Purpose:
To evaluate the short- and long-term success rates of xenogeneic-free cultivated limbal epithelial stem cell transplantation (CLET) for the treatment of limbal stem cell deficiency (LSCD).
Methods:
Thirteen patients with LSCD underwent an autologous (n = 9) or allogeneic (n = 4) CLET. The primary end point was to assess the long-term anatomical success rate of transplanted grafts at a follow-up of at least 3 years, in comparison with the short-term outcomes. Secondary end points involved reviewing functional improvement, patient-reported symptoms, and change in percentage area of corneal vascularization in both short-term and long-term.
Results:
The mean short- and long-term follow-up periods were 2.1 ± 0.38 years and 6.7 ± 1.81 years, respectively. The total anatomical success rate was 46.1% in the short-term, but it decreased to 23.1% in the long-term. A partial success rate of 30.8% was observed in both short- and long-term, and the failure rate increased from 23.1% to 46.1%. The mean percentage of vessel area decreased from 12.11% ± 5.29% preoperatively to 7.82% ± 6.70% in the short-term and increased to 8.70% ± 6.32% in the long-term. There was a significant improvement in best-corrected visual acuity (P = 0.044) in the short-term although not in the long-term (P = 0.865).
Conclusions:
This study shows that anatomical and functional success rates of CLET decrease over time. We believe that the decline of success is related to the extent of disease, cell origin, and lack of niche protection because subtotal LSCD and autologous donor cells confer a higher chance of success in the long-term.
Key Words: limbal stem cell deficiency, cultivated limbal stem cell transplantation, clinical trial, long-term outcome, cornea, limbal niche
Limbal stem cells (LSCs) are responsible for maintenance of the corneal epithelium in healthy homeostasis and after injury.1,2 The cells are located in specialized niches at the limbal junction where they are believed to maintain a physical barrier between cornea and conjunctiva.3 A wide range of diseases may result in damage to the LSCs and to their niche, leading to LSC deficiency (LSCD). When severe, this condition can result in conjunctivalization and vascularization of the cornea, chronic inflammation, poor epithelial integrity, and recurrent epithelial erosions. As a result, these patients suffer from pain, photophobia, and a decreased visual acuity.3,4 Standard corneal transplants tend to fail in these cases because they require a healthy barrier to prevent conjunctival overgrowth and a normal functioning population of stem cells to survive.5
The management of LSCD aims to optimize the ocular surface and to restore the limbal functions. The choice of treatment depends on the extent of limbal involvement and on the laterality of the disease.6 Cultivated limbal epithelial stem cell transplantation (CLET) is one treatment option indicated for severe LSCD that aims to restore the limbal barrier and to reconstruct a transparent corneal surface. The use of this technique in humans was first reported in 1997 by Pellegrini et al.7 Limbal cells are harvested from a healthy autologous or allogeneic donor limbus, grown and multiplied in a laboratory over several days, and then transplanted onto the stem cell-deficient eye. The CLET technique has been known for more than 20 years, and, to date, there is no consensus on the optimal protocol for this procedure. Cells can be cultured in either a suspension7–9 or an explant culture system,10–13 and different culture media and substrates exist. Many cell culture protocols depend on the support of animal products for nurturing and expansion of the graft. However, animal-derived culture products such as mouse fibroblast feeder layers, fetal bovine serum, and cholera toxins carry a risk of transmitting xenogeneic diseases.14 Although the reported results of CLET are generally good, the follow-up period is variable with only a limited number of studies assessing long-term outcomes of more than 3 years. In this study, we reviewed our series of CLET procedures performed over a 10-year period, comparing short- and long-term results of this technique.
MATERIALS AND METHODS
Study Design and Subjects
The trial was approved by the Antwerp University Hospital Ethical Committee (approval number: EC7/28/153; EudraCT no 2008-001543-19) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants for all of the procedures described in this study. The study design of the first 2 follow-up years was a prospective, noncomparative case series. To obtain long-term follow-up data, patients were contacted for a new follow-up control in our center. In case of loss to follow up, the most recent charts were retrospectively reviewed.
The diagnosis of LSCD was made clinically, based on the patient's history and on the slit-lamp findings. The presence of conjunctival and vascular overgrowth on the cornea, with the absence of a normal limbal architecture, was indicative of LSCD. The complete absence of limbal architecture with conjunctivalization over the entire cornea was termed total LSCD, whereas the involvement of fewer clock hours was termed subtotal LSCD.
All patients underwent ex vivo cultivated limbal epithelial transplantation for the treatment of LSCD between July 2008 and December 2012.
Inclusion criteria for this study were as follows: 1) a documented history of unilateral or bilateral LSCD; 2) when LSCD was documented, it was graded as total or subtotal. Patients with subtotal LSCD were only included if there had been at least 3 failed previous attempts at corneal pannus debridement or reconstruction with amniotic membrane. 3) Patients who underwent autologous or allogeneic ex vivo cultivated limbal transplantation; 4) postoperative follow-up data of at least 36 months; and 5) complete follow-up data.
Patients who were pregnant or lactating, had active inflammation of the eye, or had uncontrolled glaucoma were not considered for CLET grafting.
End Points and Outcome Measures
The main study end point was to examine the anatomical success rate of the transplanted limbal epithelial stem cell grafts after a follow-up period of at least 36 months and to compare these results with the short-term outcomes after an average follow-up of 2 years. The secondary end point was to assess the change in visual acuity, change in percentage area of corneal vascularization, and patient-reported outcomes including pain and photophobia.
Total anatomical success was defined as a transparent, intact corneal epithelium without central 8 mm neovascularization. Partial anatomical success was defined as a relapsed corneal superficial neovascularization, not as extensive as at the time of admission, without central 4 mm neovascularization and an intact epithelial layer. Anatomical failure was defined as recurrent epithelial defects and/or superficial corneal neovascularization or conjunctivalization encroaching on the central 4 mm of the cornea.
Data Collection and Quantification of Outcome Measures
Data retrieved from the medical records included the patient's age and sex, etiology of LSCD, degree of limbal involvement, best-corrected visual acuity (BCVA), pain scale, photophobia scale, intraocular pressure, corneal vascularization, presence or absence of epithelial defects, adverse events, and follow-up duration.
Visual acuity was recorded using the Snellen eye chart. For the purpose of this analysis, all visual acuity data were converted to LogMAR equivalents. Acuities of counting fingers at 1 m were converted to LogMAR 2.0, hand movements (HM) at 1 m to LogMAR 2.3, perception of light to LogMAR 2.75, and no perception of light to LogMAR 3.0.15,16
A numerical pain scale ranging from 0 to 10 was used to record pain caused by the stem cell-deficient eye (0 corresponding to no pain and 10 corresponding to the worst pain possible in the verbal descriptor scale). Similarly, a 4-point scale was used for the assessment of photophobia (0 = no photophobia, 1 = mild photophobia, 2 = moderate photophobia, and 3 = severe photophobia).17 The ocular surface status was documented using ocular slit-lamp photographs. All photographs were graded by 5 observers with corneal expertise (S.N.D., C.K., M.-J.T., N.Z., and J.B.). In case there was no unanimous agreement, the result with the highest votes was selected. If a consensus could not be reached, the adjudicator (J.B.) was tasked with making the final decision.
Further quantification of corneal neovascularization was performed by an external experienced blinded investigator (F.B.) at a reading center with corneal angiogenesis expertise (Cologne Ophthalmological Reading and Image Analysis Center, University Hospital Cologne). All images were analyzed morphometrically using a standardized semiautomatic image analysis software (Cell F; Olympus, Hamburg, Germany) based on gray filter sampling, as described elsewhere.18–21 Briefly, after contrast and shading optimization of each image, the region of interest was determined along the corneal limbus. The vascularized area (expressed as a percentage) was then calculated by threshold setting and normalized to the total region of interest.
Treatment Protocol
Surgical techniques, cultivation technique, and postoperative management were performed as previously described.17,22 Briefly, a superficial limbal tissue biopsy of 1 mm by 2 mm was harvested from either the superior or the inferior limbus of the donor eye under local anesthesia. The explants were cultured in a CnT-20 medium (CELLnTEC, Switzerland) supplemented with 1% human AB serum (Sigma Aldrich, Germany) on a denuded amniotic membrane. No feeder cells or other animal-derived products were used. All culture processes were performed under conditions of good manufacturing practice (GMP). After a culture period of 14 days, the composite graft was transplanted: after dissection of the pannus tissue, the graft was applied to the surface of the cornea using fibrin glue. A secondary amniotic membrane covering the whole graft was secured to the surrounding conjunctiva, serving as a temporary patch.
In case of unilateral disease, the patient's contralateral, healthy eye served as the donor eye. In case of bilateral disease, limbal donor tissue was harvested from a living relative's eye. In cases without an appropriate living-related donor, limbal tissue was harvested from a cadaveric donor eye. For all allogeneic donors, a human leukocyte antigen (HLA) match of at least 50% on loci HLA-A, HLA-B, and HLA-DR was required.
Statistical Analysis
Statistical analyses were performed using SPSS 25 (IBM Statistics Inc, Chicago, IL) at a significance level of 0.05. A Wilcoxon signed-rank test was used to compare the outcome parameters (percentage area of corneal neovascularization, visual acuity, pain, and photophobia) before and after CLET. In addition, a McNemar test was used to compare the anatomical success outcomes between the short-term and long-term period.
RESULTS
Patient Characteristics
Between July 2008 and December 2012, 21 patients underwent CLET. Eight of these patients were lost to follow up in the long-term or had incomplete follow-up data and therefore were excluded for further analysis. The remaining 13 patients were included in this study. There were 7 (53.8%) men and 6 (46.2%) women, with a mean age of 48.5 ± 16.00 years. Eleven (84.6%) patients had total LSCD, whereas 2 (15.4%) patients had subtotal LSCD. Nine (69.2%) patients received autologous LSC transplantation, and 4 (30.8%) patients received an allogeneic stem cell graft. Two (15.4%) patients underwent the procedure twice, whereas 11 (84.6%) patients were grafted only once. The etiology of LSCD included chemical burn (n = 5), aniridia (n = 2), iatrogenic damage (n = 2), keratitis (n = 1), corneal ulcer (n = 1), trauma (n = 1), and congenital microphthalmia (n = 1). All patient details are listed in Table 1.
TABLE 1.
Clinical Outcomes
The mean short-term follow-up period was 2.1 ± 0.38 years, whereas the follow-up in the long-term period was 6.7 ± 1.81 years. In the short-term, 6 of the 13 (46.1%) patients were graded as anatomical success, 4 (30.8%) patients were graded as partially successful, and 3 (23.1%) patients were deemed as failure. After a mean follow-up of 6.7 years, 3 of the 13 (23.1%) eyes retained anatomical success, whereas 4 (30.8%) eyes were graded as partially successful. Six (46.1%) eyes failed in the long-term. There was no statistically significant difference in the long-term compared with the short-term regarding anatomical outcomes (P = 0.17).
Differences were observed between the autologous and allogeneic groups regarding etiology and outcomes. Of the 4 eyes that received allogeneic CLET, 2 patients had aniridia, 1 patient suffered LSCD resulting from a bilateral chemical burn, and 1 patient had congenital microphthalmia. In the short-term, 1 eye of the allogeneic transplant subgroup was graded as total success, 2 eyes were graded as partial success, and 1 eye as failure. In the long-term, none of the allogeneic transplants were graded as total success. Both of the patients with aniridia failed in the long-term.
In the autologous subgroup (n = 9), LSCD resulted from a chemical burn in most of the cases (44.4%). Five (55.6%) and 2 (22.2%) of the 9 eyes in the autologous transplant group were graded as total and partial success, respectively, whereas 2 (22.2%) autologous cases failed in the short-term. In the long-term, these numbers were 3 (33.3%), 2 (22.2%), and 4 (44.4%) for total success, partial success, and failure, respectively. In this autologous subgroup, 2 of the 4 patients with a chemical burn were graded as total success and 2 failed in the short-term. On the long-term, the 2 patients that were initially graded as a total success were now deemed as partially successful. Representative images are displayed in Figure 1.
FIGURE 1.
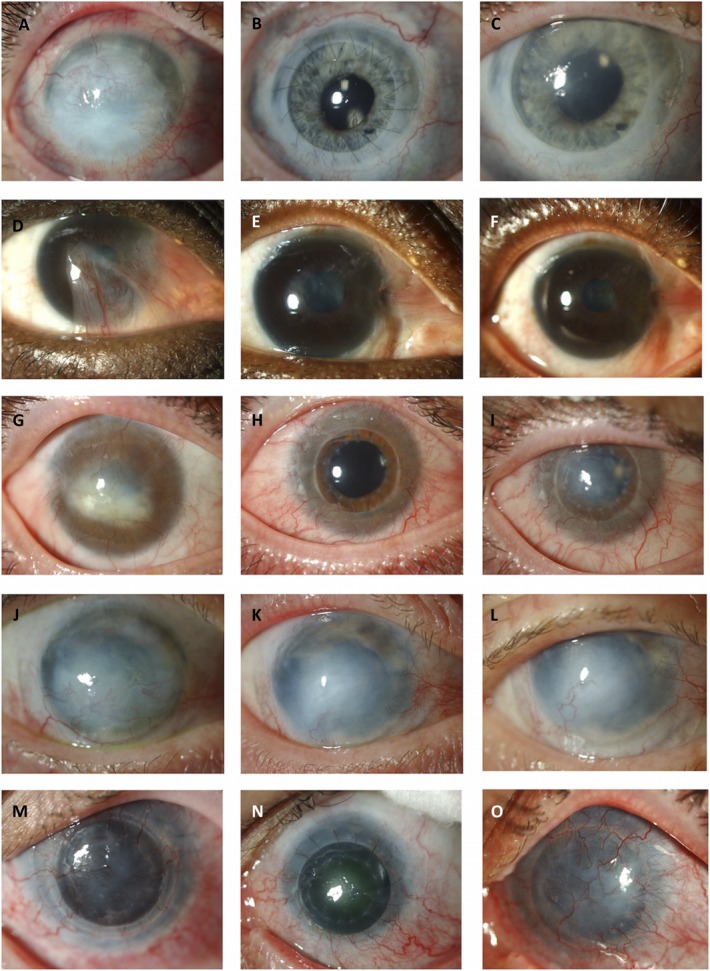
Representative anterior segment images of patients with total (A, G, J, M) and subtotal (D) LSCD preoperatively. Photographs of the short-term postoperative outcomes are graded as total (B, E, H, N) and partial (K) success. Photographs of the same patients in the long-term achieving total success (C, F), partial success (J), and failure (L, O).
Functional Outcomes
Short-term results showed a significant increase in BCVA (P = 0.044) and a significant improvement in photophobia (P = 0.014) after CLET in the total cohort. There was no significant improvement in pain (P = 0.18). Long-term results, however, showed no statistically significant difference in BCVA (P = 0.865), pain (P = 0.063), and photophobia (P = 0.288) in the total cohort (Fig. 2).
FIGURE 2.

Postoperative outcomes in the total cohort: pain (A) and photophobia (B), preoperatively (Pre-op), in the short-term and in the long-term (*P < 0.05; **P > 0.05).
When we compared the short-term with the long-term functional parameters, there was a significant decrease in BCVA (P = 0.018) and increase in pain (P = 0.028) although no statistical difference was noted for photophobia (P = 0.063). Only 2 of the 13 patients experienced an improvement in BCVA in the long-term. The highest improvement in BCVA included an improvement from HM to 0.4 and was observed in allogeneic CLET for LSCD after a bilateral chemical burn. The patient received stem cells from his mother. Five (38.5%) of the 13 patients experienced a decrease in BCVA during the course of the follow-up. One of these patients (case 5) had aniridia with a progressive aniridia-related keratopathy and progressive glaucoma. In case 3, the decrease in BCVA was contributed to the occurrence of a dense nuclear cataract, for which the patient has refused intervention to date. A decompensated corneal graft after CLET contributed to a decrease in BCVA in case 10. In case 1 and case 11, the decrease in BCVA was related to the increase in conjunctivalization and, hence, to graft failure. Case 1, however, was noncompliant with follow-up care after transplantation. A decrease in BCVA from counting fingers to HM was observed after a follow-up of 41 months in case 11, despite careful postoperative care.
Analysis of the Ocular Surface Photographs
The mean percentage of vessel area decreased from 12.11% ± 5.29% preoperatively to 7.82% ± 6.70% in the short-term and increased to 8.70% ± 6.32% in the long-term. The decrease in corneal vessel area was significant in the short-term (P = 0.037) although not in the long-term (P = 0.139), compared with the preoperative photographs. No significant difference in vascularized area was found between short- and long-term photographs (P = 0.767). There was no significant difference in the difference of vascularization per year (P = 0.200). Nine photographs of 3 patients were excluded because of technical issues.
DISCUSSION
Although the CLET technique has been evolving over the past 20 years, there is still a lack of long-term follow-up data. In this report, we present the outcomes of CLET surgeries up to 10 years postoperatively and report the effect over time. Our major finding is a decrease in both anatomical and functional success rates over time. Total or partial success was attained in 76.9% (46.1% total + 30.8% partial) of the eyes in the short-term and subsequently decreased to 53.9% (23.1% total + 30.8% partial) after an additional mean follow-up of 4.6 years. Analysis of the ocular surface photographs showed an increase in corneal vessel area in the long-term compared with the short-term although this was not statistically significant. Regarding functional results, there was a significant decrease in BCVA and an increase in pain, although not in photophobia, comparing short- and long-term data. These results demonstrate a clear reduction of the clinical effect with time.
Articles from other groups that included Kaplan–Meier survival charts indicate in general that failures mainly occur in the first 1 to 2 years after transplantation, and that, after that time, the success rate remains constant.23–27 The mean follow-up of these trials is however limited, and long-term follow-up data (mean follow-up > 3 years) are scarce in the existing literature. Sejpal et al reported a success ratio of 46.7% after a mean follow-up of 3.4 years. All patients were children with ocular surface burns.28 Sangwan et al,29 from the same group, reported a stable ocular surface in 142 of 200 (71%) eyes at a mean follow-up of 3 ± 1.6 years. In another study of Scholz et al,30 an entirely stable corneal surface was observed in 75.4% of 61 eyes transplanted after a mean follow-up of 50.8 ± 32.7 months. With a mean of 6.7 years, our follow-up period is almost twice as long compared with the aforementioned groups.
Although our patient numbers are too small to draw major conclusions, there were several observations regarding the factors influencing the outcome. In this series, none of the allogeneic transplants succeeded in the long-term. Eyes with autologous CLET grafts tend to show better outcomes compared with allogeneic cases, which is also the experience of other research groups.24,31 Reasons for failure in allogeneic cases include the risk of immunoreactivity. Diagnosis of graft rejection is however difficult because aspecific symptoms such as perilimbal congestion, epithelial haze, or epithelial breakdown are hard to attribute directly to an immune rejection rather than primary failure of the CLET graft.
In the total cohort, only 3 patients achieved total success in the long-term. Of these, all were unilateral autologous cases with different underlying etiologies including corneal ulcer, keratitis, and iatrogenic LSCD. These conditions are in general related to less severe LSCD, which is supported by the observation that 2 of these patients were graded as subtotal LSCD. We believe that the extent and etiology of LSCD affect the outcome after CLET, probably because of the presence of surviving stem cells in the less severe forms.
Also culture protocols may influence the clinical outcomes. In this trial, an explant system was used for cultivation of the cells, which is suggested to be a less efficient method in isolating LSCs compared with the suspension culture system by some laboratory studies.32–34 However, there is currently no evidence of superiority regarding clinical outcomes, and therefore, it is hard to attribute poor long-term total success to our explant culture system. Although a comparative clinical trial could answer this question, this is made difficult in LSC research by the rarity of the clinical entity and the heterogeneity of the etiologies.
Furthermore, we believe that failure could be preceded by the presence of subclinical ocular surface inflammation, resulting in a harmful environment for the transplanted cells. Identification of inflammatory biomarkers in the extracellular milieu of the ocular surface (eg, by tear sampling) that are correlated with failure could improve patient selection or help to target therapy.35
Because of the observed decrease in effect over time, we also have to question the long-term effect of CLET in general. The mechanisms by which the CLET regenerates corneal epithelium are still unclear, and there is uncertainty about the question of whether the cells continue to survive after transplantation.36,37 We speculate that a gradual loss of the transplanted cells over time may account for the decrease in clinical outcomes. This hypothesis can only be validated by labeling and tracking the cells after transplantation although this is currently not technically possible in clinical practice.38 In addition, we believe that the absence of physiological niche structures may contribute to the loss of cells and the decrease in success rates over time because there is no supporting environment to protect transplanted cells in the long-term. The limbal niche plays a major role in the biological regulation of the LSCs39 and in maintenance of the LSC pool.40 A recent study has shown that geometry alone influences stem cell differentiation in a 3D printed stem cell niche,41 which illustrates the importance of its precise microarchitecture on the LSC function. There is, however, no evidence that the normal limbal niche features reform after limbal tissue transplantation.42 We believe that recreation of the crypt structures should be addressed to increase chances of long-time survival of the LSCs. Recent progress has been made in this field, and engineered artificial stem cell niches are being designed such as the Real Architecture For 3D Tissues technique.43,44
Finally, the progressive course of nontrauma LSCD etiologies such as genetic disorders may be another reason for (late) deterioration of an initial good result, which may explain why patients with aniridia present with poor results in the long-term.
Our findings have to be considered in the context of the limitations of this study, which include its retrospective nature, the high number of patients lost to follow up, the small number of eyes studied, and the heterogeneous study population. The limited patient numbers did not allow the formation of subgroups, and because of this small sample size, P values should be interpreted with caution. Furthermore, variability in surgical techniques, culture protocols, inclusion criteria, outcome parameters, and follow-up periods among groups limits the direct comparison of the reported results. These limitations are inherently encountered by all groups working in the field.
By analyzing 2 time points over a long follow-up period, this study has shown that anatomical and functional success rates of CLET decrease over time. The extent of the disease and origin of the cells likely contribute to the success, where subtotal LSCD and autologous stem cells seem to give higher chances of successful grafting in the long-term. In general, however, our overall long-term total success rate was low. Recreation of the protective LSC niche would likely improve long-term results in the future.
ACKNOWLEDGMENTS
The authors would like to thank Sean O. Dubhghaill and Kristien Wouters for their support.
Footnotes
This work was supported by the EU Horizon2020 project ARREST BLINDNESS (grant agreement number 667400) and the “Agentschap voor Innovatie door Wetenschap en Technologie—Toegepast Biomedisch onderzoek” (IWT-TBM, project number: 130270).
N. Zakaria has been an employee at Novartis since October 2017. The remaining authors have no or conflicts of interest to disclose.
REFERENCES
- 1.Lavker RM, Tseng SC, Sun T. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. [DOI] [PubMed] [Google Scholar]
- 2.Daniels JT, Dart JK, Tuft SJ, et al. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. [DOI] [PubMed] [Google Scholar]
- 4.Dua HS, Joseph A, Shanmuganathan VA, et al. Stem cell differentiation and the effects of deficiency. Eye (Lond). 2003;17:877–885. [DOI] [PubMed] [Google Scholar]
- 5.Sepsakos L, Cheung AY, Holland EJ. Outcomes of keratoplasty after ocular surface stem cell transplantation. Cornea. 2017;36:1025–1030. [DOI] [PubMed] [Google Scholar]
- 6.Dua HS, Miri A, Said DG. Contemporary limbal stem cell transplantation - a review. Clin Exp Ophthalmol. 2010;38:104–117. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- 8.Daya SM, Watson A, Sharpe JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112:470–477. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Inatomi T, Sotozono C, et al. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology. 2006;113:1765–1772. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. [DOI] [PubMed] [Google Scholar]
- 11.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 12.Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. [DOI] [PubMed] [Google Scholar]
- 13.Sangwan VS, Vemuganti GK, Singh S, et al. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci Rep. 2003;23:169–174. [DOI] [PubMed] [Google Scholar]
- 14.Behaegel J, Ní Dhubhghaill S, Koppen C, et al. Safety of cultivated limbal epithelial stem cell transplantation for human corneal regeneration. Stem Cells Int. 2017;2017:6978253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. [DOI] [PubMed] [Google Scholar]
- 16.Lange C, Feltgen N, Junker B, et al. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–142. [DOI] [PubMed] [Google Scholar]
- 17.Zakaria N, Possemiers T, Dhubhghaill SN, et al. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bock F, Onderka J, Hos D, et al. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res. 2008;87:462–470. [DOI] [PubMed] [Google Scholar]
- 19.Cursiefen C, Bock F, Horn FK, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–1637. [DOI] [PubMed] [Google Scholar]
- 20.Bock F, Matthaei M, Reinhard T, et al. High-dose subconjunctival cyclosporine a implants do not affect corneal neovascularization after high-risk keratoplasty. Ophthalmology. 2014;121:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: the I-CAN study. Ophthalmology. 2014;121:1683–1692. [DOI] [PubMed] [Google Scholar]
- 22.Zakaria N, Koppen C, Van Tendeloo V, et al. Standardized limbal epithelial stem cell graft generation and transplantation. Tissue Eng Part C Methods. 2010;16:921–927. [DOI] [PubMed] [Google Scholar]
- 23.Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29–34. [DOI] [PubMed] [Google Scholar]
- 24.Pauklin M, Fuchsluger TA, Westekemper H, et al. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. [DOI] [PubMed] [Google Scholar]
- 25.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. [DOI] [PubMed] [Google Scholar]
- 26.Ramírez BE, Sánchez A, Herreras JM, et al. Stem cell therapy for corneal epithelium regeneration following good manufacturing and clinical procedures. Biomed Res Int. 2015;2015:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153:643–650. [DOI] [PubMed] [Google Scholar]
- 28.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–736. [DOI] [PubMed] [Google Scholar]
- 29.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–1529. [DOI] [PubMed] [Google Scholar]
- 30.Scholz S, Thomasen H, Hestermann K, et al. Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency. Ophthalmologe. 2016;113:321–329. [DOI] [PubMed] [Google Scholar]
- 31.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143:945–953. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Sun H, Tang X, et al. Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp Eye Res. 2005;80:227–233. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–2121. [PubMed] [Google Scholar]
- 34.Kim HS, Jun Song X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakaria N, Van Grasdorff S, Wouters K, et al. Human tears reveal insights into corneal neovascularization. PLoS One. 2012;7:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson TR, Coster DJ, Williams KA. The long term outcome of limbal allografts: the search for surviving cells. Br J Ophthalmol. 2001;85:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djalilian AR, Mahesh SP, Koch CA, et al. Survival of donor epithelial cells after limbal stem cell transplantation. Invest Ophthalmol Vis Sci. 2005;46:803–807. [DOI] [PubMed] [Google Scholar]
- 38.Genicio N, Gallo Paramo J, Shortt AJ. Quantum dot labeling and tracking of cultured limbal epithelial cell transplants in vitro. Invest Ophthalmol Vis Sci. 2015;56:3051–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Hayashida Y, Chen YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prina AE, Sidney L, Tromayer M, et al. Geometry alone influences stem cell differentiation in a precision 3D printed stem cell niche. bioRxiv. 2018:1–21. [Google Scholar]
- 42.Pedrotti E, Passilongo M, Fasolo A, et al. In vivo confocal microscopy 1 year after autologous cultured limbal stem cell grafts. Ophthalmology. 2015;122:1660–1668. [DOI] [PubMed] [Google Scholar]
- 43.Levis HJ, Daniels JT. Recreating the human limbal epithelial stem cell niche with bioengineered limbal crypts. Curr Eye Res. 2016;3683:1–8. [DOI] [PubMed] [Google Scholar]
- 44.Levis HJ, Kureshi AK, Massie I, et al. Tissue engineering the Cornea: the evolution of RAFT. J Funct Biomater. 2015;6:50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]