Background:
Despite marked gains in longevity attributable to antiretroviral therapy (ART), older women living with HIV (OWLH) experience substantial health challenges, and few studies addressed whether they can achieve successful aging (SA). This is among the first studies examining prevalence and psychosocial correlates of self-rated SA (SRSA) among OWLH and women at risk of HIV.
Methods:
The sample included 386 OWLH and 137 HIV-seronegative women enrolled in the Women's Interagency HIV Study (WIHS) who were aged 50 years and older and participated in the “From Surviving to Thriving” (FROST) substudy. The FROST survey included measures of SRSA and positive psychosocial constructs.
Results:
Participants were on average 57 years (SD = 5.3), 74% African American and 30% unemployed. Among OWLH, 94% were on ART and 73% were virally suppressed. Compared with OWLH, a higher proportion of HIV-seronegative women had an annual income ≤ $6000, no health insurance, and reported lower optimism and health-related quality of life. We found no differences in SRSA prevalence by HIV status: 84% of OWLH and 83% of HIV-seronegative women reported SRSA ≥7 (range = 2–10, higher scores signify better SRSA). Having SRSA ≥7 was associated with higher levels of positive psychosocial characteristics (eg, resilience and optimism) among both OWLH and HIV-seronegative women.
Conclusions:
SRSA is achievable among older women with and at risk of HIV despite health complications. Among disadvantaged women, factors other than HIV may be primary drivers of SRSA. Future research is needed to examine determinants of SRSA and to design public health interventions enhancing SA within this population.
Key Words: HIV/AIDS, successful aging, mental health, older adults, healthy aging, aging well
INTRODUCTION
Older women (age 50+) living with HIV (OWLH) remain underrepresented in research1 despite the fact that their numbers have been steadily growing. The estimated prevalence of OWLH per 100,000 US population increased from 94.7 in 2007 to 172.8 in 2015.2,3 Over 102,000 US women aged 50 years and older lived with diagnosed HIV infection at the end of 2015.3 This increase is due to the combination of new HIV diagnoses in older people and increased longevity with the advent of antiretroviral therapy (ART).4 Despite this gain in longevity, OWLH experience complications related to the interaction of HIV and aging, such as the increased likelihood of multimorbidities, frailty, and polypharmacy, as well as substance abuse, social discrimination, and stigma.5–7 Little is known about whether successful aging (SA) is achievable for this population.6
SA was initially defined by Rowe and Kahn,8 in contrast to “usual” aging as the absence or avoidance of disease and disability, retaining a high level of physical and cognitive function and active engagement with life.9 However, this definition has been criticized as unattainable for marginalized populations, such as minority women and people living with HIV (PLWH).10–13 Thus, subsequent literature shifted its focus to self-perceived or self-rated SA (SRSA) defined as individuals' own holistic appraisals of how well they are aging; it is usually measured by asking the respondents how successfully they have aged on a 1–10 scale.14,15 Although SA as defined by Rowe and Kahn8 may be harder to achieve for socially and economically disadvantaged social groups whose aging can rarely be free from disease and disability, SRSA may be possible even in the presence of comorbidities.10,16,17
In fact, Raeanne Moore et al15 suggested that SRSA is achievable among PLWH. Although HIV-seronegative participants in this study experienced significantly higher SRSA prevalence and mean scores, the prevalence of SRSA among PLWH was high at 66%. Similar results were obtained in a study by David Moore et al.18 These findings are corroborated by qualitative research, which suggests that PLWH may be less concerned with the avoidance of disease and disability in their definitions of SA but focus on staying positive, caring for others, and maintaining self-care, independence, and engaged lifestyle.19–23 Furthermore, studies found that although SRSA was not associated with any HIV disease characteristics (eg, viral load) or sociodemographic factors, better SRSA scores were significantly associated with better health-related quality of life (HRQOL) and positive psychosocial factors, such as resilience and optimism.15,18 It is important to note, however, that the aforementioned studies examining SRSA among PLWH were implemented in the samples that were predominantly men and white. Few studies address SRSA among OWLH.
Importantly, observations among mostly white male samples cannot be generalized to OWLH, who are not only demographically different but also experience unique biomedical and psychosocial challenges.24 At year-end 2015, 58% (59,417) of OWLH were African American and only 18% (18,540) were white.3 Thus, most OWLH belong to ethnic/racial minorities, which often intersects with other social vulnerabilities, such as poverty, domestic violence, racial discrimination, and barriers to health care.1 Compared with HIV-seropositive men, OWLH also experience added burdens of gender-based inequality and stigma, as well as lower levels of sexual activity and poorer sexual health.24,25 Research additionally uncovers sex disparities in HIV-related health outcomes with women generally faring worse than men, partially because of differences in quality of and access to care.26 Thus, women lag behind men in terms of ART prescription, use, and adherence as well as viral suppression, with only 48% being virally suppressed in 2014.27,28 Research shows that compared with HIV-seropositive men, women have worse HRQOL and greater HIV-related morbidity and HIV-related and all-cause mortality.26 Among aging PLWH, female sex also increases the likelihood of such geriatric outcomes as frailty and falls.29,30 Moreover, OWLH face sex-specific health challenges, such as menopause and the increased risks of low bone mineral density and postmenopausal osteoporosis, perhaps exacerbated by the interaction of HIV infection, aging, and ART.31 Given these described disparities, it is reasonable to expect that SRSA will be less prevalent among OWLH than among white HIV-seropositive men.
The aim of this study was to examine the prevalence and correlates of SRSA in a sample of OWLH and, as a comparison group, older HIV-seronegative women enrolled in the Women's Interagency HIV Study (WIHS). Based on the findings described previously,15,18 we hypothesized that OWLH will have lower prevalence and lower mean SRSA scores as compared to HIV-seronegative women. We also expect that better SRSA scores will be associated with higher levels of positive psychosocial factors.
METHODS
Research Design
WIHS is one of the key national cohorts providing opportunities for research in women aging with HIV.27 It is an observational multisite study, which was established in 1993 to investigate the progression and effects of HIV in US women.32 WIHS participants are women with and at risk of HIV infection, who were enrolled during 4 waves: 1994–95, 2001–02, 2011–12, and 2013–15.33 WIHS study visits occur every 6 months and typically consist of a centrally scripted interview, clinical examination, and laboratory testing. WIHS structured interview consists of core modules, such as medical and medication history, substance use, or psychosocial/behavioral, and may also include various substudy modules.33 WIHS research protocols are approved by institutional review boards at each participating institution. Participants are compensated for each study visit. Details of the WIHS research design have been previously published.32–34
During WIHS visit 47, which occurred between October 2, 2017, and March 30, 2018, the “From Surviving to Thriving” (FROST) substudy survey was included as an interview module at 4 WIHS sites: Atlanta, Chapel Hill, Brooklyn, and Bronx. The FROST survey was administered as part of the WIHS interview to all participants who were aged 50 or older, spoke English, and were able and willing to provide informed consent. The FROST survey took approximately 10 minutes to complete and included the SRSA assessment used in previous research15 as well as validated scales assessing positive psychosocial factors, such as resilience.
The cross-sectional analyses for this study are based on WIHS data collected from 523 women (386 OWLH and 137 HIV-seronegative) who completed the FROST survey.
Measures
SRSA
Following Moore et al,15 SRSA was assessed by the following question: “Using your own definition, where would you rate yourself in terms of “successful aging,” from “1” (least successful) to “10” (most successful)?” We also constructed a dichotomous outcome, which was coded as 1 if participants had scores ≥7 and 0 otherwise. The cut-point of ≥7 is considered to be high level of SA and has been used in previous research.18
Based on research among those without HIV, 2 questions were included as additional measures of SRSA.17,35 In a separate portion of the FROST survey, participants were asked to indicate their agreement with the statement “I am aging well,” using a 4-point Likert scale (1 = definitely false, 2 = mostly false, 3 = mostly true, and 4 = definitely true). Finally, in yet another portion of the survey, participants were asked to rate their current life on a scale from “1” (worst life possible) to “10” (best life possible).
Positive Psychosocial Constructs
The following validated scales were included in the FROST survey to assess positive psychosocial characteristics: optimism was measured by a 6-item Lifetime Orientation Test-Revised (LOT-R);36 resilience was assessed by the Connor Davidson Resilience Scale—10 Item (CD-RISC-10),37 and personal mastery was measured by a 7-item Pearlin–Schooler Personal Mastery Scale (PMS).38 Finally, coping with stress was assessed by a single question: “In the past 30 days, how effectively have you been able to cope with stress in your life? Please rate your ability to cope with stress from “0” (feel unable to cope) to “100” (I have coped extremely well).” In addition, several measures were available from the core WIHS data. The level of social support was assessed by the tangible and emotional support subscales of the Medical Outcomes Study Social Support Survey (MOS-SSS).39 The Medical Outcome Study 36-Item Short Form Health Survey (MOS SF-36) was used to assess HRQOL.40 Finally, spirituality was measured by the Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being Scale (FACIT-Sp-12).41
Mental Health and Traumatic Experiences
The following measures were available from the core WIHS data. Anxiety was measured by a 7-item General Anxiety Disorder Scale (GAD-7),42 depression—by the Center for Epidemiologic Studies Depression Scale (CES-D),43 loneliness—by a 3-item shortened version of the Revised UCLA Loneliness Scale (R-UCLA),44 and lifetime experience of discrimination—by the Everyday Discrimination Scale.45 In addition, participants' self-reports of several traumatic life events (eg, history of incarceration) were captured both in baseline and follow-up interviews.
Other Participant Characteristics
Sociodemographic characteristics (eg, marital status) and substance use (eg, current smoking) were based on participants' self-report in the 6 months before the WIHS visit. HIV RNA viral load and current CD4 cell count were determined by laboratory testing, whereas ART use and history of AIDS were self-reported. The body mass index was calculated as measured weight in kg/height2 in meters. History of hypertension was defined as systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg or as self-reported hypertension diagnosis with antihypertensive medication use. Diabetes was defined as fasting blood glucose >126 mg/dL, hemoglobin A1c >6.5%, or self-reported antidiabetic medication use combined with self-reported diabetes diagnosis.
Data Analysis
Descriptive statistics were computed, and differences between HIV serostatus groups were assessed using χ2 tests for dichotomous and t-tests for continuous variables. As a next step, we examined correlates of a dichotomized SRSA outcome by the HIV serostatus group. Unadjusted bivariate logistic regression models were first fitted to examine relationships between dichotomized SRSA and potential sociodemographic and biomedical covariates. Those covariates that were significant at P ≤ 0.10 level were included in the adjusted logistic regression models with robust standard errors to examine relationships between psychosocial variables (positive psychosocial constructs and mental health/traumatic experiences) and dichotomized SRSA. All analyses were HIV-stratified. All statistical analyses were completed using Stata 14 software.
RESULTS
Sample Description
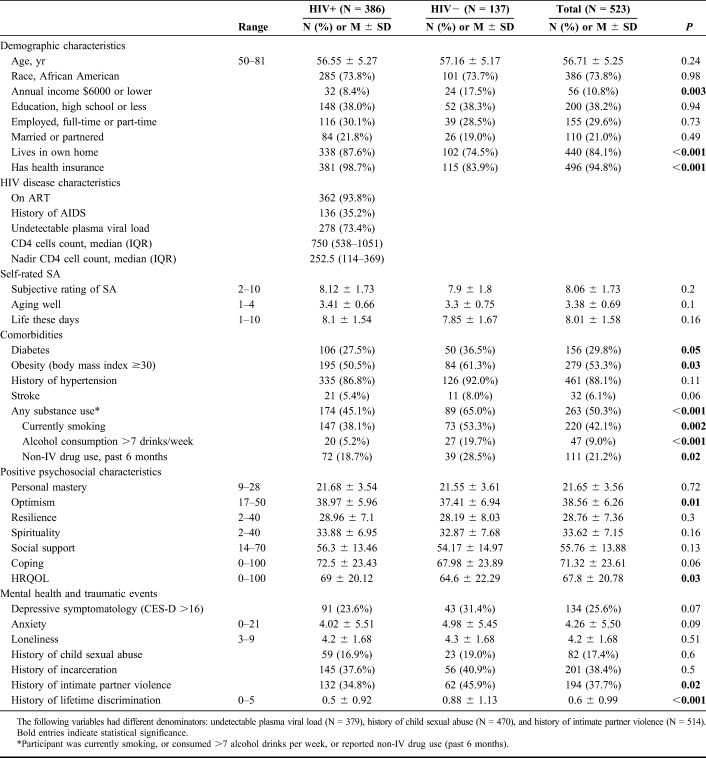
Participants were, on average, 57 years old, predominantly African American, unemployed, and not married or partnered, with no difference by HIV status (Table 1). More than one-third (38%) of participants in both groups had high school or less education. However, we found several significant group differences indicating that HIV-seronegative women in our sample may be somewhat more disadvantaged than the OWLH. A higher proportion of HIV-seronegative participants had very low annual income of $6000 or less, had no health insurance, and did not live in their own home. As compared to OWLH, a higher proportion of HIV-seronegative counterparts reported substance use, lower HRQOL, lower level of optimism, and higher level of perceived lifetime discrimination. OWLH in our sample had well-controlled HIV disease: 94% reported ART use, 73% had an undetectable viral load, and the median CD4 cell count was 750 cells/µL (IQR = 538; 1051).
Table 1.
Comparison of OWLH and HIV-Seronegative Women
SRSA Prevalence
We found no HIV group differences in any of the 3 measures of SRSA (Table 1). Moreover, the 3 measures were highly and significantly correlated; the Cronbach alpha was 0.67. Therefore, we focus our discussion on the subjective rating of SA because this is the measure that was used by previous research among PLWH. The mean score of subjective rating of SA among OWLH was 8.1 (SD = 1.73) and among HIV-seronegative participants was 7.9 (SD = 1.80). Moreover, similar proportions of OWLH and HIV-seronegative participants had scores ≥7 (83.7% vs. 82.5%; Fig. 1).
FIGURE 1.
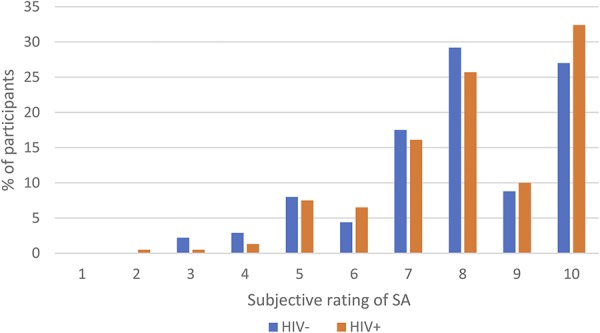
SRSA score distribution by HIV serostatus groups.
SRSA Correlates
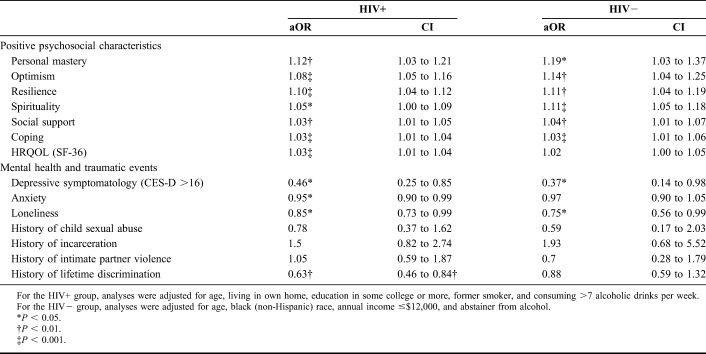
Among the OWLH subsample, the adjusted logistic regression models showed relationships between multiple psychosocial factors and dichotomized SRSA (Table 2). The increased adjusted odds of SRSA ≥7 were significantly associated with the higher levels of the following positive psychosocial factors: personal mastery, optimism, resilience, spirituality, social support, coping, and HRQOL. Conversely, higher levels of anxiety, depression, loneliness, and history of lifetime discrimination were associated with the reduced adjusted odds of SRSA ≥7. Thus, OWLH with current depressive symptomatology had 54% lower adjusted odds of SRSA $7 as compared to OWLH with no depressive symptomatology (P < 0.05). Please note that although we present results for continuous measures of anxiety, social isolation, and lifetime discrimination, as reflected in Tables 1 and 2, we also tested associations with the following dichotomous measures: moderate to high level of anxiety (GAD-7 ≥10), any lifetime discrimination (Everyday Discrimination Scale score >0), and above the median loneliness (R-UCLA score >3). These analyses produced results similar to those presented. All of the aforementioned logistic regression models were adjusted for age, living in own home, some college or higher education, former cigarette smoker status, and consuming >7 alcoholic beverages per week. Of potential sociodemographic and biomedical (including HIV disease characteristics) covariates considered, only some college or higher education was associated [odds ratio (OR) = 2.2, 95% confidence interval (CI): 1.11 to 4.23, P = 0.02] with dichotomized SRSA among OWLH, whereas living in own home, some college or higher education, former cigarette smoker status, and consuming >7 alcoholic beverages per week approached significance at P ≤ 0.10 level. Age was not associated with SRSA ≥7 but was included as a covariate in the adjusted logistic regression models because of its theoretical importance.
TABLE 2.
Adjusted Logistic Regression Analyses: Associations Between Psychosocial Factors and SRSA ≥7
Similar results were obtained in the HIV-seronegative subsample (Table 2). Of the 7 positive psychosocial characteristics considered, 6 were significantly associated with increased adjusted odds of SRSA ≥7, whereas HRQOL approached significance (adjusted OR (aOR) = 1.02, 95% CI: 1.00 to 1.04, P = 0.07). Of the mental health and traumatic events considered, having depressive symptomatology and higher levels of loneliness were associated with the reduced adjusted odds of SRSA ≥7. These analyses were adjusted for age, African American race, annual income ≤ $12,000, and abstainer from alcohol. Of all sociodemographic and biomedical variables considered as potential covariates within the HIV-seronegative subsample, only annual income ≤ $12,000 was significantly associated with SRSA ≥7 (OR = 0.31, 95% CI: 0.11 to 0.88, P = 0.03), whereas African American race and abstainer from alcohol approached significance at P ≤ 0.10 level in the unadjusted models. Similar to OWLH subsample, age was not significantly associated with SRSA ≥7 but was included as a covariate in the adjusted models because of its theoretical importance.
DISCUSSION
To the best of our knowledge, this is the first published report of SRSA among OWLH. Our findings suggest that SRSA is achievable among socially and economically disadvantaged women in the presence of HIV and comorbidities. Moreover, we found high SRSA prevalence among OWLH and no significant difference in SRSA prevalence or mean scores by HIV status. There were no strong relationships between SRSA and sociodemographic characteristics, HIV disease characteristics, and comorbidities. However, higher levels of positive psychosocial factors, such as resilience and optimism, were associated with higher odds of SRSA ≥7 among both OWLH and HIV-seronegative women. By contrast, current depressive symptomatology and higher levels of loneliness reduced the likelihood of SRSA ≥7, regardless of HIV status. Among OWLH, 2 additional negative psychosocial factors were associated with reduced odds of SRSA ≥7: higher levels of anxiety and lifetime discrimination.
SRSA prevalence of 83.7% and mean scores of 8.12 (SD = 1.73) among OWLH in our study are comparable with those of HIV-seronegative participants in the studies using predominantly white male samples.15,18 Thus, a 2018 study by Moore et al18 found that SRSA prevalence and mean scores were, respectively, 82% and 7.87 (SD = 1.82) among HIV-seronegative adults as compared to only 67% and 7.05 (SD = 2.29) among PLWH in a sample that was 76% white and 79% men. Comparing Moore et al18 study results with our findings highlights the need for future research examining SRSA sex/gender differences and their intersectionality with race among OWLH and older men living with HIV (OMLH). Both previous research15,19–22 and our findings indicate that SRSA among PLWH is associated with resilience and spirituality. African Americans and other minorities develop higher resilience than whites46 and more often cite the importance of religion in their lives. In a US population–based Pew Research Poll, 75% of African Americans stated that they consider religion very important, compared with 49% of whites.47 Given the racial composition of our sample as well as OWLH in general, resilience and spirituality may be key factors driving high prevalence of SRSA among this population as compared to white OMLH. Overall, we hypothesize that SRSA in OWLH and OMLH may be driven by different psychosocial factors. Although OWLH may experience higher levels of negative psychosocial factors, such as stigma and discrimination, OMLH (and especially white OMLH) may experience lower levels of such positive psychosocial factors as spirituality and resilience. Qualitative studies are also needed to improve our understanding of gender differences in subjective understandings of SA.
In contrast to previous research among PLWH, we also found no significant difference in SRSA prevalence and mean scores by HIV status. It is important to note that likely due to WIHS selection criteria for enrolling HIV-seronegative women who engaged in HIV risk behaviors,32–34 OWLH in our sample were better off in terms of several sociodemographic characteristics, had higher levels of HRQOL, optimism, and a lower level of perceived lifetime discrimination. We therefore hypothesize that negative effects of HIV among OWLH may have been counterbalanced by factors related to social disadvantage among HIV-seronegative participants.
Similar to other studies,15,17,18,35 we found that SRSA was associated with positive psychosocial factors and mental health but not with sociodemographic variables or HIV disease characteristics. Also congruent with the existing literature,15,18 SRSA in our study was not associated with most negative life events, that is, history of incarceration, child sexual abuse, and intimate partner violence. We found, however, strong negative effects of one specific type of negative life events among OWLH, that is, lifetime experience of discrimination. It should be noted, however, that we are not aware of studies that specifically examined the effects of lifetime discrimination on SRSA. It is possible that in difference to other negative life events, such as incarceration, which may result in acute trauma with subsequent healing and greater resilience, the effects of discrimination may be more continuous and debilitating, resulting in reduced SRSA. It is also possible that the negative association between lifetime discrimination and SRSA is specific to OWLH. Clearly, more research is needed examining the effects on SRSA of various types of negative life events and especially those that remain understudied, such as the experience of gender-, HIV-, and sexual orientation–related sigma and discrimination. There is also a paucity of research examining relationships between SRSA and comorbidities or SRSA and health behaviors (eg, substance use). Although our study did not find significant relationships between these variables, further research is needed. Currently, little is known about the effects on SRSA of multimorbidity, polypharmacy, frailty, and falls as well as diet, sleep, and exercise, all of which are extremely important for the aging PLWH.
This study had several limitations. Our analyses were based on a cross-sectional survey, which does not allow conclusions about temporal relationships between psychosocial correlates and SRSA. Future research will investigate social and psychosocial determinants of SRSA. OWLH in our sample had well-controlled HIV disease, with 73% of them being virally suppressed in contrast to only 49% of viral suppression rate among HIV-seropositive US women. It is thus unknown whether our results are generalizable to all OWLH although previous research suggests that HIV disease characteristics may not be associated with SRSA.15 In addition, participants in our sample were embedded into a long-term research study, which may have resulted in more regular contact with HIV care providers as well as increased levels of positive psychosocial factors, such as social support. It is thus possible that the prevalence and mean score among OWLH in our sample may be higher than among OWLH outside of research studies. Nevertheless, these are important findings suggesting that high levels of SRSA are achievable among OWLH.
ACKNOWLEDGMENTS
The authors thank WIHS participants who volunteered for the FROST substudy and WIHS staff who assisted with FROST administration, especially Rachael Abraham, Antonina Foster, Kayla Smith, Cherrione Davis, Catalina Ramirez, Susan Holman, Kemi Sosanya, and Christine Alden. The authors also thank Dr. Kimberly Hagen for her unwavering support throughout this research.Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): Atlanta WIHS (I.O., G.M.W., and Anandi Sheth), U01-AI-103408; Bronx WIHS (Kathryn Anastos and A.S.), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracy Wilson), U01-AI-031834; UNC WIHS (A.A.A.), U01-AI-103390; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Footnotes
This work was assisted in part by a developmental grant (A.R.R., PI) from the NIH Center for AIDS Research at Emory University (P30AI050409), Sustained Training in HIV and Aging (STAHR) training grant (R25 MH108389—Scott Letendre and Dilip Jeste, PIs), Atlanta WIHS (U01-AI-103408—I.O., G.M.W., and Anandi Sheth, PIs), Bronx WIHS (U01-AI-035004—Kathryn Anastos and A.S., PIs), Brooklyn WIHS (U01-AI-031834—D.G. and Tracy Wilson, PIs), UNC WIHS (U01-AI-103390—A.A.A., PI), WIHS Data Management and Analysis Center (U01-AI-042590—Stephen Gange and Elizabeth Golub, PIs), and Emory SCORE (U54AG062334—I.O. and Lisa Haddad, PIs). This publication resulted in part from research supported by the Penn Center for AIDS Research (CFAR) (P30 AI 045008—Ronald Collman, PI), the Penn Mental Health AIDS Research Center (PMHARC) (P30 MH 097488—Dwight Evans, PI) and the CFAR Social & Behavioral Science Research Network National Scientific Meeting (SBSRN) (R13 HD 074468—Michael Blank, PI).
Meetings at which parts of data were presented: Annual Scientific Meeting of the Gerontological Society of America; November 17, 2018; Boston, MA. HIV & Aging: from Mitochondria to the Metropolis Conference; April 12, 2019; Atlanta, GA.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Zablotsky DL. Overlooked, ignored, and forgotten. Older women at risk for HIV infection and AIDS. Res Aging. 1998;20:760–775. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Diagnoses of HIV infection among adults aged 50 years and older in the United States and Dependent Areas, 2007–2010. HIV Surveill Supplemental Rep. 2013;18 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Accessed September 15, 2016. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Diagnoses of HIV infection among adults aged 50 years and older in the United States and Dependent Areas, 2011–2016. HIV Surveill Supplemental Rep. 2018;23 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Accessed February 25, 2019. [Google Scholar]
- 4.Rueda S, Law S, Rourke SB. Psychosocial, mental health, and behavioral issues of aging with HIV. Curr Opin HIV AIDS. 2014;9:325–331. [DOI] [PubMed] [Google Scholar]
- 5.Chambers LA, Wilson MG, Rueda S, et al. Evidence informing the intersection of HIV, aging and health: a scoping review. AIDS Behav. 2014;18:661–675. [DOI] [PubMed] [Google Scholar]
- 6.Rubtsova AA, Kempf MC, Taylor TN, et al. Healthy aging in older women living with HIV infection: a systematic review of psychosocial factors. Curr HIV/AIDS Rep. 2017;14:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcagno A, Nozza S, Muss C, et al. Ageing with HIV: a multidisciplinary review. Infection. 2015;43:509–522. [DOI] [PubMed] [Google Scholar]
- 8.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. [DOI] [PubMed] [Google Scholar]
- 10.Baker TA, Buchanan NT, Mingo CA, et al. Reconceptualizing successful aging among black women and the relevance of the strong black woman archetype. Gerontologist. 2015;55:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. [DOI] [PubMed] [Google Scholar]
- 12.Kahana E, Kahana B. Successful aging among people with HIV/AIDS. J Clin Epidemiol. 2001;54(suppl 1):S53–S56. [DOI] [PubMed] [Google Scholar]
- 13.Martinson M, Berridge C. Successful aging and its discontents: a systematic review of the social gerontology literature. Gerontologist. 2015;55:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu D, Feng Q, Sautter JM, et al. Concordance and discordance of self-rated and researcher-measured successful aging: subtypes and associated factors. J Gerontol B Psychol Sci Soc Sci. 2017;72:214–227. [DOI] [PubMed] [Google Scholar]
- 15.Moore RC, Moore DJ, Thompson WK, et al. A case-controlled study of successful aging in older HIV-infected adults. J Clin Psychiatry. 2013;74:e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strawbridge WJ, Wallhagen MI, Cohen RD. Successful aging and well-being: self-rated compared with Rowe and Kahn. Gerontologist. 2002;42:727–733. [DOI] [PubMed] [Google Scholar]
- 17.Montross LP, Depp C, Daly J, et al. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. 2006;14:43–51. [DOI] [PubMed] [Google Scholar]
- 18.Moore DJ, Fazeli PL, Moore RC, et al. Positive psychological factors are linked to successful cognitive aging among older persons living with HIV/AIDS. AIDS Behav. 2018;22:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emlet CA, Harris L. Giving back is receiving: the role of generativity in successful aging among HIV-positive older adults. J Aging Health. 2018. 10.1177/0898264318804320 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Emlet CA, Harris L, Pierpaoli CM, et al. “The journey I have been through”: the role of religion and spirituality in aging well among HIV-positive older adults. Res Aging. 2018;40:257–280. [DOI] [PubMed] [Google Scholar]
- 21.Emlet CA, Harris L, Furlotte C, et al. “I′m happy in my life now, I'm a positive person”: approaches to successful ageing in older adults living with HIV in Ontario, Canada. Ageing Soc. 2017;37:2128–2151. [Google Scholar]
- 22.Solomon P, Letts L, O'Brien KK, et al. “I′m still here, I′m still alive”: understanding successful aging in the context of HIV. Int J STD AIDS. 2018;29:172–177. [DOI] [PubMed] [Google Scholar]
- 23.Fazeli PL, Montoya JL, McDavid CN, et al. Older HIV+ and HIV− adults provide similar definitions of successful aging: a mixed-methods examination. Gerontologist. 2018;73:1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durvasula R. HIV/AIDS in older women: unique challenges, unmet needs. Behav Med. 2014;40:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roger KS, Mignone J, Kirkland S. Social aspects of HIV/AIDS and aging: a thematic review. Can J Aging. 2013;32:298–306. [DOI] [PubMed] [Google Scholar]
- 26.Blackstock O, Tate J, Akgün K, et al. Sex disparities in overall burden of disease among HIV-infected individuals in the Veterans Affairs healthcare system. J Gen Intern Med. 2013;28:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoff DM, Colosi D, Rubtsova A, et al. HIV and aging research in women: definitions, models. Curr HIV/AIDS Rep. 2016;13:383–391. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. HIV Among Women. 2018. Available at: https://www.cdc.gov/hiv/group/gender/women/index.html. Accessed February 27, 2019. [Google Scholar]
- 29.Erlandson KM, Plankey MW, Springer G, et al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med. 2016;17:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levett TJ, Cresswell FV, Malik MA, et al. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr Soc. 2016;64:1006–1014. [DOI] [PubMed] [Google Scholar]
- 31.Weitzmann MN, Ofotokun I, Titanji K, et al. Bone loss among women living with HIV. Curr HIV/AIDS Rep. 2016;13:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkan SE, Melnik SL, Preston-Martin S, et al. The Women′s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 33.Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women′s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AS, Palmer BW, Rock D, et al. Associations of self-perceived successful aging in young-old versus old-old adults. Int Psychogeriatr. 2015;27:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol. 1994;67:1063–1078. [DOI] [PubMed] [Google Scholar]
- 37.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 38.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- 39.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
- 40.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 41.Brady MJ, Peterman AH, Fitchett G, et al. A case for including spirituality in quality of life measurement in oncology. Psychooncology. 1999;8:417–428. [DOI] [PubMed] [Google Scholar]
- 42.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 44.Hughes ME, Waite LJ, Hawkley LC, et al. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. 2004;26:655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams DR, Gonzalez HM, Williams S, et al. Perceived discrimination, race and health in South Africa. Soc Sci Med. 2008;67:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyes CLM. The black–white paradox in health: flourishing in the face of social inequality and discrimination. J Pers Soc Psychol. 2009;77:1677–1706. [DOI] [PubMed] [Google Scholar]
- 47.Pew Research Center. Religious Landscape Study. 2014. Available at: http://www.pewforum.org/religious-landscape-study/racial-and-ethnic-composition. Accessed March 5, 2019. [Google Scholar]