Introduction:
Bronchiolitis is a common lower respiratory tract illness in young children often caused by the respiratory syncytial virus (RSV). Antimicrobials are not recommended in infants with bronchiolitis unless there is strong evidence that a bacterial coinfection exists.
Methods:
We conducted a retrospective chart review comparing antimicrobial use and outcomes in previously healthy infants ≤24 months of age with RSV bronchiolitis at a single Canadian tertiary pediatric hospital during RSV seasons (December–April) from 2011 to 2016. An audit and feedback antimicrobial stewardship program was introduced in this hospital in August 2014.
Results:
Compared with the 2011–2012 cohort, the 2015–2016 cohort showed a decrease of 46% in mean days of therapy per 1,000 patient-days in the >28 days old age group of patients. There was also a 15.1% absolute reduction in the proportion of patients who received any antimicrobials in the hospital between the 2 cohorts (neonates included). The proportion of patients receiving antimicrobial prescriptions at discharge also decreased from 33.5% to 19%. The use of second-generation cephalosporins was eliminated in the 2016 cohort. There was a significant decrease in length of stay between the 2011–2012 and 2015–2016 cohorts, and no readmissions were documented.
Conclusions:
This study adds to the accumulating literature that antimicrobial stewardship program interventions along with guidelines and order sets can safely contribute to a reduction in antimicrobial use both in hospital and at discharge in children <2 years of age hospitalized due to RSV. Further research in identifying those who would or would not benefit from antibiotics should be promoted.
INTRODUCTION
In Canada, Bont et al1 estimated that 1.5% of children <2 years old are hospitalized for bronchiolitis annually with an average length of stay (LOS) ranging from 2 to 11 days. There are evidence-based guidelines for the management of bronchiolitis published by the Canadian Pediatric Society (CPS) and the American Academy of Pediatrics (AAP).2,3 The recommended management of bronchiolitis is supportive care with interventions, such as hydration, supplemental oxygen, nasal suctioning, and epinephrine/saline nebulization. The rate of serious bacterial infections in febrile children with confirmed respiratory syncytial virus (RSV) and clinical bronchiolitis is low overall, with rates of urinary tract infection (UTI) reported at 3.3%–6% and bacteremia between 0.6% and 1.1%.4 Despite the evidence, rates of antibiotic use in uncomplicated RSV bronchiolitis have not decreased significantly among pediatric inpatients.5,6
Approximately 60% of patients in pediatric hospitals receive at least one antimicrobial during their stay, with lower respiratory tract infection being one of the most common indications for antimicrobial therapy.7 Since RSV is a common reason for hospital admission, antimicrobial use in these patients are an important metric for antimicrobial stewardship programs (ASPs) since it is a defined clinical condition where guidelines exist.
The primary hypothesis of this study was that the implementation of general antimicrobial stewardship rounds would lead to a decrease in antimicrobial use in hospitalized children <2 years old with documented RSV bronchiolitis.
METHODS
We performed a retrospective chart review at the Children’s Hospital of Eastern Ontario (CHEO), a 125-bed urban tertiary pediatric hospital in Ottawa, Ontario, Canada, with >6,700 admissions and 70,500 emergency department (ED) visits per year. CHEO serves Eastern Ontario, Western Quebec, parts of Northern Ontario, and the Qikiqtani region of Nunavut. An ASP targeted at general pediatric patients at CHEO was instituted in August 2014 on 2 ward services with a final service added in January 2015. The program had been in place in the intensive care unit (ICU) since 2013. A pediatric infectious disease physician and a clinical pharmacist perform prospective audit and feedback 3 times a week on patients admitted to the ICU and general medicine services who were receiving antimicrobials. This audit involves identifying all patients on inpatient wards receiving antimicrobials on Mondays, Tuesdays, and Thursdays weekly. The stewardship physician and pharmacist discuss antimicrobial choice and duration for each patient with the most responsible physician or the senior resident. No other active interventions were ongoing.
Case Finding and Review
The Research Ethics Board of the CHEO Research Institute approved the study. One of the principal investigators (J.K.) developed and piloted a case report form on 10 cases for accuracy and reliability (J.K. and N.L.S., 5 cases each). The case definition was all children ≤2 years who were admitted to CHEO within the specified periods with a primary International Classification of Diseases, 10th Revision (ICD-10) diagnosis of “acute bronchiolitis due to RSV” or “bronchiolitis, unspecified” and had a LOS of ≤7 days. Testing on nasopharyngeal swabs was performed using a triplex Polymerase chain reaction (PCR) method for RSV, influenza A, and influenza B. Only patients with a documented positive RSV test were included in this study. We excluded patients if they were admitted to the ICU (pediatric or neonatal) during hospitalization or had any of the following comorbid conditions: hemodynamically significant heart disease, malignancy, hemoglobinopathy, underlying pulmonary pathology, cerebral palsy or other chronic neurological disease, upper airway mechanical problems (such as tracheostomies), genetic syndromes, primary immune deficiency, history of aspiration pneumonia, or preterm birth at <37 weeks gestational age. The CHEO inpatient electronic medical record (Sunrise) was then used to screen cases for study eligibility criteria. We chose the December 1–April 30 period as it represents the usual peak of RSV admissions. The season 1 cohort which predates the ASP (pre-ASP) was between December 1, 2011, and April 30, 2012. The post-ASP cohort was from season 5 between December 1, 2015, and April 30, 2016. To generate data for a representative sample of patients in the intervening period, we reviewed antimicrobial use data in a total of 60 cases (20 in each of the second, third, and fourth seasons). For the middle 3 periods, the complete admission lists for December to April were sent from medical records to the statistician who randomly shuffled the cases electronically. The first 60 patients who met the case definition were selected using the same inclusion and exclusion criteria.
Each case was assigned a unique study number. Data from both the electronic medical record and paper charts were collected into a digitized version of the case report form in the research database, Research Electronic Data Capture, hosted at the CHEO Research Institute.8 Information collected on all patients included demographics and antibiotic use (type, start, and stop dates in hospital and outpatient prescriptions). In the subset of the patients admitted during seasons 1 (2011–2012) and 5 (2015–2016), additional information collected included the results of investigations performed during hospitalization, the rationale for antibiotic use, days of fever (≥38.0°C), and days of supplementary oxygen requirement. The primary outcomes of the study were the proportion of patients who received antimicrobials and the average number of days of antimicrobial received. Secondary outcomes included the LOS, days of fever, days of supplementary oxygen use, coinfection rates (viral or bacterial), the rationale for antimicrobial use, and the type of antimicrobial used. We also recorded any visits to ED or admissions after the index admission.
Statistical Analysis
For the present study, we used the “date of discharge minus date of presentation to the ED plus 1” as the “true” LOS as this value includes all days upon which antibiotics could have been prescribed for a given clinical presentation (eg, presented to ED on December 14 at night, discharged on December 16 = 3 days LOS). Any dose of an antimicrobial that was received during 24 hours represented 1 day of therapy (DOT) such that if 2 antimicrobials were used on the same day, this counted as 2 DOT. The number of DOT is reported per 1,000 patient-days. Antimicrobials that were received in ED were included.
A data analysis plan was created, and data were exported from the Research Electronic Data Capture database into the R statistical program version 3.6.0. Continuous variables were summarized using median and interquartile range, and the Mann-Whitney test was used to compare pre- and post-ASP cohorts. Categorical variables were summarized using frequency and percent, and Pearson’s chi-square test or Fisher’s exact test were used, as appropriate, to test for differences between periods. We calculated each patient’s days of therapy by summing the number of days of therapy for each antimicrobial received. To take overdispersion into account, we used a negative binomial model to analyze the number of days of therapy per 1,000 days of patient stay. In each period, the mean days of therapy per 1,000 days of patient stay was estimated, together with a 95% confidence interval. The ratio of the mean days of therapy per patient day in the post-ASP compared with the pre-ASP cohort was also calculated, together with a 95% confidence interval. In a posthoc analysis, these analyses were repeated, excluding patients ≤28 days of age. All analyses were carried out using R version 3.6.0 (2019-04-26). Negative binomial models were estimated using the R package MASS version 7.3.51.4. Estimated marginal means were calculated using the R package emmeans version 1.3.4.
RESULTS
We identified a total of 579 patients through health records of which 398 (191 in the first period, 60 in the second, third, and fourth periods, and 147 in the last period) met the case definition (Fig. 1). The current study’s population of previously healthy children <2 years old hospitalized with RSV bronchiolitis represented 64% of the total number of RSV bronchiolitis admissions in both the first and last seasons. Patient characteristics are detailed in Table 1. The median (IQR) age pre-ASP was 3.6 months (1.7–9.0). In the post-ASP group, median (IQR) for age was 3.7 months (1.5–7.5). There was a statistically significant decrease in median LOS from 5 days (IQR 4.0–6.0) (range 2.0–9.0), in cohort 1, to 4 days (IQR 3.0–5.0) (range 2.0–8.0), in cohort 5 (P < 0.001). There were no patients in either group who returned to the study hospital within the week of being discharged who required antibiotic therapy.
Table 1.
Patient Characteristics
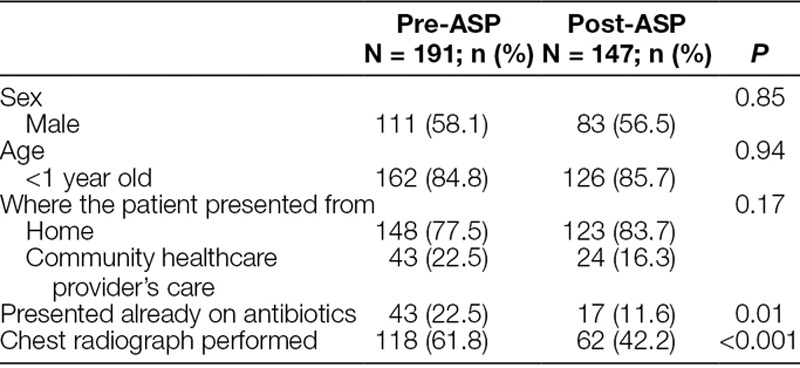
Fig. 1.
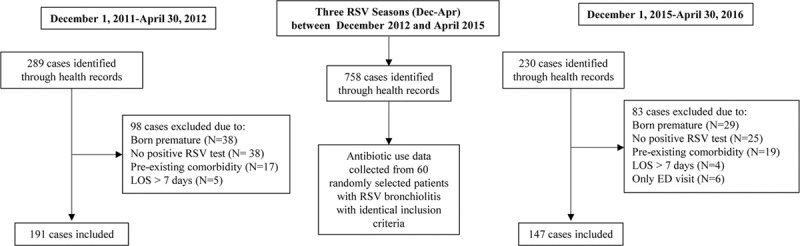
Flow chart of included/excluded cases <2 years of age admitted for bronchiolitis.
Table 2 details patient outcomes, including the proportion of patients receiving antimicrobials, the number of days requiring supplemental oxygen, and the number of days of fever. There was a 15.1% absolute reduction in the proportion of patients who received any antimicrobials [90/191 (47.1%) versus 47/147 (32%), P = 0.01]. Comparison of cohort 1 (pre-ASP) and cohort 5 (post-ASP) demonstrated a decrease in mean DOT per 1,000 patient-days from 414 DOT/1,000 patient-days [95% confidence interval (CI), 319–538] to 323 DOT/1,000 patient-days (95% CI, 237–439) with a ratio of 0.78 (95% CI, 0.52–1.17) and P-value of 0.23. Excluding patients who were ≤28 days old, the mean DOT per 1,000 patient-days were 371 DOT/1,000 patient-days (95% CI, 286–482) in cohort 1 and 201 DOT/1,000 patient-days (95% CI, 144–280) in cohort 5 with a ratio of 0.54 (95% CI, 0.35–0.82) and P-value of 0.004 (Fig. 2).
Table 2.
Patient Outcomes
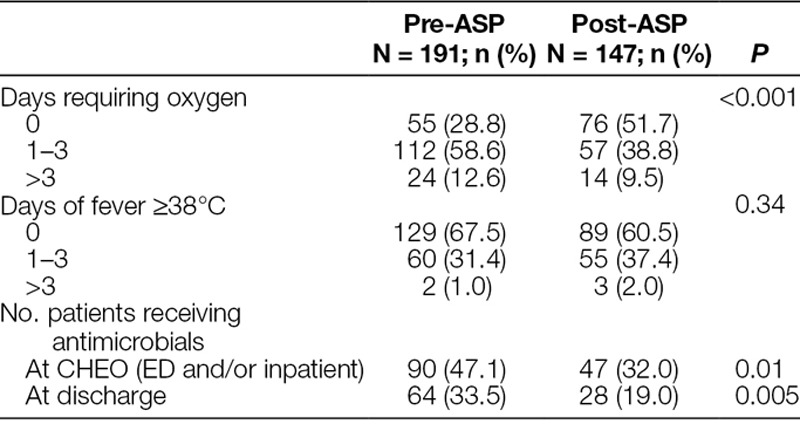
Fig. 2.
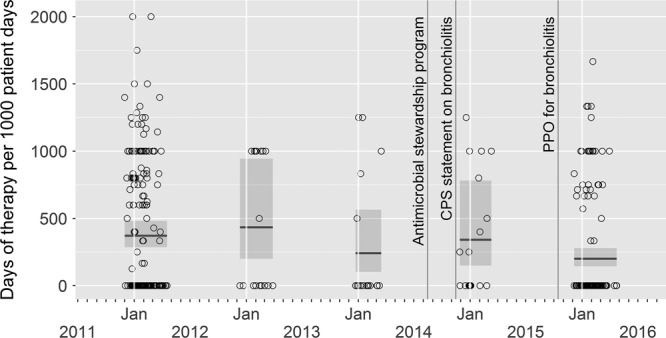
Plot of in-hospital days of therapy per 1,000 patient-days in patients older than 28 days old over 5 RSV seasons (December 1 to April 30 of each year listed). Each data point represents 1 patient’s DOT/1,000 patient-days. The mean DOT/1,000 patient-days with confidence intervals by season is also displayed. The timing of initiation of known interventions that may have contributed to antimicrobial use is indicated. Jan, January.
Seven patients in the pre-ASP cohort and 11 patients in the post-ASP cohort had 2 rationales documented, while the remainder of the patients only had 1 (rationales pre-ASP n = 97; rationales post-ASP n = 58). The top 3 diagnoses used as rationales for antimicrobial use were pneumonia [51/97 (52.6%) pre-ASP versus 16/58 (27.6%) post-ASP], Acute otitis media (AOM) [22/97 (22.7%) pre versus 17/58 (29.3%) post], and “rule out sepsis” [10/97 (10.3%) pre versus 17/58 (29.3%) post]. Of the patients who had an infiltrate reported on chest X-ray (53 patients pre-ASP, 20 patients post-ASP), 46 (86.8%) patients in the pre-ASP cohort and 14 (70%) in the post-ASP cohort patients received antimicrobials. Of the patients who had a chest X-ray performed but did not have an infiltrate reported (65 pre-ASP, 42 post-ASP), 26 patients (40%) in the pre-ASP group still received antimicrobials [8 (30.8%) for pneumonia, 10 (38.5%) for AOM, 8 (30.8%) other] versus 13 (31%) patients in the post-ASP group [2 (15.4%) for pneumonia, 5 (38.5%) for AOM, 6 (46.1%) for other]. Of the total 338 patients, 9 patients (6 pre-ASP, 3 post-ASP; 2.7%) were treated for UTI, but only 5 (1.5%) met diagnostic criteria for UTI. Only 6 (2%) patients had identified viral coinfections. Three positive blood cultures were documented, but all 3 were considered contaminants.
The top 5 most commonly used antibiotics in both pre-ASP and post-ASP cohorts in order of most used to least were: amoxicillin, ampicillin, ceftriaxone/cefotaxime, cefuroxime, and tobramycin/gentamicin. There was a significant decrease in the use of cefuroxime (26.7% versus 0%, P < 0.001) as well as a significant increase in the use of tobramycin/gentamicin (0% versus 31.9%, P < 0.001) between the pre-ASP and post-ASP periods. In the pre-ASP period, 15 patients (7.9%) were given ceftriaxone/cefotaxime compared with 5(3.4%) in the post-ASP period (P = 0.41). Very small numbers of patients were given amoxicillin-clavulanate (1 pre, 3 post), azithromycin (1 pre, 1 post), cefazolin (1 pre, 0 post), clarithromycin (1 pre, 2 post), erythromycin (1 pre, 1 post), penicillin G (1 pre, 0 post), Trimethoprim-sulfamethoxazole (TMP-SMX) (1 pre, 1 post), or vancomycin (2 pre, 0 post). No patients received cefixime, cephalexin, ciprofloxacin, clindamycin, cloxacillin, meropenem, metronidazole, penicillin V, or piperacillin-tazobactam in either cohort.
DISCUSSION
The present study examines the use of antibiotics in patients hospitalized due to RSV bronchiolitis. A previous study done at the same institution in 1999 noted that 60.5% of patients received at least 1 antibiotic during their hospitalization.9 In the present study, before the institution of ASP, 47.1% of patients received antibiotics. This practice decreased to 32% after almost 2 years of a prospective audit and feedback stewardship program. Using the entire age-range of patients, we obtained a 22% decrease in mean DOT per 1,000 patient-days between the pre- and post-ASP periods, which was not significantly different. However, in the >28 days old age group, there was a nearly one-half reduction in mean DOT per 1,000 patient-days (ratio 0.54, 95% CI, 0.35–0.82, P = 0.004). This difference in antimicrobial prescribing between the 2 age groups (≤28 days old versus >28 days old) is likely due to the usual practice of empirically giving ampicillin and tobramycin in any febrile neonate out of concern for possible sepsis.
The reduction in DOT per 1,000 patient-days in the >28 days old age group is greater than the 30% decrease in antimicrobial use for a similar population of children (excluded children <28 days old) and follow-up time where investigators used both educational initiatives aimed at both the ED and ward staff in addition to stewardship rounds in the hospital.10 This reduction in antimicrobial use appears to be on the higher end when comparing overall reduction seen inpatient pediatric settings (6%–38%) following the implementation of stewardship programs.11–13 AAP and CPS guidelines on bronchiolitis were published in 2014 and may have contributed to the willingness to discontinue antimicrobials, but literature is conflicted as to whether guidelines can significantly reduce use to the extent that we observed in our study.5,14,15 In this study, there was a more modest reduction (15%) in the proportion of patients who were started on antimicrobials compared with the overall days of therapy (46%), suggesting that ward ASP has a greater impact on shortening duration or discontinuing of antimicrobials in patients >28 days of age. The proportion of patients receiving antimicrobial prescriptions at discharge also decreased from 33.5% to 19%. Indeed, this is consistent with the trend of decreasing outpatient antimicrobial prescribing rates for children over the last 20 years.16 The decrease in use in inpatients with RSV appears to be safe as the LOS was reduced, and no patients returned to the hospital within a week although they could have been seen elsewhere as outpatients and not been captured in this study.
The ASP also likely contributed to the decreased spectrum of antimicrobials prescribed, as the use of cefuroxime decreased from 26.7% to 0% and third-generation cephalosporins from 7.9% to 3.4%. This reduction in cefuroxime use was appropriate, given the widespread vaccination of infants against Haemophilus influenzae. The increased use of “rule out sepsis” and acute otitis media as the rationale for antibiotics in the post-ASP period may explain the increased use of aminoglycosides (0% to 31.9%). This rationale is concerning given that the rate of serious bacterial infection in patients with RSV bronchiolitis has repeatedly been shown to be very low.4 The uncertainty of diagnosis or safety concerns may have prompted these actions.17
Other than the initiation of ASP rounds and the publication of guidelines, other factors could have contributed to decreased antimicrobial use and decreased LOS in the post-ASP period. First, there was a decrease in the number of patients who presented to the ED already on antimicrobials in the first cohort compared with the last. This finding may reflect an overall practice change in the wider medical community between the 2 study periods and could account for some of the decrease in antimicrobial use. Next, there was a new preprinted order set (PPO) for clinical bronchiolitis introduced in October 2015 throughout the hospital (ED and inpatient). This “hard copy” PPO did not include orders for antimicrobials as this was left to the discretion of the admitting physician. However, the order set did promote lower oxygen saturation targets by nursing staff and may have accounted for the shorter LOS overall. Prior studies of hospital-specific order sets have demonstrated that PPOs have only been modestly successful in decreasing antimicrobial use.6,18
Both the AAP and CPS guidelines do not recommend routine chest X-rays, which likely contributed to the ordering of significantly fewer X-rays in the post-ASP period compared with the pre-ASP period. This recommendation would have resulted in fewer reports of “infiltrates” and thus contributed to lower antimicrobial use for “pneumonia.” Lastly, in this institution, there was a change in viral testing methodology (antigen detection to molecular testing in 2013), but the time to test reporting did not change. Moreover, rapid viral testing has been shown to not reduce antibiotic use in pediatric patients with acute febrile respiratory illness.19
The study was limited by being a retrospective chart review done at a single pediatric hospital comparing all eligible patients in 2 viral seasons and use of a random sample for the 3 intervening seasons. A complete time series analysis would have been optimal as the small number of cases in the intervening seasons resulted in wide confidence intervals seen in Figure 2.20
Patients who are hospitalized with RSV represent a distinct subset of all children with RSV. The prevalence of secondary pneumonia in this subset is likely higher; therefore, the goal of eliminating all antimicrobial use in this subset of hospitalized patients is not realistic. However, this study does add to the accumulating literature that ASP interventions along with guidelines and order sets can safely contribute to a reduction in antimicrobial use of 25%–50% and decreased use of broad-spectrum cephalosporins particularly for children between 1 month and 2 years of age hospitalized with RSV. Further research in identifying those who would or would not benefit from antibiotics should be promoted.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online August 30, 2019.
To cite: Kalil J, Bowes J, Reddy D, Barrowman N, Saux NL. Pediatric Inpatient Antimicrobial Stewardship Program Safely Reduces Antibiotic Use in Patients with Bronchiolitis Caused by Respiratory Syncytial Virus: A Retrospective Chart Review. Pediatr Qual Saf 2019;5:e211.
REFERENCES
- 1.Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther. 2016;271–298.. doi:10.1007/s40121-016-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman JN, Rieder MJ, Walton JM; Canadian Paediatric Society, Acute Care Committee, Drug Therapy and Hazardous Substances Committee. Bronchiolitis: recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr Child Health. 2014;19:485–498.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralston SL, Lieberthal AS, Meissner HC, et al. ; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502.. [DOI] [PubMed] [Google Scholar]
- 4.Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165:951–956.. [DOI] [PubMed] [Google Scholar]
- 5.Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1–e7.. [DOI] [PubMed] [Google Scholar]
- 6.Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570–6.e3.. [DOI] [PubMed] [Google Scholar]
- 7.Handy LK, Bryan M, Gerber JS, et al. Variability in antibiotic prescribing for community-acquired pneumonia. Pediatrics. 2017;139(4):e20162331 doi:10.1542/peds.2016–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samson L, Cooke C, Macdonald N. Analysis of antibiotic use and misuse in children hospitalized with RSV infection. Paediatr Child Health. 1999;4(3):195–199.. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2828193&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 10.Quintos-Alagheband ML, Noyola E, Makvana S, et al. Reducing antibiotic use in respiratory syncytial virus-A quality improvement approach to antimicrobial stewardship. Pediatr Qual Saf. 2017;2:e046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersh AL, De Lurgio SA, Thurm C, et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics. 2015;135:33–39.. [DOI] [PubMed] [Google Scholar]
- 12.Kreitmeyr K, von Both U, Pecar A, et al. Pediatric antibiotic stewardship: successful interventions to reduce broad-spectrum antibiotic use on general pediatric wards. Infection. 2017;45:493–504.. [DOI] [PubMed] [Google Scholar]
- 13.Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatric Infect Dis Soc. 2015;4:e127–e135.. [DOI] [PubMed] [Google Scholar]
- 14.Florin TA, Byczkowski T, Ruddy RM, et al. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American academy of pediatrics bronchiolitis guidelines. J Pediatr. 2014;165(4):786–792.e1.. doi:10.1016/j.jpeds.2014.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LW, Robles J, Hudgins A, et al. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131(Suppl 1):S103–S109.. [DOI] [PubMed] [Google Scholar]
- 16.Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133:375–385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665–680.. [DOI] [PubMed] [Google Scholar]
- 18.Akenroye AT, Baskin MN, Samnaliev M, et al. Impact of a bronchiolitis guideline on ED resource use and cost: a segmented time-series analysis. Pediatrics. 2014;133(1):e227–e234.. doi:10.1542/peds.2013-1991 [DOI] [PubMed] [Google Scholar]
- 19.Doan Q, Enarson P, Kissoon N, et al. Rapid viral diagnosis for acute febrile respiratory illness in children in the emergency department (Review). Cochrane Database Syst Rev. 2014;(9):CD006452. www.cochranelibrary.com 10.1002/14651858.CD006452.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355.. [DOI] [PMC free article] [PubMed] [Google Scholar]


