Abstract
Here we investigated the influence of LPS-induced gut injury on antioxidant homeostasis, mitochondrial (mt) function and the level of mitophagy in piglets. The results showed that LPS-induced intestinal injury decreased the transepithelial electrical resistance, increased the paracellular permeability of F1TC dextran 4 kDa, and decreased the expression of claudin-1, occludin and zonula occludens-1 in the jejunum compared with the control group. LPS decreased the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and increased the content of malondialdehyde in the jejunum. Meanwhile, the expression of SOD-related genes (Cu/Zn-SOD, Mn-SOD) and GSH-Px-related genes (GPX-1, GPX-4) declined in LPS-challenged pigs compared with the control. LPS also increased TNF-α, IL-6, IL-8 and IL-1β mRNA expression. LPS induced mt dysfunction, as demonstrated by increased reactive oxygen species production and decreased membrane potential of intestinal mitochondria, intestinal content of mt DNA and activities of the intestinal mt respiratory chain. Furthermore, LPS induced an increase in expression of mitophagy related proteins, PTEN-induced putative kinase (PINK1) and Parkin in the intestinal mitochondria, as well as an enhancement of the ratio of light chain 3-II (LC3-II) to LC3-I content in the jejunal mucosa. These results suggested that LPS-induced intestinal injury accompanied by disrupted antioxidant homeostasis, caused mt dysfunction and triggered mitophagy.
Keywords: LPS, intestinal injury, mitochondrial function, mitophagy, piglets
Introduction
The intestine is not only a major digestive and absorptive organ for nutrients, is it also an effective barrier against various infectious agents.1 Damage to intestinal integrity increases gut permeability, leading to bacterial translocation and subsequent intestinal infection.2 Therefore, improving restoration of the intestinal barrier may be beneficial to the health and survival of neonatal humans and animals.
Restoration of the intestinal barrier has a high demand for energy.3 Mitochondria are the intracellular organelles that provide most of the energy consumed.4 However, mitochondria energy metabolism is simultaneously an important source of reactive oxygen species (ROS) and an important target for the damaging effects of ROS, and ultimately results in ROS overproduction by mitochondria.5 ROS overproduction contributes to mitochondrial (mt) dysfunction, as well as causing disruption to ATP synthesis and activation of cell death pathways.6 Cells have developed a defense mechanism to selectively sequestrate and degrade dysfunctional mitochondria before they can harm the cell.7 This defence mechanism is called mt autophagy, or mitophagy. It would be of great interest to determine the effect of acute intestinal disruption on mt function and the level of mitophagy, for which no data are currently available. In this study, we utilized a model that induced acute intestinal disruption by injecting LPS.8,9 We aimed to determine whether mt dysfunction and mitophagy are involved in the intestinal barrier alterations observed after LPS injection. We hypothesized that LPS injection might influence intestinal barrier function, antioxidant balance, inflammatory status and mitochondria function, and trigger mitophagy.
Materials and methods
Animals and experimental design
All procedures were approved by the Zhejiang University Animal Care and Use Committee. Twelve male Duroc × Landrace × Yorkshire 35-d-old pigs (weaned at 21 d of age), with an average body mass (BM) of 9.8 kg, were allotted to two treatments of six piglets each: a challenged group and a control group. At the beginning of the experiment, the challenged piglets were injected i.p. with Escherichia coli LPS (E. coli serotype 055: B5, Sigma Chemicals, St. Louis, MO, USA) at 100 µg/kg BM and the control group was injected with the same volume of 0.9% (w/v) NaCl solution. The dose of LPS was chosen to cause acute intestinal injury in accordance with previous studies.10,11 Pigs were individually housed in pens (1.8 × 1.1 m2) in an environmentally controlled nursery barn. The piglets had access to feed and water ad libitum.
Sample collection
All piglets were killed after 7 d according to previous studies.8,9,12 Piglets were euthanized with an intravenous injection of sodium pentobarbital (200 mg/kg BM) and the gastrointestinal tract was removed quickly. Segments of proximal jejunum were harvested immediately post mortem, and prepared for Ussing chamber studies and isolation of intestinal mitochondria. Mucosal scrapings from the adjacent jejunum were collected, frozen rapidly in liquid nitrogen and stored at –80℃.
Ex vivo Ussing Chamber to Measure Intestinal barrier function
Tissues were mounted in an EasyMount Ussing chamber system (model VCC MC6; Physiologic Instruments, San Diego, CA) as previously described.2 Briefly, data was collected automatically using Acquire and Analyze software (Physiologic Instruments, San Diego, CA, USA). Transepithelial electrical resistance (TER) was recorded at 15-min intervals over 1 h after a 15-min equilibration period. The fluxe of F1TC dextran 4 kDa (FD4) was used to evaluate the epithelial barrier function as previously described.13,14 Probe (400 µg/ml FD4; FD4-100MG; Sigma-Aldrich, St. Louis, MO) was added into the mucosal side. The concentration of FD4 in the serosal side was measured in a fluorescence microplate reader (FL × 800; BioTek Instruments, Inc., Winooski, VT, USA). The excitation and extinction wavelengths were 492 and 520 nm, respectively.
Isolation of mitochondria
All procedures for mt isolation were conducted at 4℃. Mitochondria were isolated from jejunum mucosa using a Tissue Mitochondria Isolation Kit (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer’s instructions.
Intestinal mt ROS assay
Isolated intestinal mitochondria were treated with 2′,7′-dichlorohydro-fluorescein diacetate (DCFH-DA), which can pass through the mt membrane and is hydrolyzed by intracellular esterase. The esterases cleave DCFH-DA at the two ester bonds, producing a relatively polar and cell membrane-impermeable product, H2DCF. This non-fluorescent molecule accumulates intracellularly, and subsequent oxidation yields the highly fluorescent product DCF. Accumulation of DCF may be measured by an increase in fluorescence at 528 nm when the sample is excited at 485 nm. The isolated mitochondria (0.4 mg/ml) were treated with 2 µmol/l DCFH-DA and incubated at room temperature (24℃) for 20 min. The fluorescence intensity was detected using a fluorescence microplate reader as described.15
Intestinal mt membrane potential assay
Changes in mt membrane potential (△Ψm) were measured using a mt membrane potential assay kit with the cyanine dye JC-1 (5,50,6,60-tetrachloro-1,10,3,30-tetraethylbenzimidazolylcarbocyanine iodide) (Beyotime Institute of Biotechnology) according to the manufacturer’s instructions. Briefly, isolated mitochondria were suspended in 0.5 ml medium containing 5 mmol/l JC-1. Samples were analyzed by automatic fluorescence microplate reader (FL × 800, Bio-Tek Instruments, Inc.). The values of OD at 590 nm and 530 nm were determined by a spectrofluorometry. As the △Ψm is proportional to the ratio of OD590 nm to OD530 nm, the △Ψm was expressed as OD590 nm/OD530 nm.16,17
Analysis of mtDNA content in the jejunum
The content of mtDNA relative to nuclear genomic DNA was measured by amplifying the mt D-loop and the nuclear-encoded β-actin genes using real-time PCR assay as described previously.18 Total DNA was extracted from proximally jejunal mucosa using a TIANamp Stool DNA Kit (Tiangen Biotech, Beijing, China), following the manufacturer’s instructions. The primers used are presented in Table 1. The ratio of mtDNA to genomic DNA content was calculated as ΔCt (mt CtDloop − nuclear Ctβ − actin). Relative abundance was calculated by the 2−ΔΔCt method, where ΔΔCt = ΔCtmtDNA content in LPS-challenged pigs −ΔCtmtDNA content in control pigs.
Table 1.
Primer sequences used for real-time PCR.
| Gene | 5′-Primer (F) | 3′-Primer (R) | Accession number | Length |
|---|---|---|---|---|
| β-Actin | CTGCGGCATCCACGAAACT | AGGGCCGTGATCTCCTTCTG | DQ845171.1 | 147 |
| mt D-loop1 | GATCGTACATAGCACATATCATGTC | GGTCCTGAAGTAAGAACCAGATG | AF276923 | 198 |
| CuZnSOD2 | CAGGTCCTCACTTCAATCC | CCAAACGACTTCCASCAT | NM_001190422 | 255 |
| MnSOD3 | GGACAAATCTGAGCCCTAACG | CCTTGTTGAAACCGAGCC | NM_214127 | 159 |
| GPx 14 | TGGGGAGATCCTGAATTG | GATAAACTTGGGGTCGGT | NM_214201 | 183 |
| GPx 45 | GATTCTGGCCTTCCCTTGC | TCCCCTTGGGCTGGACTTT | NM_214407.1 | 172 |
1mt D-loop = Mitochondria DNA loop.
2CuZnSOD = Copper and zinc superoxide dismutase.
3MnSOD = Manganese-containing superoxide dismutase.
4GPx1 = Glutathione peroxidase 1.
5GPx4 = Glutathione peroxidase 4.
Determination of the activities of respiratory chain complexes I–IV in intestinal mitochondria
According to a previous report,19 mt respiratory chain complexes activities quantitative determination kits were used to evaluate the enzyme activities of complexes I (NADH-CoQ reductase), II (succinate-CoQ reductase), III (CoQ-cytochrome c reductase), and IV (cytochrome c oxidase) according to the manufacturer’s instructions (GenMed Scientifics, Shanghai, China).
mRNA expression analysis by RT-PCR
The mRNA levels of copper and zinc superoxide dismutase (Cu/Zn-SOD), manganese-containing superoxide dismutase (Mn-SOD), glutathione peroxidase 1 (GPX-1) and glutathione peroxidase 4 (GPX-4) were analyzed as described by a previous study.20 The sequence of primers used for the qPCR are shown in Table 1. Total RNA was extracted from jejunal mucosa using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s guidelines. The concentration and purity of all RNA samples were measured using a Nano Drop spectrophotometer (ND-2000; NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription using the PrimeScripte RT reagent kit (TaKaRa Biotechnology, Dalian, China) was carried out following the manufacturer’s manual. Quantitative analysis of PCR was carried out in a StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR Green Master mix (Promega, Madison, WI, USA), according to the manufacturer’s specification. Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. The 2–ΔΔCt method was used to analyze the relative expression (fold changes), calculated relative to the values from the control group. ΔΔCT was computed for each target gene from the treatment groups by subtracting the average ΔCT for the control group. The final fold differences were computed as 2–ΔΔCt for each target gene. All samples were run in triplicate. Our results showed that β-actin exhibited no difference among different time points.
Protein expression analysis by Western blot
Western blot analysis was performed according to the procedures outlined by Hu et al. and Larson-Casey et al.2,21 Briefly, after electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary Ab at 4℃ for 10 h and then with the secondary Ab for 2 h at room temperature. The primary Abs [occludin, claduin-1, zonula occludens-1 (ZO-1), light chain 3-I (LC3-I), LC3-II, Parkin, (PTEN-induced putative kinase) Pink1, VADC, β-actin] were purchased from Santa Cruz Technology Inc. (Santa Cruz, CA, USA). The secondary Ab was HRP-conjugated anti-rabbit Ab (Cell Signaling Technology, Danvers, MA, USA). Western blot was detected with an enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL, USA), photographed by a ChemiScope 3400 (Clinx Science Instruments, Shanghai, China) and analyzed using Quantity One software. β-Actin and VDAC were used as internal controls, and exhibited no differences bwteen the groups. The relative abundances of intestinal target proteins and mt target proteins were expressed as target protein/β-actin, target protein/VDAC protein ratio, respectively. The protein expression of all samples was expressed as fold changes, calculated relative to the control group.
Statistical analysis
One-way analysis of variance (ANOVA) was conducted using SPSS 20.0 statistical package (SPSS Inc., Chicago, IL). Differences among means were tested using Student’s t-test. Effects were considered significant at P < 0.05.
Results
Intestinal barrier function and tight junction expression
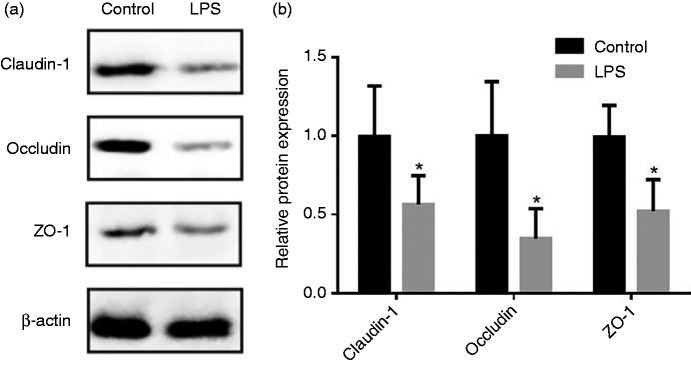
The data for jejunal barrier function of piglets are summarized in Table 2. Compared with the control group, the pigs challenged with LPS reduced (P < 0.05) TER in the jejunum, and also increased the mucosal-to-serosal flux of FD4. Figure 1 shows the protein expression of occludin, claudin-1 and ZO-1 in the jejunal mucosa. Compared with the control group, LPS challenge decreased (P < 0.05) protein levels of occludin, claudin-1 and ZO-1 in the jejunal mucosa.
Table 2.
Effect of LPS injection on jejunal barrier function of piglets.
| Items | Control1 | LPS2 | P Value |
|---|---|---|---|
| TER, Ω·cm2 | 61.64 ± 10.01 | 48.16 ± 9.73 | 0.040 |
| FD4 flux, µg cm–2 h–1 | 1.37 ± 0.60 | 2.25 ± 0.30 | 0.014 |
Data are presented as fold changes, calculated relative to the control group (means ± SD (n = 6)). TER: Transepithelial electrical resistance; FD4: F1TC dextran (4 kDa).
1Injected i.p. with 0.9% (w/v) NaCl solution.
2Injected i.p. with LPS at 100 µg/kg BM.
Figure 1.
Effect of LPS injection on the expression of tight junction (TJ) proteins of piglets in jejunal mucosa. (a) Representative blots of claudin-1, occludin, ZO-1 and β-actin in the jejunal mucosa of piglets. (b) Summary of Western blots for n = 6 pigs per treatment in LPS-injected pigs and control pigs. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. LPS group (gray bars), injected with LPS at 100 µg/kg BM. The protein expression of all samples is shown.
Activities of SOD, GSH-Px, and malondialdehyde, and expression of antioxidant enzymes-related genes in the jejunum
The activities of SOD, GSH-Px and content of malondialdehyde (MDA) in intestinal mucosa are shown in Table 3. Compared with the control group, LPS injection resulted in reducing the activities of SOD, GSH-Px in jeunum. And then, a greater (P < 0.05) content of MDA in the jejunal mucosa was observed in LPS group compared with control group. Table 4 shows the expression of antioxidant enzymes-related genes in the jejunum. Compared with the control group, the levels of Cu/Zn-SOD, MnSOD, GPX-1, GPX-4 decreased markedly (P < 0.05) after LPS challenged.
Table 3.
Effects of LPS injection on antioxidant enzyme activities and MDA content in jejunal mucosa of piglets.
| Items | Control1 | LPS2 | P Value |
|---|---|---|---|
| SOD, U/mg protein | 95.85 ± 3.13 | 63.12 ± 2.97 | < 0.001 |
| GSH-Px, U/mg protein | 72.11 ± 3.51 | 53.99 ± 3.26 | < 0.001 |
| MDA, nmol/g protein | 0.95 ± 0.16 | 1.51 ± 0.25 | < 0.001 |
Data are presented as means ± SD (n = 6). SOD: Superoxide dismutase; GSH-Px: glutathione peroxidases; MDA: malondialdehyde
1Injected i.p. with 0.9% (w/v) NaCl solution.
2Injected i.p. with LPS at 100 µg/kg BM.
Table 4.
Effects of LPS injection on expression of antioxidant enzyme-related genes in the jejunal mucosa of piglets.
| Items | Control1 | LPS2 | P Value |
|---|---|---|---|
| Cu/Zn-SOD | 1.00 ± 0.34 | 0.33 ± 0.14 | 0.003 |
| MnSOD | 1.00 ± 0.26 | 0.42 ± 0.22 | 0.002 |
| GPX-1 | 1.00 ± 0.24 | 0.52 ± 0.15 | 0.003 |
| GPX-4 | 1.00 ± 0.33 | 0.59 ± 0.22 | 0.031 |
Data are presented as means ± SD (n = 6). CuZnSOD: Copper and zinc superoxide dismutase; MnSOD: manganese-containing superoxide dismutase; GPx1: glutathione peroxidase 1; GPx4: glutathione peroxidase 4.
1Injected i.p. with 0.9% (w/v) NaCl solution.
2Injected i.p. with LPS at 100 µg/kg BM.
Pro-inflammatory cytokine mRNA
The mRNA abundance of cytokines (TNF-α, IL-6, IL-8 and IL-1β) in the jejunal mucosa of piglets is shown in Table 5. Compared with the control group, LPS increased (P < 0.05) the mRNA abundance of TNF-α, IL-6, IL-8 and IL-1β.
Table 5.
Effects of LPS injection on cytokine mRNA levels on the jejunal mucosa of piglets.
| Items | Control1 | LPS2 | P Value |
|---|---|---|---|
| TNF-α | 1.00 ± 0.21 | 2.83 ± 0.50 | < 0.0001 |
| IL-6 | 1.00 ± 0.18 | 2.71 ± 0.57 | < 0.0001 |
| IL-8 | 1.00 ± 0.26 | 3.44 ± 0.41 | < 0.0001 |
| IL-Iβ | 1.00 ± 0.14 | 1.74 ± 0.34 | 0.0006 |
Data are presented as means ± SD (n = 6).
1Injected i.p. with 0.9% (w/v) NaCl solution.
2Injected i.p. with LPS at 100 µg/kg BM.
Intestinal mt ROS production and △Ψm
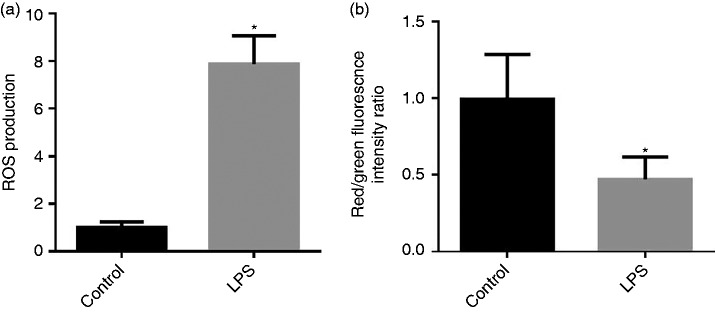
Mitochondrial ROS production and mt △Ψm in the jejunum are presented in Figure 2a and b. Compared with the control group, LPS injection increased (P < 0.05) the mt ROS production and deceased (P < 0.05) mt membrane potentials (△Ψm) in the jejunum.
Figure 2.
(a) Effect of LPS injection on intestine mitochondrial (mt) reactive oxygen species (ROS) production of piglets. (b) Effect of LPS injection on intestinal mt membrane potential change (△Ψm) of piglets. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. LPS group (gray bars), injected with LPS at 100 µg/kg BM. The ROS production and mt △Ψm were expressed as fold changes, calculated relative to the control group.
Mitochondria DNA content in jejunum and activities of intestinal mt respiratory chain complexes I–IV
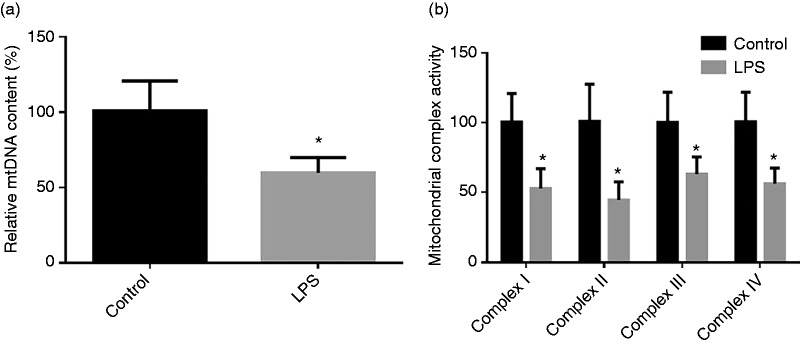
The mtDNA content in jejunum and activities of intestinal mt respiratory chain complexes after injecting LPS are shown in Figure 3. Compared with the control group, LPS injection resulted in decreased (P < 0.05) the mtDNA content in jejunum and activities of intestinal mt respiratory chain complex I–IV.
Figure 3.
(a) Effect of LPS injection on intestine content of mtDNA of piglets. (b) Effect of LPS injection on intestinal mt complex activity of piglets. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. LPS group (gray bars), injected with LPS at 100 µg/kg BM.
Expression of mitophagy related proteins
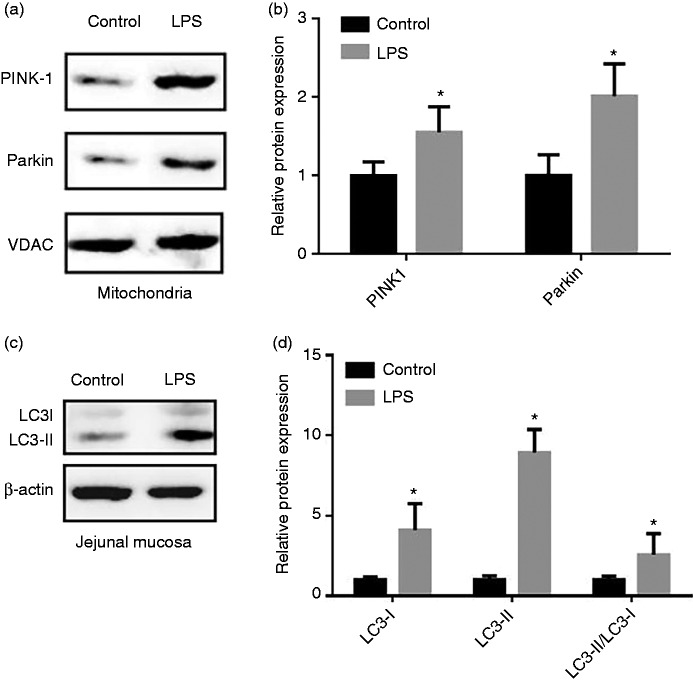
Figure 4 presents the expression of mitophagy related proteins in the mt and jejunal mucosa of piglets. In comparison with the control group, LPS challenge increased (P < 0.05) expression of PINK1 and Parkin in the jejunal mitochondria. Correspondingly, the results showed that administration of LPS enhanced (P < 0.05) both the abundance of LC3-I and LC3-II and the ratio of LC3-II to LC3-I content in the jejunal mucosa.
Figure 4.
Effect of LPS injection on the level of mitophagy-related protein of piglets. (a) Representative blots of PINK1, Parkin in the intestinal mitochondria. (b) Shows relative PINK1 and Parkin expression. (c) Representative blots of LC3-I and LC3-II in the jejunum mucosa. (d) Shows relative LC3-I and LC3-II expression. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. LPS group (gray bars), injected with LPS at 100 µg/kg BM. The protein expression of all samples was expressed as fold changes, calculated relative to the control group.
Discussion
Intestinal mucosa plays a vital role in the digestion and absorption of nutrients. It also provides a physical barrier to prevent the diffusion of pathogens, toxins and allergens from the external environment into tissues.1 Dysfunction of the intestinal barrier results in the translocation of luminal antigens into subepithelial tissues, inciting mucosal and systemic inflammatory responses.2 Hence, improving the restoration of intestinal barrier may be beneficial for the health and survival of neonatal humans and animals. In the present study, we took advantage of a model for inducing gut injury in pigs by injecting E. coli LPS.9,11,22 LPS-induced intestinal injury in piglets is a well-established animal model for studying infant nutrition and gastrointestinal physiology.8,9
An intact intestinal barrier plays a central role in preventing the penetration by luminal bacteria and dietary allergens into the mucosa.1 The intestinal epithelial barrier is the first line of defense against a hostile environment within the intestinal lumen.13 In the present experiment, the Ussing chamber technique was used to monitor intestinal barrier function in terms of TER and flux of FD4. The TER is considered to reflect the opening of TJs between epithelial cells and the paracellular permeability of the intestinal mucosa.13 The flux of intact FD4 across the intestinal epithelium occurs mainly through paracellular pathways.14 A decreased TER and increased flux of FD4 reflects an impaired intestinal barrier.13 We found that LPS injection disrupted intestinal mucosal barrier function. Similarly, previous studies demonstrated that LPS-injection results in destruction of intestinal barrier as demonstrated by high activity of diamine oxidase in the serum of piglets.23,24 The intestinal barrier is formed mainly by a layer of epithelial cells joined together by TJs that are composed of a layer of columnar epithelium and interepithelial TJs.3 Our present results showed that protein abundance of occludin, claudin-1, and ZO-1 of the LPS group was decreased compared with the control group. This result is consistent with the increased intestinal epithelial paracellular permeability.
In the current study, the redox status of jejunum was determined by evaluating the product of oxidative injury, activity of antioxidative enzymes and expression of related genes. SOD supplies an effective disproportionation of superoxide anions to H2O2 and O2– which protects the organism from oxidative damage.25 We found that SOD activity in the jejunum after injection with LPS was decreased compared with the control group. Furthermore, we found that LPS decreased the mRNA level of SOD-related genes (Cu/Zn-SOD, MnSOD), which was consistent with the jejunum SOD activity alteration pattern. The GSH-Px takes charge of the elimination of H2O2 and lipid hydroperoxides.25 The present results showed that LPS decreased the activity of GSH-Px compared with the control, which was consistent with the expression of GPX-1 and GPX-4 in the jejunal mucosa. Additionally, the product of lipid peroxidation, i.e. MDA content in jejuna mucosa, increased significantly after administration with LPS, consistent with a previous report.26 The lower antioxidant enzymes and greater MDA content verified the imbalance of oxidative and antioxidant systems induced by LPS.
Cytokines also play an important role in regulation of intestinal barrier integrity.2,23 Overproduction of pro-inflammatory cytokines has a negative influence on gut integrity and epithelial function.27 In the present study, pro-inflammatory cytokines (TNF-α, IL-6, IL-8 and IL-1β) gene expression was elevated in the jejunal mucosa of piglets challenged with LPS, which was consistent with previous studies.22,28 LPS is recognized by, and binds to, TLR4, thereby inducing production of pro-inflammatory cytokines IL-8 and IL-1β.29 In turn, these cytokines stimulate the production, or potentiate the action, of other pro-inflammatory cytokines such as TNF-α and IL-6 produced by APCs.30
Actually, mt ROS drive pro-inflammatory cytokine production. Moreover, the mitochondrion has a central role in energy metabolism homeostasis through the mt respiratory chain. Meanwhile, the mt respiratory chain is the major source of intracellular ROS generation, but is also an important target for the damaging effects of ROS.5 Mitochondrial insults, including oxidative damage itself, can cause an imbalance between ROS production and removal, resulting in ROS overproduction.4 So far, information regarding ROS production by intestinal mitochondria in acute intestinal disruption of piglets is unavailable. In the present study, we demonstrated, for the first time, that ROS production by intestinal mitochondria increased significantly in LPS-treated piglets. West et al. had shown that the level of mt ROS production increased significantly when RAW 264.7 macrophages were stimulated with LPS.31 Mitochondrial ROS also contribute to macrophage bactericidal activity, through mechanisms linking innate immune signalling.31 Lee et al. reported that TNF-α/IFN-γ potentiates iNOS/NO induction, ROS production, and loss of ΔΨm synergistically, playing an important role in promoting the apoptosis and liver injury induced by LPS.32 A possible reason for the high level of ROS of mitochondria may be that the antioxidative enzyme system was disrupted after administration of LPS,33 so the ROS degradation process was not sufficiently maintained, resulting in an excess of ROS. When ROS produced by the electron-transport chain accumulates up to a threshold level, it triggers the opening of the inner membrane anion channel (IMAC). The opening of the IMAC releases ROS from the mt matrix, resulting in mt membrane depolarization.34 However, no data is available related to the influence of intestinal injury on polarized state of mitochondria in piglets. In the current study, we demonstrated, for the first time, that LPS-induced intestinal injury significantly decreased the △Ψm of intestinal mt in piglets. Yuan et al. had discovered that LPS-mediated p53 activation, loss of mt membrane potential, caspase-3 activation, decrease of Bcl-2 expression in RAW264.7 macrophage.35 We speculate that increased intestinal mt ROS production caused by LPS may cause the opening of mt permeability transition, leading to the depolarization of intestinal mt membrane.
The mitochondrion has a pivotal role in energy metabolism through its respiratory chain, which is the primary source of ROS.34 The major target of ROS is mtDNA, since this is close to the mt respiratory chain and lacks protective histones.36 Mitochondrial DNA damage may result in cell death through disrupted electron transport, depolarized mt membrane, and disturbed energy production.36 Therefore, mtDNA content is a potential biomarker of mt dysfunction.37 So far, information regarding mtDNA content in LPS-induced intestinal injury of piglets is unavailable. Therefore, we determined, for the first time, that the content of jejunal mtDNA was markedly lower in LPS-challenged pigs. Deng et al. had shown that LPS-induced mtDNA release causes acute lung injury and systemic inflammation through TLR9 in mice.38 Liu et al. reported that LPS treatment decreased hepatic ATP and NADH levels and the expression levels of most mtDNA-encoded genes as well as mt complex I, IV and V activities.39 A possible reason for the low content of mtDNA in jejunum in the present experiment may be that the antioxidative enzyme system was disrupted after LPS injection, so that ROS degradation was not sufficiently maintained, resulting in disruption and mutation of mtDNA. Furthermore, mtDNA encodes multiple polypeptide subunits comprising the oxidative phosphorylation complexes (I, III, IV), except for complex II, which is encoded by the nuclear genome.40 Since the mutations of mtDNA would subsequently lead to impairment of oxidative phosphorylation complexes, overproduction of ROS and production of pro-apoptotic protein would ultimately lead to cell death and tissue damage. Nevertheless, no information is available related to the influence of LPS-induced intestinal injury on the activity of the mt oxidative phosphorylation complexes of pigs. Therefore, in the current study, we demonstrated for the first time that, after challenge with LPS, the activity of mitochondria oxidative phosphorylation complexes I, II, III, IV was dramatically decreased. Noh et al. reported that systemic injection of LPS induces region-specific neuroinflammation and mt oxidative phosphorylation complex II, III dysfunction in mouse brain.41 Zhou et al. found a significant decrease of activity of the mt respiratory chain under endotoxin-induced intestinal injury in rats.42 Sun et al. demonstrated that LPS-induced human umbilical vein endothelial cell apoptosis occurs via decreasing the activities of mt respiratory chain complexes (I, II, III, IV, and V).43 We speculated that LPS injection led to overwhelming superoxide and mt depolarization (decrease in mt membrane potential), which disrupt the activity of mt oxidative phosphorylation complexes, resulting in a decline in energy generation and disruption of intestinal integrity.
In response to damaged mitochondria, cells have developed a self-protection mechanism to degrade the dysfunctional mitochondrion before it causes activation of cell death.44 This process is known as mt autophagy, or mitophagy, and is triggered by ROS overproduction and depolarization of the mt membrane.45 Nevertheless, no information was available regarding mitophagy during the LPS-induced intestinal injury in piglets. To explore whether mitophagy is involved in intestinal disruption induced by LPS, we determined whether injection with LPS influenced the expression level of mitophagy-related proteins in piglets. It was demonstrated that PINK1 and parkin play an important role in mediating mitophagy.45,46 PINK1 is expressed in healthy, polarized mitochondria, and then degraded rapidly by proteolysis and maintained at very low levels.46 PINK1 accumulation in damaged mitochondria is necessary for Parkin recruitment to induce mitophagy after mt depolarization stemming from overwhelming oxidative damage.47 In the present research, we revealed that LPS-injection increased PINK1 and Parkin protein abundance in the mitochondrion. During initial phagophore formation in the mitophagy process, LC3-I is modified and converted to LC3-II, which is translocated to the membrane of autophago- and autolysosomes.48 Our results showed that LPS promoted the conversion of LC3-II from LC3-I, indicating that the autophagosome contained dysfunctional mt formation in the jejunum. Mitophagy in this study may protect cells against cell death caused by the LPS challenge in the intestine of piglets due to damaged mitochondria being taken up by autophagosomes and subsequently degraded by lysosomes, which contributes to intestinal homeostasis.
In summary, LPS challenge disrupted intestinal barrier function, impaired intestinal antioxidant balance, and caused mt dysfunction in pigs. Furthermore, levels of mitophagy-related proteins were up-regulated in intestinal mitochondria in response to LPS-induced intestinal injury in pigs.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial supportfor the research, authorship, and/or publication of this article:This research was supported by the National Key Research and Development Program (2016YFD0501210), the National Natural Science Foundation of China (grant number 31472103), the Special Fund for Agro-scientific Research in the Public Interest (grant number 201403047) and Dabeinong Funds for Discipline Development and Talent Training in Zhejiang University.
References
- 1.Xiao K, Song ZH, Jiao LF, et al. Developmental changes of TGF-beta 1 and Smads signaling pathway in intestinal adaption of weaned pigs. PloS one 2014; 9: e104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu CH, Xiao K, Luan ZS, et al. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci 2013; 91: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 3.Pi DA, Liu YL, Shi HF, et al. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem 2014; 25: 456–462. [DOI] [PubMed] [Google Scholar]
- 4.Marcu R, Zheng Y, Hawkins BJ. Mitochondria and angiogenesis. Adv Exp Med Biol 2017; 982: 371–406. [DOI] [PubMed] [Google Scholar]
- 5.Jeong EM, Chung J, Liu H, et al. Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc 2016; 5: pii: e00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palikaras K, Lionaki E, Tavernarakis N. Mitophagy: in sickness and in health. Mol Cell Oncol 2016; 3: e1056332–e1056332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun 2013; 4: 1410–1410. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Chen F, Odle J, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr 2012; 142: 2017–2024. [DOI] [PubMed] [Google Scholar]
- 9.Xiao K, Jiao L, Cao S, et al. Whey protein concentrate enhances intestinal integrity and influences transforming growth factor-beta1 and mitogen-activated protein kinase signalling pathways in piglets after lipopolysaccharide challenge. Br J Nutr 2016; 115: 984–993. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Hou Y, Yang S, et al. Transcriptional profiling of mammary gland in Holstein cows with extremely different milk protein and fat percentage using RNA sequencing. BMC Genomics 2014; 15: 226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng W, Liu Y, Shi H, et al. Aspartate alleviates liver injury and regulates mRNA expressions of TLR4 and NOD signaling-related genes in weaned pigs after lipopolysaccharide challenge. J Nutr Biochem 2014; 25: 592–599. [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Liu YL, Zhu HL, et al. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun 2013; 19: 504–515. [DOI] [PubMed] [Google Scholar]
- 13.Wijtten PJA, van der Meulen J, Verstegen MWA. Intestinal barrier function and absorption in pigs after weaning: a review. Brit J Nutr 2011; 105: 967–981. [DOI] [PubMed] [Google Scholar]
- 14.Jiao LF, Ke YL, Xiao K, et al. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs. J Anim Sci 2015; 93: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 15.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, et al. PPAR gamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 2012; 153: 329–338. [DOI] [PubMed] [Google Scholar]
- 16.Li WJ, Chen Y, Nie SP, et al. Ganoderma atrum polysaccharide induces anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. J Cell Biochem 2011; 112: 860–871. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Nie S, Chen Y, et al. Enhancement of cyclophosphamide-induced antitumor effect by a novel polysaccharide from Ganoderma atrum in sarcoma 180-bearing mice. J Agric Food Chem 2011; 59: 3707–3716. [DOI] [PubMed] [Google Scholar]
- 18.Huang Q, Xu W, Bai KW, et al. Protective effects of leucine on redox status and mitochondrial-related gene abundance in the jejunum of intrauterine growth-retarded piglets during early weaning period. Arch Anim Nutr 2017; 71: 93–107. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Tian H, Huang YQ, et al. Alterations of mitochondrial protein assembly and jasmonic acid biosynthesis pathway in Honglian (HL)-type cytoplasmic male sterility rice. J Biol Chem 2012; 287: 40051–40060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J, Wu MM, Xiao H, et al. Development of an antioxidant system after early weaning in piglets. J Anim Sci 2014; 92: 612–619. [DOI] [PubMed] [Google Scholar]
- 21.Larson-Casey JL, Deshane JS, Ryan AJ, et al. Macrophage akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 2016; 44: 582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao K, Cao ST, Jiao LF, et al. Anemonin improves intestinal barrier restoration and influences TGF-beta1 and EGFR signaling pathways in LPS-challenged piglets. Innate Immun 2016; 22: 344–352. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Liu Y, Wang X, et al. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun 2016; 22: 577–587. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Pi D, Leng W, et al. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway. Innate Immun 2017; 23: 546–556. [DOI] [PubMed] [Google Scholar]
- 25.Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J 2012; 5: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CM, Park JY, Noh KH, et al. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-kappaB modulation in RAW 264.7 cells. J Ethnopharmacol 2011; 133: 834–842. [DOI] [PubMed] [Google Scholar]
- 27.Bomba L, Minuti A, Moisa SJ, et al. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct Integr Genomics 2014; 14: 657–671. [DOI] [PubMed] [Google Scholar]
- 28.Upadhaya SD, Kim JC, Mullan BP, et al. Vitamin E and omega-3 fatty acids independently attenuate plasma concentrations of proinflammatory cytokines and prostaglandin E3 in Escherichia coli lipopolysaccharide-challenged growing-finishing pigs. J Anim Sci 2015; 93: 2926–2934. [DOI] [PubMed] [Google Scholar]
- 29.Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 2009; 30: 30–45. [DOI] [PubMed] [Google Scholar]
- 30.Korneev KV, Atretkhany KN, Drutskaya MS, et al. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017; 89: 127–135. [DOI] [PubMed] [Google Scholar]
- 31.West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011; 472: 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Oh YK, Rhee M, et al. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J Mol Biol 2007; 369: 967–984. [DOI] [PubMed] [Google Scholar]
- 33.Jiao N, Wu ZL, Ji Y, et al. L-Glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr 2015; 145: 2258–2264. [DOI] [PubMed] [Google Scholar]
- 34.Venditti P, Di Stefano L, Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013; 13: 71–82. [DOI] [PubMed] [Google Scholar]
- 35.Yuan ZH, Liang ZE, Wu J, et al. A potential mechanism for the anti-apoptotic property of koumine involving mitochondrial pathway in LPS-mediated RAW 264.7 macrophages. Molecules 2016; 21: pii: E1317–pii: E1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto M, Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med 2015; 85: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013; 13: 481–492. [DOI] [PubMed] [Google Scholar]
- 38.Deng SY, Ai YH, Zhang LM, et al. LPS-induced mitochondrial DNA release causes acute lung injury and systemic inflammation through toll-like receptor 9 in mice. Chest 2016; 149: A168–A168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Guo J, Sun H, et al. Alpha-lipoic acid attenuates LPS-induced liver injury by improving mitochondrial function in association with GR mitochondrial DNA occupancy. Biochimie 2015; 116: 52–60. [DOI] [PubMed] [Google Scholar]
- 40.Moran M, Moreno-Lastres D, Marin-Buera L, et al. Mitochondrial respiratory chain dysfunction: Implications in neurodegeneration. Free Radical Bio Med 2012; 53: 595–609. [DOI] [PubMed] [Google Scholar]
- 41.Noh H, Jeon J, Seo H. Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem Int 2014; 69: 35–40. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Ruan Z, Zhou LL, et al. Chlorogenic acid decreased intestinal permeability and ameliorated intestinal injury in rats via amelioration of mitochondrial respiratory chain dysfunction. Food Sci Biotechnol 2016; 25: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun ZW, Lan XY, Ahsan A, et al. Phosphocreatine protects against LPS-induced human umbilical vein endothelial cell apoptosis by regulating mitochondrial oxidative phosphorylation. Apoptosis 2016; 21: 283–297. [DOI] [PubMed] [Google Scholar]
- 44.Hirota Y, Aoki Y, Kanki T. Mitophagy: selective degradation of mitochondria by autophagy. Seikagaku 2011; 83: 126–130. [PubMed] [Google Scholar]
- 45.Springer MZ, Macleod KF. In Brief: Mitophagy: mechanisms and role in human disease. J Pathol 2016; 240: 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 2015; 33: 95–101. [DOI] [PubMed] [Google Scholar]
- 47.Lazarou M, Sliter DA, Kane LA, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015; 524: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin J, Duan JL, Cui ZJ, et al. Hydrogen peroxide-induced oxidative stress activates NF-kappa B and Nrf2/Keap1 signals and triggers autophagy in piglets. Rsc Adv 2015; 5: 15479–15486. [Google Scholar]