Abstract
Each year millions of neonates die due to vaccine preventable infectious diseases. Our study seeks to develop novel neonatal vaccines and improve immunogenicity of early childhood vaccines by incorporating TLR agonist-adjuvant combinations that overcome the inherent neonatal Th2 bias and stimulate Th1 polarizing response from neonatal APCs. We systematically stimulated cord blood mononuclear cells with single and multiple combinations of TLR agonists and measured levels of IL-12p70, IFN-γ, IFN-α, IL-10, IL-13, TNF-α, IL-6 and IL-1β from cell culture supernatants. APC-specific surface expression levels of costimulatory markers CD40, CD83 and PD-L1 were assessed by flow cytometry. Whole blood assays were included to account for the effect of plasma inhibitory factors and APC intracellular TNF-α and IL-12p40 secretions were measured. We found robust Th1 polarizing IL-12p70, IFN-γ and IFN-α responses when cord blood APCs were stimulated with TLR agonist combinations that contained Poly I:C, Monophosphoryl Lipid A (MPLA) or R848. Addition of class A CpG oligonucleotide (ODN) to Th1 polarizing TLR agonist combinations significantly reduced cord blood IL-12p70 and IFN-γ levels and addition of a TLR2 agonist induced significantly high Th2 polarizing IL-13. Multi-TLR agonist combinations that included R848 induced lower inhibitory PD-L1 expression on cord blood classical dendritic cells than CpG ODN-containing combinations. Incorporation of combination adjuvants containing TLR3, TLR4 and TLR7/8 agonists to neonatal vaccines may be an effective strategy to overcome neonatal Th2 bias.
Keywords: Neonatal innate immunity, IL-12, IFN-γ, TLR synergy, neonatal vaccines
Introduction
Globally neonatal deaths account for 45% of under-five-yr-old deaths and nearly half of all neonatal deaths are caused by infectious diseases.1 Vaccination is the most effective measure to prevent infection at birth and in early life. However, neonates often do not generate effective immune responses to vaccines due to distinct immune adaptations of early life.2–4
One of the features of the neonatal immune system is poor ability of neonatal monocytes and dendritic cells (DCs) to generate Th1 polarizing cytokines such as IL-12p70 and IFN-γ in response to microbial stimuli.5–14 This leads to suboptimal adaptive immune responses making neonates vulnerable to bacterial and viral pathogens. Bacille Calmette-Guerin (BCG) vaccine is widely given to newborns and it elicits robust Th1 responses as well as enhancing T and B cell responses to non-specific Ags.15–21 BCG is a live attenuated vaccine and it contains inherent adjuvant activity stimulating TLR2, 4 and 9.22,23 Therefore, by utilizing the plasticity of the neonatal immune system, innate responses can be modulated with synergistic combinations of TLR agonists that may lead to mature-like Th1 responses.
Over the last decade, TLR agonists have emerged as novel vaccine adjuvants24 but their application with neonatal vaccines faces roadblocks of altered neonatal TLR responsivity.3,5,8,9 Neonatal APCs appear to have similar basal TLR expression and signaling levels as adults.25 However, in general, neonatal cord blood mononuclear cells (CBMCs) produce less TNF-α and IL-1β but more IL-10 and IL-6 in response to TLR ligand stimulation compared to adult cells.8 IL-12p70 and IFN-α levels only gradually increase in the first yr of life.5,6,9 Neonatal peculiarities of APCs are multifactorial, characterized by low basal expression of costimulatory molecules (CD40, 80 and 86), defective production of Th1 polarizing cytokines such as IL-12, decreased ability to stimulate allogeneic responses and reduced endocytic activity.26 Prior research has shown that strong neonatal Th1 responses can be elicited by IL-12 secreting adult APCs suggesting that the maturity of APC populations is a key rate-limiting step in neonatal Th1 function.14 Considering the minimal effects of maternal Abs on T cell responses, deficiencies in numbers of DCs and their functional incompetence likely are crucial factors limiting the capacity in neonates (and infants) to generate T effector memory responses.27 Despite suboptimal levels in neonates, DC-derived IL-12 has been shown to modulate Ag-specific T cell responses by inducing T cell:IFN-γ secretion.16 In cord blood, individual TLR agonists such as those for TLR3, 4, 7 and 9 have been shown to be suboptimal to stimulate polarizing cytokine (IL-12, IFN-α) responses that favor Th1 differentiation.28 TLR8 agonists such as imidazoquinoline and single stranded viral RNAs induce production of TNF-α and IL-12/23p40 and up-regulation of CD40 on neonatal DCs but not equivalent to adult DCs.29,30 Similarly CpG oligonucleotides (ODNs) have been shown to enhance neonatal Th1 responses in mouse models.31,32 Recent studies demonstrated that simultaneous activation of C-type lectin receptor and TLRs induce robust IL-12p70 secretion by neonatal monocyte-derived DCs.33,34 Thus, optimal induction of vaccine-specific T cell memory in neonates/early life likely requires strategies designed to compensate for APC functional peculiarities.
Previous studies using adult human PBMCs showed that synergistic stimulation of DCs with two or more TLR agonists induces sustained IL-12p70 secretion causing significantly higher naïve T cell Th1 polarization compared to single TLR agonist stimulation.35 Triggering specific combinations of TLRs in DCs has been shown to induce synergistic production of Th1 polarizing cytokines, up-regulation of DC costimulatory markers and down-regulation of inhibitory programmed death-ligand 1 (PD-L1) expression.36
Neonatal APCs can be induced with appropriate microenvironmental signals such as TLR agonists or adjuvants. In order to identify efficient combinations of TLR signals that would potentiate neonatal innate immunity to prime Th1 responses, we hypothesized that TLR agonist combinations which simultaneously activate the MyD88 (TLR2, 4, 9), TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent (TLR3 or 4) pathways together with endosomal receptor TLRs (TLR7/8) could lead to synergistic activation of IL-12p70 production. In this study, using cord blood as a source of neonatal immune cells, we systematically studied single and combinations of TLR agonists that could favorably modulate Th1 immune responses from neonatal CBMCs.
Materials and methods
TLR agonists
PAM3CSK4 (TLR2/1 agonist), polyinosine-polycytidylic acid (Poly I:C Low Molecular Weight (LMW), TLR3 agonist), Escherichia coli LPS O111:B4 or monophosphoryl lipid A (MPLA, TLR4 agonists), resiquimod (R848, TLR7/8 agonist), imiquimod (R837, TLR7 agonist) and class A CpG ODN (ODN 2336, TLR9 agonist) were purchased from Invivogen (San Diego, CA). TLR agonists were formulated according to the manufacturer’s instructions.
Blood processing and in vitro cell culture conditions
The study was approved by the Institutional Ethics Review Board at Rochester General Hospital. Non-identifiable cord blood samples from normal full-term healthy deliveries and de-identified adult blood samples were collected with informed consent into sodium heparin-containing vacutainers (BD Biosciences, San Diego, CA). CBMCs and adult PBMCs were isolated from buffy coat using Ficoll-Paque gradient (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s instructions. Cells were washed in PBS and re-suspended at a concentration of 1 × 107 cells/ml in recovery cell culture freezing media (Life Technologies, Carlsbad, CA) and frozen in liquid nitrogen until use. Prior to stimulation, cells were quick-thawed in 37℃ water bath followed by slowly adding culture medium (RPMI 1640 supplemented with 10% of FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin). After thawing and washing, cells were counted, washed and plated (2 × 106 cells/ml) at 200 µl/well in 96-well round-bottom plates and rested for 1 h at 37℃ in 5% CO2 incubator. At the end of 1 h incubation, Poly I:C (10 µg/ml) and ODN 2336 (5 µM) were added to respective wells and incubated for 2 h followed by addition of PAM3CSK4 (1 µg/ml), E. coli LPS (50 ng/ml) or MPLA (1 µg/ml) or R848 (1 µg/ml) and further incubated for an additional 22 h at 37℃ in 5% CO2 incubator. The doses of TLR agonists used in this study were selected based on several dose-response/kinetic experiments. We selected the doses that induced maximum cytokine response for a particular ligand by itself and in combination without inducing > 20% cell death.
After 24 h culture, cells were centrifuged, and supernatants were collected and stored at −80℃ until Luminex analysis. An identical set of samples were plated for 24 h in 96-well flat-bottom plates for phenotyping and assessing the maturation/activation status by flow cytometry.
Flow cytometry analysis
Post stimulation, cells were washed once before incubating them with live-dead fixable aqua dead cell stain (Life Technologies) in PBS for 30 min. Cells were then washed, and surface stained along with FcR blocking solution (human TruStain FcX, BioLegend, San Diego, CA) for 20 min in the dark at 4℃. The different combinations of surface stains used (all from BioLegend unless otherwise stated) are phycoerythrin (PE) conjugated anti-CD303 (BDCA-2; 201A), PE-CF594 anti-HLA-DR (G46-6; BD Biosciences, San Jose, CA), peridinin chlorophyll A protein-Cy5.5 (PerCP-Cy5.5) anti-CD16 (3G8), Brilliant Violet 605 anti-CD14 (M5E2), Alexa Fluor 647 anti-CD11c (BU 15; AbD Serotec, UK), Alexa Fluor 700 anti-CD86 (FUN-1; BD Biosciences), Alexa Fluor 488 anti-CD83 (HB15e), PE-Cy7 anti-CD40 (5C3), Brilliant Violet 421 anti-CD274 (PD-L1; 29E.2A3) and allophycocyanin-Cy7 anti-CD3 (HIT3a), CD19 (HIB19), CD20 (2H7) and CD56 (HCD56) as lineage cocktail (Supplementary Figure 2). After the wash step, cells were acquired on a BD LSR II flow cytometer.
Whole blood intracellular cytokine assays
For whole blood intracellular cytokine assays, we adopted the method reported by Jansen et al.37 Briefly, neonatal cord blood was mixed 1:1 with sterile prewarmed RPMI 1640 medium and 200 µl was added to each well of a 96-well flat-bottom plate. Whole blood suspension rested for 1 h at 37℃ in 5% CO2 incubator were stimulated first with Poly I:C and ODN 2336 and subsequently after 2 h with PAM3CSK4, E. coli LPS or MPLA or R848 for an additional 6 h. Brefeldin-A (BFA; Cell Signaling, Danvers, MA) was added at 10 µg/ml for the last 5 h at 37℃ in 5% CO2 incubator. For the last 10 min of incubation, 2 mM EDTA was added to each well to dislodge the cells. Cells were centrifuged at the end of incubation and re-suspended in 200 µl of 1 × BD FACS lysing solution and stored in a −80℃ freezer until stained. For whole blood intracellular staining, cells were thawed and centrifuged at 300 g for 5 min and permeabilized with BD Cytofix/Cytoperm solution for 10 min at room temperature (20–22℃) and washed two times. After the washes, surface and intracellular Ab cocktail was added and incubated for 30 min at room temperature. Intracellular stains used were FITC IL-12p40/70 (C11.5; BD Biosciences), PE-Cy7 anti-TNF-α (MAb11) and V450 anti-IFN-α2b (7N4-1; BD Biosciences). Plates were washed subsequently, and cells were acquired on BD LSR II flow cytometer. Data were analyzed using FlowJo software (FlowJo, Ashland, OR).
Cytokine measurement
Supernatants stored at −80℃ were thawed at room temperature and IL-12p70, IFN-γ, IFN-α, TNF-α, IL-1β, IL-6, IL-13 and IL-10 levels were measured from undiluted samples using Milliplex human high sensitivity cytokine/chemokine MAP kit (EMD Millipore, Billerica, MA) according to the manufacturer’s instructions. Prepared samples were run on a Bio-Plex 200 system with Luminex xMAP technology (Bio-Rad).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 7.03 (GraphPad Software, La Jolla, CA) using an ordinary one-way ANOVA with Tukey’s or Dunnett’s post hoc correction for multiple comparisons. Multiplicity adjusted P values of < 0.05 were considered significant.
Results
Multi-TLR agonist combinations elicit significant neonatal IL-12p70 response
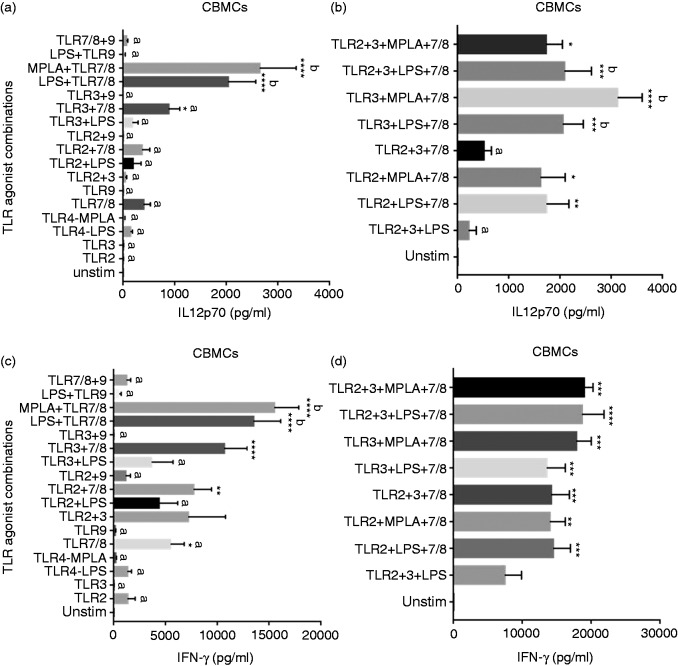
Single and multiple combinations of TLR agonists (TLR2, 3, 4, 7/8 and 9) were systematically studied to determine the best TLR agonist combinations that would induce robust Th1 polarizing IL-12p70 and IFN-γ cytokines from CBMCs. When single TLR agonists and combinations of two TLR agonists were compared to unstimulated CBMCs for IL-12p70 secretion, only dual agonist combinations of TLR3 + 7/8 and TLR4 + 7/8 (either E. coli LPS or MPLA with R848) induced significant IL-12p70 (Figure 1a). Both TLR4 (LPS or MPLA) + 7/8 agonist combinations induced IL-12p70 levels significantly higher than all single and other double TLR agonist combinations (including TLR3 + 7/8) (Figure 1a). MPLA + R848 induced the highest IL-12p70 levels among all single and double TLR agonist combinations and R848 induced the highest IL-12p70 levels among the single TLR agonists (Figure 1a).
Figure 1.
Neonatal IL-12p70 and IFN-γ cytokine response to multi-TLR agonist stimulations. Cord blood (n = 13) mononuclear cells were stimulated as indicated. After 24 h, supernatants were tested for (a) and (b) IL-12p70 and (c) and (d) IFN-γ by Luminex multiplex ELISA. Results are expressed as mean ± SE analyzed using one-way ANOVA with Tukey’s post hoc correction for multiple comparisons. Bar graphs with different letters are significantly different from each other. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 denotes significance compared to unstimulated control.
Addition of TLR2 or TLR3 agonists or both to a TLR4 + 7/8 combination induced significantly higher IL-12p70 levels compared to no stimulation (Figure 1b). TLR3 + LPS + 7/8, TLR3 + MPLA + 7/8 and TLR2 + 3 + LPS + 7/8 agonist combinations along with TLR4 + 7/8 (E. coli LPS or MPLA) (Figure 1a and b) induced the highest IL-12p70 levels from CBMCs and these were significantly higher than multiple TLR agonist combinations containing TLR2, 3, 4 and 9 and TLR2 + 3 + 7/8. TLR2 + LPS + 7/8 agonist combination induced IL-12p70 levels that were significantly different compared to unstimulated and single TLR agonist stimulations, and were not significantly different from any double or multiple TLR agonist combinations containing TLR2, 3, LPS and 9 (Figure 1a and b).
The immunomodulatory role of neonatal plasma is well established.38 In order to account for the regulatory effects of human neonatal plasma on the cytokine response we measured, neonatal whole blood (without Ficoll separation) were stimulated with various TLR agonist combinations (see ‘Materials and methods’ section) for intracellular IL-12p40 and TNF-α levels from classical DCs (cDCs) (Supplementary Figure 1a and b). The percentage of cDCs that were positive for IL-12p40 was significantly increased with TLR7/8 agonist alone or in combinations that included TLR7/8 agonist compared to unstimulated cells (Supplementary Figure 1a). Although few TLR9 + TLR7/8 agonist-containing combinations induced significant cDC IL-12p40, the data were similar to total cytokine levels measured from PBMCs (Supplementary Figure 1a).
Multi-TLR agonist combinations elicit significant neonatal IFN-γ response
CBMC IFN-γ responses to TLR7/8 and TLR2 + 7/8 agonists were similar to IL-12p70 responses with a few notable exceptions. As seen with IL-12p70, TLR4 + 7/8 agonist combinations (E. coli LPS or MPLA) produced the highest IFN-γ levels compared to all single TLR agonists and most dual combinations (Figure 1c). Except for TLR2 + 3 + 4 agonist combination, all TLR3 and/or TLR4 (LPS or MPLA) agonist combinations also containing TLR7/8 agonist induced significantly increased IFN-γ from CBMCs compared to unstimulated controls (Figure 1d). TLR2 + 3 + LPS or MPLA + 7/8 agonist combination induced significantly more IFN-γ levels compared to TLR7/8 and TLR2 + 7/8 (Figure 1c and d).
TLR9 agonist down-modulates neonatal Th1 cytokine response
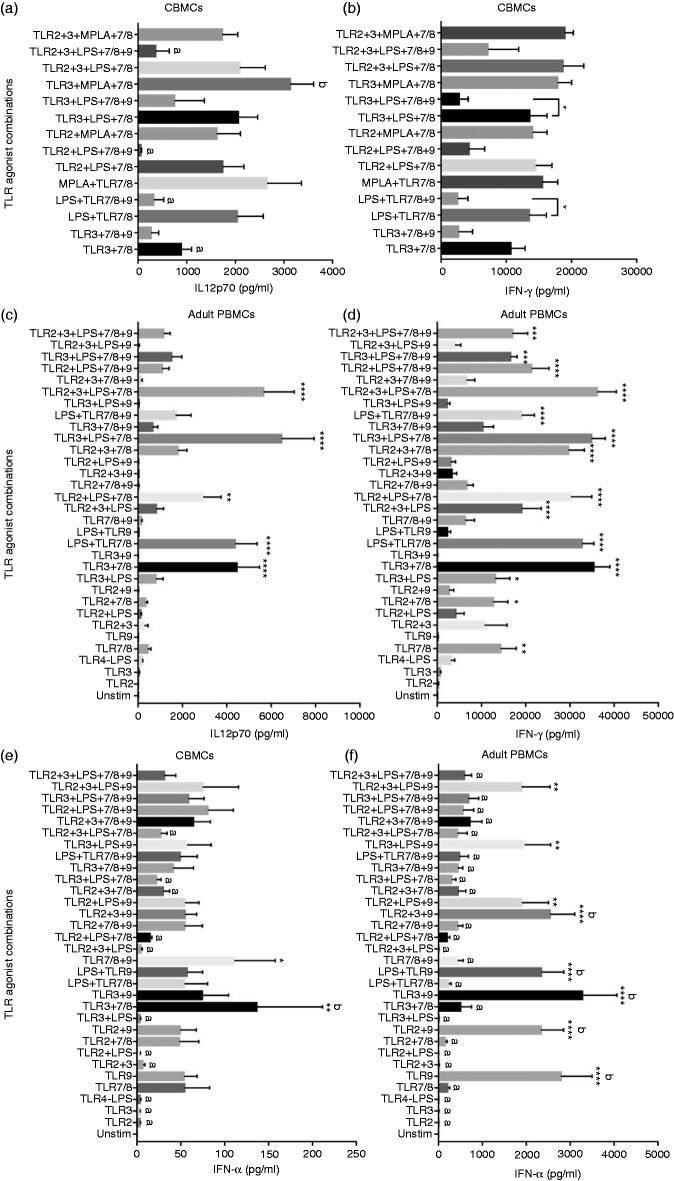
Stimulation of TLR9 is known to induce a Th1 polarizing response from human PBMCs.11,39 When TLR9 agonist ODN 2336, with known preference for human TLR9, was used alone to stimulate CBMCs, it did not elicit significant increases in IL-12p70 or IFN-γ levels (Figure 1a and b). Addition of TLR9 agonist to various combinations of TLR2, 3, LPS and 7/8 agonists reduced the total average secretion of IL-12p70 and IFN-γ cytokines from CBMCs (Figure 2a and b). The negative impact of TLR9 agonist on IL-12p70 and IFN-γ cytokine levels was significant when added to LPS + 7/8 or TLR3 + LPS + 7/8 agonist combinations (Figure 2a and b). ODN 2336 induced type I IFN-α secretion similar to levels induced by TLR7/8 agonist and higher than other single TLR agonists (Figure 2e). When combined with TLR7/8 agonist, ODN 2336 induced significant IFN-α compared to unstimulated control (Figure 2e). While ODN 2336 did not suppress IFN-α secretion from CBMCs in combination with other TLR agonists as seen with IL-12p70 or IFN-γ, it generally did not show an additive effect on total IFN-α secretion (except with TLR7/8; Figure 2e). TLR3 + 7/8 agonist combination elicited the highest IFN-α levels among the multi-TLR agonist combinations although these were not significantly higher than the TLR7/8 + 9 combination (Figure 2e).
Figure 2.
Comparison of neonatal and adult Th1 response to multi-TLR agonist stimulations. Cord blood (n = 13) and adult (n = 6) mononuclear cells were stimulated as indicated. (a) Effect of ODN 2336 addition to best neonatal IL-12p70 inducing multi-TLR agonist combinations. (b) Effect of ODN 2336 addition to best neonatal IFN-γ inducing multi-TLR agonist combinations. (c) and (d) Adult PBMC IL-12p70 and IFN-γ response to multi-TLR agonist combinations with or without ODN 2336, respectively. (e) and (f) Neonatal and adult IFN-α response to multi-TLR agonist combinations, respectively. Twenty-four h post stimulation, supernatants were analyzed by Luminex multiplex ELISA. Results are expressed as mean ± SE and analyzed using one-way ANOVA with Dunnett’s post hoc correction ((a) and (b)) or Tukey’s post hoc correction ((c) and (f)) for multiple comparisons. Bar graphs with different letters are significantly different from each other. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 denotes significance compared to unstimulated control.
Multi-TLR agonist stimulation of adult PBMCs
Although at higher levels, similar patterns of Th1 cytokine (IL-12p70 and IFN-γ) production as observed with CBMCs were measured from human adult PBMCs when stimulated with R848 agonist containing TLR agonist combinations (Figure 2c and d). R848 alone did not induce significant IL-12p70 compared to unstimulated cells. However, TLR3 + 7/8, TLR4 + 7/8 (with E. coli LPS; MPLA was not tested in adult PBMCs), TLR2 + LPS + 7/8, TLR3 + LPS + 7/8 and TLR2 + 3 + LPS + 7/8 agonist combinations induced significant IL-12p70 levels compared to unstimulated cells. As observed with CBMCs, when TLR9 agonist was added to each of the corresponding combinations that induced significant IL-12p70 (ex: TLR3 + 7/8 versus TLR3 + 7/8 + 9), IL-12p70 production decreased significantly (except for TLR2 + LPS + 7/8; Figure 2c and Supplementary Table 1). While some of the multi-TLR agonist combinations containing both TLR7/8 and TLR9 agonists were successful in inducing significant IFN-γ secretion from adult PBMCs (Figure 2d), addition of TLR9 to TLR7/8 agonist-containing combinations significantly decreased IFN-γ levels compared to combinations that had only TLR7/8 agonist (except for TLR2 + LPS + 7/8 + 9; Figure 2d and Supplementary Table 1). Contrary to the observations with CBMCs, adult PBMCs stimulated with TLR9 agonist or combinations that contained TLR9 agonist but not TLR7/8 agonist induced significant IFN-α secretion (Figure 2f).
Most TLR agonist combinations induced pro-inflammatory TNF-α and IL-1β secretion from neonates
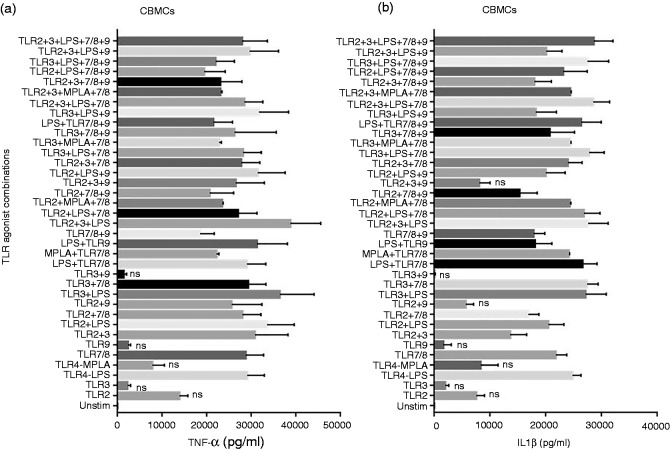
We examined the effect of TLR agonist combinations in inducing pro-inflammatory cytokines from CBMCs as shown in Figure 3. Among the single TLR agonists, E. coli LPS and R848 induced significant TNF-α secretion while TLR2, 3, MPLA and TLR9 agonists failed to induce significant TNF-α compared to unstimulated control. All the dual and multiple TLR agonist combinations induced significant TNF-α levels except for TLR3 + 9 (Figure 3a, only non-significant comparisons to unstimulated control are marked). Similar TNF-α results were observed from neonatal whole blood cDCs (Supplementary Figure 1b, only non-significant comparisons to unstimulated control are marked).
Figure 3.
Neonatal TNF-α and IL-1β response to multi-TLR agonist stimulations. Cord blood (n = 13) mononuclear cells were stimulated as indicated. After 24 h, supernatants were tested for (a) TNF-α and (b) IL-1β by Luminex multiplex ELISA. Results are expressed as mean ± SE and analyzed using one-way ANOVA with Tukey’s post hoc correction for multiple comparisons. ns, not significant.
IL-1β levels induced by single and multiple TLR agonists mirrored the pattern of TNF-α levels with TLR2 + 9 also not inducing significant levels compared to unstimulated control (Figure 3b, only non-significant comparisons to unstimulated control are marked). IL-6 levels were also tested, and the pattern resembled that of TNF-α and IL-1β (data not shown). Adult PBMCs produced similar levels of IL-1β as that of CBMCs, whereas adult PBMCs produced higher levels of TNF-α (data not shown).
TLR2 agonist alone and in combination induces significant neonatal Th2 cytokine response
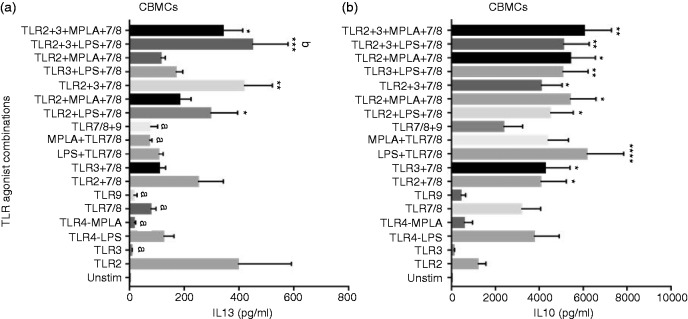
Th2 polarizing cytokine IL-13 levels from CBMCs were significantly induced by TLR2 agonist compared to unstimulated controls (Figure 4a). Moreover, TLR2 in combination with LPS + TLR7/8 and TLR3 + LPS or MPLA + 7/8 also induced significant IL-13 levels from CBMCs. IL-13 levels induced by TLR2 agonist alone and TLR2 + 3 + LPS + 7/8 combination were significantly higher than those induced by TLR3, MPLA, TLR7/8, TLR9, TLR7/8 + 9 and MPLA + 7/8.
Figure 4.
Neonatal IL-13 and IL-10 response to TLR agonist combinations that induce Th1 cytokines. Cord blood mononuclear cells were stimulated as indicated. After 24 h, supernatants were tested for (a) IL-13 (n = 7) and (b) IL-10 (n = 13) by Luminex multiplex ELISA. Results are expressed as mean ± SE and analyzed using one-way ANOVA with Tukey’s post hoc correction for multiple comparisons. Bar graphs with different letters are significantly different from each other. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 denotes significance compared to unstimulated control.
Studies have shown that newborns produce high levels of anti-inflammatory cytokine IL-10 at birth.8 We found that TLR agonist combinations that contain TLR7/8 induce significantly high IL-10 compared to unstimulated control (Figure 4b). Among the single agonists, E. coli LPS and R848 induced the highest IL-10 levels.
Single as well as multi-TLR combinations induce neonatal DC maturation
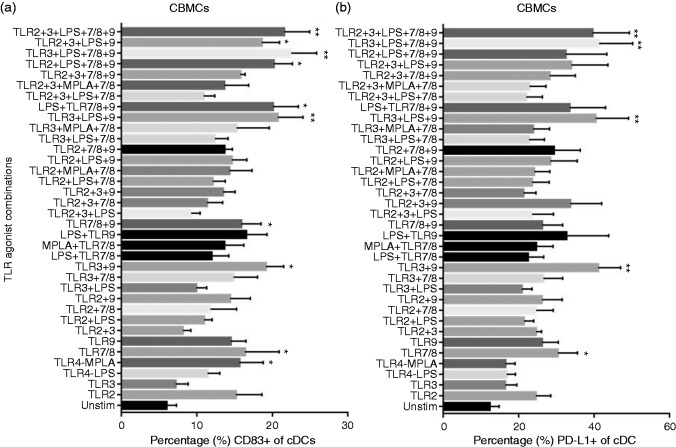
To assess the maturation status of neonatal DCs after TLR stimulation, CBMCs cultured in the absence or presence of single or multiple TLR agonists were tested for CD83 and CD40 expression levels. Exposure to MPLA or R848 alone stimulated significant CD83 expression compared to unstimulated cells (Figure 5a). TLR2 and TLR9 agonists alone and many multi-TLR agonist combinations also up-regulated CD83 expression. Among the multi-TLR combinations, TLR9 agonist-containing combinations elicited significant increased expression of CD83 compared to unstimulated controls (Figure 5a). CD40 levels had a similar pattern of expression (data not shown).
Figure 5.
Expression of costimulatory markers on neonatal classical dendritic cells in response to TLR stimulation. Cord blood (n = 8) mononuclear cells were stimulated as indicated. After 24 h, surface expression of (a) costimulatory marker CD83 and (b) inhibitory marker PD-L1 (CD274) were measured on neonatal classical dendritic cells by flow cytometry. Results are expressed as mean ± SE and analyzed using one-way ANOVA with Tukey’s post hoc correction for multiple comparisons. *P < 0.05, **P < 0.01, denotes significance compared to unstimulated control.
Multi-TLR combinations containing TLR9 induce PD-L1 expression on neonatal DCs
PD-L1 is described as a key negative regulator and inducer of tolerance.40 We evaluated whether synergistic stimulation with multi-TLR agonists down-modulated DC surface expression of inhibitory molecule PD-L1. TLR7/8 agonist R848 induced significant PD-L1 expression compared to unstimulated control (Figure 5b), while multi-TLR agonist combinations that contained R848-induced PD-L1 levels did not reach significant levels. Interestingly, most of the TLR agonist combinations that included TLR9 agonist induced significantly high PD-L1 expression compared to unstimulated control (Figure 5b).
Discussion
Cord blood is readily available and used extensively to study neonatal immune responses to vaccine Ags and immunomodulators. However, cord blood may carry adult cells from the mother at very low frequencies. Therefore, adult PBMCs were used as a comparator and the results demonstrate that cord blood-induced cytokine levels after TLR stimulation are much lower than adult PBMC-induced levels, suggesting neonatal-like response. In this study, we used human CBMCs as a model system to study TLR immune modulation of neonates to potentiate Th1 responses since newborns elicit mainly a Th2 response. Together, human neonatal monocytes and cDCs express TLR2, 4, 8 and 9 with plasmacytoid DCs (pDCs) expressing only TLR7 and 9,41 while TLR3 is mainly expressed by NK cells.41 By using multiple TLR agonists that stimulate TLRs 2, 3, 4, 7, 8 and 9 we studied the broadest TLR repertoire expressed by innate cells seeking the best TLR agonist combination that could induce Th1 response from newborns.
cDCs and pDCs are the major producers of IL-12p70 and IFN-α respectively with NK cells playing a predominant role in producing IFN-γ and IFN-α as part of the innate response.12 We found that TLR agonist combinations that contain R848 and E. coli LPS or MPLA and/or Poly I:C induced the highest IL-12p70 and IFN-γ response from CBMCs and adult PBMCs (Figures 1 and 2c and d). Addition of more agonists (>3) did not further increase IL-12 or IFN-γ responses significantly.
R848 induced the highest IL-12 response among the single TLR agonists and multi-TLR agonist synergy was most dramatic with IL-12p70 secretion on addition of LPS or MPLA or Poly I:C to R848. TLR7 agonist R837 did not induce IL-12 or IFN-γ response from CBMCs or adult PBMCs (data not shown), suggesting the involvement of R848-TLR8 signaling in agreement with published results from other groups.8,42 We observed an early effect of R848 on IL-12 production. R848 alone and combinations including R848 induced the highest IL-12p40 response from whole blood similar to that observed with CBMCs. Due to the lack of availability of reliable fluorescently conjugated IL-12p70 markers, we could not assess IL-12p70 directly from whole blood DCs. The whole blood intracellular cytokine stimulation protocol difference to PBMC cytokine measurement precludes direct comparison between the two to demonstrate this neonatal plasma effect on stimulations. Increased IL-12p40 could also indicate IL-23 production. We did test IL-23 and IL-17 using Luminex cytokine panels but could not detect both the cytokines from the CBMC supernatants with the kits used.
This suggests that plasma inhibitory factors have minimal effect on selected TLR combination-induced synergistic neonatal Th1 polarizing cytokine responses.
We found that combinations of TLR3 and 7/8 agonists or TLR4 and 7/8 agonists induced higher IL-12 and IFN-γ, pointing to synergistic interaction between MyD88 and TRIF intracellular signaling pathways. Of particular interest is the observation that MPLA with R848 agonist combination induced highest IL-12 and IFN-γ among dual combinations in neonatal or adult PBMCs even compared to E. coli LPS + R848 with the same TLR specificity (Figures 1a and c and 2c and d). Both E. coli LPS and MPLA signal through MyD88 and TRIF.43 MPLA, with its very low toxicity is attractive as an adjuvant and is US Food and Drug Administration approved for vaccines. Prior work has suggested that MPLA stimulates an immune response biased towards TRIF-associated signaling rather than MyD88-associated signaling, compared to E. coli LPS and perhaps contributes towards synergy with R848 through the MyD88 signaling pathway.43 TLR3 agonist Poly I:C also utilizes the TRIF signaling pathway44 explaining our result of higher IL-12 and IFN-γ with Poly I:C and R848 combination compared to TLR2 agonist PAM3CSK4 and R848 combination, both of which signal only through MyD88. TLR3 is predominantly expressed by NK cells in human PBMCs41 leading us to speculate that Poly I:C might be inducing a synergistic effect in IFN-γ secretion by stimulating NK cells as well.
R848 also targets pDCs through TLR7 and it has been shown that following stimulation, pDCs produce substantial amounts of IFN-α mediated through the TLR7-MyD88-IRF7 pathway.45–48 pDCs also express TLR9 and induce large amounts of pDC:IFN-α secretion.41 Similar to pDCs, B cells express marked levels of both TLR7 and 9.41 In this study we used human specific type A CpG (ODN 2336) which specifically targets pDC to induce IFN-α but not from B cells.8 Multiple studies had demonstrated that IFN-α produced by pDCs may interact with cDCs in an autocrine-paracrine feedback loop to augment the transcription of IL-12 by regulating IL-12p35 mRNA accumulation.49 However, when TLR9 agonist ODN 2336 was added to the TLR agonist combinations that induced significant IL-12p70 and IFN-γ secretion, there was a significant decrease in both IL-12p70 and IFN-γ levels in CBMCs and adult PBMCs (Figure 2a and d). TLR9 agonists have been shown to induce Th1 responses. We did not expect ODN 2336 to stimulate IL-12p70 and IFN-γ responses as it mainly induces pDC to produce IFN-α (Figure 2e and f). However, we expected a synergistic response with TLR7/8 containing combinations as seen with TLR3 agonist, Poly I:C (Figure 1a and c). Both Poly I:C and ODN 2336 were added to the respective CBMC cultures 2 h prior to PAM3CSK4 or LPS or MPLA or R848 stimulation to accommodate for different kinetics of TLR stimulation as demonstrated by others.35,37 After initial TLR stimulation, cells may become refractory to subsequent stimulation by the same or different TLR agonists. Napolitani et al.35 has demonstrated that for a synergistic response of TLR3 agonist with combinations containing TLR4 and TLR7/8 agonists, TLR3 needs to be added at least 2–3 h before TLR4 and 7/8. Additionally, Jansen et al.37 had shown that TLR3 and 9 requires 3–4 h in cell culture before addition of BFA because of the transport requirements of the ligands and their receptors to and from endoplasmic reticulum to endosome for TLR stimulation response. Similar observations in our hands also led us to add TLR3 and 9 agonists 2 h prior to addition of other ligands to cell culture. We speculate that addition of ODN 2336 before R848 might have affected the distribution of endosomal TLR8 and prevented efficient R848 signaling through MyD88 to produce IL-12. In contrast early TLR3 stimulation might not have affected R848 kinetics in a similar fashion and by using the TRIF pathway contributed to the observed synergy. This model partially explains why adult IFN-α secretion levels are significantly high only with TLR9 agonist- or ODN 2336-containing combinations compared to R848-containing combinations (Figure 2f) while both R848 and ODN 2336 are known to induce large amounts of pDC:IFN-α. However, in neonates synergistic IFN-α response, albeit at a lower level, was observed with R848 and ODN 2336 as well as R848 and Poly I:C dual combinations (Figure 2e) and an inhibitory effect on IFN-α production was not observed when R848 and ODN 2336 were used together. Whether this is because of lower IFN-α production potential of neonatal pDCs and NK cells needs further investigation.
Most of the double and multi-TLR agonist combinations induced significant TNF-α and IL-1β levels from CBMCs. Assessing TNF-α and IL-1β production in response to multi-TLR agonist combinations therefore did not yield meaningful information on TLR synergy. Among the single TLR agonists, Poly I:C and ODN 2336 induced the least TNF-α and IL-1β.
While TLR2 agonist PAM3CSK4 induced significant IL-12p70 and IFN-γ with LPS/MPLA + R848 or Poly I:C + LPS/MPLA + R848 agonist combinations (Figure 1a and d), it also induced significant amounts of the Th2 cytokine IL-13 (Figure 4a). Since PAM3CSK4 did not add synergy to the combinations that induced best Th1 responses but instead induced Th2 cytokine response, using TLR2 agonists as adjuvants may not be a desirable approach to enhancing neonatal Th1 responses to vaccines.
It is generally accepted that neonates have an enhanced capacity to produce IL-10 and levels steadily decline to reach adult levels by age 2 yr old.8,50 Most of the double and multi-TLR agonist combinations induced significant IL-10 from CBMCs (Figure 4b). When developing novel vaccine adjuvants that potentiate the immune response it is essential to avoid harmful inflammatory responses. Therefore, it is important to note that the TLR agonist combinations that significantly induced IL-12 and IFN-γ levels also induced high anti-inflammatory cytokine IL-10 levels (Figure 4b). IL-10 is also known to favorably contribute directly to B cell responses and promote Ab responses. Therefore, a balanced response is observed with the best Th1 polarizing TLR agonist combinations we studied in generating favorable vaccine responses and perhaps avoiding potentially harmful side effects.
Despite significant induction of Th1 polarizing cytokines, up-regulation of DC activation markers (CD83 and CD40) were not synergistically induced by multi-TLR agonist combinations compared to single TLRs (Example: MPLA or R848; Figure 5a). Perhaps DC activation and cytokine secretion have different thresholds of induction. However, when we studied DC:PD-L1 expression levels—a costimulatory molecule that can inhibit the T cell responses—many of the ODN 2336-containing multi-TLR agonist combinations induced significantly high PD-L1 expression levels compared to unstimulated control whereas R848-containing multi-TLR agonist combinations did not (Figure 5b). Although R848 by itself induced significant DC:PD-L1 expression, multi-TLR agonist combinations that included R848 but not ODN 2336 induced lower average (not significantly different) PD-L1 expression levels compared to R848 only stimulation. Biologically, this suggests that only a synergistic stimulation by multi-TLR agonists mimicking a natural infection can ensure a robust immune response to vaccination.
Among the promising TLR agonists that promote neonatal Th1 response, Poly I:C has been used in human clinical trials (NCT01012700, NCT01079741, NCT01299662) with a good safety record. It has been shown to promote DC cross-priming of CD8 T cells by inducing IFN-α in respiratory infections and is considered as a good mucosal adjuvant.44 MPLA in combination with aluminium hydroxide is safe to use in humans and is included in licensed vaccines such as Cervarix (GlaxoSmithKline).51 R848 has been used safely in human clinical trials (NCT01737580, NCT00175435) as a topical immune-modulating agent with intradermal flu vaccines and hepatitis B vaccines. Recent infant macaque models have shown R848 in formulations as a safe vaccine adjuvant.52
Developing methods to safely increase the immunogenicity of vaccines so they might be administered to neonates has been long sought. It is increasingly clear that fully mature adaptive immune responses can be generated in neonates.2 Whole cell vaccines provide intrinsic adjuvant activity but reactogenicity limits their use as seen with whole cell pertussis vaccines.53 For optimal immunogenicity subunit vaccines with purified microbial products require addition of adjuvants. Adjuvants may boost responses to Ags by shaping the type and magnitude of vaccine responses. Current alum-based vaccines do not induce Th1 responses.24
In conclusion, TLR agonist combinations that contain Poly I:C, MPLA and R848 synergistically induce Th1 polarizing cytokines IL-12p70, IFN-γ and IFN-α from CBMCs while addition of class A CpG ODN 2336 significantly decrease IL-12p70 and IFN-γ secretion and increase DC:PD-L1 expression. Addition of TLR2 agonist PAM3CSK4 to synergistic Th1 polarizing TLR agonist combinations induced significant Th2 responses. Dual or triple TLR agonist combinations containing TLR3, TLR4 and TLR7/8 induced a synergistic CBMC Th1 response and reduced inhibitory cDC:PD-L1 expression compared to single TLR agonists. Combinations containing > 3 TLR agonists did not contribute towards synergistic Th1 response. Significant expression of costimulatory markers (CD83) does not predict IL-12p70 or IFN-γ response patterns. Our data support using multi-TLR agonist combinations as novel vaccine adjuvants for neonates. However, vaccine development containing multiple TLR agonists is still in its infancy due to limited understanding of molecular mechanisms of TLR synergy.
Supplemental Material
Supplemental material for TLR agonist combinations that stimulate Th type I polarizing responses from human neonates by Naveen Surendran, Andrea Simmons and Michael E Pichichero in Innate Immunity
Acknowledgments
We thank Jareth Wischmeyer and Yousuf Khalil for technical help.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health/National Institute of Allergy and Infectious Diseases grant R03AI117700 awarded to N Surendran.
References
- 1.WHO. Neonatal Mortality Rate (SDG 3.2.2). In: The Global Strategy for Women’s, Children’s and Adolescents’ Health (2016–2030). Report, WHO, 2015.
- 2.Mohr E, Siegrist CA. Vaccination in early life: standing up to the challenges. Curr Opin Immunol 2016; 41: 1–8. [DOI] [PubMed] [Google Scholar]
- 3.Morris MC, Surendran N. Neonatal vaccination: challenges and intervention strategies. Neonatology 2016; 109: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol 2014; 10: 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burl S, Townend J, Njie-Jobe J, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One 2011; 6: e18185–e18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 2010; 5: e15041–e15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goriely S, Van Lint C, Dadkhah R, et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med 2004; 199: 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183: 7150–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HH, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med 2008; 205: 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7: 379–390. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen M, Leuridan E, Zhang T, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One 2010; 5: e10407–e10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol 2009; 39: 26–35. [DOI] [PubMed] [Google Scholar]
- 13.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol 2009; 30: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upham JW, Rate A, Rowe J, et al. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun 2006; 74: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van den Biggelaar AH, Prescott SL, Roponen M, et al. Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J Allergy Clin Immunol 2009; 124: 544–550, 550 e1–2. [DOI] [PubMed] [Google Scholar]
- 16.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol 1999; 163: 2249–2255. [PubMed] [Google Scholar]
- 17.Liu E, Law HK, Lau YL. BCG promotes cord blood monocyte-derived dendritic cell maturation with nuclear Rel-B up-regulation and cytosolic I kappa B alpha and beta degradation. Pediatr Res 2003; 54: 105–112. [DOI] [PubMed] [Google Scholar]
- 18.Aaby P, Andersen A, Ravn H, et al. Co-administration of BCG and diphtheria-tetanus-pertussis (DTP) vaccinations may reduce infant mortality more than the WHO-schedule of BCG first and then DTP. A re-analysis of demographic surveillance data from rural Bangladesh. EBioMedicine 2017; 22: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol 2014; 15: 895–899. [DOI] [PubMed] [Google Scholar]
- 20.Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on Ab and cytokine responses to human neonatal vaccination. J Immunol 2002; 168: 919–925. [DOI] [PubMed] [Google Scholar]
- 21.Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol 2001; 31: 1531–1535. [DOI] [PubMed] [Google Scholar]
- 22.Jang S, Uematsu S, Akira S, et al. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol 2004; 173: 3392–3397. [DOI] [PubMed] [Google Scholar]
- 23.Pompei L, Jang S, Zamlynny B, et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol 2007; 178: 5192–5199. [DOI] [PubMed] [Google Scholar]
- 24.Dowling DJ, Levy O. Pediatric vaccine adjuvants: components of the modern vaccinologist’s toolbox. Pediatr Infect Dis J 2015; 34: 1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy O, Zarember KA, Roy RM, et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol 2004; 173: 4627–4634. [DOI] [PubMed] [Google Scholar]
- 26.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol 2006; 121: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegrist CA. Mechanisms by which maternal Abs influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 2003; 21: 3406–3412. [DOI] [PubMed] [Google Scholar]
- 28.Fransen F, Boog CJ, Van Putten JP, et al. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect Immun 2007; 75: 5939–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012; 130: 195–204 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol 2007; 68: 813–822. [DOI] [PubMed] [Google Scholar]
- 31.Pihlgren M, Tougne C, Schallert N, et al. CpG-motifs enhance initial and sustained primary tetanus-specific Ab secreting cell responses in spleen and bone marrow, but are more effective in adult than in neonatal mice. Vaccine 2003; 21: 2492–2499. [DOI] [PubMed] [Google Scholar]
- 32.Higgins D, Rodriguez R, Milley R, et al. Modulation of immunogenicity and allergenicity by controlling the number of immunostimulatory oligonucleotides linked to Amb a 1. J Allergy Clin Immunol 2006; 118: 504–510. [DOI] [PubMed] [Google Scholar]
- 33.Lemoine S, Jaron B, Tabka S, et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J Allergy Clin Immunol 2015; 136: 1355–1368 e1–15. [DOI] [PubMed] [Google Scholar]
- 34.Van Haren SD, Dowling DJ, Foppen W, et al. Age-specific adjuvant synergy: dual TLR7/8 and Mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol 2016; 197: 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napolitani G, Rinaldi A, Bertoni F, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 2005; 6: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Egelston C, Gagnon S, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest 2010; 120: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen K, Blimkie D, Furlong J, et al. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods 2008; 336: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belderbos ME, Levy O, Meyaard L, et al. Plasma-mediated immune suppression: a neonatal perspective. Pediatr Allergy Immunol 2013; 24: 102–113. [DOI] [PubMed] [Google Scholar]
- 39.Gold MC, Donnelly E, Cook MS, et al. Purified neonatal plasmacytoid dendritic cells overcome intrinsic maturation defect with TLR agonist stimulation. Pediatr Res 2006; 60: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfle SJ, Strebovsky J, Bartz H, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol 2011; 41: 413–424. [DOI] [PubMed] [Google Scholar]
- 41.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002; 168: 4531–4537. [DOI] [PubMed] [Google Scholar]
- 42.Levy O, Suter EE, Miller RL, et al. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 2006; 108: 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 2008; 65: 3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 2009; 206: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawai T, Sato S, Ishii KJ, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 2004; 5: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 46.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol 2004; 5: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 47.Gujer C, Sandgren KJ, Douagi I, et al. IFN-alpha produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. J Leukoc Biol 2011; 89: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Lepelley A, Azria E, et al. Neonatal plasmacytoid dendritic cells (pDCs) display subset variation but can elicit potent anti-viral innate responses. PLoS One 2013; 8: e52003–e52003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 2005; 201: 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kollmann TR, Levy O, Montgomery RR, et al. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 52.Dowling DJ, Van Haren SD, Scheid A, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2017; 2: e91020–e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eberhardt CS, Siegrist CA. What is wrong with pertussis vaccine immunity? Inducing and recalling vaccine-specific immunity. Cold Spring Harb Perspect Biol 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for TLR agonist combinations that stimulate Th type I polarizing responses from human neonates by Naveen Surendran, Andrea Simmons and Michael E Pichichero in Innate Immunity