking analysis system (NanoSight LM10, Malvern Instruments Ltd., UK) was used to determine particle size and particle concentration per milliliter.
IEC-6 cells
IEC-6 cells were purchased from Sigma-Aldrich. RPMI 1640 medium (Mediatech Inc., Manassas, VA) was used in all experiments, supplemented with 8 mM glutamate, 1% penicillin/streptomycin, and 5% FCS. IEC-6 cells were grown to 80% confluence. Cells were seeded at density of 3 × 106 and plated on six-well tissue culture dishes. Experimental cells were used between passages 8 and 12. Cells were treated with 100 or 200 µM doses of H2O2 and varying concentrations of breast milk-derived exosomes. IEC-6 cells were imaged using an Olympus inverted microscope.
Cell count
An Invitrogen Countess automated cell counter was used to measure cell viability. In brief, 10 µl of trypan blue 0.4% solution was added to the same volume of sample and mixed. Ten μL of the mixture was added to the slide and cells were counted.
Protein concentration assay
The Bio-Rad DC Protein Assay was used to determine the protein concentrations of the samples. The absorbencies were read on a spectrophotometer at 750 nm.
Western blot
Cells were triturated and pelleted then lysed with radioimmunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, pH 8.0). Fifteen ml of protein was collected and run on an SDS 12% gel. Samples were run at 120 V for 60 min. Gel was transferred to nitrocellulose using the Trans Blot Semi-Dry apparatus for 30 min at 15 V. The blot was washed three times with TBS–0.1% Tween (TBST). The membrane was blocked for 60 min with 3% milk solution. Primary Ab (CD81 and clathrin) was added at 1:1000 dilutions in TBST, incubated for 1 h at 21℃ (room temperature) on a rotator, and then washed three times. Biotin anti-rabbit or anti-mouse secondary Ab (Invitrogen), along with streptavidin HRP (Invitrogen) at a 1:1000 dilution of each in TBST, was incubated for 1 h at room temperature on a rotator. The blot was washed three times for 15 min each and developed using the Novex ECL Chemiluminescent and film with Kodak developer.
Statistics
Two-tailed Student’s t test and one-way ANOVA with Dunnett’s multiple-comparisons post hoc test were used to analyze data (GraphPad Prism 7).
Results
Characterization of human breast milk-derived exosomes
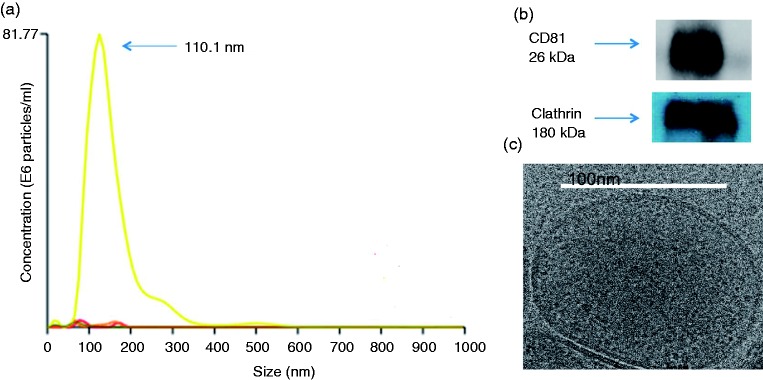
Purified exosomes had a mean size less than 200 nm (Figure 1a) as seen by NanoSight tracking analysis. Western blot analysis was performed in the samples to determine if markers of exosomes were in this fraction. CD81 and clathrin were both present in the purified sample (Figure 1b). Particles were in the range of 3–9 × 108 particles per ml; mean size was 74.6 ± 48.0 nm.
Figure 1.
Characteristics of human breast milk. (a) Average size of exosome particles plotted as the number of particles per ml. (b) Western blot representation of CD81 and clathrin represented at approximately 26 and 180 kDA, respectively. (c) A 109 sample of exosomes was prepared for EM. Samples for analysis were processed at the UAB Cryo-EM facility. The core EM facility uses an FEI Tecnai F20 200 kV field-emission gun transmission electron microscope. Images were collected using a 4 k × 4 k-pixel Gatan charge-coupled device camera.
Human breast milk-derived exosomes and oxidative stress
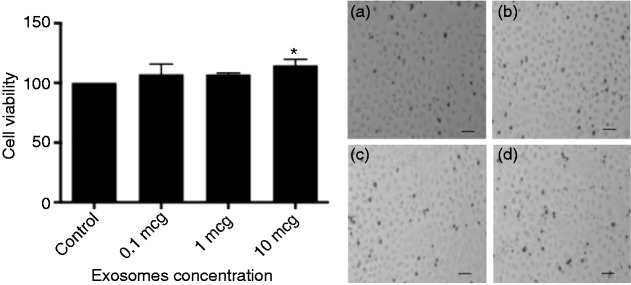
The direct effect of exosome viability on IECs was not observed until exosome concentrations were at 10 µg with a statistical significance of P < 0.0403 (Figure 2). To assess whether human breast milk-derived exosomes could protect against oxidative stress, we used H2O2 at 100 µM. There was minimal cell death with 100 µM H2O2 and 0.1 µg of exosomes, but a protective trend at both 1 and 10 µg of exosomes (Figure 3). We increased the concentration to 200 µM H2O2 and observed 50% cell death without exosomes, which was statistically significant compared with control. Cells treated with 1 and 10 µg of exosomes in the presence of H2O2 had complete recovery and these results were statistically significant, P < 0.05 for 1 µg and P < 0.01 for 10 µg (Figure 4).
Figure 2.
Viability of IEC-6 cells treated with increasing doses of human breast milk-derived exosomes. *P < 0.05 using post hoc Dunnett's test. Bright-field images of IEC-6 cells were taken at 10× magnification. (a) Control, (b) exosome concentration of 0.1 µg, (c) exosome concentration of 1 µg, (d) exosome concentration of 10 µg.
Figure 3.
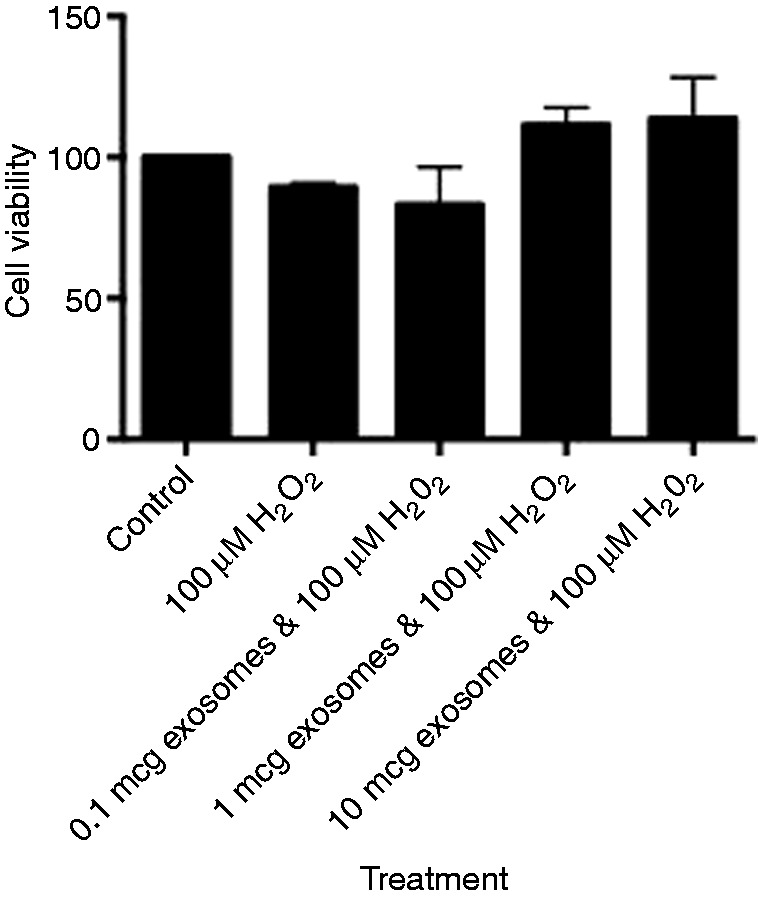
Viability of IEC-6 cells treated with 100 μM H2O2 with increasing concentrations of human breast milk-derived exosomes.
Figure 4.
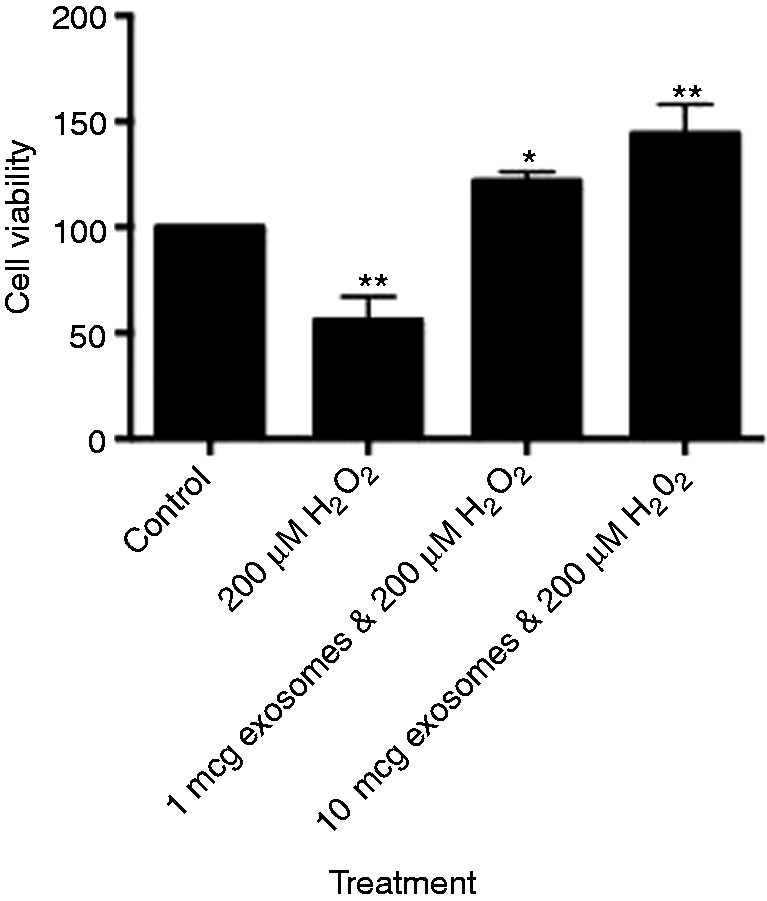
Viability of IEC-6 cells treated with 200 μM H2O2 with increasing concentrations of human breast milk-derived exosomes. *P < 0.05, **P < 0.01 using post hoc Dunnett's test.
Protective mechanism and protein synthesis
One potential mechanism involved in exosome protection of IEC-6 cells could be linked to gene regulation requiring new protein synthesis. A well-known inhibitor of protein synthesis (cycloheximide) was used in similar experiments as above. Results were similar to experiments without cycloheximide. Concentrations at 1 and 10 µg had the most dramatic effect, but were only significant at 10 µg (Figure 5).
Figure 5.
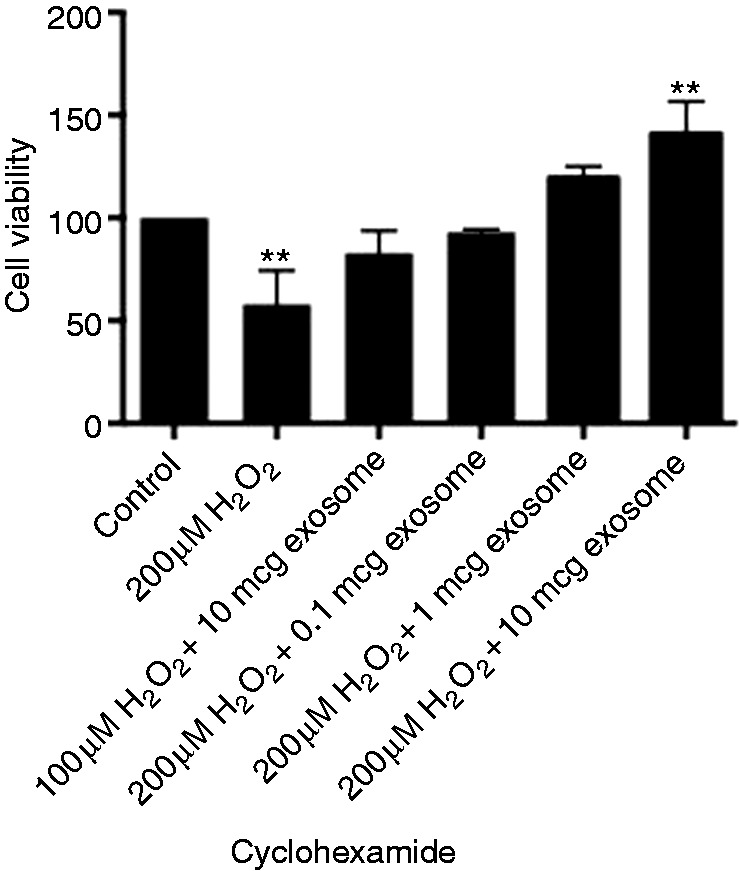
Viability of IEC-6 cells treated with 100 or 200 μM H2O2 with increasing concentrations of human breast milk-derived exosomes and cycloheximide. **P < 0.01 using post hoc Dunnett's test.
Discussion
The work from this study demonstrates that human breast milk-derived exosomes reduce oxidative stress-related injury on IECs. The use of IEC-6 cells is a well-characterized and reliable means to clarify epithelial/host defense mechanisms and the effect of oxidative stress on immune dysfunction. In the present system, we used H2O2 as an inducer of oxidative stress in the intestinal epithelium. Oxidative stress on the intestinal epithelial compartment is one potential mechanism leading to NEC in neonates.25 For the first time, we have shown that human breast milk-derived exosomes are a potential therapy to decrease cell toxicity in IECs. The study also confirms the ability to isolate exosomes from stored breast milk samples and demonstrates their functionality. The protective effect of exosomes can be found in several cell types. The specifics are incompletely understood, but an emerging body of work suggests that IECs themselves also have the potential to secrete exosomes.26 It is unclear if breast milk-derived and intestine-derived exosomes have the same immune profile. It is also not known what effect breast milk-derived exosomes have on IEC secretion of endogenous exosomes. These subjects are the focus of ongoing research.
In the context of NEC pathogenesis, many of the protective properties of exosomes on the intestinal epithelial compartment make it an ideal therapeutic target. Exosome-mediated delivery of epigenetic modifications have been found to have an impact on barrier function,27 pathogenic microbial luminal sensing,28 and the up-regulation of antimicrobial peptides in intestinal crypts.29 All of these factors have been implicated in NEC pathogenesis, including oxidative stress.30,31 In the present study, we used H2O2 to induce oxidative stress on IECs. The focus in our laboratory has been to better understand the protective mechanisms of oxidative stress-induced cellular damage.32 Oxidative stress has also been studied as a potential mechanism in NEC pathogenesis.33,34 These mechanisms are not clear, making the protective role of breast milk-derived exosomes appealing. The IEC-6 cell line is a small IEC line. In NEC, the small intestine is the primary location of intestinal injury.35 It is unclear if breast milk-derived exosomes are able to provide protective benefits directly to the intestinal crypt or if their effects are mediated through other intestinal cell types. Future studies in animal models will further clarify this.
The biology of exosomes is in its infancy and it is unclear if exosome production changes during cellular stress. Murine breast exosomes have been shown to promote IEC growth.24 Here, we show that intestinal cells can be protected by human breast milk-derived exosomes. We also show that, despite the species difference, human exosomes were still able to protect murine IECs. The conserved protection offered by human exosomes suggests that this mechanism could be a powerful defense mechanism against injury. The protective effect is most likely multifactorial, but we do show that the effect appears to be able to acutely protect cells. When protein synthesis was inhibited with cycloheximide, the protective effect persisted. This is of particular interest because it argues that the exosome has what it needs to offer this protection without altering any protein levels. Therefore, to determine which single or multiple factor is able to confer protection is of great importance.
Exosomes have fundamental roles in delivering functional messages to cells both in an autocrine and paracrine manor. Stressed cells may have different messages to other cells or may have decreased trophic factors emitted. It is unclear if supplemental exosomes restore this system. Breast milk-derived exosomes offer a potential therapy that may improve early gut integrity. The importance of exosomes in early development is not well understood. Thus, understanding their role in cytoprotection could lead to alternative treatment options. The concentration of exosomes delivered and the timing of their delivery will be vital to understanding this process.
An alternative protective mechanism of exosomes on IECs is the regulation of apoptosis. Oxidative stress is one of the major factors causing cell apoptosis resulting from increased expression of p53.36 The effect of oxidative stress on p53-regulated epithelial cell apoptosis is well established;37 however, the effect of the tumor suppressor p53 on NEC pathogenesis is not clear. A recent study by Hackam et al. demonstrated that p53 regulates epithelial damage in in a TLR4-dependent mechanism.38 Exosome administration may provide a targeted therapy for this protective mechanism. The means by which exogenous exosomes may regulate p53 could be facilitated by microRNAs, cellular fragments that prevent the binding of a particular protein. Milk exosomes deliver microRNA-125b, which can target and inactivate the apoptosis-inducer p53.39 microRNA-148a promotes growth and survival of IECs through promoter demethylation. Exosomes have been shown to increase villus height in a murine model.40 This may be a unexplored mechanism of NEC prevention and will be the focus of future exploration. In addition, breast milk-derived miRNA-125b, miRNA-30d, and miRNA-25 have been shown to alter cellular kinetics, apoptosis, and metabolism.41
In conclusion, our study confirms that breast milk-derived exosomes are protective against cell toxicity induced by H2O2. The study also shows that protein synthesis does not play the only role in the observed protection. This helps us to understand that the exosomes are capable of protecting cells with their own cargo. Human breast milk offers a potentially plentiful source of exosomes that could one day be used in neonates with intestinal injury. The exosomes in this work were given at the time of the injury and could give the same benefit clinically. It is not known which patients will develop intestinal pathologies such as NEC, so the treatment would most likely be after the injury or at the time the injury is occurring. Even after the onset of NEC, patients are not given breast milk. Along this line of thinking, exosomes could be delivered at any time to the neonate directly into the gastrointestinal tract. More studies will need to be done, but this is a promising line of research and utilizes a natural and readily available source of protection.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santulli TV, Schullinger JN, Heird WC, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics 1975; 55: 376–387. [PubMed] [Google Scholar]
- 3.Tanner SM, Berryhill TF, Ellenburg JL, et al. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 2015; 185: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol 2003; 8: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisk PM, Lovelady CA, Dillard RG, et al. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007; 27: 428–433. [DOI] [PubMed] [Google Scholar]
- 6.So S, Patterson C, Gold A, et al. Early neurodevelopmental outcomes of infants with intestinal failure. Early Hum Dev 2016; 101: 11–16. [DOI] [PubMed] [Google Scholar]
- 7.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol 2008; 42 Suppl 2: S46–S52. [DOI] [PubMed] [Google Scholar]
- 8.Wu SF, Caplan M, Lin HC. Necrotizing enterocolitis: old problem with new hope. Pediatr Neonatol 2012; 53: 158–163. [DOI] [PubMed] [Google Scholar]
- 9.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990; 336: 1519–1523. [DOI] [PubMed] [Google Scholar]
- 10.Schanler RJ, Abrams SA. Postnatal attainment of intrauterine macromineral accretion rates in low birth weight infants fed fortified human milk. J Pediatr 1995; 126: 441–447. [DOI] [PubMed] [Google Scholar]
- 11.Schanler RJ, Burns PA, Abrams SA, et al. Bone mineralization outcomes in human milk-fed preterm infants. Pediatr Res 1992; 31: 583–586. [DOI] [PubMed] [Google Scholar]
- 12.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999; 103: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 13.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005; 135: 1–4. [DOI] [PubMed] [Google Scholar]
- 14.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 2007; 61: 2–8. [DOI] [PubMed] [Google Scholar]
- 15.Verhasselt V, Milcent V, Cazareth J, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med 2008; 14: 170–175. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579. [DOI] [PubMed] [Google Scholar]
- 17.Stremersch S, Marro M, Pinchasik BE, et al. Identification of individual exosome-like vesicles by surface enhanced raman spectroscopy. Small 2016; 12: 3292–3301. [DOI] [PubMed] [Google Scholar]
- 18.Stremersch S, Vandenbroucke RE, Van Wonterghem E, et al. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release 2016; 232: 51–61. [DOI] [PubMed] [Google Scholar]
- 19.Sims B, Farrow AL, Williams SD, et al. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int J Nanomedicine 2017; 12: 4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims B, Gu L, Krendelchtchikov A, et al. Neural stem cell-derived exosomes mediate viral entry. Int J Nanomedicine 2014; 9: 4893–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasser C, Alikhani VS, Ekstrom K, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 2011; 9: 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Herwijnen MJ, Zonneveld MI, Goerdayal S, et al. Comprehensive proteomic analysis of human milk-derived extracellular vesicles unveils a novel functional proteome distinct from other milk components. Mol Cell Proteomics 2016; 15: 3412–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rager TM, Olson JK, Zhou Y, et al. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg 2016; 51: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hock A, Miyake H, Li B, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg 2017; 52: 755–759. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Zani A, Martin Z, et al. Intestinal epithelial cell injury is rescued by hydrogen sulfide. J Pediatr Surg 2016; 51: 775–778. [DOI] [PubMed] [Google Scholar]
- 26.Smythies LE, Smythies JR. Exosomes in the gut. Front Immunol 2014; 5: 104–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guichard A, Cruz-Moreno B, Aguilar B, et al. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe 2013; 14: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Cacho E, Gallego M, Lillehoj HS, et al. Tetraspanin-3 regulates protective immunity against Eimeria tenella infection following immunization with dendritic cell-derived exosomes. Vaccine 2013; 31: 4668–4674. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Gong AY, Roth AL, et al. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 2013; 9: e1003261–e1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm Infant. Neoreviews 2011; 12: e517–e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitkamp JH, Koyama T, Rock MT, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2012; 62: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crockett S, Clarke M, Reeves S, et al. Cystine glutamate exchanger upregulation by retinoic acid induces neuroprotection in neural stem cells. Neuroreport 2011; 22: 598–602. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Christley S, Alverdy JC, et al. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg Infect (Larchmt) 2012; 13: 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Wang Q, Evers BM, et al. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediat Res 2005; 58: 1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Ortega G, Camp M, et al. Necrotizing enterocolitis requiring surgery: outcomes by intestinal location of disease in 4371 infants. J Pediatr Surg 2011; 46: 1475–1481. [DOI] [PubMed] [Google Scholar]
- 36.Simon K, Mukundan A, Dewundara S, et al. Transcriptional profiling of the age-related response to genotoxic stress points to differential DNA damage response with age. Mech Ageing Dev 2009; 130: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budanov AV. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid Redox Signal 2011; 15: 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neal MD, Sodhi CP, Jia H, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 2012; 287: 37296–37308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le MT, Shyh-Chang N, Khaw SL, et al. Conserved regulation of p53 network dosage by microRNA-125b occurs through evolving miRNA-target gene pairs. PLoS Genet 2011; 7: e1002242–e1002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen T, Xie MY, Sun JJ, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep 2016; 6: 33862–33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodo C, Melnik GS. DNA methyltransferase 1-targeting miRNA-148a of dairy milk: a potential bioactive modifier of the human epigenome. Functional Foods in Health and Disease 2017; 7: 671–687. [Google Scholar]