Objective
This study aimed at evaluating efficacy and safety of thrombus aspiration and intracoronary-targeted thrombolysis on coronary thrombus burden in ST-elevation myocardial infarction (STEMI) patients undergoing primary percutaneous catheterization, comparing their effects on myocardial perfusion through index of microcirculatory resistance (IMR) and single-photon emission computed tomography (SPECT).
Participants and methods
From January 2017 to January 2018, STEMI patients with high thrombus burden undergoing primary catheterization were enrolled and randomly assigned to receiving thrombus aspiration (TA group) or intracoronary thrombolysis (IT group). IMR, SPECT, and other conventional measurements were adopted to assess myocardial perfusion. Major adverse cardiovascular events (MACEs) and complications were recorded over a 90-day follow-up and a 12-month follow-up after the procedure.
Results
The study consisted of 38 patients in the IT group and 33 in the TA group. After recanalization, thrombus burden score, corrected thrombolysis in myocardial infarction (TIMI) frame count, the proportion of TIMI myocardial perfusion 3 grade, and IMR in the IT group were significantly better than those of the TA group (P<0.05). During the 90-day follow-up, no difference was observed in cardiac function and MACEs. During the 12-month follow-up, there were significant differences in infarct size of SPECT (18.56±8.56 vs. 22.67±7.66, P=0.046), left ventricular ejection fraction of echocardiography (58.13±5.92 vs. 55.17±5.68, P=0.043), and the composite MACEs between the two groups (P=0.034).
Conclusion
Thrombus aspiration and intracoronary-targeted thrombolysis are effective and safe strategies in managing high coronary thrombus burden in STEMI patients. Compared with aspiration, intracoronary-targeted thrombolysis is more beneficial in improving myocardial microcirculation perfusion.
Keywords: aspiration, index of microcirculatory resistance, intracoronary thrombolysis, myocardial perfusion, percutaneous coronary catheterization, single-photon emission computed tomography, ST-segment elevation myocardial infarction, thrombus burden
Introduction
ST-segment elevation myocardial infarction (STEMI), a syndrome that results from coronary plaque rupture and acute thrombus formation, has become one of the leading causes of mortality all around the world in recent years. For patients with STEMI, the vital principle is to perform reperfusion therapy as soon as possible by reopening infarct-related artery (IRA) to restore effective myocardial tissue perfusion, inhibit infarction extension, and save ischemic myocardium. Despite the thrombolysis in myocardial infarction (TIMI) 3-grade epicardial blood flow after recanalization, there is an urgent need to address the significance of myocardial microcirculation perfusion restoration and protection, which are closely associated with prognosis and occurrence of cardiac events [1]. During catheterization, unsuitable management of thrombus and direct stent implantation have been confirmed to contribute to the distal embolization, bringing about a second strike to the coronary microvascular circulation and cardiac myocytes [2,3]. Consequently, for those STEMI patients with high thrombus burden, it is not recommended to implement stents directly. Therefore, to solve and take precautions against this issue, a set of strategies has been advocated, including thrombus aspiration and medication pretreatment. Considering the result of total and present guidelines, routine thrombus aspiration has not been carried out as an option in primary intervention with a recommendation level of III-A [4,5]. For patients with large residual thrombus burden, however, aspiration is considered an effective method in emergent recanalization. In addition, intracoronary thrombolysis has been proved to be an efficient alternative in coping with residual thrombus burden in clinical practice. In spite of the lack of the recommendation, its positive therapeutic effects have been investigated in patients with stent thrombosis, left main thrombus, Kawasaki disease, failed aspiration, and other complex circumstances [6–8]. Recently, intracoronary-targeted thrombolysis has become popular and efficient in handling coronary thrombotic lesions. Aspiration and intracoronary thrombolysis are two potential approaches for managing the large residual intracoronary burden; however, a comparison of their effects on myocardial perfusion is still under discussion. Since 2003, the index of microcirculatory resistance (IMR) has been described and applied gradually as a novel invasive indicator of the microvascular flow [9]. Compared with other noninvasive or invasive method, IMR exhibits advantages of good reproducibility, specificity, and independence of epicardial vascular stenosis and dynamics. Therefore, combined with other approaches, this novel index apparently enhances the accuracy in evaluating the myocardial tissue level perfusion. In this study, the efficacy and safety of aspiration and intracoronary-targeted thrombolysis on coronary thrombus burden in STEMI patients undergoing primary catheterization were evaluated, comparing their effects on myocardial microvascular perfusion using of IMR and single-photon emission computed tomography (SPECT).
Participants and methods
Study participants
In this prospective, randomized, control study, patients admitted to the Cardiology Department of the Second Hospital of Hebei Medical University from January 2017 to January 2018 were enrolled consecutively. All the enrolled participants met the following criteria: (i) patients were diagnosed with STEMI according to the guideline-recommended standard; (ii) patients were scheduled for primary percutaneous coronary intervention (PCI) or percutaneous transluminal coronary angioplasty in emergent catheterization laboratory within 12 h from chest pain onset to admission; (iii) patients presented with high intracoronary thrombus burden under angiographic imaging; (iv) patients agreed to accept IMR examination during procedure and resting SPECT during hospitalization. However, patients who met the following descriptions were excluded from this study: having received intravenous thrombolytic agents, cardiac shock, refusing primary operation or planning a selective intervention, myocardial infarction in situ or with a history of coronary artery bypass grafting, failing to reopen IRA, developing dissection and mechanical complications during procedure, severe hepatic or renal insufficiency, malignant tumor, contraindication of antithrombotic, and anticoagulation therapy.
STEMI was diagnosed on the basis of the following conditions [4]: (i) typical chest pain symptoms lasted for more than 30 min without relief, (ii) ST-segment elevated 0.1 mV in at least two contiguous leads or new left bundle brunch block in ECG examination, and (iii) high sensitive positive troponin with increased myocardial biomarker values.
Feature of high thrombus burden was defined according to Yip’s standards [10]: estimating diameter of IRA lumen of at least 4.0 mm, liner thrombus with linear dimension three-fold greater than diameter, cutoff occlusion, thrombus accumulation on the proximal occlusion (>5.0 mm), thrombus floating on proximal end, and sustaining dye stasis on distal obstruction.
All chosen patients provided signed consent. Study protocol and procedures performed were approved by the local ethics committee and in accordance with the Helsinki Declaration.
Study protocols
Upon admission, patients’ brief history and basic physical examination were immediately taken. An 18-lead ECG was accomplished within 10 min. All patients were prescribed with loading doses of aspirin (300 mg) and ticagrelor (180 mg) upon STEMI diagnose. Venous blood samples were collected for laboratory tests, including blood routine, biochemical assay (high sensitive C-reactive protein, hepatic and renal function, glucose, lipid, electrolyte), myocardial biomarkers (creatine kinase-MB isozyme), troponin I, as well as D-dimer. All participants signed the operation informed consents.
The interventional procedure was performed by at least two qualified cardiologists in accordance with the standard clinical practice. As soon as the guidewire crossing or balloon inflating occthe culprit lesions, the thrombus burden of IRA was analyzed and scored. After evaluation, eligible participants were divided into two groups, namely, the intracoronary thrombolysis group (IT group) and the thrombus aspiration group (TA group), at a ratio of 1: 1 according to random number table.
Patients in the IT group received intravascular targeted thrombolysis. Finecross microcatheter (NC-F863A; Terumo, Tokyo, Japan) was applied to inject intracoronary fibrinolytic agent from the distal end of the IRA. The microcatheter was withdrawn while injecting the drug slowly, reinforcing the targeted thrombolytic effects by promoting thrombus exposure to the agent. In this study, 10 mg of Recombinant Human Prourokinase (Pro-UK, 5 mg; Tasly Pharmaceuticals, Shanghai, China), a known urokinase precursor and specific plasminogen activator, was administered. Variations in vital signs and intracoronary thrombolysis were closely observed during the agent injection. Furthermore, an additional 5 or 10 mg Pro-UK would be given if residual thrombus existed and the total dose of drug applied was 20 mg or less. The patients were excluded from this study if the retrograde fibrinolysis failed to improve coronary perfusion within 10 min.
Patients in the TA group underwent thrombus aspiration with Export AP catheter (Medtronic Cardiovascular, Santa Rosa, California, USA) for several repetitions. If the aspiration catheter could not pass through the lesions, the patients would be transferred to the IT group receiving intracoronary thrombolysis. If thrombus failed to be aspirated entirely or epicardial flow could not be restored, the patients were dropped out to be subjected to other treatments.
Angiographic analysis and index of microcirculatory resistance measurement
Coronary stenosis severity was measured by the Quantitative Coronary Analysis system. If the severity degree was over 75%, stenting was considered as useful primary reperfusion therapy. All patients received intravenous unfractionated heparin of 50–70 U/kg to maintain the activated clotting time of 250–300 s. Anticoagulant doses were adjusted in terms of individual details and the use of glycoprotein inhibitor. Routine medical equipment (stents, balloons, catheters, and wires) were determined by the operators. Reperfusion time data, including symptom onset to balloon time and first medical contact to balloon time, were carefully recorded. When guidewire or balloons passed the lesions, TIMI flow grade, TIMI myocardial perfusion grade (TMPG) and thrombus scores of IRA were evaluated depending on the conventional approach [11–13]. After revascularization, the TIMI flow grade, TMPG, and corrected TIMI frame count (cTFC) of the artery were assessed again [14]. The relevant scoring rules were put forward as described previously. ECG would be measured again 1 h after intervention for the degree of ST-segment resolution on corresponding leads.
After stenting, the patients were subjected to IMR measurement with the pressure wire (PressureWire Certus, C12008; St Jude Medical System AB, Uppsala, Sweden). The wire together with pressure/temperature sensor on the head was placed on the distal end of the vessel. After device calibration, 3 ml saline on room temperature was injected three times through guiding catheter to collect the baseline data. ATP was administered through intravenous transfusion at a speed of 140 μg/kg/min to achieve coronary hyperemia. The hyperemic mean transit time was obtained through repeated saline injection. The value of coronary flow reserve and fractional flow reserve displayed on the screen were carefully recorded. IMR values after the catheterization were calculated using the formula, in which IMR equals to the distant artery pressure multiplied by the hyperemic mean transit time, without considering the coronary wedge pressure. Pressure wire was kept remaining in the same position during the IMR assessment to guarantee the reliability of the results [15].
Echocardiography and single-photon emission computed tomography
Two-dimension transthoracic echocardiography was performed within 2 days after the procedure to evaluate the cardiac function and remodeling using Simpson’s method. The parameters including End-Diastolic Volume Index (EDVI), End Systolic Volume Index (ESVI), left ventricular ejection fraction (LVEF), and Wall Motion Score Index (WMSI), were collected. Body surface area was calculated based on Stevenson’s formula: body surface area=0.0061×height (cm)+0.0128×weight (kg)−0.1529. The wall motion was calculated with a 16-segment model, with the scoring of WSMI being described using five grades (5=aneurismal, 4=dyskinetic, 3=akinetic, 2=hypokinetic, 1=normal wall motion).
All the enrolled patients underwent rest SPECT imaging (Discovery NM670; GE Healthcare, Milwaukee, Wisconsin, USA) within 1 week after intervention. Before the examination, 925–1100 MBq (25–30 mCi) 99mTc-sestamibi ((Xinkesida Pharmaceutical Technique Co. Ltd, Beijing, China) was administered intravenously. SPECT was implemented 90 min after administration of 99mTc-sestamibi. Images were acquired with a dual-head rotating gamma camera system over a 180 arc from right anterior oblique 45° to left posterior oblique 45°, with 3 arcs taking one frame. After collection, images were cast into short axis, horizontal long axis, and a vertical long axis. From the slices, myocardial quantification and reconstruction were implemented using Emory Cardiac Toolbox software to establish the bull’s eye polar analysis, from which to access left ventricular perfusion. The perfusion status was recognized as different digital counts and illustrated in corresponding colors. The region of interest (ROI) was sketched automatically when the counts ratio reached the threshold value of 40%.
Endpoints and clinical follow-up
The primary endpoint was the combination of TMPG and IMR values after the intervention. The secondary endpoints were ROI, LVEF, and incidence of major adverse cardiac events (MACEs) during follow-up. At the 90-day and 12-month follow-ups, echocardiography was carried out again to estimate changes of LVEF and ventricular remodeling parameters. Rest SPECT was performed to assess the infarction area again at the 12-month follow-up. MACEs included cardiac death, worsening heart failure, malignant ventricular arrhythmia, and target vessel revascularization. Worsening heart failure was defined as follows: (i) Killip classification advanced during hospitalization, for instance, lung rales extended; (ii) New emerging congestive heart failure symptoms or signs requiring treatment after discharge. The incidence of major or minor bleeding complications was also recorded according to TIMI classification [16]. Major bleeding was defined as intracranial, gastrointestinal, or another organic hemorrhage with a decrease of hemoglobin of more than 5 g/dl. Minor bleeding was defined by suspicious bleeding with a decrease of hemoglobin of more than 3 g/dl.
Sample size calculation
On the basis of our pre-experiment result and previously published literature, the percentage of TMPG grade 3 in STEMI patients after primary PCI was nearly 50% [17]. When receiving intracoronary fibrinolysis and aspiration, this percentage would rise to 95 and 70%, respectively. A sample size of this study was calculated with PASS 11.0 software (NCSS LLC., Kaysville, Utah, USA). The power of the test was set at 0.80, with α error set at 0.05 and statistical significance level (double-sided) at 0.05, finally, 33 patients in each group were required [18].
Statistical analysis
Statistical analysis was carried out with SPSS 23.0 Package (IBM Inc., Chicago, Illinois, USA). Continuous variables were tested for normal distribution using Kolmogorov–Smirnov’s test. Normally distributed data were presented as mean±SD and were compared by Student’s t-test. Non-normally distributed data were presented as a median (first quartile, third quartile) and compared by Mann–Whitney U-test. Categorical variables were reported as a percentage and compared using χ2 or Fisher’s exact test. A two-sided P value of less than 0.05 was considered to be statistically significant.
Results
Baseline characteristics
From January 2017 to January 2018, a total of 189 patients were consecutively scheduled for primary intervention. Among them, 108 cases were excluded from further investigation, including five with cardiogenic shock, nine with previous myocardial infarction or PCI history, 16 receiving intravenous fibrinolysis, four with non-ST-segment elevation acute myocardial infarction, 69 with low angiographically visible thrombus, and five refusing IMR or SPECT measurement. As a result, this study comprised a total of 81 patients.
The selected patients were divided into the IT and TA groups. Of which, the IT group consisted of 41 patients whereas the TA group had 40 patients. For the IT group patients, five cases were eliminated from the study because of the subsequent aspiration while three cases in the TA group were transferred to the IT group because of the failure of catheter passing through the lesions. In addition, other four individuals in the IT group were excluded because of unsuccessful recanalization of the aspiration and following thrombolysis. Finally, 38 patients were assigned to the IT group and 33 to the TA group (Fig. 1).
Fig. 1.
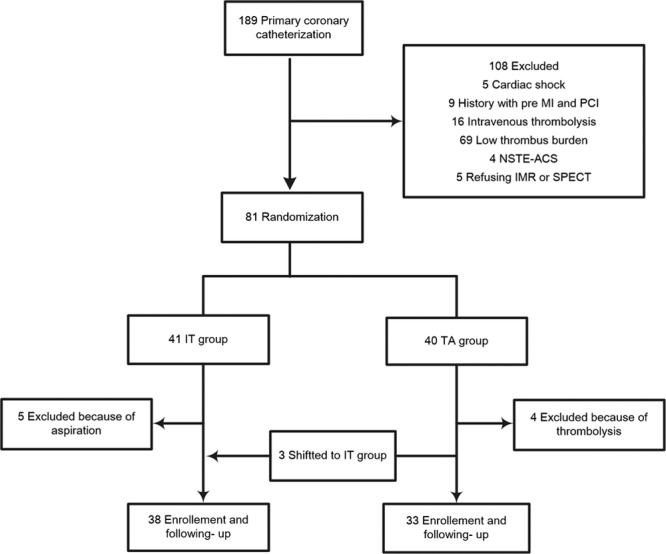
Flow chart of study protocol IMR, index of microcirculatory resistance; MI, myocardial infarction; NEST-ACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SPECT, single-photon emission computed tomography.
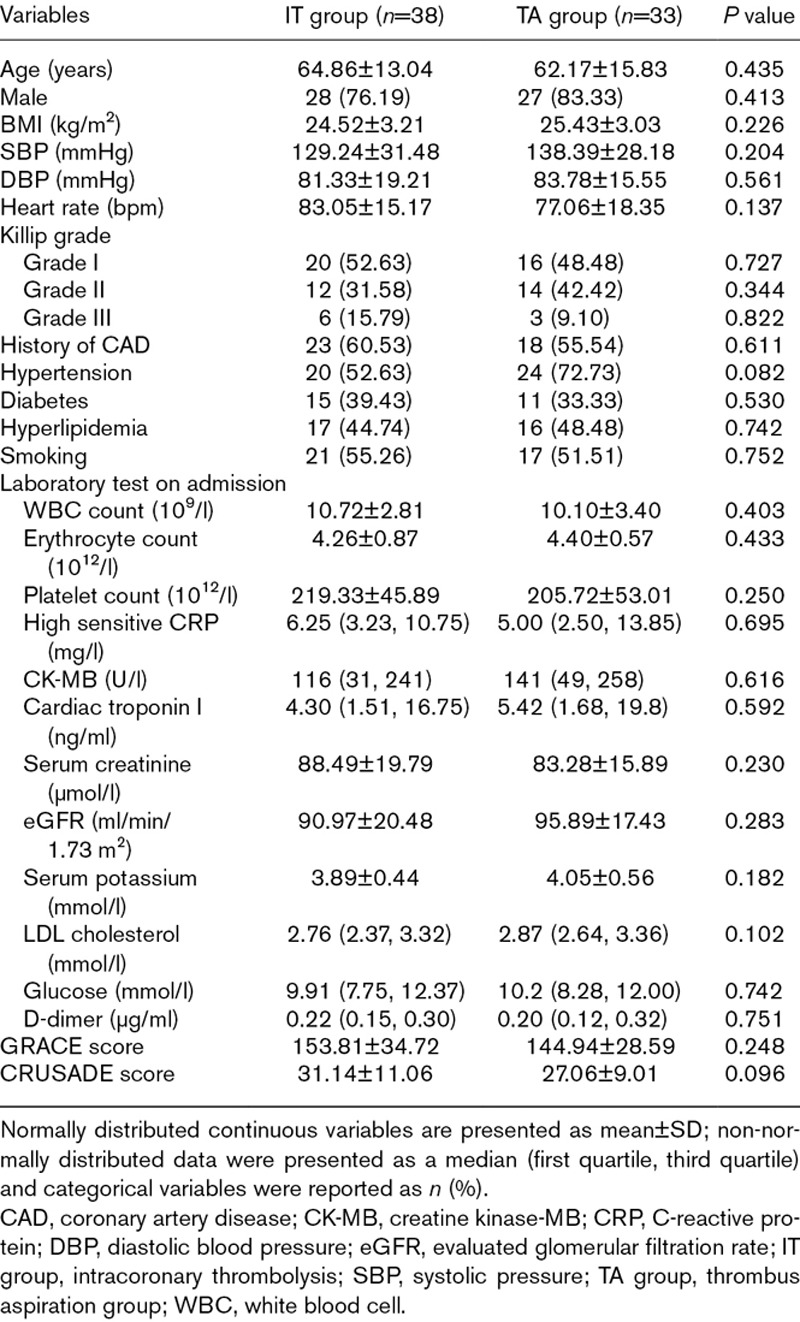
Demographic data, baseline clinical characteristic, and preoperative laboratory test of the studied participants are presented in Table 1. No significant difference was observed between the two groups on following parameters, such as sex, age, BMI, vital signs, Killip class, risk factors, related scores, blood routine test, myocardial biomarkers, biochemical test, and D-dimer (all P>0.05). Therefore, the samples selected in this study were in accordance with the requirement for statistical analysis.
Table 1.
Baseline clinical characteristics between two groups
Angiographic analysis and invasive measures of microvascular perfusion
The interventional characteristics of the participants assigned to the IT and TA groups are summarized in Table 2. After comparing the data obtained from the two groups, no statistical difference was found regarding symptom onset to balloon time, first medical contact to balloon time, IRA distribution, stenting details, and procedural medications (all P>0.05).
Table 2.
Procedural and angiographic features between two groups
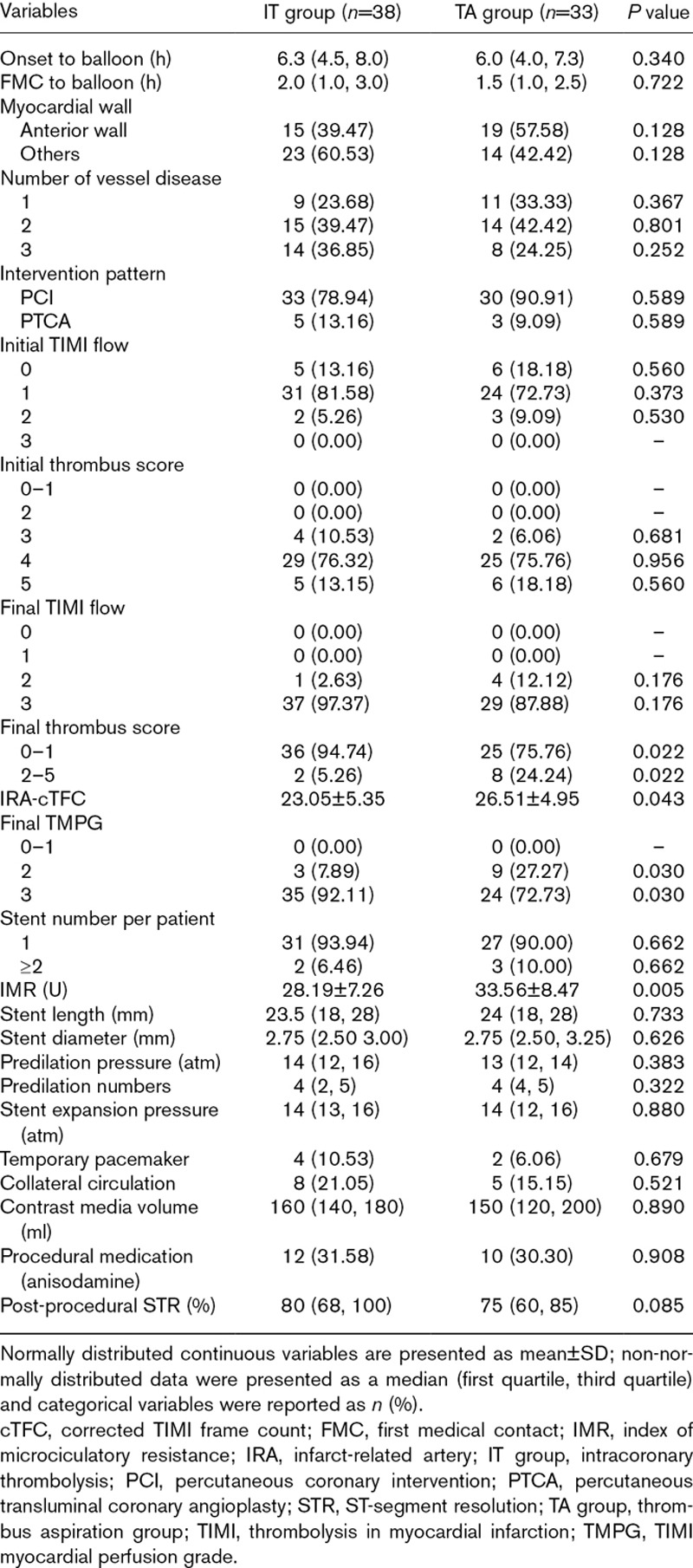
The thrombus burden was assessed in patients of the IT and TA groups as well as myocardial perfusion and IMR after angiographic analysis. The results indicated that the initial TIMI grade and thrombus burden score were comparable for both groups of patients. After successful intervention, thrombus burden score, cTFC, and the percentage of TMPG grade 3 were found to be significantly different between the two groups (final thrombus score 2–5 point: 5.26 vs. 24.24%, P=0.022; cTFC: 23.05±5.35 vs. 26.51±4.95, P=0.043; proportion of final TMPG grade 3: 92.11 vs. 72.73%, P=0.030), whereas no obvious difference in TIMI flow was observed in the two groups of patients. Postprocedure IMR value of the IT group was lower than that of the TA group (28.19±7.26 vs. 33.56±8.47, P=0.005). The two groups showed no statistical difference in ST-segment resolution 1 h after the intervention. The related results were listed in Table 2.
Echocardiography and single-photon emission computed tomography
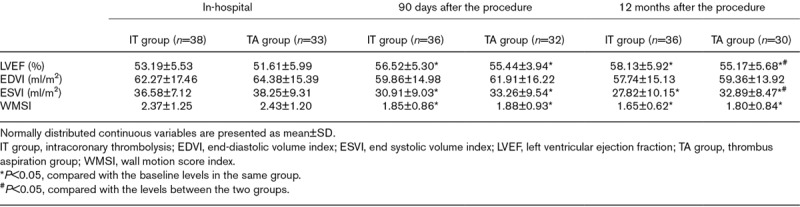
Analysis of the baseline cardiac function of the patients in the two groups revealed that there was no significant difference in terms of LVEF, EDVI, ESVI, and WMSI. After 90 days of intervention, the LVEF, ESVI, and WMSI values were shown to be obviously improved in the respective group (both P<0.05). However, no distinct difference was observed on LVEF between the two groups of participants. At the 12-month follow-up, the parameters were assessed again. LVEF value was higher in the IT group with a statistical difference (58.13±5.92 vs. 55.17±5.68, P=0.043). EDVI, ESVI, and WMSI values decreased within the same group compared with the baseline information. There was an obvious difference in ESVI between two groups (27.82±10.15 vs. 32.89±8.47, P=0.033). The measurement results of echocardiography are manifested in Table 3.
Table 3.
Echocardiography examination between two groups in following-up
Within 7 days after intervention, rest SPECT imaging was carried out for all studied population to evaluate myocardial perfusion. The results indicated that, although ROI value (%) of the IT group was lower than that of the TA group (21.29±9.65 vs. 24.82±8.87, P=0.115), the difference had no statistical significance. At the 12-month follow-up, ROI was tested again. Values within the same group decreased without a significant difference (IT group: 21.29±9.65 vs. 18.56±8.56, P=0.200; TA group: 24.82±8.87 vs. 22.67±7.66, P=0.309), whereas there was an obvious difference of ROI values (%) between the two groups (18.56±8.56 vs. 22.67±7.66, P=0.046).
Clinical follow-up
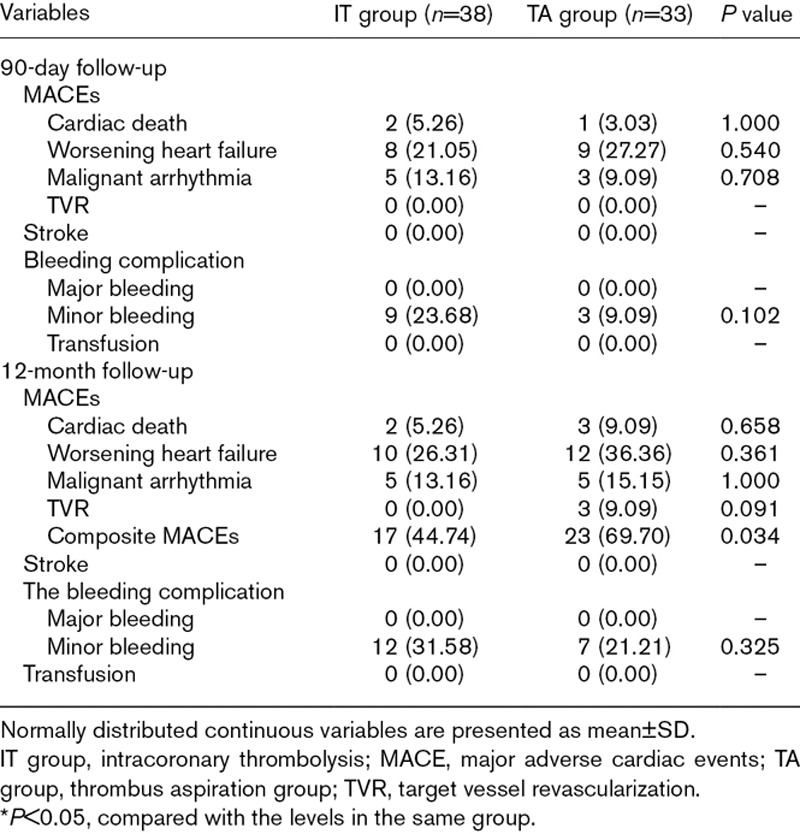
The 90-day and the 12-month follow-ups after operation were performed, during which MACEs alongside the accompanying complications were recorded and no case was lost. The results are listed in Table 4. At the 90-day follow-up, none of those differences on the events and complications were shown to be statistically significant between the two groups. At the 12-month follow-up, in spite no difference was noticed between the two groups, the composite endpoint of the IT group was lower than the TA group (44.74 vs. 69.7%, P=0.034). In terms of complications, the incidence of ischemic stroke, major or minor bleeding and transfusion, had no statistical difference between the two groups (all P>0.05).
Table 4.
Major adverse cardiovascular events, stroke, bleeding complications during follow-up
Discussion
Acute coronary thrombus formation secondary to unstable endothelial plaque rupture, ulcers and erosion plays a crucial role in the pathophysiology of STEMI. According to existing research data, the intracoronary high thrombus is quite common [19]. Sianos et al. [20] reported that among the STEMI patients undergoing primary recanalization, almost 70% were diagnosed with large intracoronary thrombus burden. EMERALD (Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris) trial held by Stone et al. [21] showed that visible thrombus debris could be retrieved in nearly 73% of patients with STEMI. In addition to its prevalence, thrombus lesion is also hard to manage in emergent intervention. Repeated, inappropriate mechanical inflating and stenting would result in obstruction of microvascular distal end as well as aggravating injury on the microcirculation. Henriques et al. [22] pointed out that during the process of primary PCI, up to 15% of patients would develop distal embolization. Therefore, managing the thrombus burden properly has raised controversy in clinical practice in recent years. This study aimed to investigate the safety and efficacy of two possible approaches, namely, thrombus aspiration and intracoronary thrombolysis, and their potential protective effects on myocardial perfusion, which is directly associated with clinical benefits and prognosis.
On the basis of former trials, like TAPAS, INFUSE-AMI, and TASTE, intracoronary thrombus aspiration seemed to be an appropriate method to improve the prognosis of patients with STEMI. Nonetheless, the result of TOTAL (Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI) was frustrated so that routine coronary thrombus aspiration was no longer recommended according to latest guideline [5]. However, for those with high thrombus burden, aspiration would be an alternative method to minimize residual thrombus in the culprit artery. Aspiration catheter was also applied to inject intracoronary drugs to improve myocardial perfusion [23,24].
Apart from mechanical aspiration, intracoronary drug administration has turned into a rewarding attempt, which proved potential in dealing with high thrombus burden in STEMI patients [25]. Kelly evaluated intracoronary tenecteplase in 34 patients who developed severe thrombus events during PCI, including no-reflow, distal embolization, or visible intracoronary thrombus. The results showed that intracoronary tenecteplase was successful in dissolving angiographic thrombus in 91% of patients without increasing the incidence of hemorrhage [26]. However, randomized large scale controlled trials for evaluating the routine intracoronary thrombolysis for STEMI patients are still not common, whereas present studies are mainly case reports or small sample studies.
Although the above mentioned methods are beneficial in resolving thrombus burden and reopening the culprit artery, their effects on coronary microcirculation are not clear. In this paper, not only the conventional reference standards (TIMI blood flow, cTFC, TMPG, and ST-segment resolution) were employed, but IMR and SPECT were adopted to determine the microvascular perfusion. IMR, a thermodilution-derived quantitative measurement of coronary microvascular resistance, was first proposed by Fearon et al. [9]. Porcine model has been investigated the correlation between the calculated IMR value and true distal resistance, validating the feasibility of this innovative technique in estimating microvascular resistance [9]. Different from other current physiological and functional assessment, like coronary flow reserve and fractional flow reserve, the application of IMR is independent of epicardial stenosis, reproducibility, and hemodynamic instability. Except being related to the perfusion status of myocardial tissue, IMR has also been shown one prediction reference of prognosis in the setting of STEMI [9,27,28]. Apart from those invasive methods, SPECT, a noninvasive imaging technique, was utilized to show the myocardial perfusion as well. Since 1970s, great advances in nuclear cardiology have interrogated cardiac physiology and pathophysiology, providing novel insights into understandings of myocardial perfusion, metabolism, and ventricular function, which improve the diagnosis and treatment. The most widely employed nuclear cardiac procedure is SPECT of myocardial perfusion imaging [29,30]. During perfusion estimation a bull’s eye polar analysis illustrating the perfusion status at different colors was used in this study. Depending on the computer program processing, the ROI area was calculated and reflected as a perfusion defect. Typically, rest imaging in the acute stage would overestimate infarct size in the presence of stunning and hibernating. Therefore, SPECT was performed again at the 12-month follow-up to explore the improvement of myocardial perfusion. These approaches combining together drastically contributes to enhancing the accuracy of the results, suggesting the availability of using these methods together in evaluating the perfusion status of STEMI patients and the beneficial effects of intracoronary-targeted thrombolysis.
Results in this study revealed that the IT group patients exhibited less residual thrombus after the procedure than the TA group ones. Compared with the TA group participants, cTFC, the proportion of TMPG grade 3 and IMR value of the IT group seemed to be more satisfactory. At a long-term follow-up, patients receiving retrograde thrombolysis have less MACEs occurrence and better cardiac function. These results suggested that, intracoronary-targeted thrombolysis was better in improving myocardial microvascular perfusion than aspiration, possibly because of the following reasons: First, instead of injection through guiding catheter, fibrinolytic agent was administered through Finecross microcatheter placed on the distal end of artery, which increased the contact of drug and thrombus and the success probability of intracoronary-targeted fibrinolysis. Second, it has been verified that intravenous thrombolysis with Pro-UK, the precursor of urokinase, is capable of an opening occluded artery and is reliable with rare bleeding complications. As an inactive agent, Pro-UK transforms into urokinase once contacting the surface of thrombus, functioning as a fibrin-specific plasminogen activator without interfering with the fibrinolytic system and increasing the risk of bleeding [31,32]. Third, intracoronary fibrinolytic agents resolve the thrombus in epicardial as well as a microvascular artery, providing an alternative to promote the myocardial perfusion after infarction. Whereas, microemboli cannot be removed when extracting large clots outside. Moreover, improper aspiration of the left descending artery or the left circumflex artery would draw the thrombus to the left main artery, inducing acute occlusion and deadly events.
As described in Table 4, the incidence of composite endpoint seemed higher, and the potential reason lied in the definition of worsening heart failure. During hospitalization, some patients had an advanced Killip classification and lung rale extension after the catheterization, which might possibly be on account of reperfusion injury, excess contrast amount, and liquid intake. Under close monitoring, those participants have not developed cardiac function deterioration and apparent symptoms. These patients might add the incidence of heart failure and the composite MACEs during follow-up. After long-term observation, cardiac parameters raised significantly in both the groups suggesting the importance of coronary catheterization in improving cardiac function. At 12-month after the procedure, the significant differences in LVEF and ROI between the two groups certified the effects of intracoronary thrombolysis in improving myocardial perfusion.
The definition of a high thrombus burden was another issue that needed to be addressed in this study. The most commonly chosen standard was established by Gibson et al. [13], defining the thrombus with a score of at least 4 as a high thrombus burden. However, on account of the clinical practice and former data, after guidewire or balloon passing through the occluded lesions in the coronary angiographic procedure, only 0.4% patients were left with a score of 5, whereas nearly 30% sharing a score of 4 [20]. Consequently, Yip’s principle was utilized for identifying coronary high thrombus burden subsequent to guidewire passing or small balloon inflation [10].
Some limitations in this study are present to be further solved. First, the sample size of this single-center trial was small; more participants are required to be enrolled in subsequent studies to improve the experimental quality. Second, except for SPECT, other assessing techniques, for instance, cardiac MRI and PET, have been shown accurately in evaluating infarct size and microvascular perfusion [1,33]. Limited by the economic cost, however, it is SPECT that was applied in this study. Furthermore, in real-world experiences, unlike the single therapy in this study, an integrated combination of various techniques would be put into use when coping with a high thrombus burden. Future trials are required to explore other complementary treatments in the setting of high coronary thrombus burden.
Conclusion
Thrombus aspiration and intracoronary-targeted thrombolysis are effective and safe strategies in managing high coronary thrombus burden in STEMI patients. Compared with aspiration, intracoronary-targeted thrombolysis is more beneficial in improving myocardial microcirculation perfusion.
Acknowledgements
This work was partially funded and supported by the National Key R&D Program of China (No. 2016YFC1301100).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016; 37:1024–1033 [DOI] [PubMed] [Google Scholar]
- 2.Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009; 120:1822–1836 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS, Burke A, Farb A, Kaye D, Lesser JR, Henry TD, et al. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol. 2009; 54:2167–2173 [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39:119–177 [DOI] [PubMed] [Google Scholar]
- 5.Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015; 372:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boscarelli D, Vaquerizo B, Miranda-Guardiola F, Arzamendi D, Tizon H, Sierra G, et al. Intracoronary thrombolysis in patients with ST-segment elevation myocardial infarction presenting with massive intraluminal thrombus and failed aspiration. Eur Heart J Acute Cardiovasc Care. 2014; 3:229–236 [DOI] [PubMed] [Google Scholar]
- 7.Schieman G, Cohen BM, Kozina J, Erickson JS, Podolin RA, Peterson KL, et al. Intracoronary urokinase for intracoronary thrombus accumulation complicating percutaneous transluminal coronary angioplasty in acute ischemic syndromes. Circulation. 1990; 82:2052–2060 [DOI] [PubMed] [Google Scholar]
- 8.Gürkan U, Tatlisu MA, Aruğaslan E, Bolca O. Successful management of left main coronary artery thrombus with intracoronary thrombolysis. Turk Kardiyol Dern Ars. 2014; 42:475–477 [DOI] [PubMed] [Google Scholar]
- 9.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003; 107:3129–3132 [DOI] [PubMed] [Google Scholar]
- 10.Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, Fang CY, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002; 122:1322–1332 [DOI] [PubMed] [Google Scholar]
- 11.Ganz W. The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med. 1985; 313:1018. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000; 101:125–130 [DOI] [PubMed] [Google Scholar]
- 13.Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001; 103:2550–2554 [DOI] [PubMed] [Google Scholar]
- 14.Gibson CM, Cannon CP, Daley WL, Dodge JT, Alexander B, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996; 93:879–888 [DOI] [PubMed] [Google Scholar]
- 15.Fearon WF, Kobayashi Y. Invasive assessment of the coronary microvasculature: the index of microcirculatory resistance. Circ Cardiovasc Interv. 2017; 10:e005361. [DOI] [PubMed] [Google Scholar]
- 16.Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial – phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988; 11:1–11 [DOI] [PubMed] [Google Scholar]
- 17.Kunadian V, James SK, Wojdyla DM, Zorkun C, Wu J, Storey RF, et al. Angiographic outcomes in the PLATO Trial (Platelet Inhibition and Patient Outcomes). JACC Cardiovasc Interv. 2013; 6:671–683 [DOI] [PubMed] [Google Scholar]
- 18.Chow SC, Shao J, Wang H. Sample size calculations in clinical research. 2008. 2nd ed, Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- 19.Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008; 358:557–567 [DOI] [PubMed] [Google Scholar]
- 20.Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, van Domburg RT, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007; 50:573–583 [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005; 293:1063–1072 [DOI] [PubMed] [Google Scholar]
- 22.Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van ‘t Hof AW, Hoorntje JC, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002; 23:1112–1117 [DOI] [PubMed] [Google Scholar]
- 23.Liu CP, Lin MS, Chiu YW, Lee JK, Hsu CN, Hung CS, et al. Additive benefit of glycoprotein IIb/IIIa inhibition and adjunctive thrombus aspiration during primary coronary intervention: results of the Initial Thrombosuction and Tirofiban Infusion (ITTI) trial. Int J Cardiol. 2012; 156:174–179 [DOI] [PubMed] [Google Scholar]
- 24.Gatto L, Di LA, Romagnoli E, Marco V, Russo C, Pawlowski T, et al. A comparison of intracoronary treatment strategies for thrombus burden removal during primary percutaneous coronary intervention: a COCTAIL II substudy. Coron Artery Dis. 2018; 29:186–193 [DOI] [PubMed] [Google Scholar]
- 25.Geng W, Zhang Q, Liu J, Tian X, Zhen L, Song D, et al. A randomized study of prourokinase during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J Interv Cardiol. 2018; 31:136–143 [DOI] [PubMed] [Google Scholar]
- 26.Kelly RV, Crouch E, Krumnacher H, Cohen MG, Stouffer GA. Safety of adjunctive intracoronary thrombolytic therapy during complex percutaneous coronary intervention: initial experience with intracoronary tenecteplase. Catheter Cardiovasc Interv. 2005; 66:327–332 [DOI] [PubMed] [Google Scholar]
- 27.Ng MK, Yong AS, Ho M, Shah MG, Chawantanpipat C, O’Connell R, et al. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. 2012; 5:515–522 [DOI] [PubMed] [Google Scholar]
- 28.Murai T, Yonetsu T, Kanaji Y, Usui E, Hoshino M, Hada M, et al. Prognostic value of the index of microcirculatory resistance after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Catheter Cardiovasc Interv. 2018; 92:1063–1074 [DOI] [PubMed] [Google Scholar]
- 29.Buechel RR, Kaufmann BA, Tobler D, Wild D, Zellweger MJ. Non-invasive nuclear myocardial perfusion imaging improves the diagnostic yield of invasive coronary angiography. Eur Heart J Cardiovasc Imaging. 2015; 16:842–847 [DOI] [PubMed] [Google Scholar]
- 30.Udelson JE, Dilsizian V, Bonow RO. Mann D, Zipes D, Libby P, Bonow R. Nuclear cardiology. Braunwald’s heart disease: a textbook of cardiovascular medicine. 10th ed, Philadelphia, PA: Elesevier Medicine; 261–300 [Google Scholar]
- 31.Zhao L, Zhao Z, Chen X, Li J, Liu J, Li G. Safety and efficacy of prourokinase injection in patients with ST-elevation myocardial infarction: phase IV clinical trials of the prourokinase phase study. Heart Vessels. 2018; 33:507–512 [DOI] [PubMed] [Google Scholar]
- 32.Han YL, Liu JN, Jing QM, Ma YY, Jiang TM, Pu K, et al. The efficacy and safety of pharmacoinvasive therapy with prourokinase for acute ST-segment elevation myocardial infarction patients with expected long percutaneous coronary intervention-related delay. Cardiovasc Ther. 2013; 31:285–290 [DOI] [PubMed] [Google Scholar]
- 33.Bulluck H, White SK, Fröhlich GM, Casson SG, O’Meara C, Newton A, et al. Quantifying the area at risk in reperfused ST-segment-elevation myocardial infarction patients using hybrid cardiac positron emission tomography-magnetic resonance imaging. Circ Cardiovasc Imaging. 2016; 9:e003900. [DOI] [PMC free article] [PubMed] [Google Scholar]


